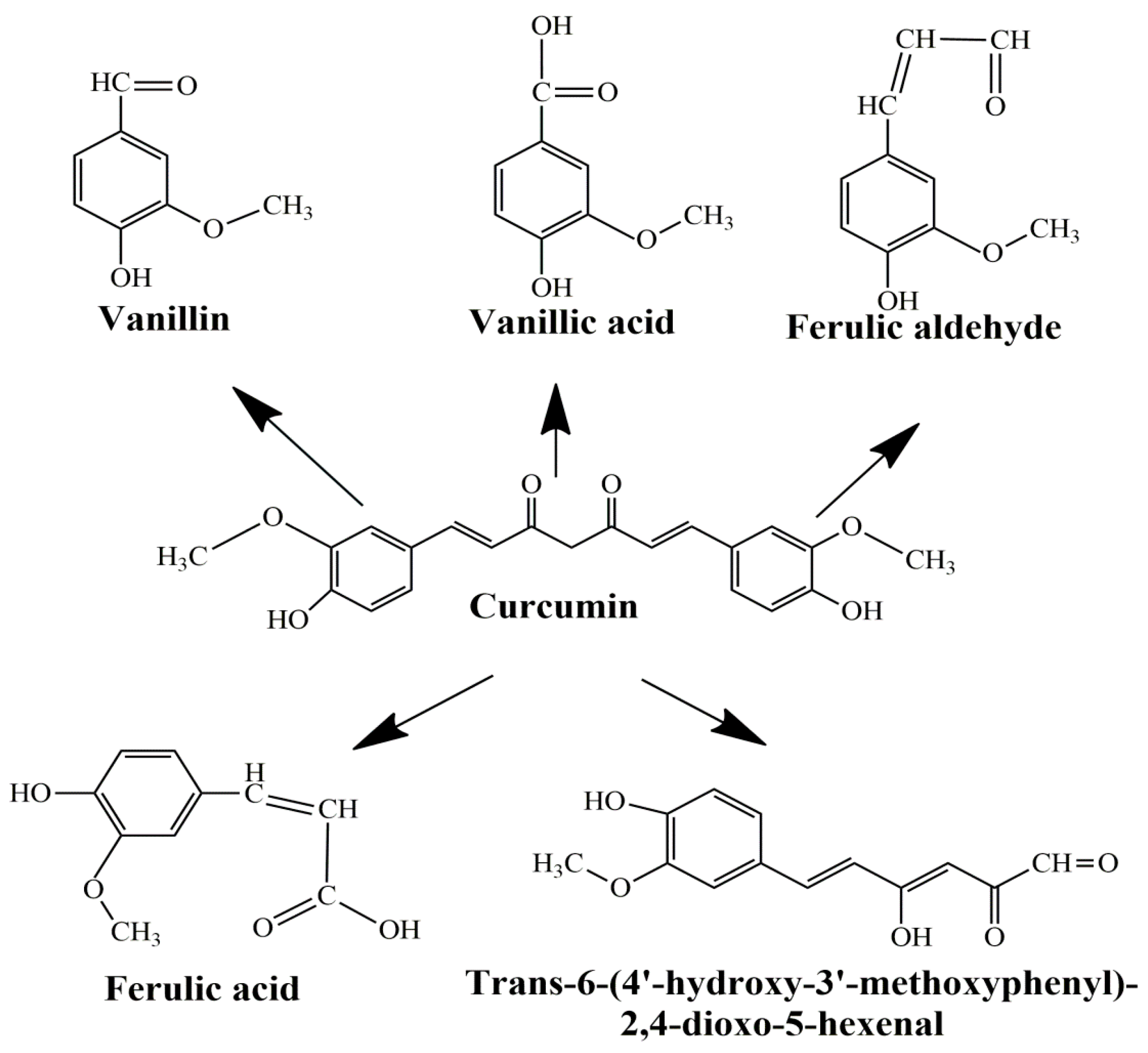
Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin
Abstract
1. Introduction
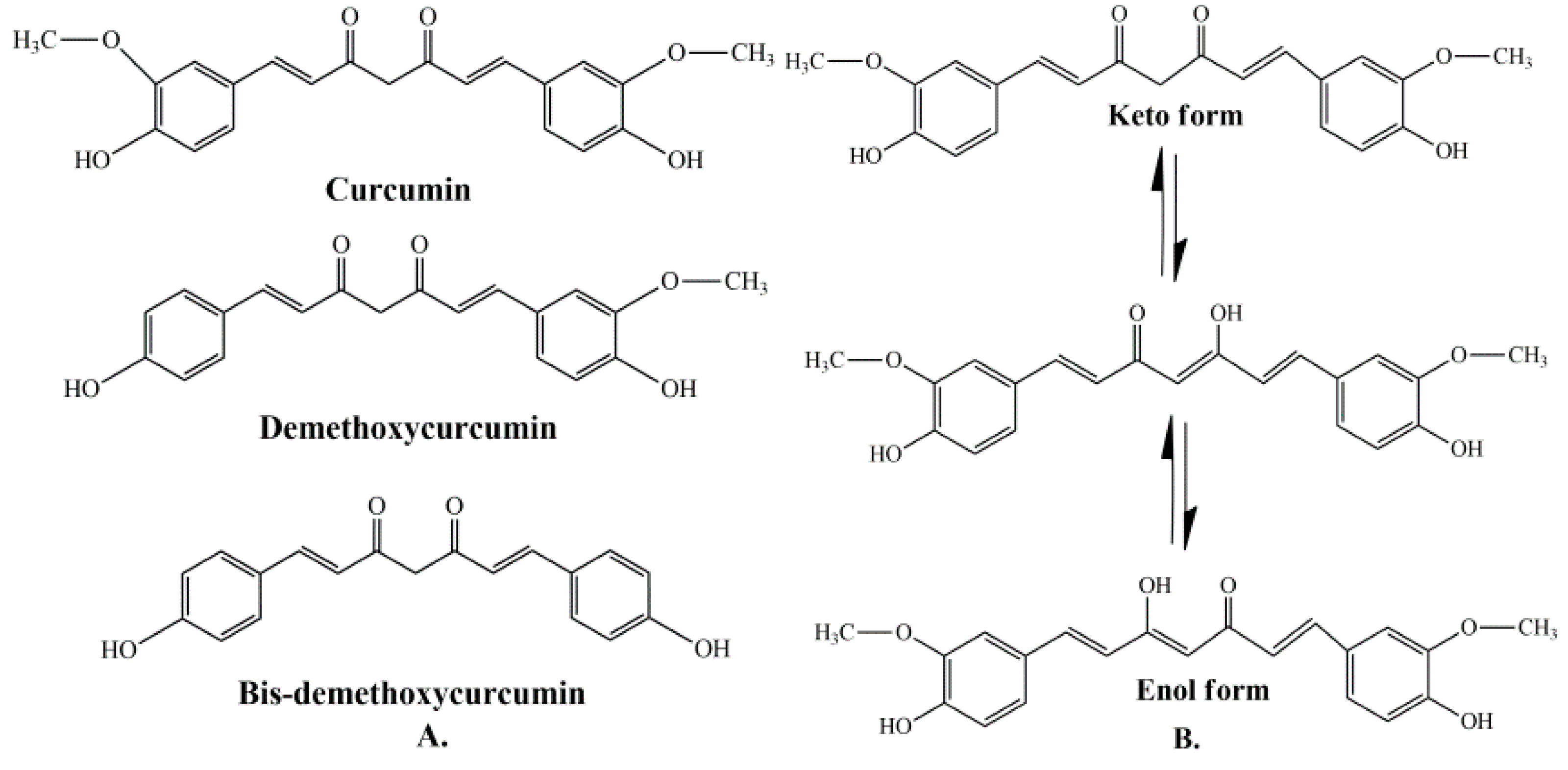
2. Biological Actions of Curcumin
2.1. General Bioactivities and Limitations
2.2. Antibacterial Properties
2.3. Antifungal Effects
2.4. Antiviral Actions
2.5. Antioxidant and Anti-Inflammatory Action of Curcumin
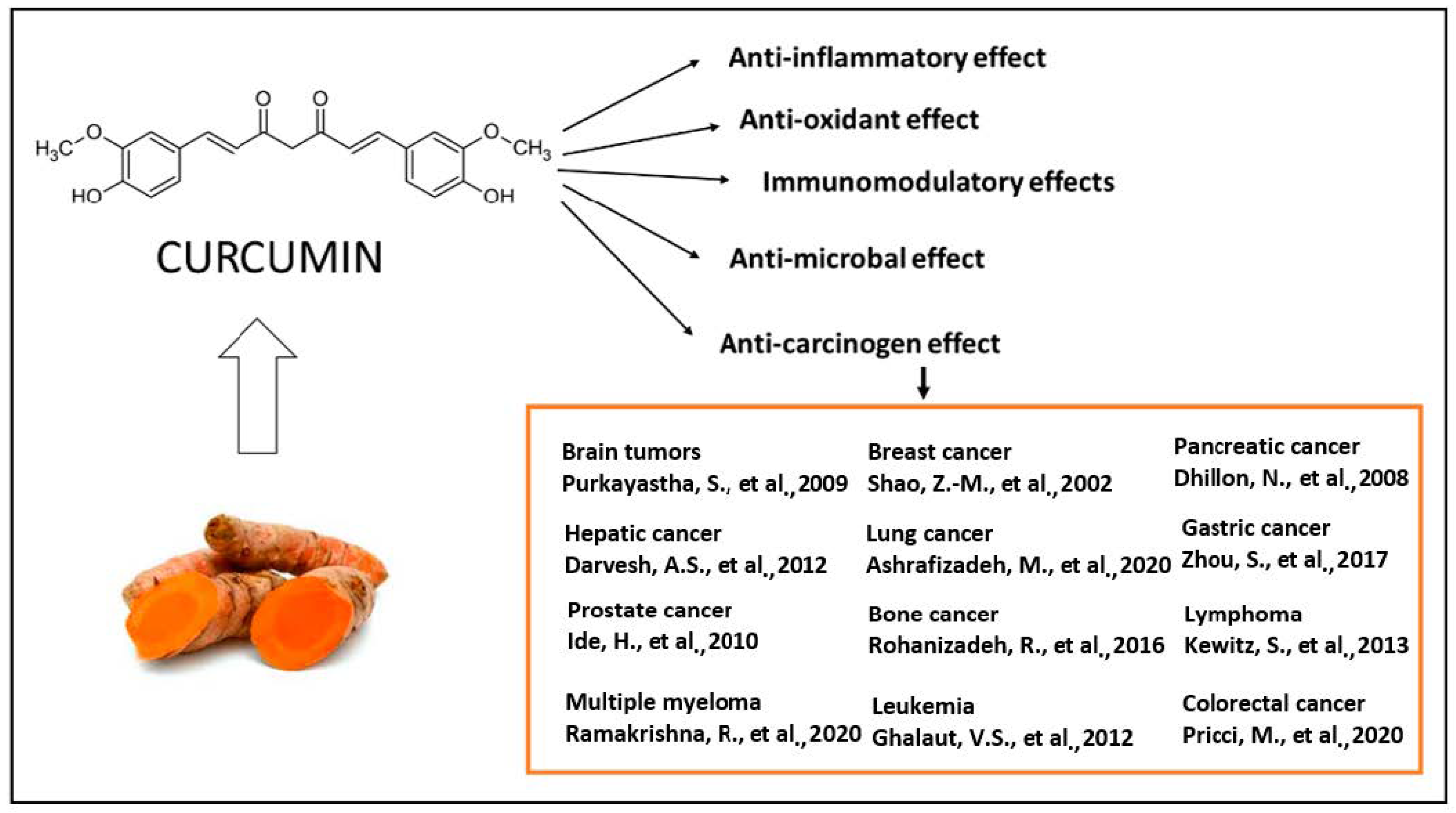
2.6. Antitumoral Effects of Curcumin
3. Curcumin Bioavailability Improving
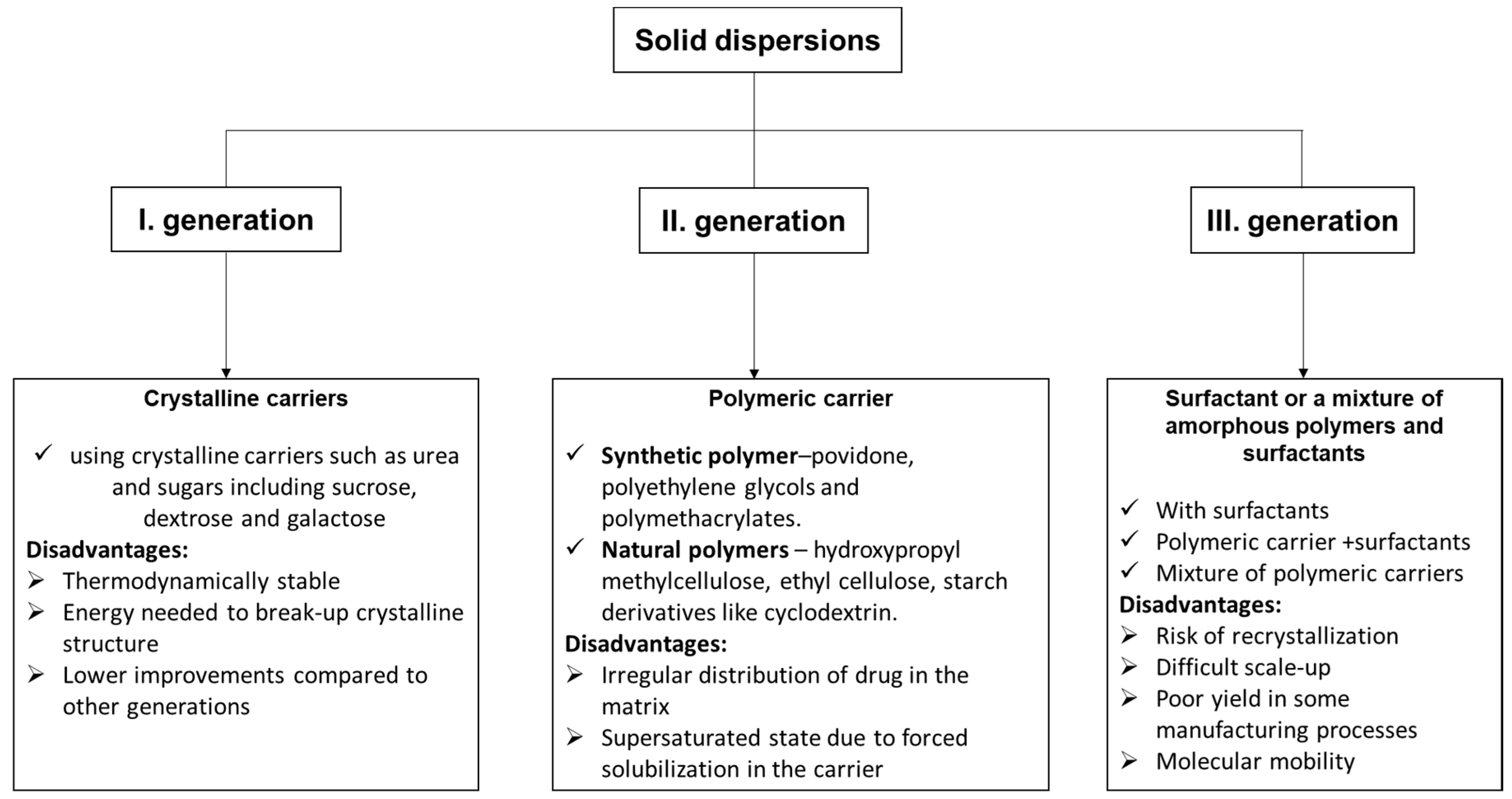
3.1. Curcumin-Based Solid Dispersions
3.2. Curcumin Complexes with Proteins
3.2.1. Using Fluorescence Spectroscopy to Monitor the Interaction between CCM and Proteins
3.2.2. Using Circular Dichroism for Monitoring the Protein’s Secondary Structure
3.2.3. Molecular Docking for Modelling the Binding between Curcumin and Protein
3.2.4. Infrared Spectroscopy
3.3. Combination with Bioactive Molecules
4. Challenges and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dwivedy, A.K.; Singh, V.K.; Das, S.; Chaudhari, A.K.; Upadhyay, N.; Singh, A.; Singh, A.; Dubey, N.K. Biodiversity Bioprospection with Respect to Medicinal Plants. In Ethnopharmacology and Biodiversity of Medicinal Plants, 1st ed.; Patra, J.K., Das, G., Kumar, S., Thatoi, H., Eds.; Apple Academic Press: New York, NY, USA, 2019; pp. 3–30. [Google Scholar]
- Poletto, P.; Alvarez-Rivera, G.; Torres, T.M.S.; Mendiola, J.A.; Ibañez, E.; Cifuentes, A. Compressed fluids and phytochemical profiling tools to obtain and characterize antiviral and anti-inflammatory compounds from natural sources. TrAC Trend. Anal. Chem. 2020, 129, 115942. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- EL-Meghawry EL-Kenawy, A.; Hassan, S.M.A.; Mohamed, A.M.M.; Mohammed, H.M.M. Tumeric or Curcuma longa Linn. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Sanches Silva, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 447–453. [Google Scholar]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Degot, P.; Huber, V.; Touraud, D.; Kunz, W. Curcumin extracts from Curcuma longa–Improvement of concentration, purity, and stability in food-approved and water-soluble surfactant-free microemulsions. Food Chem. 2021, 339, 128140. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Benediktsdottir, B.E.; Baldursson, O.; Gudjonsson, T.; Tonnesen, H.H.; Masson, M. Curcumin, bisdemethoxycurcumin and dimethoxycurcumin complexed with cyclodextrins have structure specific effect on the paracellular integrity of lung epithelia in vitro. Biochem. Biophys. Rep. 2015, 4, 405–410. [Google Scholar] [CrossRef][Green Version]
- Akter, J.; Hossain, A.; Sano, A.; Takara, K.; Islam, Z.; Hou, D.-X. Antifungal activity of various species and strains of turmeric (Curcuma spp.) against Fusarium Solani Sensu Lato. Pharm. Chem. J. 2018, 52, 320–325. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Villaflores, O.B.; Chen, Y.J.; Chen, C.P.; Yeh, J.M.; Wu, T.Y. Effects of curcumin and demethoxycurcumin on amyloid-β precursor and tau proteins through the internal ribosome entry sites: A potential therapeutic for Alzheimer’s disease. Taiwan J. Obstet. Gynecol. 2012, 51, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Alves Zanetti, T.; Biazi, B.I.; Castello Coatti, G.; Baranoski, A.; Areal Marques, L.; Corveloni, A.C.; Mantovani, M.S. Dimethoxycurcumin reduces proliferation and induces apoptosis in renal tumor cells more efficiently than demethoxycurcumin and curcumin. Chem. Biol. Interact. 2021, 338, 109410. [Google Scholar] [CrossRef] [PubMed]
- Hatamipour, M.; Ramezani, M.; Tabassi, S.A.S.; Johnston, T.P.; Sahebkar, A. Demethoxycurcumin: A naturally occurring curcumin analogue for treating non-cancerous diseases. J. Cell. Physiol. 2019, 234, 19320–19330. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Shin, E.M.; Zhou, H.Y.; Jung, J.W.; Kim, Y.S. Comparison of suppressive effects of demethoxycurcumin and bisdemethoxycurcumin on expressions of inflammatory mediators in vitro and in vivo. Arch. Pharm. Res. 2008, 31, 490–496. [Google Scholar] [CrossRef]
- Huang, C.; Lu, H.-F.; Chen, Y.-H.; Chen, J.-C.; Chou, W.-H.; Huang, H.-C. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin induced caspase-dependent and -independent apoptosis via Smad or Akt signaling pathways in HOS cells. BMC Complement. Med. Ther. 2020, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Nantasenamat, C.; Simeon, S.; Hafeez, A.; Prachayasittikul, V.; Worachartcheewan, A.; Songtawee, N.; Srungboonmee, K.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, S.; Prachayasittikul , V. Elucidating the structure-activity relationship of curcumin and its biological activities. In Curcumin: Synthesis, Emerging Role in Pain Management and Health Implications; Pouliquen, D.L., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 49–86. [Google Scholar]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef]
- Rege, S.A.; Arya, M.; Momin, S.A. Structure activity relationship of tautomers of curcumin: A review. Ukr. Food J. 2019, 8, 45–60. [Google Scholar] [CrossRef]
- Gunnam, A.; Nangia, A.K. Solubility improvement of curcumin with amino acids. CrystEngComm 2021, 23, 3398–3410. [Google Scholar] [CrossRef]
- Thadakapally, R.; Aafreen, A.; Aukunuru, J.; Habibuddin, M.; Jogala, S. Preparation and characterization of PEG-albumin-curcumin nanoparticles intended to treat breast cancer. Indian J. Pharm. Sci. 2016, 78, 65–72. [Google Scholar] [PubMed]
- Purkayastha, S.; Berliner, A.; Fernando, S.S.; Ranasinghe, B.; Ray, I.; Tariq, H.; Banerjee, P. Curcumin blocks brain tumor formation. Brain Res. 2009, 1266, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-M.; Shen, Z.-Z.; Liu, C.-H.; Sartippour, M.R.; Go, V.L.; Heber, D.; Nguyen, M. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int. J. Cancer 2002, 98, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Najafi, M.; Makvandi, P.; Zarrabi, A.; Farkhondeh, T.; Samarghandian, S. Versatile role of curcumin and its derivatives in lung cancer therapy. J. Cell Physiol. 2020, 235, 9241–9268. [Google Scholar] [CrossRef] [PubMed]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and colorectal cancer: From basic to clinical evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Ghalaut, V.S.; Sangwan, L.; Dahiya, K.; Ghalaut, P.S.; Dhankhar, R.; Saharan, R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2012, 18, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Kewitz, S.; Volkmer, I.; Staege, M.S. Curcuma contra cancer? Curcumin and Hodgkin’s Lymphoma. Cancer Growth Metastasis 2013, 6, 35–52. [Google Scholar] [CrossRef]
- Rohanizadeh, R.; Deng, Y.; Verron, E. Therapeutic actions of curcumin in bone disorders. BoneKEy Rep. 2016, 5, 793. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Diamond, T.H.; Alexander, W.; Manoharan, A.; Golombick, T. Use of curcumin in Multiple Myeloma patients intolerant of steroid therapy. Clin. Case Rep. 2020, 8, 739–744. [Google Scholar] [CrossRef]
- Zhou, S.; Yao, D.; Guo, L.; Teng, L. Curcumin suppresses gastric cancer by inhibiting gastrin-mediated acid secretion. FEBS Open Bio 2017, 7, 1078–1084. [Google Scholar] [CrossRef]
- Darvesh, A.S.; Aggarwal, B.B.; Bishayee, A. Curcumin and liver cancer: A review. Curr. Pharm. Biotechnol. 2012, 13, 218–228. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In Advances in Experimental Medicine and Biology; Aggarwal, B.B., Surh, Y.J., Shishodia, S., Eds.; The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Greer, SC, USA, 2007; Volume 595, pp. 105–125. [Google Scholar] [CrossRef]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ahmadabady, S.; Beheshti, F.; Shahidpour, F.; Khordad, E.; Hosseini, M. A protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem. Biophys. Rep. 2021, 25, 100908. [Google Scholar] [CrossRef] [PubMed]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of curcumin using coconut milk whey and gum arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Bhattacharjee, P. Promising role of curcumin against viral diseases emphasizing COVID-19 management: A review on the mechanistic insights with reference to host-pathogen interaction and immunomodulation. J. Funct. Foods 2021, 82, 104503. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Parks, R.J. Curcumin as an antiviral agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory effects of curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Hajighasemi, S.; Kiaie, N.; Rosano, G.M.C.; Sathyapalan, T.; Al-Rasadi, K.; Sahebkar, A. Anti-fibrotic effects of curcumin and some of its analogues in the heart. Heart Fail. Rev. 2020, 25, 731–743. [Google Scholar] [CrossRef]
- Nemmar, A.; Subramaniyan, D.; Ali, B.H. Protective effect of curcumin on pulmonary and cardiovascular effects induced by repeated exposure to diesel exhaust particles in mice. PLoS ONE 2012, 7, e39554. [Google Scholar] [CrossRef]
- Dourado, D.; Freire, D.T.; Pereira, D.T.; Amaral-Machado, L.; Alencar, E.E.N.; Branco de Barros, A.L.; Egito, E.S.T. Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials? Biomed. Pharmacother. 2021, 139, 111578. [Google Scholar] [CrossRef]
- Manoharan, Y.; Haridas, V.; Vasanthakumar, K.C.; Muthu, S.; Thavoorullah, F.F.; Shetty, P. Curcumin: A wonder drug as a preventive measure for COVID19 management. Indian J. Clin. Biochem. 2020, 35, 373–375. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Jankun, J.; Wyganowska-Swiatkowska, M.; Dettlaff, K.; Jelinska, A.; Surdacka, A.; Watrobska-Swietlikowska, D.; Skrzypczak-Jankun, E. Determining whether curcumin degradation/condensation is actually bioactivation (Review). Int. J. Mol. Med. 2016, 37, 1151–1158. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-L.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Paciello, F.; Fetoni, A.R.; Mezzogori, D.; Rolesi, R.; Di Pino, A.; Paludetti, G.; Grassi, C.; Troiani, D. The dual role of curcumin and ferulic acid in counteracting chemoresistance and cisplatin-induced ototoxicity. Sci. Rep. 2020, 10, 1063. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Soares, A.K.; de Sousa, D.P. Overview of the role of vanillin on redox status and cancer development. Oxid. Med. Cell. Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef]
- Wright, L.E.; Frye, J.B.; Gorti, B.; Timmermann, B.N.; Funk, J.L. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr. Pharm. Des. 2013, 19, 6218–6225. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Gurudutt, K.N.; Srinivasan, K. Degradation of bioactive spice compound: Curcumin during domestic cooking. Eur. Food Res. Technol. 2008, 228, 807–812. [Google Scholar] [CrossRef]
- Appiah-Opong, R.; Commandeur, J.N.M.; van Vugt-Lussenburg, B.; Vermeulen, N.P.E. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology 2007, 235, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Niamsa, N.; Sittiwet, C. Antimicrobial activty of Curcuma longa aqueous extract. Br. J. Pharmacol. Toxicol. 2009, 4, 173–177. [Google Scholar] [CrossRef]
- Lawhavinit, O.A.; Kongkathip, N.; Kongkathip, B. Antimicrobial activity of curcuminoids from Curcuma longa L. on pathogenic bacteria of shrimp and chicken. Kasetsart J.—Nat. Sci. 2010, 44, 364–371. [Google Scholar]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef]
- Shariati, A.; Asadian, E.; Fallah, F.; Azimi, T.; Hashemi, A.; Yasbolaghi Sharahi, J.; Taati Moghadam, M. Evaluation of nano-curcumin effects on expression levels of virulence genes and biofilm production of multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infection in Tehran, Iran. Infect. Drug. Resist. 2019, 12, 2223–2235. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Marathe, S.A.; Kumar, R.; Ajitkumar, P.; Nagaraja, V.; Chakravortty, D. Curcumin reduces the antimicrobial activity of ciprofloxacin against Salmonella Typhimurium and Salmonella Typhi. J. Antimicrob. Chemother. 2013, 68, 139–152. [Google Scholar] [CrossRef]
- Kim, M.-K.; Choi, G.-J.; Lee, H.-S. Fungicidal property of Curcuma longa L. rhizome-derived curcumin against phytopathogenic fungi in a greenhouse. J. Agric. Food Chem. 2003, 51, 1578–1581. [Google Scholar] [CrossRef]
- Sharma, M.; Manoharlal, R.; Negi, A.S.; Prasad, R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 2010, 10, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Manoharlal, R.; Puri, N.; Prasad, R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 2010, 30, 391–404. [Google Scholar] [CrossRef]
- Sharma, R.K.; Cwiklinski, K.; Aalinkeel, R.; Reynolds, J.L.; Sykes, D.E.; Quaye, E.; Oh, J.; Mahajan, S.D.; Schwartz, S.A. Immunomodulatory activities of curcumin-stabilized silver nanoparticles: Efficacy as an antiretroviral therapeutic. Immunol. Investig. 2017, 46, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Divya, C.S.; Pillai, M.R. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol. Carcinog. 2006, 45, 320–332. [Google Scholar] [CrossRef]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Antihyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int. J. Endocrinol. Metab. 2014, 12, e18081. [Google Scholar] [CrossRef]
- Mirzabeigia, P.; Mohammadpour, A.H.; Salarifar, M.; Gholami, K.; Mojtahedzadeh, M.; Javadi, M.R. The effect of curcumin on some of traditional and nontraditional cardiovascular risk factors: A pilot randomized, double-blind, placebo-controlled trial. Iran J. Pharm. Res. 2015, 14, 479–486. [Google Scholar]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Chuang, S.-E.; Cheng, A.-L.; Lin, J.-K.; Kuo, M.-L. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem. Toxicol. 2000, 38, 991–995. [Google Scholar] [CrossRef]
- Kazemi-Andalib, F.; Mohammadikish, M.; Divsalar, A.; Sahebi, U. Hollow microcapsule with pH-sensitive chitosan/polymer shell for in vitro delivery of curcumin and gemcitabine. Eur. Polym. J. 2022, 162, 110887. [Google Scholar] [CrossRef]
- Movileanu, C.; Anghelache, M.; Turtoi, M.; Voicu, G.; Neacsu, I.A.; Ficai, D.; Trusca, R.; Oprea, O.; Ficai, A.; Andronescu, E.; et al. Folic acid-decorated PEGylated magnetite nanoparticles as efficient drug carriers to tumor cells overexpressing folic acid receptor. Int. J. Pharm. 2022, 625, 122064. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Chin. Med. 2011, 6, 31. [Google Scholar] [CrossRef]
- Mazzarino, L.; Silva, L.F.C.; Curta, J.C.; Licínio, M.A.; Costa, A.; Pacheco, L.K.; Siqueira, J.M.; Montanari, J.; Romero, E.; Assreuy, J.; et al. Curcumin-loaded lipid and polymeric nanocapsules stabilized by nonionic surfactants: An in vitro and in vivo antitumor activity on B16-F10 melanoma and macrophage uptake comparative study. J. Biomed. Nanotechnol. 2011, 7, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Bhavsar, Z.; Ko, C.-Y.; Koppolu, B.; Cui, W.; Tang, L.; Nguyen, K.T. Multifunctional particles for melanoma-targeted drug delivery. Acta Biomater. 2012, 8, 2996–3004. [Google Scholar] [CrossRef]
- Jain, B. A spectroscopic study on stability of curcumin as a function of pH in silica nanoformulations, liposome and serum protein. J. Mol. Struct. 2017, 1130, 194–198. [Google Scholar] [CrossRef]
- Mehryar, L.; Esmaiili, M.; Zeynali, F.; Imani, M.; Sadeghi, R. Fabrication and characterization of sunflower protein isolate nanoparticles, and their potential for encapsulation and sustainable release of curcumin. Food Chem. 2021, 355, 129572. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Manchanda, S.; Chandra, A.; Ali, J.; Deb, P.K. Overview of different carrier systems for advanced drug delivery. In Drug Delivery Systems. Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 179–233. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Stasilowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-cyclodextrin as an effective carrier of curcumin–piperine nutraceutical system with improved enzyme inhibition properties. J. Enzyme Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef]
- Teixeira, C.C.C.; Mendonca, L.M.; Bergamaschi, M.M.; Queiroz, R.H.; Souza, G.E.; Antunes, L.M.; Freitas, L.A.P. Microparticles containing curcumin solid dispersion: Stability, bioavailability and anti-inflammatory activity. AAPS PharmSciTech 2016, 17, 252–261. [Google Scholar] [CrossRef]
- Shabaninejad, Z.; Pourhanifeh, M.H.; Movahedpour, A.; Mottaghi, R.; Nickdasti, A.; Mortezapour, E.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; Sadeghian, M.; et al. Therapeutic potentials of curcumin in the treatment of glioblstoma. Eur. J. Med. Chem. 2020, 188, 112040. [Google Scholar] [CrossRef]
- Huang, B.B.; Liu, D.X.; Liu, D.K.; Wu, G. Application of solid dispersion technique to improve solubility and sustain release of emamectin benzoate. Molecules 2019, 24, 4315. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Dannenfelser, R.-M.; He, H.; Joshi, Y.; Bateman, S.; Serajuddin, A.T.M. Development of clinical dosage forms for a poorly water soluble drug I: Application of polyethylene glycol-polysorbate 80 solid dispersion carrier system. J. Pharm. Sci. 2004, 93, 1165–1175. [Google Scholar] [CrossRef]
- Tekade, A.R.; Yadav, J.N. A review on solid dispersion and carriers used therein for solubility enhancement of poorly water soluble drugs. Adv. Pharm. Bull. 2020, 10, 359–369. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.B.; Hai, N.T.; Son, P.K. Developing and evaluating in vitro effect of poly (ethylene glycol) conjugated CCM on human cancer cell lines. Curr. Drug Discov. Technol. 2016, 13, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. PEGylation: An approach for drug delivery. A review. Crit. Rev. Ther. Drug Carrier Syst. 2008, 25, 403–447. [Google Scholar] [CrossRef]
- Murphy, C.J.; Tang, H.; Van Kirk, E.A.; Shen, Y.; Murdoch, W.J. Reproductive effects of a pegylated curcumin. Reprod. Toxicol. 2012, 34, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Allawadi, D.; Singh, N.; Singh, S.; Arora, S. Solid dispersions: A review on drug delivery system and solubility enhancement. Int. J. Pharm. Sci. Res. 2013, 4, 2094–2105. [Google Scholar]
- Mittal, A.; Kumar, N.; Chauhan, N.S. Curcumin encapsulated PEGylated nanoliposomes: A potential anti-infective therapeutic agent. Indian J. Microbiol. 2019, 59, 336–343. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yang, C.; Wang, P.; Oelschlager, D.K.; Zheng, Y.; Tian, D.-A.; Grizzle, W.E.; Buchsbaum, D.J.; Wan, M. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol. Pharmacol. 2009, 76, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Friedman, J.M.; Nacharaju, P. Fabrication of biodegradable PEG-PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Castro Frabel do Nascimento, T.; Meza Casa, D.; Facco Dalmolin, L.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Aydoğmuş, Z.; Senkal, F.; Turan, G. Investigation of curcumin water solubility through emulsifying with biocompatible polyethylene glycol–based polymers. Food Anal. Methods 2019, 12, 2129–2138. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Li, M.; Ma, Y.; Ngadi, M.O. Binding of curcumin to β-lactoglobulin and its effect on antioxidant characteristics of curcumin. Food Chem. 2013, 141, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano encapsulated curcumin: And its potential for biomedical applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef] [PubMed]
- Solghi, S.; Emam-Djomeh, Z.; Fathi, M.; Farahani, F. The encapsulation of curcumin by whey protein: Assessment of the stability and bioactivity. J. Food Process Eng. 2020, 43, e13403. [Google Scholar] [CrossRef]
- Camargo, L.E.A.; Brustolin Ludwig, D.; Tominaga, T.T.; Carletto, B.; Favero, G.M.; Mainardes, R.M.; Khalil, N.M. Bovine serum albumin nanoparticles improve the antitumour activity of curcumin in a murine melanoma model. J. Microencapsul. 2018, 35, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Sneharani, A.H. Curcumin-sunflower protein nanoparticles-A potential antiinflammatory agent. J. Food. Byochem. 2019, 43, e12909. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Salami, M.; Moghadam, M.; Amirsalehi, A.; Emam-Djomeh, Z. Mung bean protein as a promising biopolymeric vehicle for loading of curcumin: Structural characterization, antioxidant properties, and in vitro release kinetics. J. Drug Deliv. Sci. Technol. 2021, 61, 102148. [Google Scholar] [CrossRef]
- Chen, F.-P.; Li, B.-S.; Tang, C.-H. Nanocomplexation of soy protein isolate with curcumin: Influence of ultrasonic treatment. Food Res. Int. 2015, 75, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-P.; Liu, L.-L.; Tang, C.-H. Spray-drying microencapsulation of curcumin nanocomplexes with soy protein isolate: Encapsulation, water dispersion, bioaccessibility and bioactivities of curcumin. Food Hydrocoll. 2020, 105, 105821. [Google Scholar] [CrossRef]
- Erickson, P.S.; Kalscheur, K.F. Nutrition and feeding of dairy cattle. In Animal Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 157–180. [Google Scholar] [CrossRef]
- Rahimi Yazdi, S.; Corredig, M. Heating of milk alters the binding of curcumin to casein micelles. A fluorescence spectroscopy study. Food Chem. 2012, 132, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef]
- Jiang, S.; Altaf Hussain, M.; Cheng, J.; Jiang, Z.; Geng, H.; Sun, Y.; Sun, C.; Hou, J. Effect of heat treatment on physicochemical and emulsifying properties of polymerized whey protein concentrate and polymerized whey protein isolate. LWT–Food Sci. Technol. 2018, 98, 134–140. [Google Scholar] [CrossRef]
- Qi, P.X.; Onwulata, C.I. Physical properties, molecular structures, and protein quality of texturized whey protein isolate: Effect of extrusion temperature. J. Agric. Food Chem. 2011, 59, 4668–4675. [Google Scholar] [CrossRef]
- Kevij, T.H.; Salami, M.; Mohammadian, M.; Khodadadi, M. Fabrication and investigation of physicochemical, food simulant release, and antioxidant properties of whey protein isolate-based films activated by loading with curcumin through the pH-driven method. Food Hydrocoll. 2020, 108, 106026. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Nadaud, P.; Krochta, J.M. Water vapor permeability, solubility, and tensile properties of heat-denatured versus native whey protein films. J. Food Sci. 1999, 64, 1034–1037. [Google Scholar] [CrossRef]
- Silva, K.S.; Fonseca, T.M.R.; Amado, L.R.; Mauro, M.A. Physicochemical and microstructural properties of whey protein isolate-based films with addition of pectin. Food Packag. Shelf Life 2018, 16, 122–128. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Chillo, S.; Buonocore, G.G.; Massaro, A.; Conte, A.; Bevilacqua, A.; Del Nobile, M.A. Influence of spelt bran on the physical properties of WPI composite films. J. Food Eng. 2009, 92, 467–473. [Google Scholar] [CrossRef]
- Nourbakhsh, H.; Madadlou, A.; Emam-Djomeh, Z.; Wang, Y.-C.; Gunasekaran, S. One-pot nanoparticulation of potentially bioactive peptides and gallic acid encapsulation. Food Chem. 2016, 210, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Akkermans, C.; Van der Goot, A.J.; Venema, P.; Van der Linden, E.; Boom, R.M. Properties of protein fibrils in whey protein isolate solutions: Microstructure, flow behaviour and gelation. Int. Dairy J. 2008, 18, 1034–1042. [Google Scholar] [CrossRef]
- Ng, S.K.; Nyam, K.L.; Nehdi, I.A.; Chong, G.H.; Lai, O.M.; Tan, C.P. Impact of stirring speed on β-lactoglobulin fibril formation. Food Sci. Biotechnol. 2016, 25, 15–21. [Google Scholar] [CrossRef]
- Araujo Mantovani, R.; de Figueiredo Furtado, G.; Netto, F.M.; Lopes Cunha, R. Assessing the potential of whey protein fibril as emulsifier. J. Food Eng. 2018, 223, 99–108. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Cold-set hydrogels made of whey protein nanofibrils with different divalent cations. Int. J. Biol. Macromol. 2016, 89, 499–506. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of curcumin-loaded emulsion using high pressure homogenization: Impact of oil phase and concentration on physicochemical stability. LWT–Food Sci. Technol. 2017, 84, 34–46. [Google Scholar] [CrossRef]
- McClements, D.J. Delivery by Design (DbD): A Standardized approach to the development of efficacious nanoparticle- and microparticle-based delivery systems. Compr. Rev. Food Sci. Food Saf. 2018, 17, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing curcumin oral bioavailability through nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Colloid Interface Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; McClements, D.J. Formulation of more efficacious delivery systems using colloid science: Enhanced solubility, stability, and bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Woo, M.W.; Selomulya, C. Modification of molecular conformation of spray-dried whey protein microparticles improving digestibility and release characteristics. Food Chem. 2019, 280, 255–261. [Google Scholar] [CrossRef]
- Ye, Q.; Ge, F.; Wang, Y.; Woo, M.W.; Wu, P.; Chen, X.D.; Selomulya, C. On improving bioaccessibility and targeted release of curcumin-whey protein complex microparticles in food. Food Chem. 2021, 346, 128900. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Safarionpour, S.; Diosady, L.L. Curcumin, a potent therapeutic nutraceutical and its enhanced delivery and bioaccessibility by Pickering emulsions. Drug Deliv. Transl. Res. 2022, 12, 124–157. [Google Scholar] [CrossRef]
- Brodkorb, A.; Croguennec, T.; Bouhallab, S.; Kehoe, J.J. Heat-Induced denaturation, aggregation and gelation of whey proteins. In Advanced Dairy Chemistry; McSweeney, P., O’Mahony, J., Eds.; Springer: New York, NY, USA, 2016; pp. 155–178. [Google Scholar]
- Alavi, F.; Momen, S.; Emam-Djomeh, Z.; Salami, M.; Moosavi-Movahedi, A.A. Radical cross-linked whey protein aggregates as building blocks of non-heated cold-set gels. Food Hydrocoll. 2018, 81, 429–441. [Google Scholar] [CrossRef]
- McClements, D.J. Recent progress in hydrogel delivery systems for improving nutraceutical bioavailability. Food Hydrocoll. 2017, 68, 238–245. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral drug delivery for regional targeting in the gastrointestinal tract–influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharm. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jauregi, P. Protective effect of beta-lactoglobulin against heat induced loss of antioxidant activity of resveratrol. Food Chem. 2018, 266, 101–109. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine whey proteins–Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Edwards, P.J.B.; Jameson, G.B. Structure and stability of whey proteins. In Milk Proteins, 2nd ed.; Singh, H., Thompson, A., Boland, M., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 201–242. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey Proteins and its derivatives: Bioactivity, functionality, and current applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Albani, J.R.; Vogelaer, J.; Bretesche, L.; Kmiecik, D. Tryptophan 19 residue is the origin of bovine beta-lactoglobulin fluorescence. J. Pharm. Biomed. Anal. 2014, 91, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Taulier, N.; Chalikian, T.V. Characterization of pH-induced transitions of beta-lactoglobulin: Ultrasonic, densimetric, and spectroscopic studies. J. Mol. Biol. 2001, 314, 873–889. [Google Scholar] [CrossRef]
- Sridharan, R.; Zuber, J.; Connelly, S.M.; Mathew, E.; Dumont, M.E. Fluorescent approaches for understanding interactions of ligands with G protein coupled receptors. Biochim. Biophys. Acta 2014, 1838, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Kevij, H.T.; Mohammadian, M.; Salami, M. Complexation of curcumin with whey protein isolate for enhancing its aqueous solubility through a solvent-free pH-driven approach. J. Food Process. Preserv. 2019, 43, e14227. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.M.; Abdelhameed, A.S. A spectroscopic approach to investigate the molecular interactions between the newly approved irreversible ErbB blocker “Afatinib” and bovine serum albumin. PLoS ONE 2016, 11, e0146297. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H. Nanocomplexation of proteins with curcumin: From interaction to nanoencapsulation (A review). Food Hydrocoll. 2020, 109, 106106. [Google Scholar] [CrossRef]
- Rajabi, M.; Farhadian, S.; Shareghi, B.; Asgharzadeh, S.; Momeni, L. Noncovalent interactions of bovine trypsin with curcumin and effect on stability, structure, and function. Colloids Surf. B Biointerfaces 2019, 183, 110287. [Google Scholar] [CrossRef]
- Sneharani, A.H.; Karakkat, J.V.; Singh, S.A.; Rao, A.G. Interaction of curcumin with beta-lactoglobulin-stability, spectroscopic analysis, and molecular modeling of the complex. J. Agric. Food Chem. 2010, 58, 11130–11139. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Abbastabr, B.; Azizi, M.H.; Nabavi, S.R. Curcumin microparticles produced by electrospraying technique with whey protein isolate and β-cyclodextrin complex. J. Agr. Sci. Technol. 2020, 22, 709–722. [Google Scholar]
- Alavi, F.; Emam-Djomeh, Z.; Yarmand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold gelation of curcumin loaded whey protein aggregates mixed with kcarrageenan: Impact of gel microstructure on the gastrointestinal fate of curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Hu, Y.; He, C.; Jiang, C.; Liao, Y.; Xiong, H.; Zhao, Q. Complexation with whey protein fibrils and chitosan: A potential vehicle for curcumin with improved aqueous dispersion stability and enhanced antioxidant activity. Food Hydrocoll. 2020, 104, 105729. [Google Scholar] [CrossRef]
- Su, J.; Wang, L.; Dong, W.; Wei, J.; Yan, J.; Ren, F.; Yuan, F.; Wang, P. Fabrication and characterization of ultra-high-pressure (uhp)-induced whey protein isolate/k-carrageenan composite emulsion gels for the delivery of curcumin. Front. Nutr. 2022, 9, 839761. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.; El-Sherbiny, I.; Enomoto, T.; Salem, A.; Nagai, E.; Askar, A.; Abady, G.; Abdel-Hamid, M. Improving the functional activities of curcumin using milk proteins as nanocarriers. Foods 2020, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Cuartas, C.; Granda-Restrepo, D.; Sobral, P.J.A.; Castro, W. Determination of mechanical properties of whey protein films during accelerated aging: Application of FTIR profiles and chemometric tools. J. Food Process Eng. 2021, 44, e13477. [Google Scholar] [CrossRef]
- Vieira Piovezana Gomes, J.; Alves de Oliveira, L.; d’Almeida Francisquini, J.; Anunciação, P.C.; Stephani, R.; de Oliveira, L.F.C.; Perrone, I.T.; de Carvalho, A.F.; Mattos Della Lucia, C. Morphological characterization of whey protein concentrate admixture of microencapsulated curcumin by spray drying. J. Food Process Eng. 2021, 45, e15141. [Google Scholar] [CrossRef]
- Takbirgou, H.; Salami, M.; Emam-Djomeh, Z.; Waly, M.I.; Momen, S.; Ghasemi, A.; Moosavi-Movahedi, A.A. A tailored nanostructure design to protect camel casein-curcumin complex against the upper gastrointestinal tract hydrolysis using aggregated whey proteins in order to increase its antioxidant activity. Int. J. Food Prop. 2020, 23, 1874–1885. [Google Scholar] [CrossRef]
- Meena, S.; Prasad, W.; Khamrui, K.; Mandal, S.; Bhat, S. Preparation of spray-dried curcumin microcapsules using a blend of whey protein with maltodextrin and gum arabica and its in-vitro digestibility evaluation. Food Biosci. 2021, 41, 100990. [Google Scholar] [CrossRef]
- Vieira Piovezana Gomes, J.; Alves de Oliveira, L.; Michelin Santana Pereira, S.; Rosignoli da Conceicao, A.; Anunciacao, P.C.; Gomes de Souza, E.C.; Tuler Perrone, I.; da Silva Junqueira, M.; Pinheiro Sant’Ana, H.M.; Mattos Della Lucia, C. Comparison of bioactive compounds and nutrient contents in whey protein concentrate admixture of turmeric extract produced by spray drying and foam mat drying. Food Chem. 2021, 345, 128772. [Google Scholar] [CrossRef]
- Rosignoli da Conceicao, A.; Dias, K.A.; Michelin Santana Pereira, S.; Saraiva, L.C.; Alves Oliveira, L.; Gomes de Souza, E.C.; Vilela Goncalves, R.; Pinto da Matta, S.L.; Natali, A.J.; Stampini, D.; et al. Protective effects of whey protein concentrate admixtured of curcumin on metabolic control, inflammation and oxidative stress in Wistar rats submitted to exhaustive exercise. Br. J. Nutr. 2022, 127, 526–539. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, Q. Insight into Curcumin-Loaded β-Lactoglobulin nanoparticles: Incorporation, particle disintegration, and releasing profiles. J. Agric. Food Chem. 2014, 62, 8837–8847. [Google Scholar] [CrossRef]
- Aditya, N.P.; Yang, H.; Kim, S.; Ko, S. Fabrication of amorphous curcumin nanosuspensions using β-lactoglobulin to enhance solubility, stability, and bioavailability. Colloids Surf. B Biointerfaces 2015, 127, 114–121. [Google Scholar] [CrossRef]
- Lin, D.; Su, J.; Chen, S.; Wei, J.; Zhang, L.; Li, X.; Yuan, F. Formation mechanism of binary complex based on β-lactoglobulin and propylene glycol alginate with different molecular weights: Structural characterization and delivery of curcumin. Front. Nutr. 2022, 9, 965600. [Google Scholar] [CrossRef]
- Yousefi, A.; Ahrari, S.; Panahi, F.; Ghasemi, Y.; Yousefi, R. Binding analysis of the curcumin-based synthetic alpha-glucosidase inhibitors to beta-lactoglobulin as potential vehicle carrier for antidiabetic drugs. J Iran. Chem. Soc. 2022, 19, 489–503. [Google Scholar] [CrossRef]
- Maity, S.; Pal, S.; Sardar, S.; Sepay, N.; Parvej, H.; Chkraborty, J.; Halder, U.C. Multispectroscopic analysis and molecular modeling to investigate the binding of beta lactoglobulin with curcumin derivatives. RCS Adv. 2016, 6, 112175. [Google Scholar] [CrossRef]
- Jahanshahtalab, M.; Kamshad, M.; Rezaei, S.; Beigoli, S.; Rad, A.S.; Mehrzad, J.; Moghadam, S.K.; Mokaberi, P.; Gharebaghi, S.; Saberi, M.R.; et al. New insights into alpha-lactalbumin behavior upon interaction with resveratrol and curcumin by spectroscopic and molecular modeling techniques: Binary and ternary system comparison. J. Iran. Chem. Soc. 2019, 16, 1311–1326. [Google Scholar] [CrossRef]
- Wang, R.; Wen, Q.-H.; Zeng, X.-A.; Lin, J.-W.; Li, J.; Xu, F.-Y. Binding affinity of curcumin to bovine serum albumin enhanced by pulsed electric field pretreatment. Food Chem. 2022, 377, 131945. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Campos de Paula, H.M.; Dias Ferreira, G.M.; Dias Ferreira, G.M.; do Carmo Hespanhol, M.; Mendes da Silva, L.H.; Dos S Pires, A.C. Thermodynamic and kinetic analyses of curcumin and bovine serum albumin binding. Food Chem. 2018, 242, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, W.; Li, Z.; Liu, B.; Hu, Y.; Chen, S.; de Vries, R.; Yuan, Y.; Erazo Quintero, L.E.; Hou, G.; et al. The stability and bioavailability of curcumin loaded α-lactalbumin nanocarriers formulated in functional dairy drink. Food Hydrocoll. 2022, 131, 107807. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in selfassembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Soltaninejad, H.; Ghorani-Azam, A.; Nafsi-Moghadam, R.; Haddadzadegan, N.; Ansari, M.; Saeed-Banadaki, S.H.; Sobhan, M.R.; Mozafari, S.; Zahedi, M. Slow release curcumin-containing soy protein nanoparticles as anticancer agents for osteosarcoma: Synthesis and characterization. Prog. Biomater. 2022, 11, 311–320. [Google Scholar] [CrossRef]
- Jin, B.; Zhou, X.; Zhou, S.; Liu, Y.; Zhen, Z.; Liang, Y.; Chen, S. Nano-encapsulation of curcumin using soy protein hydrolysates–tannic acid complexes regulated by photocatalysis: A study on the storage stability and in vitro release. J. Microencapsul. 2019, 36, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Zhao, C.; Zhang, H.; Chi, Y.; Wang, L.; Zhang, H.; Bai, S.; Zhang, X. Entrapment of curcumin in soy protein isolate using the pH-driven method: Nanoencapsulation and formation mechanism. LWT-Food Sci. Technol. 2022, 153, 112480. [Google Scholar] [CrossRef]
- Tapal, A.; Tiku, P.K. Complexation of curcumin with soy protein isolate and its implications on solubility and stability of curcumin. Food Chem. 2012, 130, 960–965. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, R.; Xu, X.; Du, M.; Zhu, B.; Wu, C. Structural interplay between curcumin and soy protein to improve the water-solubility and stability of curcumin. Int. J. Biol. Macromol. 2021, 193, 1471–1480. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Wang, W.; Dong, K. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Qi, Z.; Zhao, R.; Wang, C.; Zhang, T. Pea protein based nanocarriers for lipophilic polyphenols: Spectroscopic analysis, characterization, chemical stability, antioxidant and molecular docking. Food Res. Int. 2022, 160, 111713. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Salami, M.; Hajikhani, M.; Emam-Djomeh, Z.; Aghakhani, A.; Ghasemi, A. Electrospray production of curcumin-walnut protein nanoparticles. Food Biophys. 2021, 16, 15–26. [Google Scholar] [CrossRef]
- Xu, P.; Qian, Y.; Wang, R.; Chen, Z.; Wang, T. Entrapping curcumin in the hydrophobic reservoir of rice proteins toward stable antioxidant nanoparticles. Food Chem. 2022, 387, 132906. [Google Scholar] [CrossRef] [PubMed]
- Brotons-Canto, A.; Gonzales-Navarro, C.J.; Gil, A.G.; Asin-Prieto, E.; Saiz, M.J.; Llabres, J.M. Zein nanoparticles improve the oral bioavailability of curcumin in wistar rats. Pharmaceutics 2021, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Fereydouni, N.; Movaffagh, J.; Amiri, N.; Darroudi, S.; Gholoobi, A.; Goodarzi, A.; Hashemzadeh, A.; Darroudi, M. Synthesis of nano-fibers containing nano-curcumin in zein corn protein and its physicochemical and biological characteristics. Sci. Rep. 2021, 11, 1902. [Google Scholar] [CrossRef]
- Hasankhan, S.; Tabibiazar, M.; Hosseini, S.M.; Ehsani, A.; Ghorbani, M. Fabrication of curcumin-zein-ethyl cellulose composite nanoparticles using antisolvent co-precipitation method. Int. J. Biol. Macromol. 2020, 163, 1538–1545. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of curcumin in zein/ caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Ren, G.; He, Y.; Liu, C.; Ni, F.; Luo, X.; Shiu, J.; Song, Y.; Li, T.; Huang, M.; Shen, Q.; et al. Encapsulation of curcumin in ZEIN-HTCC complexes: Physicochemical characterization, in vitro sustained release behavior and encapsulation mechanism. LWT–Food Sci. Technol. 2022, 155, 112909. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Ren, F.; Liu, N.; Tong, Y.; Li, Y.; Liu, A.; Wu, L.; Wang, P. Delivery of Curcumin using zein-gum arabic-tannic acid composite particles: Fabrication, characterization, and in vitro release properties. Front. Nutr. 2022, 9, 842850. [Google Scholar] [CrossRef] [PubMed]
- Mokaberi, P.; Babayan-Mashhadi, F.; Zadeh, Z.A.T.; Saberi, M.R.; Chamani, J. Analysis of the interaction behavior between Nano-Curcumin and two human serum proteins: Combining spectroscopy and molecular stimulation to understand protein-protein interaction. J. Biomol. Struct. Dyn. 2021, 39, 3358–3377. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, U.K.; Shah, N.N.; Muley, A.B.; Singhal, R.S. Complexation of curcumin using proteins to enhance aqueous solubility and bioaccessibility: Pea protein vis-à-vis whey protein. J. Food Eng. 2021, 292, 110258. [Google Scholar] [CrossRef]
- Zhao, R.; Qin, X.; Zhong, J. Interaction between Curcumin and β-casein: Multi-spectroscopic and molecular dynamics simulation methods. Molecules 2021, 26, 5092. [Google Scholar] [CrossRef]
- Mohammadi, F.; Bordbar, A.-K.; Divsalar, A.; Mohammadi, K.; Saboury, A.A. Interaction of curcumin and diacetylcurcumin with the lipocalin member beta-lactoglobulin. Protein J. 2009, 28, 117–123. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Probing the binding sites of resveratrol, genistein, and curcumin with milk beta-lactoglobulin. J. Biomol. Struct. Dyn. 2013, 31, 1455–1466. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Zhang, Y.; Du, Z.; Qiu, X.; Kong, L.; Zhang, H. Binding behaviors and structural characteristics of ternary complexes of β-lactoglobulin, curcumin, and fatty acids. RSC Adv. 2017, 7, 45960–45967. [Google Scholar] [CrossRef]
- Mohammadi, F.; Moeeni, M. Study on the interactions of trans-resveratrol and curcumin with bovine α-lactalbumin by spectroscopic analysis and molecular docking. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 358–366. [Google Scholar] [CrossRef]
- Lelis, C.A.; Moreira Nunes, N.; Campos de Paula, H.M.; Coelho, Y.L.; Mendes da Silva, L.H.; dos Santos Pires, A.C. Insights into protein-curcumin interactions: Kinetics and thermodynamics of curcumin and lactoferrin binding. Food Hydrocoll. 2020, 105, 105825. [Google Scholar] [CrossRef]
- Hao, C.; Xu, G.; Wang, T.; Lv, Z.; Zhu, K.; Li, B.; Chen, S.; Sun, R. The mechanism of the interaction between curcumin and bovine serum albumin using fluorescence spectrum. Russ. J. Phys. Chem. 2017, 11, 140–145. [Google Scholar] [CrossRef]
- Sahoo, B.K.; Ghosh, K.S.; Dasgupta, S. Investigating the binding of curcumin derivatives to bovine serum albumin. Biophys. Chem. 2008, 132, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Kanakis, C.D.; Tarantilis, P.; Pollissiou, M.G.; Tajmir-Riahi, H.A. Resveratrol, genistein, and curcumin bind bovine serum albumin. J. Phys. Chem. B 2010, 114, 3348–3354. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, D.; Kapil, C.; Jairajpuri, M.A.; Sen, P. Inter-domain helix h10DOMI-h1DOMII is important in the molecular interaction of bovine serum albumin with curcumin: Spectroscopic and computational analysis. Eur. Biophys. J. 2015, 44, 139–148. [Google Scholar] [CrossRef]
- Montgomery, A.; Adeyeni, T.; San, K.; Heuertz, R.M.; Ezekiel, U.R. Curcumin sensitizes silymarin to exert synergistic anticancer activity in colon cancer cells. J. Cancer 2016, 7, 1250–1257. [Google Scholar] [CrossRef]
- Owatari, M.S.; Fernandez Alves Jesus, G.; Brum, A.; Pereira, S.A.; Breda Lehmann, N.; de Padua Pereira, U.; Martins, M.L.; Pedreira Mourino, J.L. Sylimarin as hepatic protector and immunomodulator in Nile tilapia during Streptococcus agalactiae infection. Fish Shellfish Immunol. 2018, 82, 565–572. [Google Scholar] [CrossRef]
- MacDonald-Ramos, K.; Michan, L.; Martinez-Ibarra, A.; Cerbon, M. Silymarin is an ally against insulin resistance: A review. Ann Hepatol. 2021, 23, 100255. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Que, L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006, 63, 288–294. [Google Scholar] [CrossRef]
- Abdel-Magied, N.; Elkady, A.A. Possible curative role of curcumin and silymarin against nephrotoxicity induced by gamma-rays in rats. Exp. Mol. Pathol. 2019, 111, 104299. [Google Scholar] [CrossRef]
- Rácz, L.; Tomoaia-Cotisel, M.; Rácz, C.-P.; Bulieris, P.; Grosu, I.; Porav, S.; Ciorîță, A.; Filip, X.; Martin, F.; Serban, G.; et al. Curcumin-whey protein solid dispersion system with improved solubility and cancer cell inhibitory effect. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 209–224. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Medina Neto, A.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Racz, C.P.; Pasca, R.-D.; Szabolcs, S.; Kacso, I.; Tomoaia, G.; Mocanu, A.; Tomoaia-Cotisel, M. Inclusion complex of β-cyclodextrin and quercetin. Thermodynamic approach. Rev. Chim. 2011, 62, 992–997. [Google Scholar]
- Racz, C.P.; Borodi, G.; Pop, M.M.; Kacso, I.; Santa, S.; Tomoaia-Cotisel, M. Structure of the inclusion complex of β-cyclodextrin with lipoic acid from laboratory powder diffraction data. Acta Crystallogr. B 2012, B68, 164–170. [Google Scholar] [CrossRef]
- Racz, C.-P.; Santa, S.; Tomoaia-Cotisel, M.; Borodi, G.; Kacso, I.; Pirnau, A.; Bratu, I. Inclusion of α-lipoic acid in β-cyclodextrin. Physical-chemical and structural characterization. J. Incls. Phenom. Macrocycl. Chem. 2013, 76, 193–199. [Google Scholar] [CrossRef]
- Floare, C.G.; Bogdan, M.; Tomoaia-Cotisel, M.; Mocanu, A. 1H NMR spectroscopic characterization of inclusion complex of desferrioxamine Bchelator and β-cyclodextrin. J. Mol. Struct. 2022, 1248, 131477. [Google Scholar] [CrossRef]
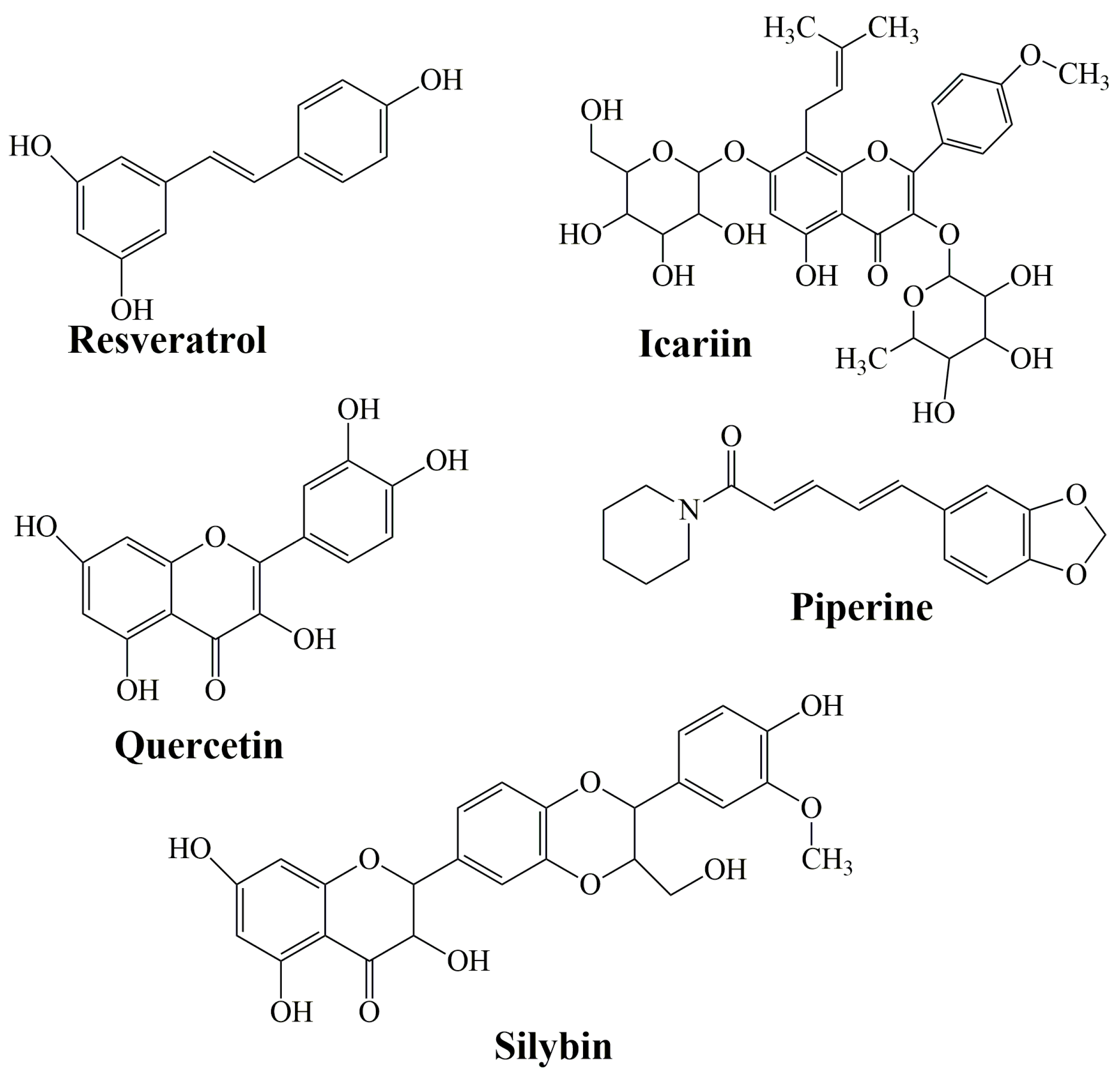
- Lu, Y.-F.; Xu, Y.-Y.; Jin, F.; Wu, Q.; Shi, J.-S.; Liu, J. Icariin is a PPARalpha activator inducing lipid metabolic gene expression in mice. Molecules 2014, 19, 18179–18191. [Google Scholar] [CrossRef]
- Wang, N.; Fu, Q.; Yang, G. Determination of the solubility, dissolution enthalpy and entropy of icariin in water, ethanol, and methanol. Fluid Ph. Equilibria 2012, 324, 41–43. [Google Scholar] [CrossRef]
- Li, D.; Yuan, T.; Zhang, X.; Xiao, Y.; Wang, R.; Fan, Y.; Zhang, X. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Cartil. 2012, 20, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, Z.; Sun, E.; Li, S.; Jia, X. Statistically designed enzymatic hydrolysis of an icariin/β-cyclodextrin inclusion complex optimized for production of icaritin. Acta Pharm. Sin. B. 2012, 2, 83–89. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: From dietary sources to human microRNA modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Rotunno, M.; Lubin, J.H.; Wacholder, S.; Consonni, D.; Pesatori, A.C.; Bertazzi, P.A.; Chanock, S.J.; Burdette, L.; Goldstein, A.M.; et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis 2010, 31, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Lin, M.-T.; Zhou, M.-J.; Yi, T.; Tang, Y.-N.; Tang, S.-L.; Yang, Z.-J.; Zhao, Z.Z.; Chen, H.B. Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 cells in vitro. Molecules 2015, 20, 11524–11534. [Google Scholar] [CrossRef] [PubMed]
- Altundag, E.M.; Yilmaz, A.M.; Serdar, B.S.; Jannuzzi, A.T.; Kocturk, S.; Yalcin, A.S. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) Cells: II. Signal transduction pathways involved. Nutr. Cancer 2021, 73, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Lund, K.C.; Pantuso, T. Combination effects of quercetin, resveratrol and curcumin on in vitro intestinal absorption. J. Restor. Med. 2014, 3, 112–120. [Google Scholar] [CrossRef]
- Shindikar, A.; Singh, A.; Nobre, M.; Kirolikar, S. Curcumin and resveratrol as promising natural remedies with nanomedicine approach for the effective treatment of triple negative breast cancer. J. Oncol. 2016, 2016, 9750785. [Google Scholar] [CrossRef]
- Mazzanti, G.; Di Giacomo, S. Curcumin and resveratrol in the management of cognitive disorders: What is the clinical evidence? Molecules 2016, 21, 1243. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Di Stefano, E.; Fantini, M.; Mattera, R.; Benvenuto, M.; Marzocchella, L.; Sacchetti, P.; Focaccetti, C.; Bernardini, R.; Tresoldi, I.; et al. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget 2014, 5, 10745–10762. [Google Scholar] [CrossRef]
- Du, Q.; Hu, B.; An, H.-M.; Shen, K.-P.; Xu, L.; Deng, S.; Wei, M.-M. Synergistic anticancer effects of curcumin and resveratrol in Hepa1-6 hepatocellular carcinoma cells. Oncol. Rep. 2013, 29, 1851–1858. [Google Scholar] [CrossRef]
- Setyaningsih, D.; Santoso, Y.A.; Hartini, Y.S.; Murti, Y.B.; Hinrichs, W.L.J.; Patramurti, C. Isocratic high-performance liquid chromatography (HPLC) for simultaneous quantification of curcumin and piperine in a microparticle formulation containing Curcuma longa and Piper nigrum. Heliyon 2021, 7, e06541. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, Y.; Ustundas, M.; Bayraktar, O.; Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J. 2018, 26, 323–334. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Piperine and its role in chronic diseases. In Advances in Experimental Medicine and Biology; Gupta, S., Prasad, S., Aggarwal, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 928, pp. 173–183. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Dudhatra, G.B.; Mody, S.K.; Awale, M.M.; Patel, H.B.; Modi, C.M.; Kumar, A.; Kamani, D.R.; Chauhan, B.N. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Sci. World J. 2012, 2012, 637953. [Google Scholar] [CrossRef]
- Racz, L.Z.; Paltinean, G.-A.; Petean, I.; Tomoaia, G.; Pop, L.C.; Arghir, G.; Levei, E.; Mocanu, A.; Racz, C.-P.; Tomoaia-Cotisel, M. Curcumin and whey protein binding and structural characteristics of their complex evidenced by atomic force microscopy. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 61–74. [Google Scholar] [CrossRef]
- Racz, L.Z.; Racz, C.-P.; Horovitz, O.; Tomoaia, G.; Mocanu, A.; Kacso, I.; Sarkozi, M.; Dan, M.; Porav, S.; Borodi, G.; et al. Complexation of curcumin using whey proteins to enhance aqueous solubility, stability and antioxidant property. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 75–99. [Google Scholar] [CrossRef]
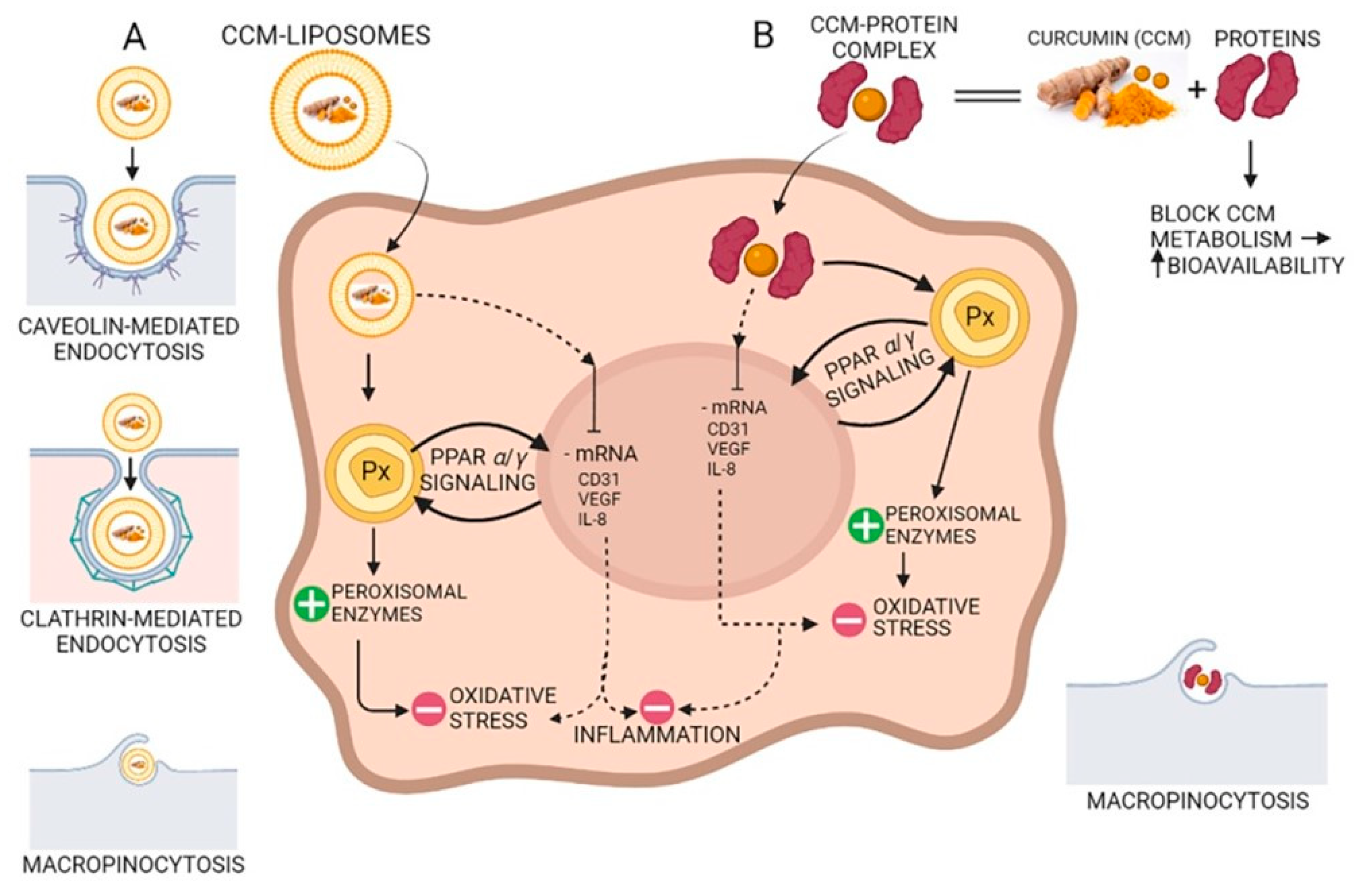
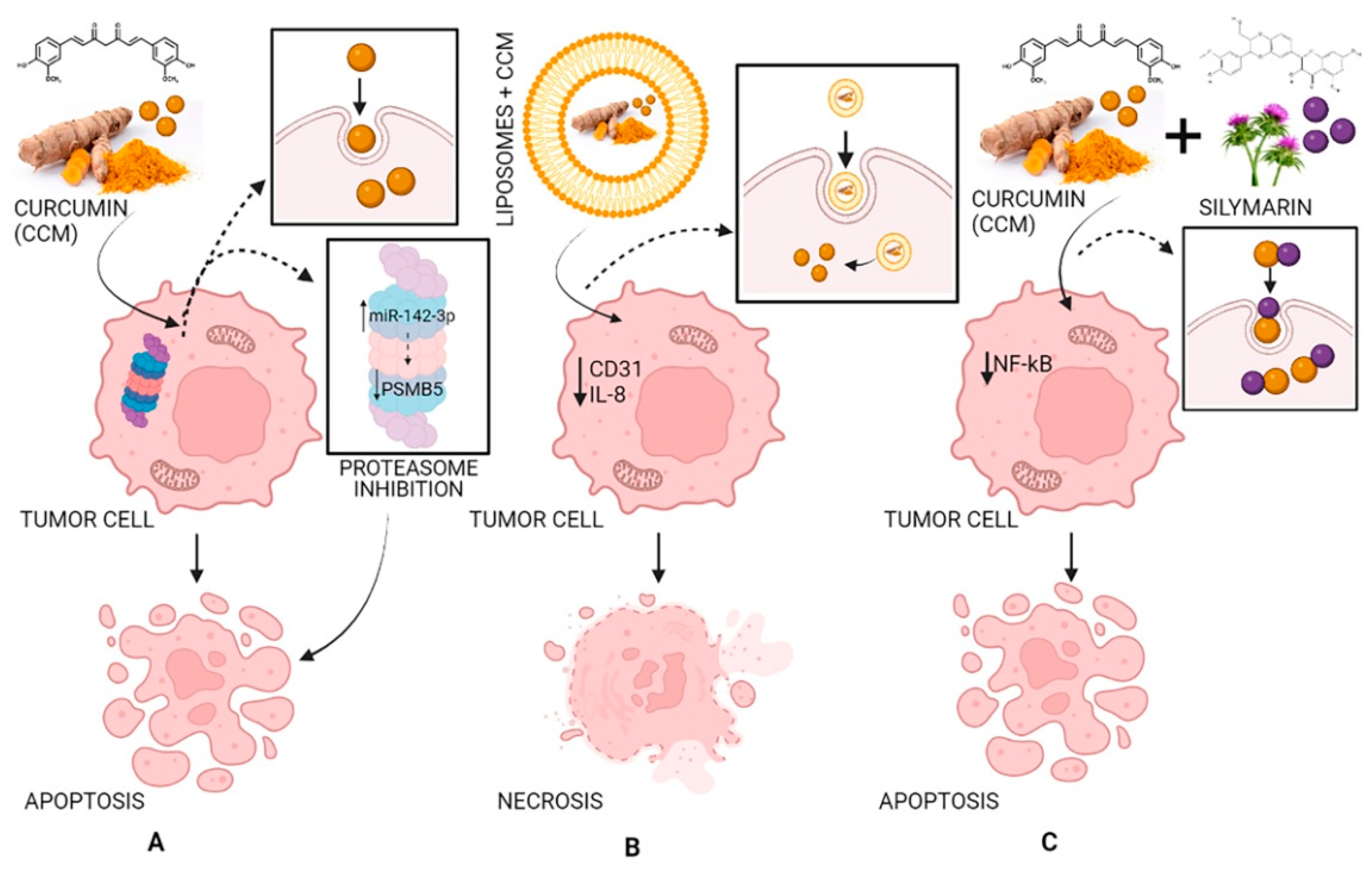
| Complex of CCM and Various Proteins | Experimental Condition | Equation to Describe the Interaction between CCM and Different Type of Proteins | Thermodynamic Parameters | Ref |
|---|---|---|---|---|
| Curcumin and β-lactoglobulin CCM-β-Lg complex | Room temperature pH 7.0 | KS: binding constant n: binding number [CCM]: curcumin concentration F0: fluorescence intensity of protein in the absence of quencher (CCM) F: fluorescence intensity of protein in the presence of quencher | KS: 1.33 × 105 M−1 n = 1.10 : −28.7 kJ/mol | [101] |
| Curcumin and bovine serum albumin complex CCM-BSA complex | 298 K pH 7.4 | Ka: binding constant [Qt]: concentration of curcumin [Pt]: concentration of BSA | Ka: (0.724 ± 0.01) × 105 L/mol n = 1.110 ± 0.038 : −23.05 ± 0.02 kJ/mol | [171] |
| Curcumin and bovine serum albumin CCM-BSA complex | 25 °C pH 7.0 | Kb: binding constant | Kb: 1.95 × 105 L·mol−1 n = 1.11 : −30.19 kJ·mol−1 | [172] |
| Curcumin and soy protein CCM-SP complex | Room temperature pH 12.0 | KSV: Stern-Volmer constant [Q]: molar concentration of curcumin | KSV: 6.3 × 104 M−1 : −26.90 kJ⋅mol−1 | [180] |
| Curcumin and pea protein isolate CCM-PPI complex | 298 K pH 7.0 | Ka: binding constant [CUR]: concentration of curcumin | Ka: 4.74 × 104 L/M n = 0.97 : −26.67 kJ·M−1 | [182] |
| Curcumin and rice protein CCM-RP complex | 298 K pH 12.0 | KSV: Stern–Volmer constant [Q]: molar concentration of curcumin | KSV: 2.17 × 105 M−1 : −30.3 kJ/mol | [184] |
| Curcumin and human serum albumin CCM-HSA complex | 298 K pH 7.4 | Kb: binding constant [Q]: quencher concentration n: binding number | Kb: (1.73 ± 0.32) × 107 M−1 : −41.29 kJ·mol−1 | [191] |
| Curcumin and holo-transferrin CCM-HTR complex | Kb: (1.81 ± 0.32) × 106 M−1 : −36.46 kJ·mol−1 | |||
| Curcumin and human serum albumin and holo-transferrin CCM-HSA-HTR complex | Kb: (3.19 ± 0.32) × 106 M−1 : −37.98 kJ·mol−1 | |||
| Curcumin and whey protein isolate CCM-WPI complex | 308 K pH 7.0 | Ka: binding constant | Ka: 1.08 × 106 L·mol−1 n = 1.30 : −37.08 kJ·mol−1 | [192] |
| Curcumin and pea protein isolate CCM-PPI complex | Ka: 7.68 × 106 L·mol−1 n = 1.44 : −41.00 kJ·mol−1 | |||
| Curcumin and β-casein CCM-β-casein complex | 298 K pH 7.4 | Kb: binding constant | Kb: (3.98 ± 0.26) × 104 L·mol−1 n = 1.59 ± 0.14 : −26.2 ± 0.09 kJ·mol−1 | [193] |
| Curcumin and β-lactoglobulin CCM-β-Lg complex | 25 °C pH 7.4 | Kd: dissociation constant; the reciprocal of Kd is Ka Ka: binding constant; [Q]a is the concentration of added CCM : fluorescence of the fully complexed protein | Ka: 1.19 × 104 M−1 : −23.2 kJ·mol−1 | [196] |
| Curcumin and bovine lactoferrin CCM-β-Lacto complex | 299.15 K pH 7.4 | Kb: binding constant | Kb: (3.01 ± 0.16) × 104 M−1 n = 1.03 ± 0.16 : −25.65 kJ·mol−1 | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racz, L.Z.; Racz, C.P.; Pop, L.-C.; Tomoaia, G.; Mocanu, A.; Barbu, I.; Sárközi, M.; Roman, I.; Avram, A.; Tomoaia-Cotisel, M.; et al. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules 2022, 27, 6854. https://doi.org/10.3390/molecules27206854
Racz LZ, Racz CP, Pop L-C, Tomoaia G, Mocanu A, Barbu I, Sárközi M, Roman I, Avram A, Tomoaia-Cotisel M, et al. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules. 2022; 27(20):6854. https://doi.org/10.3390/molecules27206854
Chicago/Turabian StyleRacz, Levente Zsolt, Csaba Pal Racz, Lucian-Cristian Pop, Gheorghe Tomoaia, Aurora Mocanu, Ioana Barbu, Melinda Sárközi, Ioana Roman, Alexandra Avram, Maria Tomoaia-Cotisel, and et al. 2022. "Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin" Molecules 27, no. 20: 6854. https://doi.org/10.3390/molecules27206854
APA StyleRacz, L. Z., Racz, C. P., Pop, L.-C., Tomoaia, G., Mocanu, A., Barbu, I., Sárközi, M., Roman, I., Avram, A., Tomoaia-Cotisel, M., & Toma, V.-A. (2022). Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules, 27(20), 6854. https://doi.org/10.3390/molecules27206854