Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient
Abstract
1. Introduction
2. Materials and Methods
3. Compositional and Toxicological Data on Coffee Silver Skin
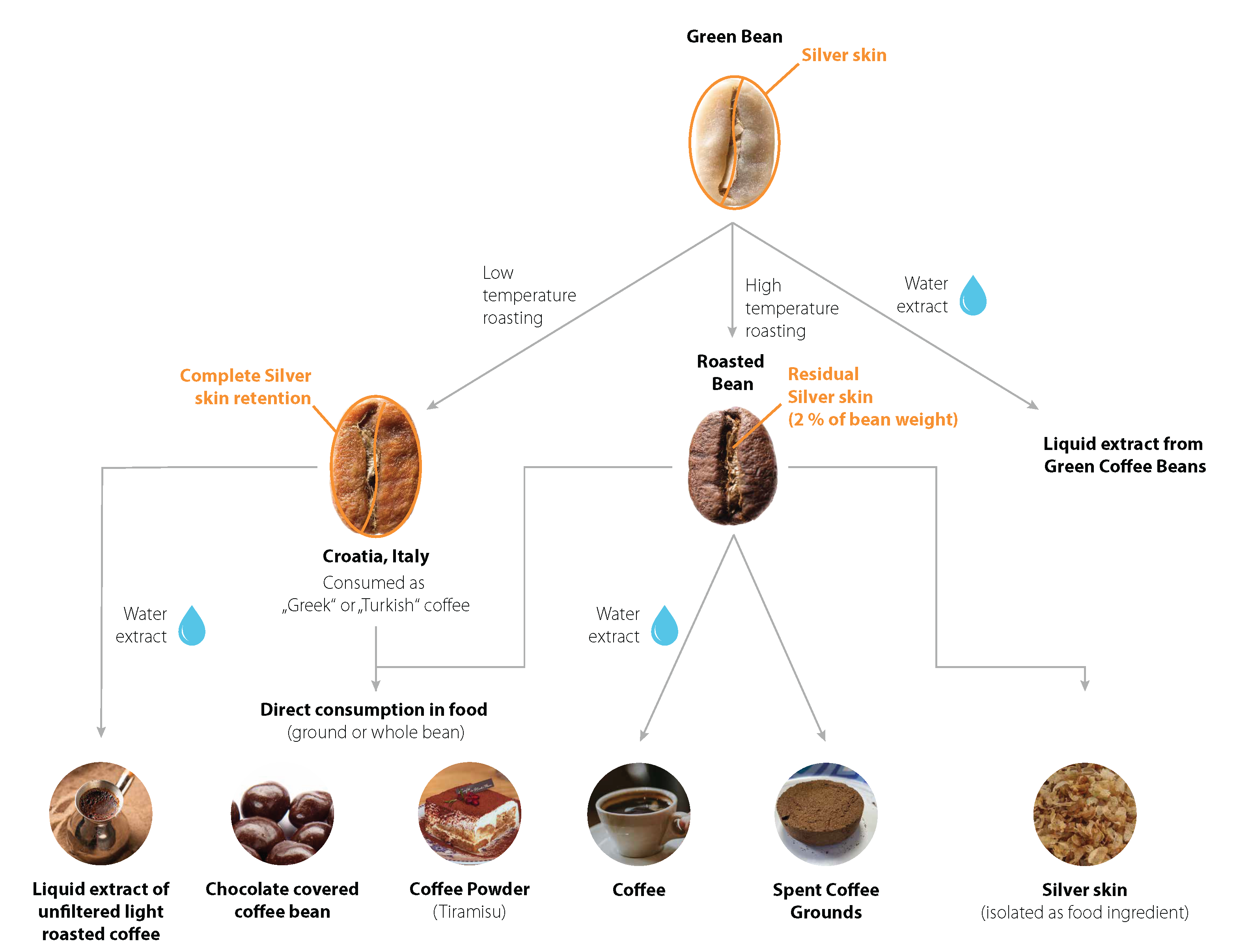
3.1. Description of Coffee Silver Skin
3.1.1. Macro Nutrients
3.1.2. Vitamins and Minerals
3.1.3. Plant Secondary Compounds
3.1.4. Contaminants
3.2. Use History and Intake
3.3. Adsorption, Distribution, Metabolism, Excretion and Nutritional Data
3.3.1. Adsorption, Distribution, Metabolism, Excretion
3.3.2. Nutritional Data
3.4. Toxicological Data
3.4.1. Human Skin
3.4.2. Acute Toxicology
3.4.3. Genotoxicity and Other Toxicological Relevant Cell Lines
3.4.4. Other Cell Lines
3.4.5. Chronic Exposure
3.4.6. Other Animal Data
3.4.7. Human Data
3.4.8. Allergenicity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 14C-FRU | C-Fructose |
| 3H-DG | H-Deoxy-D-Glucose |
| 3T3-L1 | Mouse preadipocytes |
| ATP | Adenosine triphosphate |
| bw | Body weight |
| Caco-2 | Human intestinal epithelial cells |
| DGE | Deutsche Gesellschaft für Ernährung |
| DNA | Deoxyribonucleic acid |
| EFSA | European Food Safety Authority |
| ELISA | Enzyme-linked immunosorbent assay |
| EU | European Union |
| EtOH | Ethanol |
| GAE | Gallic acid equivalent |
| GPx | Glutathione peroxidase |
| GR | Gutathione reductase |
| HaCaT | Human immortalized non-tumorigenic keratinocyte cell line |
| HCE | Human corneal epithelial |
| HepG2 | Human hepatocellular carcinoma cells |
| HFF-1 | Human foreskin fibroblasts |
| IC50 | Inhibitory concentration 50 |
| IEC-6 | Rat small intestine epithelial cells |
| ICP-MS | Inductively Coupled Plasma—Mass Spectrometry |
| INS-1E | Rat insulinoma cell line |
| INSR | Insulin receptor signal transduction |
| LD50 | Lethal Dose 50 |
| LDH | Lactate dehydrogenase |
| mM | Millimol |
| MeOH | Methanol |
| mRNA | Messenger ribonucleic acid |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromid |
| OECD | The Organisation for Economic Co-operation and Development |
| ORAC | Oxygen Radical Absorbance Capacity |
| PGC-1 | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| POP | Phytosterol oxidation products |
| RAW264.7 M | Murine-leukemic monocyte-macrophage cell line |
| RE | Rutin equivalent |
| ROS | Reactive oxygen species |
| SH-SY5Y | Human neuroblastoma cell line |
| TWI | Tolerable weekly intake |
References
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine war on global food security: Towards more sustainable and resilient food systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Trollman, H.; Trollman, F.; Garcia-Garcia, G.; Parra-López, C.; Duong, L.; Martindale, W.; Munekata, P.E.S.; Lorenzo, J.M.; Hdaifeh, A.; et al. The Russia-Ukraine conflict: Its implications for the global food supply chains. Foods 2022, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Breitling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.W.; Bunzel, M. Coffee silver skin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization. Coffee Market Report July 2022. 2022. Available online: www.ico.org (accessed on 12 August 2022).
- Clarke, R. Coffee: Volume 1: Chemistry; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- International Coffee Organization. Trade Statistics Tables. 2021. Available online: www.ico.org (accessed on 29 September 2022).
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Piofczyk, T. Untersuchung zur Röstung von Kaffeebohnen und Dabei Entstehender Emissionen in der Wirbelschicht. Ph.D. Thesis, Otto von Guericke University Library, Magdeburg, Germany, 2009. [Google Scholar]
- Tritsch, N.; Steger, M.C.; Segatz, V.; Blumenthal, P.; Rigling, M.; Schwarz, S.; Zhang, Y.; Franke, H.; Lachenmeier, D.W. Risk assessment of caffeine and epigallocatechin gallate in coffee leaf tea. Foods 2022, 11, 263. [Google Scholar] [CrossRef]
- European Food Safety Authority. Technical report on the notification of cherry pulp from Coffea arabica L. and Coffea canephora Pierre ex A. Froehner as a traditional food from a third country following Article 14 of Regulation (EU) 2015/2283. EFSA J. 2021, 18, 6657E. [Google Scholar] [CrossRef]
- European Food Safety Authority. Technical Report on the notification of infusion from coffee leaves (Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner) as a traditional food from a third country pursuant to Article 14 of Regulation (EU) 2015/2283. EFSA Support. Publ. 2020, 17, 1783E. [Google Scholar] [CrossRef]
- Denmark, Danish Veterinary and Food Administration (DVFA). Consultation Request to Determine The status of Spent Coffee Grounds, Defatted Spent Coffee Grounds and Defatted Unused Coffee Grounds Pursuant to Article 4(2) of Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Ref. Ares(2021)2011778—22/03/2021. 2021. Available online: https://food.ec.europa.eu/system/files/2021-03/novel-food_consult-status_coffee-grounds.pdf (accessed on 29 September 2022).
- Durán-Aranguren, D.D.; Robledo, S.; Gomez-Restrepo, E.; Arboleda Valencia, J.W.; Tarazona, N.A. Scientometric overview of coffee by-products and their applications. Molecules 2021, 26, 7605. [Google Scholar] [CrossRef]
- Rodrigues, F.; Matias, R.; Ferreira, M.; Amaral, M.H.; Oliveira, M.B.P. In vitro and in vivo comparative study of cosmetic ingredients coffee silverskin and hyaluronic acid. Exp. Dermatol. 2016, 25, 572–574. [Google Scholar] [CrossRef]
- Russo, M.E.; Procentese, A.; Montagnaro, F.; Marzocchella, A. Effect of enzymes adsorption on enzymatic hydrolysis of coffee silverskin: Kinetic characterization and validation. Biochem. Eng. J. 2022, 180, 108364. [Google Scholar] [CrossRef]
- Renaudie, M.; Dumas, C.; Vuilleumier, S.; Ernst, B. New way of valorization of raw coffee silverskin: Biohydrogen and acetate production by dark fermentation without exogenous inoculum. Bioresour. Technol. Rep. 2022, 17, 100918. [Google Scholar] [CrossRef]
- Hejna, A. Coffee silverskin as a potential bio-based antioxidant for polymer materials: Brief review. Proceedings 2021, 69, 20. [Google Scholar] [CrossRef]
- Engling, F.P.; Wiedenroth, H.; Rösmann, P.; Halle, I.; Denißen, J.; Meyer, A.; Radewahn, P.; Töpper, A.; Kampf, D.; Ausmeier, S.; et al. Positivliste für Einzelfuttermittel, 14th ed.; Zentralausschuss der Deutschen Landwirtschaft: Berlin, Germany, 2021.
- Thangavelu, K.P.; Tiwari, B.; Kerry, J.P.; Álvarez, C. A comparative study on the effect of ultrasound-treated apple pomace and coffee silverskin powders as phosphate replacers in irish breakfast sausage formulations. Foods 2022, 11, 2763. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. J. Food Meas. Charact. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Gocmen, D.; Sahan, Y.; Yildiz, E.; Coskun, M.; Aroufai, İ.A. Use of coffee silverskin to improve the functional properties of cookies. Int. J. Food Sci. Technol. 2019, 56, 2979–2988. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef]
- McDonald, K.; Langenbahn, H.J.; Miller, J.D.; McMullin, D.R. Phytosterol oxidation products from coffee silverskin. J. Food Sci. 2022, 87, 728–737. [Google Scholar] [CrossRef]
- Vimercati, W.C.; da Silva, A.C.; Macedo, L.L.; Pimenta, C.J. Optimal extraction condition for the recovery of bioactive compounds and antioxidants from coffee silverskin. J. Food Process Eng. 2022, 45, e14009. [Google Scholar] [CrossRef]
- Vimercati, W.C.; Araújo, C.D.; Macedo, L.L.; Correa, J.L.; Pimenta, C.J. Encapsulation of coffee silverskin extracts by foam mat drying and comparison with powders obtained by spray drying and freeze-drying. J. Food Sci. 2022, 87, 1767–1779. [Google Scholar] [CrossRef]
- Germany. Consultation on the Determination of the Status of a Novel Food under Article 4 (2) of Regulation (EU) 2015/2283, Ref. Ares(2022)4778355—30/06/2022. Available online: https://food.ec.europa.eu/system/files/2022-06/novel-food_consult-status_2022-4778355.pdf (accessed on 29 September 2022).
- Angeloni, S.; Scortichini, S.; Fiorini, D.; Sagratini, G.; Vittori, S.; Neiens, S.D.; Steinhaus, M.; Zheljazkov, V.D.; Maggi, F.; Caprioli, G. Characterization of odor-active compounds, polyphenols, and fatty acids in coffee silverskin. Molecules 2020, 25, 2993. [Google Scholar] [CrossRef]
- Bobková, A.; Poláková, K.; Demianová, A.; Belej, L.; Bobko, M.; Jurčaga, L.; Gálik, B.; Novotná, I.; Iriondo-DeHond, A.; Castillo, M.D.d. Comparative analysis of selected chemical parameters of Coffea arabica, from cascara to silverskin. Foods 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioproc. Tech. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Cantele, C.; Tedesco, M.; Ghirardello, D.; Zeppa, G.; Bertolino, M. Coffee silverskin as a functional ingredient in vegan biscuits: Physicochemical and sensory properties and in vitro bioaccessibility of bioactive compounds. Foods 2022, 11, 717. [Google Scholar] [CrossRef]
- Nasti, R.; Galeazzi, A.; Marzorati, S.; Zaccheria, F.; Ravasio, N.; Bozzano, G.L.; Manenti, F.; Verotta, L. Valorisation of coffee roasting by-products: Recovery of silverskin fat by supercritical CO2 extraction. Waste Biomass Valorization 2021, 12, 6021–6033. [Google Scholar] [CrossRef]
- Prandi, B.; Ferri, M.; Monari, S.; Zurlini, C.; Cigognini, I.; Verstringe, S.; Schaller, D.; Walter, M.; Navarini, L.; Tassoni, A.; et al. Extraction and chemical characterization of functional phenols and proteins from coffee (Coffea arabica) by-products. Biomolecules 2021, 11, 1571. [Google Scholar] [CrossRef]
- Beltrán-Medina, E.A.; Guatemala-Morales, G.M.; Padilla-Camberos, E.; Corona-González, R.I.; Mondragón-Cortez, P.M.; Arriola-Guevara, E. Evaluation of the use of a coffee industry by-product in a cereal-based extruded food product. Foods 2020, 9, 1008. [Google Scholar] [CrossRef]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of coffee industry wastes: Comprehensive physicochemical characterization of coffee silverskin and multipurpose recycling applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Machado, S.; Costa, A.S.; Pimentel, F.; Oliveira, M.B.P.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Schwarz, S.; Rieke-Zapp, J.; Cantergiani, E.; Rawel, H.; Martín-Cabrejas, M.A.; Martuscelli, M.; Gottstein, V.; Angeloni, S. Coffee by-products as sustainable novel foods: Report of the 2nd international electronic conference on foods—“Future Foods and Food Technologies for a Sustainable World”. Foods 2022, 11, 3. [Google Scholar] [CrossRef]
- Wang, X.; Hong, D.F.; Hu, G.L.; Li, Z.R.; Peng, X.R.; Shi, Q.Q.; Qiu, M.H. Morphological changes and component characterization of coffee silverskin. Molecules 2021, 26, 4914. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of coffee silver skin as potential food-safe ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef]
- Bessada, S.M.; Alves, R.C.; Oliveira, M.B.P.P. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 (1). Off. J. Eur. Union 2011, L304, 18–63. [Google Scholar] [CrossRef]
- Nolasco, A.; Squillante, J.; Esposito, F.; Velotto, S.; Romano, R.; Aponte, M.; Giarra, A.; Toscanesi, M.; Montella, E.; Cirillo, T. Coffee silverskin: Chemical and biological risk assessment and health profile for its potential use in functional foods. Foods 2022, 11, 2834. [Google Scholar] [CrossRef]
- da Silva, M.R.; Bragagnolo, F.S.; Carneiro, R.L.; Pereira, I.d.O.C.; Ribeiro, J.A.A.; Rodrigues, C.M.; Jelley, R.E.; Fedrizzi, B.; Funari, C.S. Metabolite characterization of fifteen by-products of the coffee production chain: From farm to factory. Food Chem. 2022, 369, 130753. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Castillo, M.; Fernandez-Gomez, B.; Martínez Sáez, N.; Iriondo-DeHond, A.; Martirosyan, D.M.; Mesa, M.D. Coffee Silverskin Extract for Aging and Chronic Diseases; Functional Food Center Inc.: San Diego, CA, USA, 2016; Volume 2, pp. 386–409. [Google Scholar]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Lund, M.N.; Tiwari, B.K. Ultrasound processing of coffee silver skin, brewer’s spent grain and potato peel wastes for phenolic compounds and amino acids: A comparative study. J. Food Sci. Technol. 2021, 58, 2273–2282. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Fernández-Gómez, B.; Martínez Sáez, N.; Martirosyan, D.M.; Mesa, M.D.; Castillo, M. Coffee silverskin: A low-cost substrate for bioproduction of high-value health promoting products. Ann. Nutr. Food Sci. 2017, 1, 1005. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Aroufai, İA.; Sabuncu, M.; Dülger Altiner, D.; Sahan, Y. Antioxidant properties and bioaccessibility of coffee beans and their coffee silverskin grown in different countries. J. Food Meas. Charact. 2022, 16, 1873–1888. [Google Scholar] [CrossRef]
- Lachenmeier, D.; Rajcic de Rezende, T.; Schwarz, S. An update on sustainable valorization of coffee by-products as novel foods within the European Union. Biol. Life Sci. Forum. 2021, 6, 37. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Commission Regulation (EU) 2021/1317 of 9 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs. Off. J. Eur. Union 2021, L286, 1–4. [Google Scholar]
- European Parliament and Council of the European Union. Commission Regulation (EU) 2021/1323 of 10 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in certain foodstuffs. Off. J. Eur. Union 2021, L288, 13–18. [Google Scholar]
- European Food Safety Authority. Flavouring Group Evaluation 66 (FGE. 66): Consideration of furfuryl alcohol and related flavouring substances evaluated by JECFA (55th meeting) structurally related to furfuryl and furan derivatives with and without additional side chain substituents and heteroatoms evaluated by EFSA in FGE. 13 (2005). EFSA J. 2009, 7, 752. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.J.; del Castillo, M.D. Use of coffee silverskin and stevia to improve the formulation of biscuits. Polish J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Commission Regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, L304, 24–44. [Google Scholar]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of the extension of use of plant sterol esters as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e06135. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. Int. Food Res. J. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; Del Castillo, M.D.; Morales, P. Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef]
- Il Presidente Della Repubblica. Decreto del Presidente della Repubblica, 16 Febbraio 1973, n. 470. In Gazzetta Ufficiale della Republica Italiana; Il Presidente Della Repubblica: Rome, Italy, 1963. [Google Scholar]
- European Parliament and Council of the European Union. Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials. Off. J. Eur. Union 2013, L29, 1–64. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Mastrocola, D. The role of coffee silver skin against oxidative phenomena in newly formulated chicken meat burgers after cooking. Foods 2021, 10, 1833. [Google Scholar] [CrossRef]
- European Food Safety Authority. The EFSA Comprehensive European Food Consumption Database. 2021. Available online: https://www.efsa.europa.eu/en/data-report/food-consumption-data (accessed on 13 July 2022).
- Tores de la Cruz, S.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; Del Castillo, M.D. An assessment of the bioactivity of coffee silverskin melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Ángeles Martín, M.; Mesa, M.D.; del Castillo, M.D. Insights on the health benefits of the bioactive compounds of coffee silverskin extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Ernährung e.v. 2022. Available online: https://www.dge.de/ (accessed on 16 July 2022).
- Lachenmeier, D.W.; Schwarz, S.; Teipel, J.; Hegmanns, M.; Kuballa, T.; Walch, S.G.; Breitling-Utzmann, C.M. Potential antagonistic effects of acrylamide mitigation during coffee roasting on furfuryl alcohol, furan and 5-hydroxymethylfurfural. Toxics 2019, 7, 1. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pereira, C.; Pimentel, F.; Alves, R.; Ferreira, M.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P. Are coffee silverskin extracts safe for topical use? An in vitro and in vivo approach. Ind. Crops Prod. 2015, 63, 167–174. [Google Scholar] [CrossRef]
- Rodrigues, F.; de Oliveira, A.P.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Coffee silverskin: A possible valuable cosmetic ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef]
- Juan-García, A.; Caprioli, G.; Sagratini, G.; Mañes, J.; Juan, C. Coffee silverskin and spent coffee suitable as neuroprotectors against Cell Death by beauvericin and α-zearalenol: Evaluating strategies of treatment. Toxins 2021, 13, 132. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Ramos, S.; Goya, L.; Mesa, M.D.; del Castillo, M.D.; Ángeles Martín, M. Coffee silverskin extract improves glucose-stimulated insulin secretion and protects against streptozotocin-induced damage in pancreatic INS-1E beta cells. Food Res. Int. 2016, 89, 1015–1022. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Andrade, N.; Machado, S.; Costa, A.S.; Puga, H.; Oliveira, M.B.P.; Martel, F.; Alves, R.C. Valorizing coffee silverskin based on its phytochemicals and antidiabetic potential: From lab to a pilot scale. Foods 2022, 11, 1671. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Ozmen-Togay, S.; Gulkun, G.; Degirmencioglu, N.; Guldas, M.; Yildiz, E.; Sahan, Y.; Gurbuz, O. Impact of coffee silverskin on in-vitro viability of kefir culture during storage. Mljekarstvo/Dairy 2022, 72, 22–32. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; Del Castillo, M.D. Coffee silverskin extract: Nutritional value, safety and effect on key biological functions. Nutrients 2019, 11, 2693. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Jang, J.P.; Jang, J.H.; Jin, D.H.; Kim, Y.S.; Jin, H.J. Identification of molecules from coffee silverskin that suppresses myostatin activity and improves muscle mass and strength in mice. Molecules 2021, 26, 2676. [Google Scholar] [CrossRef]
- El-Anany, A.M.; Ali, R.F.M. Hypolipidemic effect of coffee silver skin in rats fed a high-fat diet. Food Sci. Hum. Wellness 2018, 7, 252–259. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Herrera, T.; Del Castillo, M.D. Health benefits of silverskin. In Food Wastes and By-Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; Chapter 12; pp. 353–371. [Google Scholar] [CrossRef]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and their effects on muscle atrophy and muscle health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef]
- Thligene, N.; Mezzapesa, G.; Mondelli, D.; Trani, A.; Veronico, P.; Melillo, M.; Dumontet, S.; Miano, T.; Sasanelli, N. Effect of coffee silver skin and brewers’ spent grain in the control of root-knot nematodes. Helminthologia 2019, 56, 30–41. [Google Scholar] [CrossRef]
- Osimani, A.; Ferrocino, I.; Corvaglia, M.R.; Roncolini, A.; Milanović, V.; Garofalo, C.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Jamshidi, E.; et al. Microbial dynamics in rearing trials of Hermetia illucens larvae fed coffee silverskin and microalgae. Int. Food Res. J. 2021, 140, 110028. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; Del Castillo, M.D. Coffee silverskin extract protects against accelerated aging caused by oxidative agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Thayer, P.S.; Palm, P.E.; Flamm, G. A current assessment of the mutagenic and teratogenic effects of caffeine. CRC Crit. Rev. Toxicol. 1975, 3, 345–369. [Google Scholar] [CrossRef]
- Silva, I.D.; Rodrigues, A.; Gaspar, J.; Mala, R.; Laires, A.; Rueff, J. Mutagenicity of kaempferol in V79 cells: The role of cytochromes P450. Teratog. Carcinog. Mutagen. 1996, 16, 229–241. [Google Scholar] [CrossRef]
- Maruta, A.; Enaka, K.; Umeda, M. Mutagenicity of Quercetin and kaempferol on cultured mammalian cells. Gan Kagaku Ryoho 1979, 70, 273–276. [Google Scholar] [CrossRef]
- Yamada, J.; Tomita, Y. Antimutagenic activity of caffeic acid and related compounds. Biosci. Biotechnol. Biochem. 1996, 60, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.S.; Davidović, S.; Živković, J.; Glamočlija, J.; Ćirić, A.; Stevanović, M.; Ferreira, I.C.; Soković, M. Comparative evaluation of antimutagenic and antimitotic effects of Morchella esculenta extracts and protocatechuic acid. Front. Life Sci. 2013, 7, 218–223. [Google Scholar] [CrossRef]
- Nehlig, A.; Debry, G. Potential genotoxic, mutagenic and antimutagenic effects of coffee: A review. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1994, 317, 145–162. [Google Scholar] [CrossRef]
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016, 17, 877–878. [Google Scholar] [CrossRef]
- Griebel, C. Beiträge zur mikroskopischen Untersuchung der Kaffee-Ersatzstoffe. Zeitschr. Unters. Nahr. Genussmittel 1918, 35, 272–277. [Google Scholar] [CrossRef]
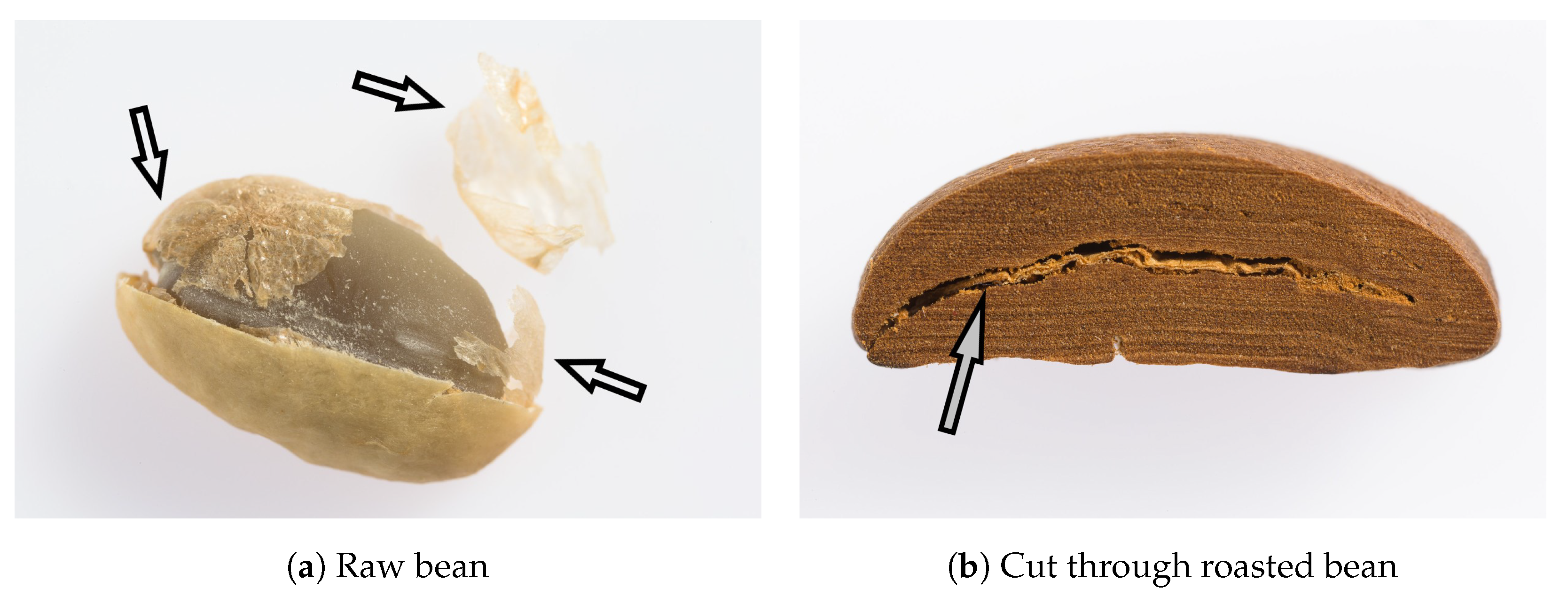

| Nutrient | Amount (%) | Reference |
|---|---|---|
| Fats a | 1.6–3.0 | [3,27,28,29,30,31,32] |
| Protein | 7.1–22.0 | [3,29,31,33] |
| Carbohydrates | 9.5–14.5 (70.2) b | [31,34] |
| fiber (total) | 34.7–68.5 | [28,29,31,34,35] |
| – soluble | 8.2–11.0 | [3,29] |
| – insoluble | 46.0–56.0 | [3,29] |
| Moisture | 2.0–7.0 | [30,31,36] |
| Ashes | 5.4–9.5 | [3,29,30] |
| Ingredient | Amount | Possible Effect | Reference |
|---|---|---|---|
| Caffeine | 0.65–1% | stimulant, diuretic, antioxidant | [3,31] |
| Polyphenols, including: | 1–6 mg GAE /g | antibacterial, enzyme inhibitory, antioxidant | [33,47,48] |
| Chlorogenic acids | 1.1–6.8% | antioxidant, antidiabetic, antiinflammatory | [49] |
| Vannilic acid | 0.088–0.147% | antioxidant, flavouring | [27] |
| Syringic acid | 0.009–0.036% | antioxidant | [27] |
| Flavonoids, including: | 0.18–2.35 mg RE /g | antioxidant | [46] |
| Rutin | 0.001–0.005% | antioxidant | [27] |
| Kaempferol- 3-glucoside | 0.003–0.007% | antioxidant | [27] |
| Melanoidins | 17–23% | antioxidant, anticancer, anticholesterol | [47] |
| Impurity | Amount | Reference | Regulatory Standard |
|---|---|---|---|
| Lead | <1–2.63 mg/kg | [35,42,44] | 0.2 mg/kg (wheat) [55] |
| Mercury | 0.05 mg/kg | [42,44] | TWI 4 g/kg bw [58] |
| Cadmium | 0.07 mg/kg | [42,44] | 0.1 mg/kg (wheat) [56] |
| 5-Hydroxymethylfurfural | 0.57 mg/kg | [36] | Threshold 1.5 g/person/d [57] |
| Furfuryl alcohol | n.d. | [3] | Threshold 1.5 g/person/d [57] |
| Polycyclic aromatic hydrocarbons | traces | [44] | 1 g/kg Benzo(a)pyrene in babyfood [59] |
| Furan | n.d. | [44] | Threshold 1.5 g/person/d [57] |
| Methylfuran | n.d. | [44] | Threshold 1.5 g/person/d [57] |
| Pesticides | n.d. | [44] | depending on individual substance |
| Acrylamide | 11.42 g/L (Extract) | [3,35,40,60] | Benchmark: |
| <20–161 g/kg | 400 g/g | ||
| 720 g/kg | (coffee) [61] | ||
| Phytosterol oxidation products (POP) | 2.1–8.8 mg/kg | [23] | safe intake: 0.64 mg/kg bw/day [62] |
| Ochratoxin A | <4 g/kg | [63] | 5 g/kg (coffee) [59] |
| 18.7–34.4 g/kg | [64] | ||
| Aflatoxins | B1 < 0.20 ppb | [34] | 2 g/kg (wheat) [59] |
| B2 < 0.06 ppb | Sum of all | ||
| G1 < 0.20 ppb | 4 g/kg (wheat) [59] | ||
| G2 < 0.06 ppb |
| Source | Concentration [%] | Estimated Intake of Source [g/kg bw/Day] | Maximum Worst-Case Intake from Source [g/kg bw/Day] |
|---|---|---|---|
| Flat Bread | 5 | 0.89 | 0.045 |
| Cakes | 7 | 0.83 | 0.058 |
| Biscuits | 3 | 0.60 | 0.018 |
| Cookies | 5 | 0.99 | 0.050 |
| Yoghurt | 6 | 2.99 | 0.179 |
| Burger Patties | 3 | 0.79 | 0.024 |
| Total assumed worst-case intake | 0.374 g/kg bw/day | ||
| Component | Amount | Evaluation |
|---|---|---|
| Macro nutrients | ||
| Fats | 1.6–3.0% [3,27,28,29,30,31,32] | Low fat food |
| Carbohydrates | 9.5–14.5% [31,34] | Low carbohydrate food |
| Proteins | 15.0–22.0% [3,29,31] | All essential amino acids except methionine [3], relevant protein source |
| Fiber | 34.7–68.5% [28,29,31,34,35] | Fiber source for nutrition, reduces deficiency |
| Micro nutrients | ||
| Vitamin E | 4.17 mg/100 g [36,41] | No relevant part of reference dose [43] |
| Zinc | 0.7–2.2 mg/100 g [29,35,36,44] | No relevant part of reference dose [73] |
| Potassium | 5000 mg/100 g [29,36,44] | Relevant amount of daily dose, no concern [73] |
| Calcium | 500–1000 mg/100 g [29,36,44] | Relevant amount of daily dose, reduces deficiency [73] |
| Magnesium | 200–2000 mg/100 g [29,36,44] | Above average intake, no concern [73] |
| Iron | 8–80 mg/100 g [29,36,44] | Exceeding reference dose, low absorption [73], no concern |
| Contaminants | ||
| Aflatoxins | n.d. [34] | No concern [59] |
| POP | 2.1–8.8 mg/kg [23] | No significant contribution to daily intake [62], no concern |
| Ochratoxin A | <4–34.4 g/kg [63,64] | No concern if ≤5 g/kg [59] |
| Acrylamide | < 20–720 g/kg [3,35,40,60] | No concern for low content [61], limit would be useful |
| Mercury | 0.05 mg/kg [42,44] | Inorganic, limited adsorption, no concern [58] |
| Cadmium | 0.07 mg/kg [42,44] | Below limit for wheat, no concern [56] |
| Lead | ≤0.36 mg/kg 2.63 mg/kg [42,44,44] | Low lead qualities available, content must be mitigated [42,55] |
| Caffeine | 0.65–1% [3,31] | Not exceeding intake guidelines [50], no concern |
| Effect Assessed | Test System | Cell Line | Outcome | Source |
|---|---|---|---|---|
| Cytotoxicity | MTT assay | HepG2 | not cytotoxic up to 10,000 g/mL | [65] |
| Cytotoxicity | LDH assay | HaCaT and HFF-1 | negative up to 1000 g/mL | [76] |
| Cytotoxicity | MTT assay | SH-SY5Y | not cytotoxic for all extracts tested | [77] |
| DNA damage | Comet assay | HepG2 | negative up to 1000 g/mL | [65] |
| Antioxidant capacity | Hemolysis | Erythrocytes | protective against oxidative stress at 25 g/L | [36] |
| Reduction of intracellular reactive oxygen species | ORAC Assay | IEC-6 | reduction at 0.004, 0.04, and 0.4 mg/mL | [71] |
| Modulation of insulin secretion | ELISA | pancreatic INS-1E | enhanced secretion | [78] |
| Uptake of sugars | non standard assay | Caco-2 | reduced uptake | [79] |
| Obesity effects | non standard assay | 3T3-L1 RAW264.7 M | reduction of obesity benchmarks | [80] |
| Effect Assessed | Animal Species | Duration (d) | Outcome | Source |
|---|---|---|---|---|
| Toxicity of aqueous extract | rat | 28 | no toxic effects a 1 g/kg bw per day | [82] |
| Toxicity of melanoidin rich extract fraction | rat | 28 | no toxicity at 1 g/kg bw per day, slight effect of high fiber content | [71] |
| Effect of silver skin extracts on muscle growth | mouse | 29 | bigger forelimb muscle, more grip strength | [83] |
| Hypolipidemic effect of silver skin | rat | 56 | reduced effect of high fat nutrition | [84] |
| Effect of silver skin extract on streptozotocin induced damage | rat | 35 | reduction of damage | [72] |
| Effect of extract on total cholesterol and triglyceride plasma levels | rat | 45 | reduction, inhibition of pancreas lipase | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorbeer, L.; Schwarz, S.; Franke, H.; Lachenmeier, D.W. Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient. Molecules 2022, 27, 6839. https://doi.org/10.3390/molecules27206839
Lorbeer L, Schwarz S, Franke H, Lachenmeier DW. Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient. Molecules. 2022; 27(20):6839. https://doi.org/10.3390/molecules27206839
Chicago/Turabian StyleLorbeer, Liane, Steffen Schwarz, Heike Franke, and Dirk W. Lachenmeier. 2022. "Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient" Molecules 27, no. 20: 6839. https://doi.org/10.3390/molecules27206839
APA StyleLorbeer, L., Schwarz, S., Franke, H., & Lachenmeier, D. W. (2022). Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient. Molecules, 27(20), 6839. https://doi.org/10.3390/molecules27206839








