Biosourced Poly(lactic acid)/polyamide-11 Blends: Effect of an Elastomer on the Morphology and Mechanical Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Theoretical Prediction of Interfacial Tension
2.2. Morphological Observation
2.3. Mechanical Properties
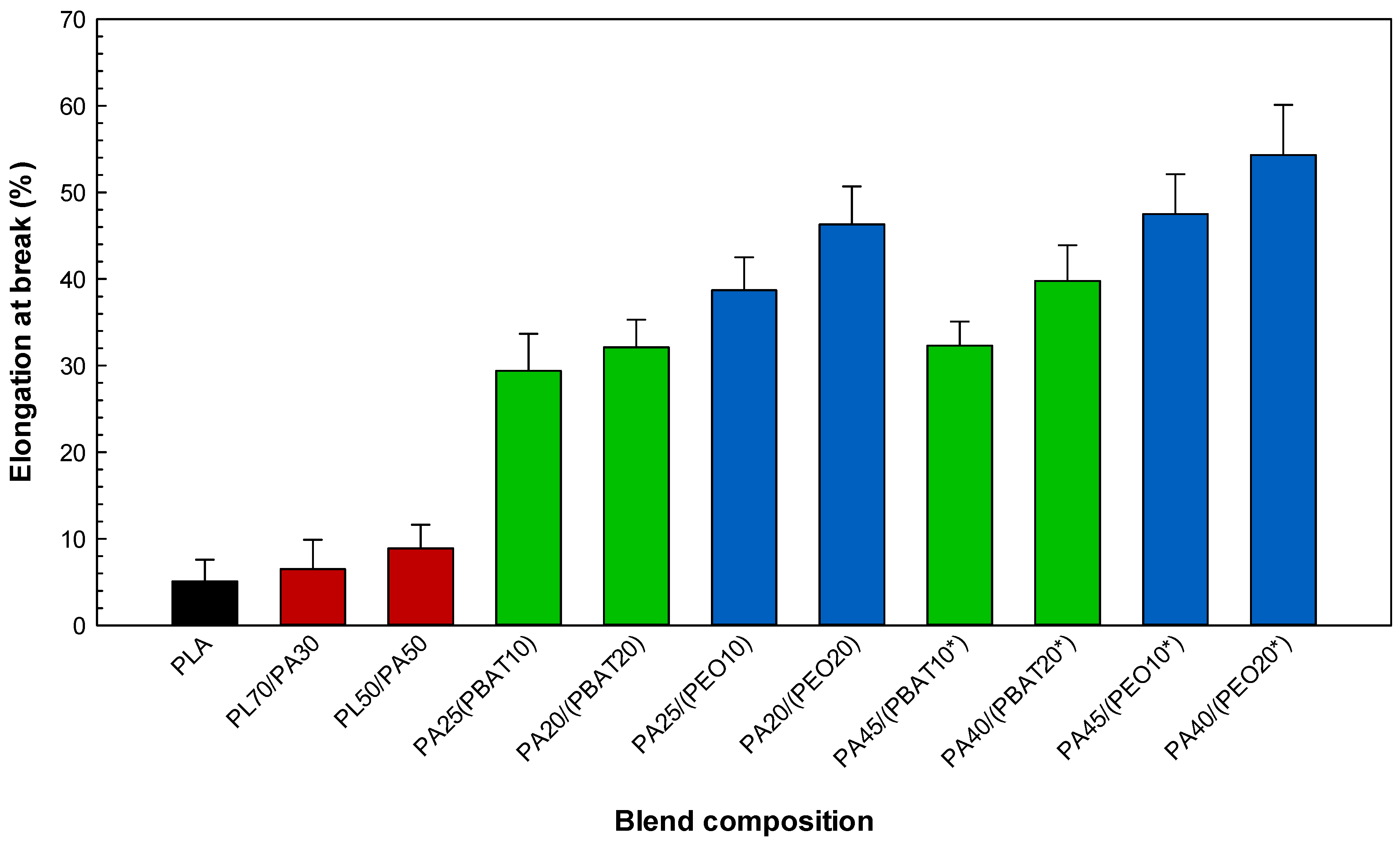
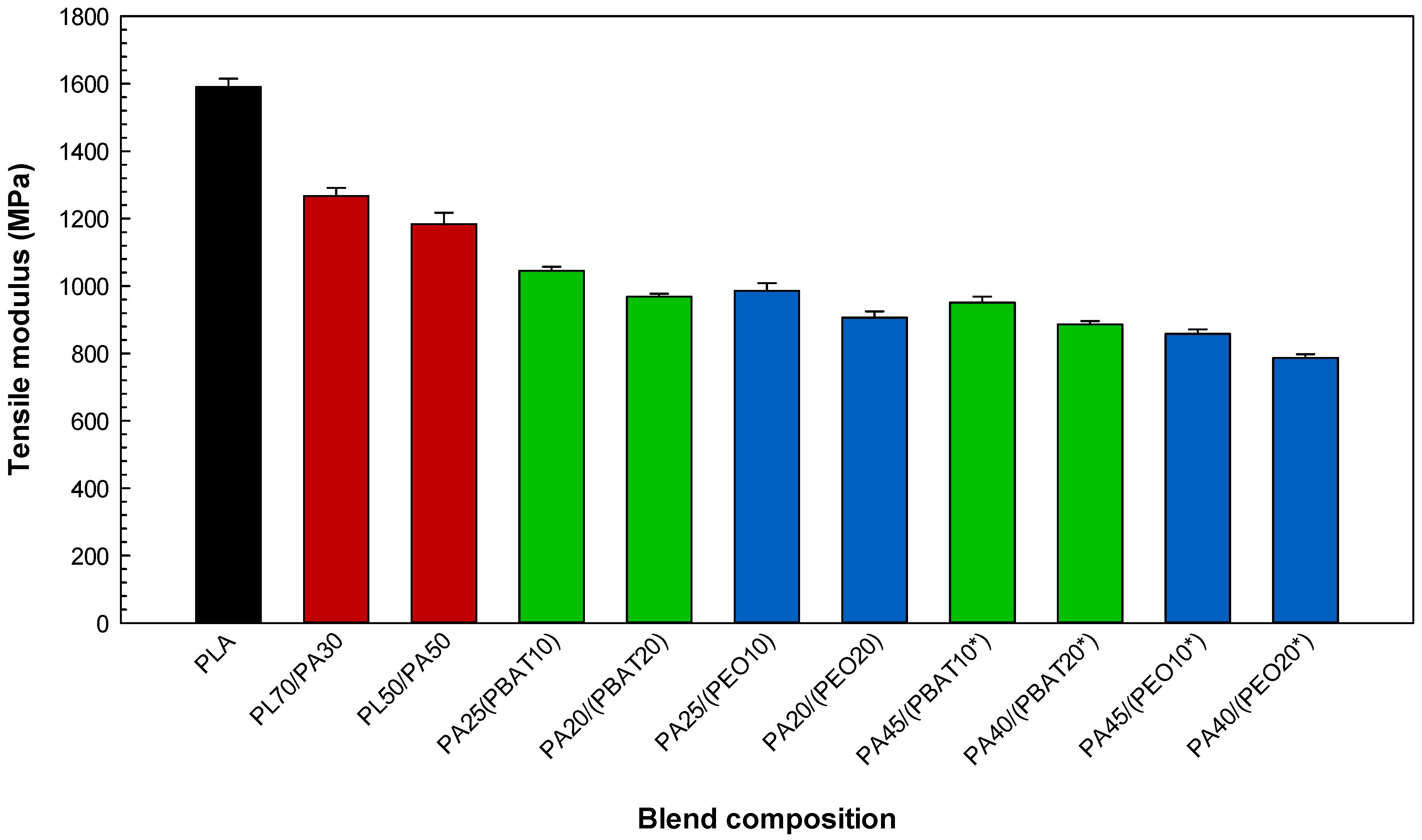
2.3.1. Tensile Properties
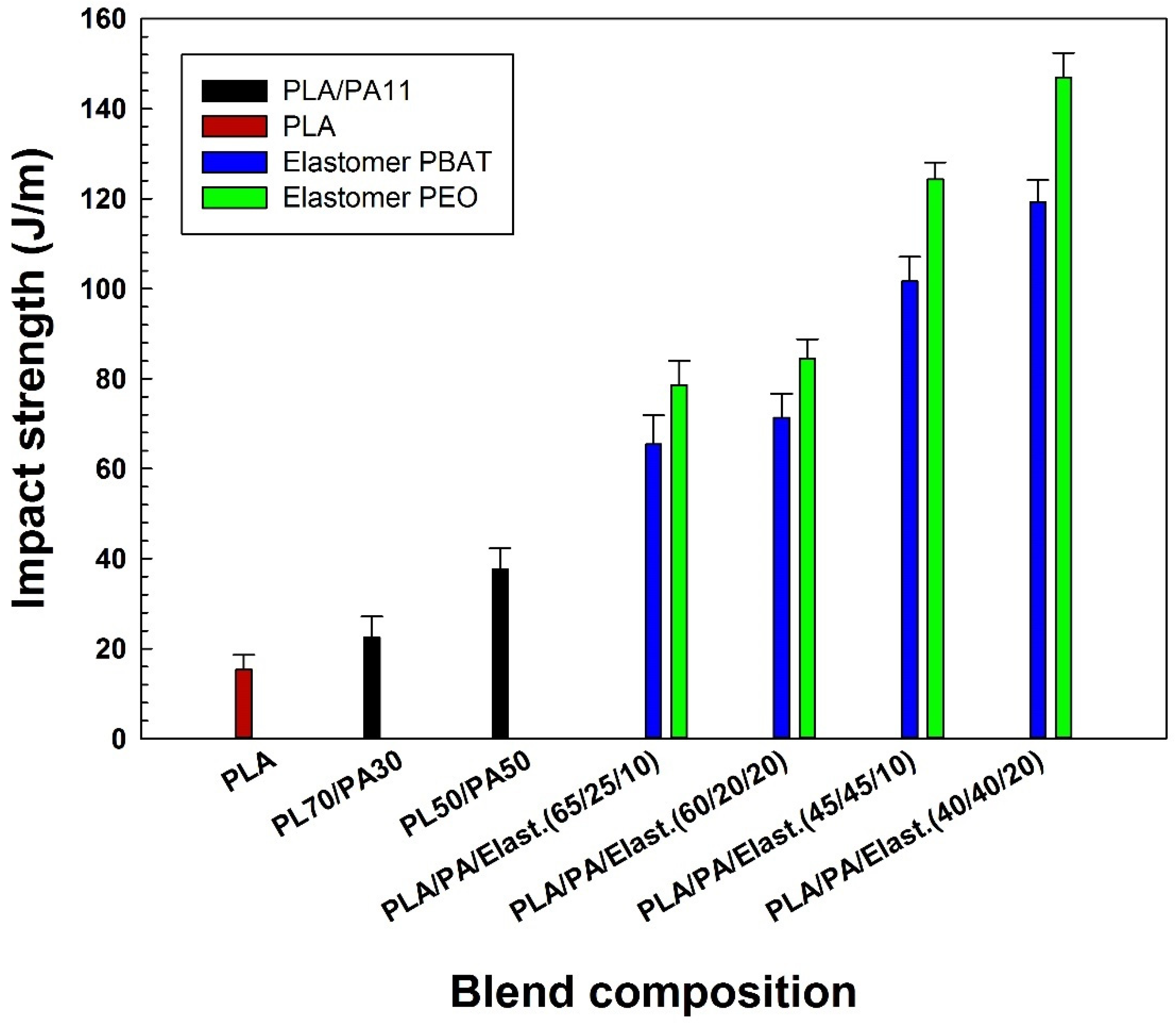
2.3.2. Impact Properties
3. Materials and Methods
3.1. Materials
3.2. Processing
3.3. Characterization
3.3.1. Contact Angle Measurements
3.3.2. Morphological Observation
3.3.3. Mechanical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Coudane, J.; Van Den Berghe, H.; Mouton, J.; Garric, X.; Nottelet, B. Poly (Lactic Acid)-Based Graft Copolymers: Syntheses Strategies and Improvement of Properties for Biomedical and Environmentally Friendly Applications: A Review. Molecules 2022, 27, 4135. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Del. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Cusson, E.; Mougeot, J.-C.; Maho, M.; Lacroix, F.; Fazli, A.; Rodrigue, D. Poly (Lactic Acid)(PLA)/Recycled Styrene Butadiene Rubber (rSBR) Composites. Adv. Environ. Eng. Res. 2022, 3, 1. [Google Scholar] [CrossRef]
- Laaziz, S.A.; Raji, M.; Hilali, E.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Bio-composites based on polylactic acid and argan nut shell: Production and properties. Int. J. Biol. Macromol. 2017, 104, 30–42. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, M.; Liu, G.; Wang, J.; Chen, Y.; Zhang, Z. Alkylated lignin with graft copolymerization for enhancing toughness of PLA. J. Mater. Sci. 2022, 57, 8687–8700. [Google Scholar] [CrossRef]
- Heshmati, V.; Favis, B.D. High performance poly (lactic acid)/bio-polyamide11 through controlled chain mobility. Polymer 2017, 123, 184–193. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Balart, R.; Ivorra-Martinez, J.; Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L. Improvement of impact strength of polylactide blends with a thermoplastic elastomer compatibilized with biobased maleinized linseed oil for applications in rigid packaging. Molecules 2021, 26, 240. [Google Scholar] [CrossRef]
- Burzic, I.; Pretschuh, C.; Kaineder, D.; Eder, G.; Smilek, J.; Másilko, J.; Kateryna, W. Impact modification of PLA using biobased biodegradable PHA biopolymers. Eur. Polym. J. 2019, 114, 32–38. [Google Scholar] [CrossRef]
- Ma, P.; Spoelstra, A.; Schmit, P.; Lemstra, P. Toughening of poly (lactic acid) by poly (β-hydroxybutyrate-co-β-hydroxyvalerate) with high β-hydroxyvalerate content. Eur. Polym. J. 2013, 49, 1523–1531. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation behavior of poly (butylene adipate-co-terephthalate)(PBAT), poly (lactic acid)(PLA), and their blend in freshwater with sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Xue, B.; He, H.-Z.; Huang, Z.-X.; Zhu, Z.; Xue, F.; Liu, S.; Liu, B. Fabrication of super-tough ternary blends by melt compounding of poly (lactic acid) with poly (butylene succinate) and ethylene-methyl acrylate-glycidyl methacrylate. Compos. B Eng. 2019, 172, 743–749. [Google Scholar] [CrossRef]
- Heshmati, V.; Zolali, A.M.; Favis, B.D. Morphology development in poly (lactic acid)/polyamide11 biobased blends: Chain mobility and interfacial interactions. Polymer 2017, 120, 197–208. [Google Scholar] [CrossRef]
- Rashmi, B.J.; Prashantha, K.; Lacrampe, M.-F.; Krawczak, P. Toughening of poly (lactic acid) without sacrificing stiffness and strength by melt-blending with polyamide 11 and selective localization of halloysite nanotubes. In Proceedings of the 31st International Conference of the Polymer Processing Society, Jeju Island, Korea, 7–11 June 2015; AIP Publishing LLC: Huntington, NY, USA, 2016. [Google Scholar]
- Feng, F.; Ye, L. Structure and property of polylactide/polyamide blends. J. Macromol. Sci. Part B Phys. 2010, 49, 1117–1127. [Google Scholar] [CrossRef]
- Stoclet, G.; Seguela, R.; Lefebvre, J.-M. Morphology, thermal behavior and mechanical properties of binary blends of compatible biosourced polymers: Polylactide/polyamide11. Polymer 2011, 52, 1417–1425. [Google Scholar] [CrossRef]
- Patel, R.; Ruehle, D.A.; Dorgan, J.R.; Halley, P.; Martin, D. Biorenewable blends of polyamide-11 and polylactide. Polym. Eng. Sci. 2014, 54, 1523–1532. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly (lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef]
- Mazidi, M.M.; Edalat, A.; Berahman, R.; Hosseini, F.S. Highly-toughened polylactide-(PLA-) based ternary blends with significantly enhanced glass transition and melt strength: Tailoring the interfacial interactions, phase morphology, and performance. Macromolecules 2018, 51, 4298–4314. [Google Scholar] [CrossRef]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened renewable PLA reactive multiphase blends system: Phase morphology and performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar] [CrossRef]
- Zolali, A.M.; Favis, B.D. Toughening of cocontinuous polylactide/polyethylene blends via an interfacially percolated intermediate phase. Macromolecules 2018, 51, 3572–3581. [Google Scholar] [CrossRef]
- Zolali, A.M.; Heshmati, V.; Favis, B.D. Ultratough co-continuous PLA/PA11 by interfacially percolated poly (ether-b-amide). Macromolecules 2017, 50, 264–274. [Google Scholar] [CrossRef]
- Li, Y.; Shimizu, H. Improvement in toughness of poly (l-lactide)(PLLA) through reactive blending with acrylonitrile–butadiene–styrene copolymer (ABS): Morphology and properties. Eur. Polym. J. 2009, 45, 738–746. [Google Scholar] [CrossRef]
- Fu, Y.; Fodorean, G.; Navard, P.; Peuvrel-Disdier, E. Study of the partial wetting morphology in polylactide/poly [(butylene adipate)-co-terephthalate]/polyamide ternary blends: Case of composite droplets. Polym. Int. 2018, 67, 1378–1385. [Google Scholar] [CrossRef]
- Ravati, S.; Beaulieu, C.; Zolali, A.M.; Favis, B.D. High performance materials based on a self-assembled multiple-percolated ternary blend. AICHE J. 2014, 60, 3005–3012. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; CRC Press—Routledge: Boca Raton, FL, USA, 1982. [Google Scholar]
- Torza, S.; Mason, S. Three-phase interactions in shear and electrical fields. J. Colloid Interface Sci. 1970, 33, 67–83. [Google Scholar] [CrossRef]
- Harkins, W.D.; Feldman, A. Films. The spreading of liquids and the spreading coefficient. J. Am. Chem. Soc. 1922, 44, 2665–2685. [Google Scholar] [CrossRef]
- Kolahchi, A.R.; Ajji, A.; Carreau, P.J. Surface morphology and properties of ternary polymer blends: Effect of the migration of minor components. J. Phys. Chem. B 2014, 118, 6316–6323. [Google Scholar] [CrossRef]
- Zolali, A.; Favis, B.D. Compatibilization and toughening of co-continuous ternary blends via partially wet droplets at the interface. Polymer 2017, 114, 277–288. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhang, Z.; Peng, S.; Chen, H.; Zhao, X. High-performance fully bio-based poly (lactic acid)/polyamide11 (PLA/PA11) blends by reactive blending with multi-functionalized epoxy. Polym. Test. 2019, 78, 105980. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Rheological, morphological and mechanical studies of sustainably sourced polymer blends based on poly (lactic acid) and polyamide 11. Polymers 2016, 8, 61. [Google Scholar] [CrossRef]
- Zolali, A.M.; Favis, B.D. Partial to complete wetting transitions in immiscible ternary blends with PLA: The influence of interfacial confinement. Soft Matter 2017, 13, 2844–2856. [Google Scholar] [CrossRef] [PubMed]
- Fazli, A.; Rodrigue, D. Thermoplastic Elastomer based on Recycled HDPE/Ground Tire Rubber Interfacially Modified with an Elastomer: Effect of Mixing Sequence and Elastomer Type/Content. Polym. Plast. Technol. Eng. 2022, 61, 1021–1038. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Moraes, E.A.; Marconcini, J.M.; Mattoso, L.H.C.; Glenn, G.M.; Medeiros, E.S. Properties of poly (lactic acid) and poly (ethylene oxide) solvent polymer mixtures and nanofibers made by solution blow spinning. J. Appl. Polym. Sci. 2013, 129, 3672–3681. [Google Scholar] [CrossRef]
- Halldén, Å.; Ohlsson, B.; Wesslén, B. Poly (ethylene-graft-ethylene oxide)(PE-PEO) and poly (ethylene-co-acrylic acid)(PEAA) as compatibilizers in blends of LDPE and polyamide-6. J. Appl. Polym. Sci. 2000, 78, 2416–2424. [Google Scholar] [CrossRef]
- Buddhiranon, S.; Kim, N.; Kyu, T. Morphology development in relation to the ternary phase diagram of biodegradable PDLLA/PCL/PEO blends. Macromol. Chem. Phys. 2011, 212, 1379–1391. [Google Scholar] [CrossRef]
- Khanteesa, R.; Threepopnatkul, P.; Sittattrakul, A. Effect of poly (ethylene oxide) on the properties of poly (lactic acid)-based blends. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Henry, S.; De Vadder, L.; Decorte, M.; Francia, S.; Van Steenkiste, M.; Saevels, J.; Vanhoorne, V.; Vervaet, C. Development of a 3D-printed dosing platform to aid in Zolpidem withdrawal therapy. Pharmaceutics 2021, 13, 1684. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmus, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H. Toughening of poly (l-lactide) modified by a small amount of acrylonitrile-butadiene-styrene core-shell copolymer. J. Appl. Polym. Sci. 2015, 132, 42554. [Google Scholar] [CrossRef]
- Kanzawa, T.; Tokumitsu, K. Mechanical properties and morphological changes of poly (lactic acid)/polycarbonate/poly (butylene adipate-co-terephthalate) blend through reactive processing. J. Appl. Polym. Sci. 2011, 121, 2908–2918. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Super toughened poly (lactic acid)-based ternary blends via enhancing interfacial compatibility. ACS Omega 2019, 4, 1955–1968. [Google Scholar] [CrossRef]
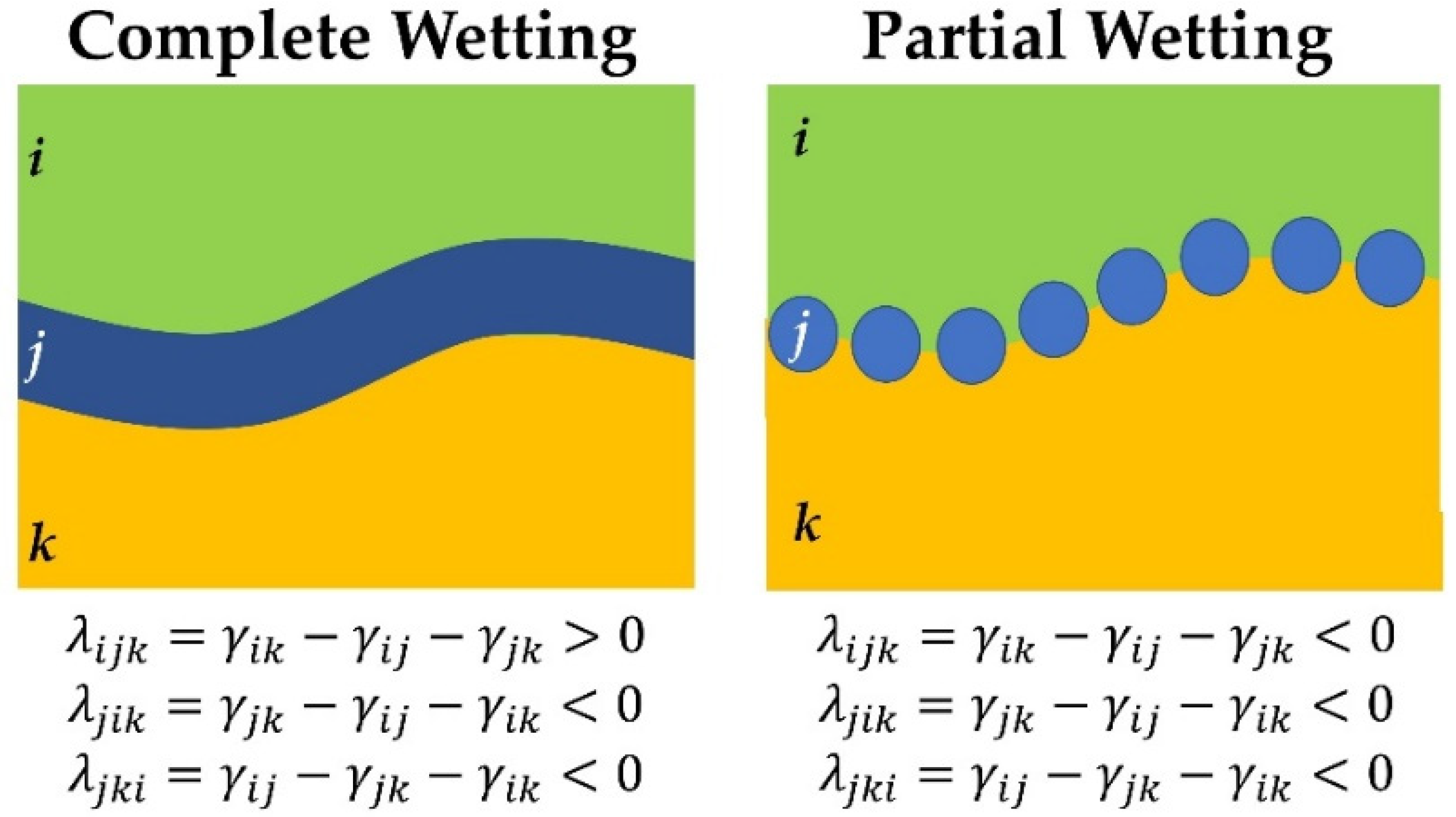

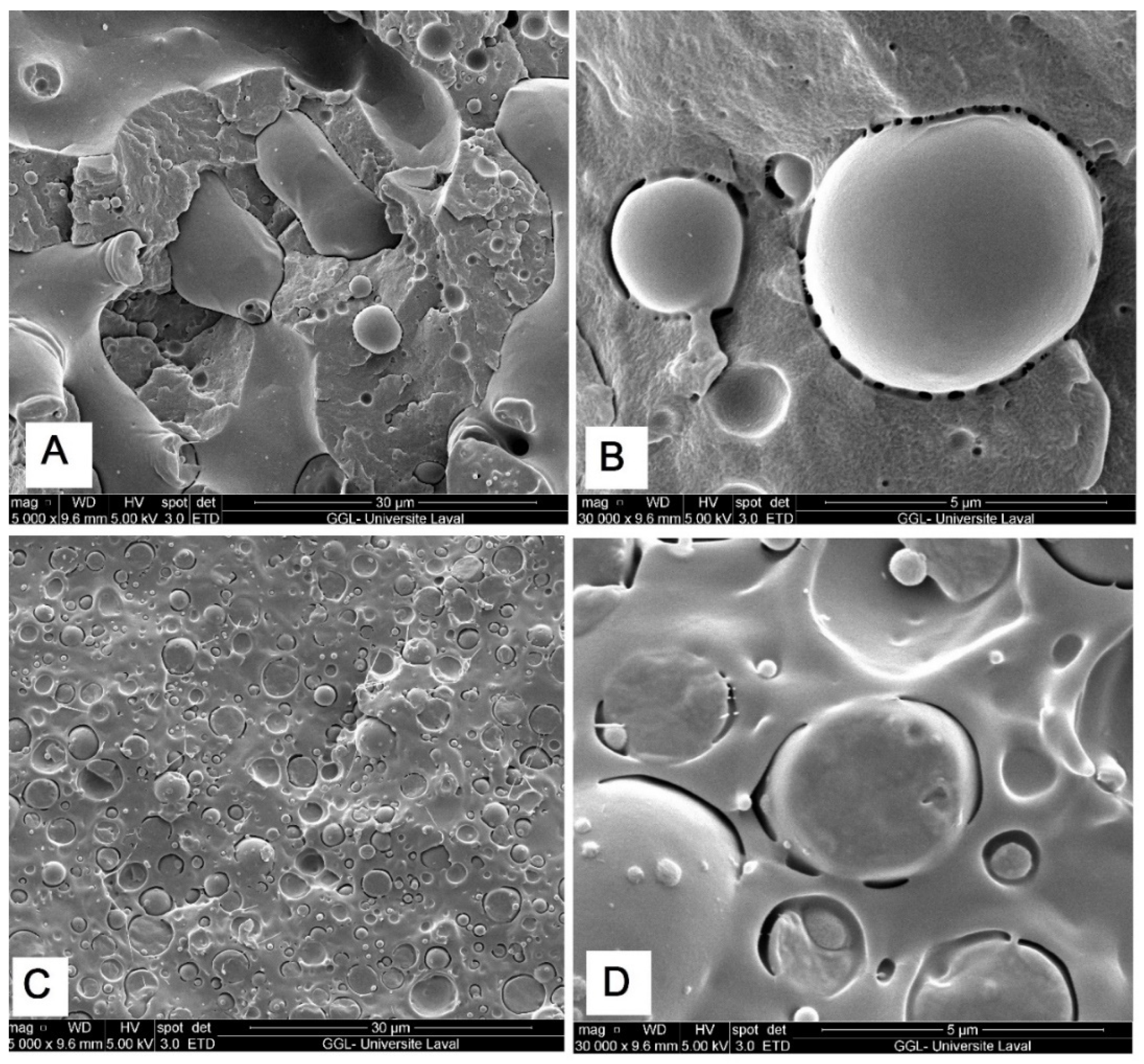
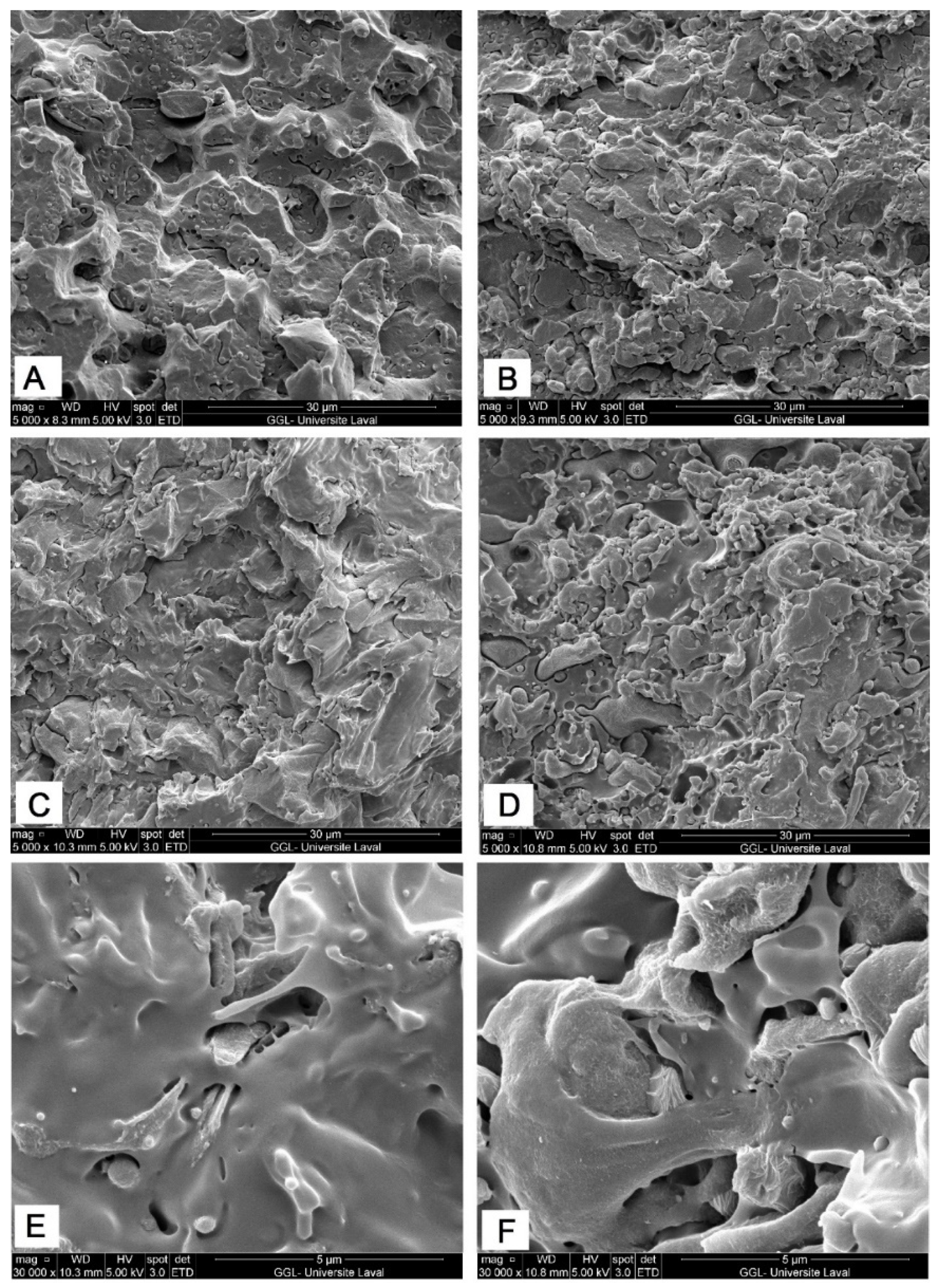

| Polymer | γ | γd | γp | Interfacial Tension | Spreading Coefficient (λ) | |
|---|---|---|---|---|---|---|
| PLA | 39.7 ± 0.3 | 26.3 ± 0.2 | 13.4 ± 0.1 | γPLA/PA11 = 3.65 ± 0.7 | λijk = −2.71 | λjih = −3.16 |
| PA11 | 42.0 ± 0.4 | 35.4 ± 0.2 | 6.6 ± 0.2 | γPLA/PBAT = 2.87 ± 0.6 | λjik = −4.59 | λijh = −4.14 |
| PEO | 33.7 ± 0.2 | 25.1 ± 0.1 | 8.6 ± 0.1 | γPA11/PBAT = 2.38 ± 0.7 | λikj = 0.56 | λihj = −1.6 |
| PBAT | 53.1 ± 0.3 | 40.1 ± 0.2 | 13.0 ± 0.1 | γPLA/PEO = 1.17 ± 0.5 | Complete wetting | Partial wetting |
| γPA11/PEO = 2.10 ± 0.6 | PLA (i), PA11 (j), PEO (k) and PBAT (h) | |||||
| Sample Code | Tensile Strength (MPa) | Tensile Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PLA | 64.5 (0.4) | 1190 (25) | 6.1 (2.5) |
| PA11 | 48.3 (0.3) | 1024 (11) | 194.7 (7.3) |
| PBAT | 19.7 (0.5) | 105 (7) | 486.1 (11.5) |
| PEO | 24.6 (0.3) | 74 (5) | 516.4 (9.1) |
| Polymer | Supplier | Grade | Tm (°C) | Density (g/cm3) | MFI (g/10 min) | Chemical Structure |
|---|---|---|---|---|---|---|
| PLA | Nature Works | 2003D | 175 | 1.24 | 6 | 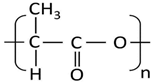 |
| PA11 | Arkema | Rilsan BMNO | 178 | 1.03 | 11 | 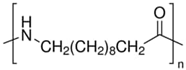 |
| PBAT | TUHNE | TH801T | 116 | 1.21 | 4.5 |  |
| PEO | Dow Chemicals | Polyox WSR-N10 | 65 | 1.13 | 2.5 | 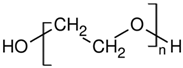 |
| Sample Code | PLA (wt %) | PA11 (wt %) | PBAT (wt %) | PEO (wt %) |
|---|---|---|---|---|
| PLA | 100 | - | - | - |
| PL70/PA30 | 70 | 30 | - | - |
| PL50/PA50 | 50 | 50 | - | - |
| PA25(PBAT10) | 65 | 25 | 10 | - |
| PA20/(PBAT20) | 60 | 20 | 20 | - |
| PA25/(PEO10) | 65 | 25 | - | 10 |
| PA20/(PEO20) | 60 | 20 | - | 20 |
| PA45/(PBAT10*) | 45 | 45 | 10 | - |
| PA40/(PBAT20*) | 40 | 40 | 20 | - |
| PA45/(PEO10*) | 45 | 45 | - | 10 |
| PA40/(PEO20*) | 40 | 40 | - | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazli, A.; Rodrigue, D. Biosourced Poly(lactic acid)/polyamide-11 Blends: Effect of an Elastomer on the Morphology and Mechanical Properties. Molecules 2022, 27, 6819. https://doi.org/10.3390/molecules27206819
Fazli A, Rodrigue D. Biosourced Poly(lactic acid)/polyamide-11 Blends: Effect of an Elastomer on the Morphology and Mechanical Properties. Molecules. 2022; 27(20):6819. https://doi.org/10.3390/molecules27206819
Chicago/Turabian StyleFazli, Ali, and Denis Rodrigue. 2022. "Biosourced Poly(lactic acid)/polyamide-11 Blends: Effect of an Elastomer on the Morphology and Mechanical Properties" Molecules 27, no. 20: 6819. https://doi.org/10.3390/molecules27206819
APA StyleFazli, A., & Rodrigue, D. (2022). Biosourced Poly(lactic acid)/polyamide-11 Blends: Effect of an Elastomer on the Morphology and Mechanical Properties. Molecules, 27(20), 6819. https://doi.org/10.3390/molecules27206819







