Comparison of the Main Metabolites in Different Maturation Stages of Camelliavietnamensis Huang Seeds
Abstract
1. Introduction
2. Results
2.1. Metabolite Detection in Seeds from C. vietnamensis at Different Maturation Stages
2.2. Multivariate Analysis of Seeds in C. vietnamensis
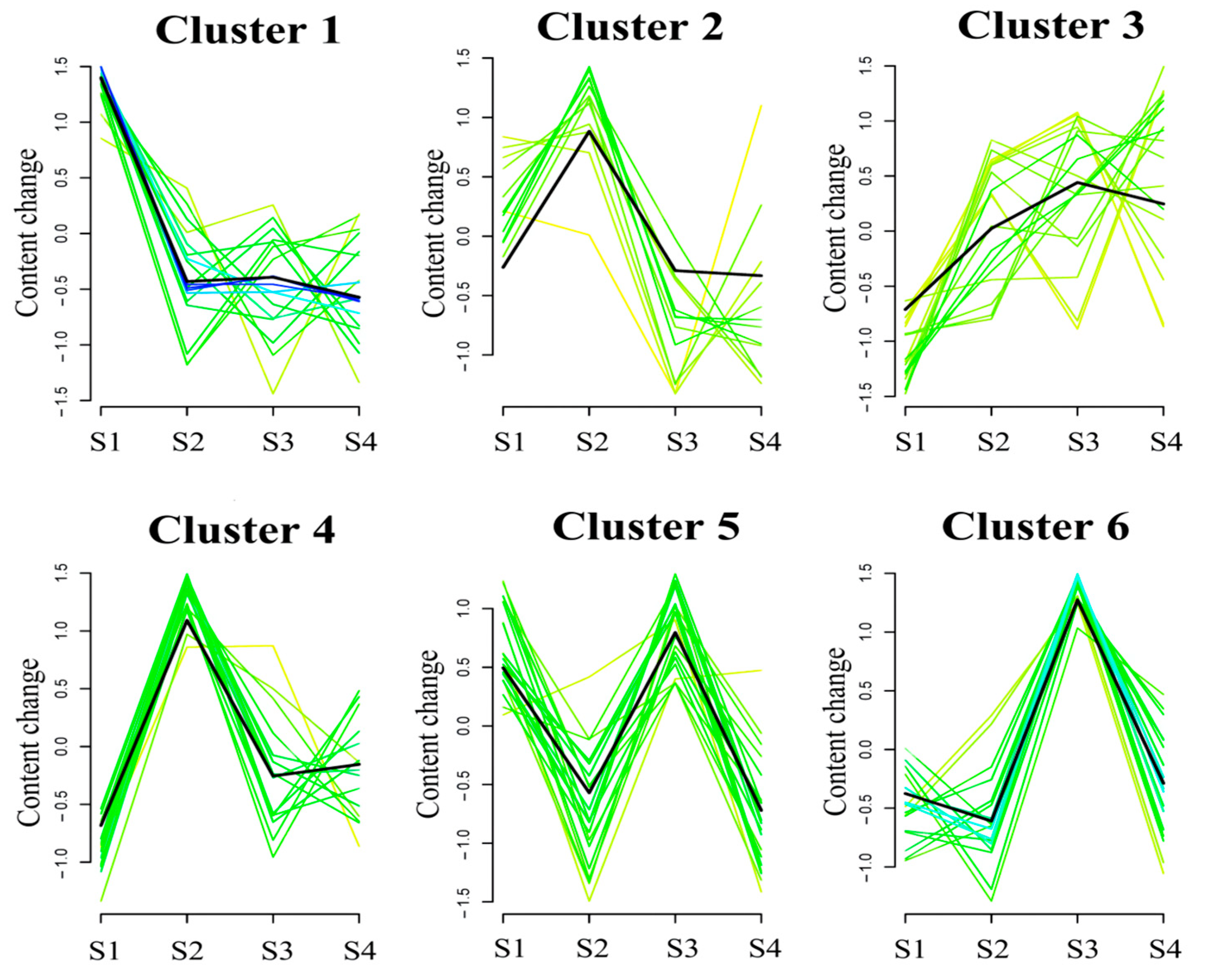
2.3. Global Trend Analysis of Metabolites during Seed Maturation in C. vietnamensis
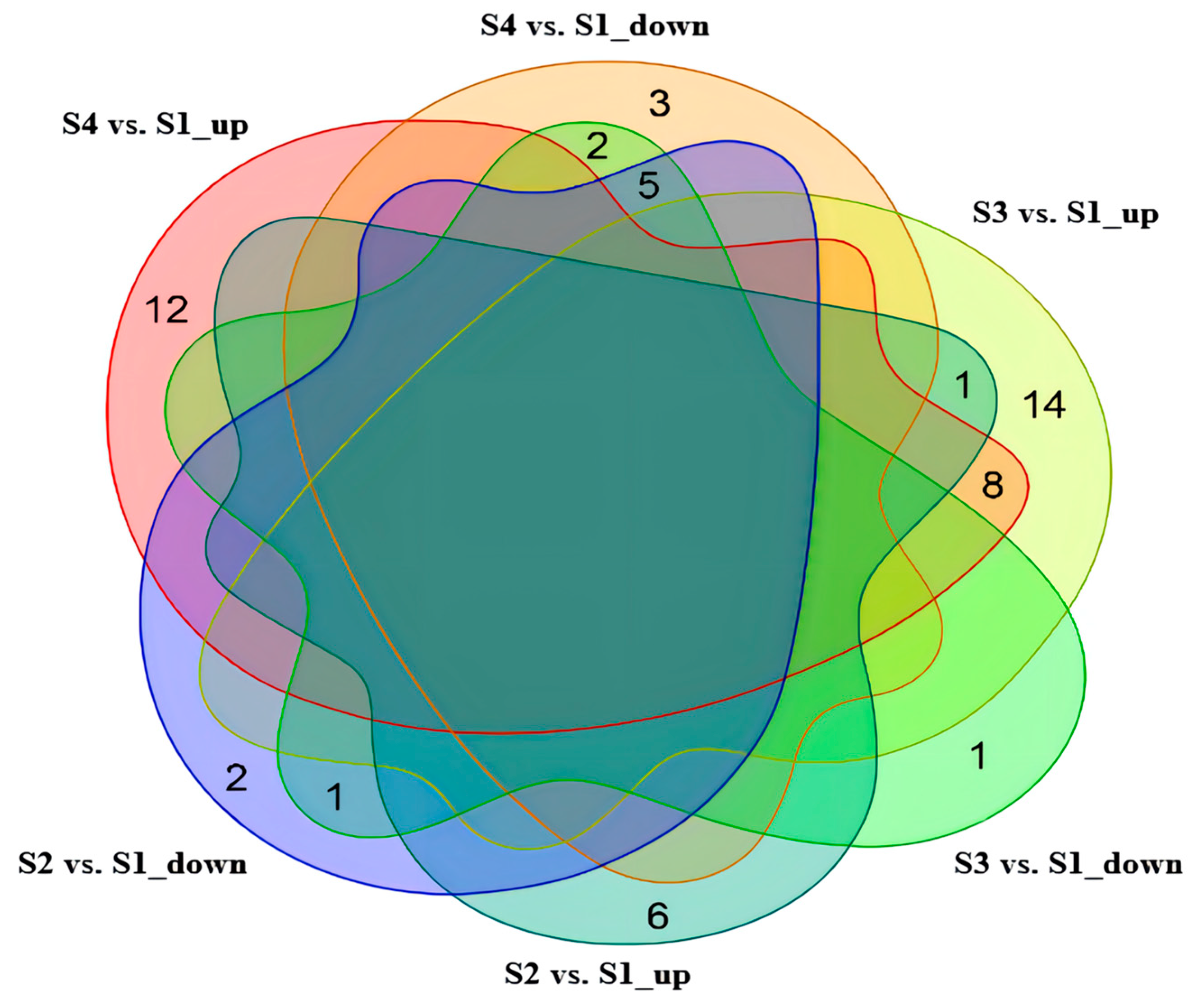
2.4. Identification of Differential Metabolites
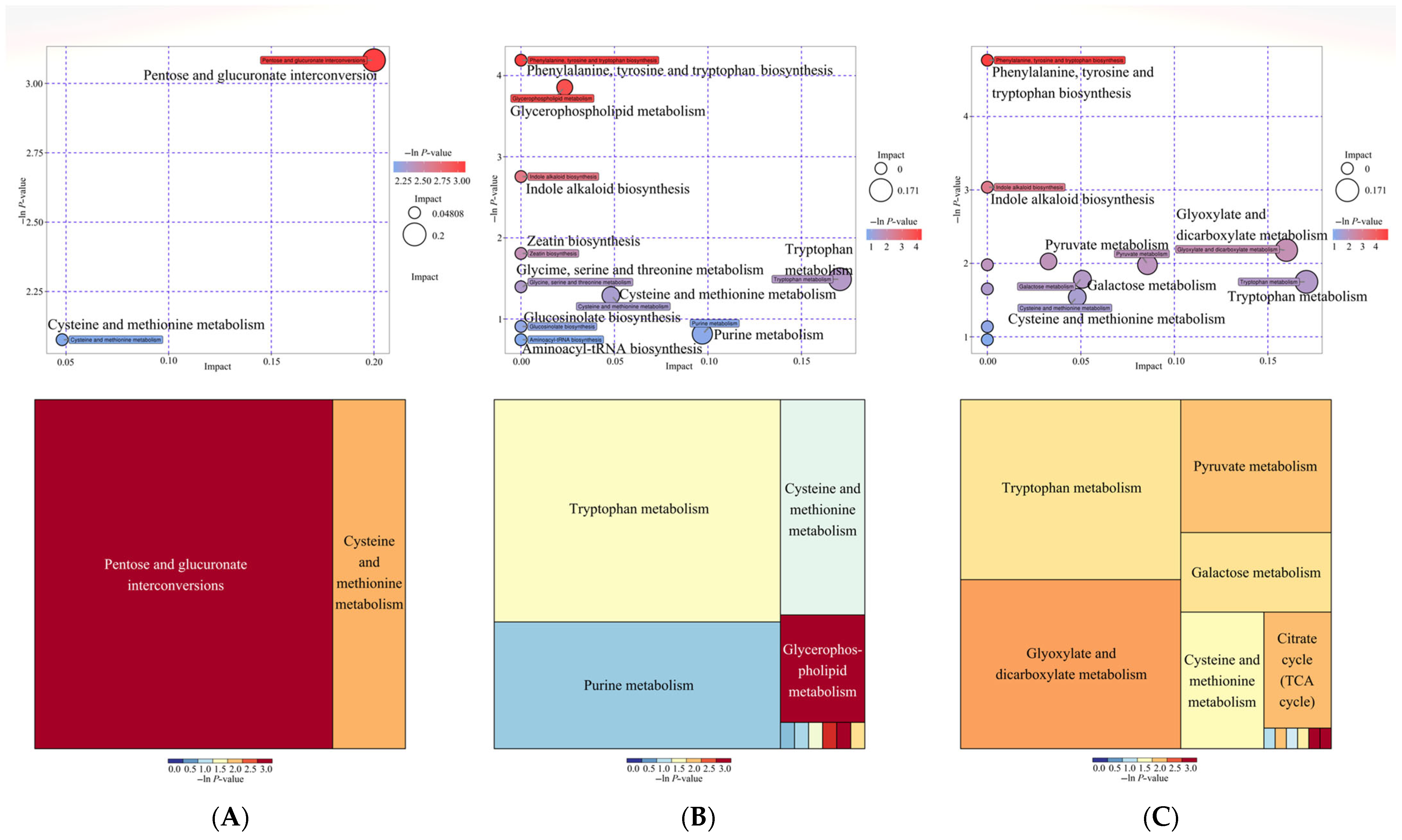
2.5. Analysis of Differential KEGG Pathway
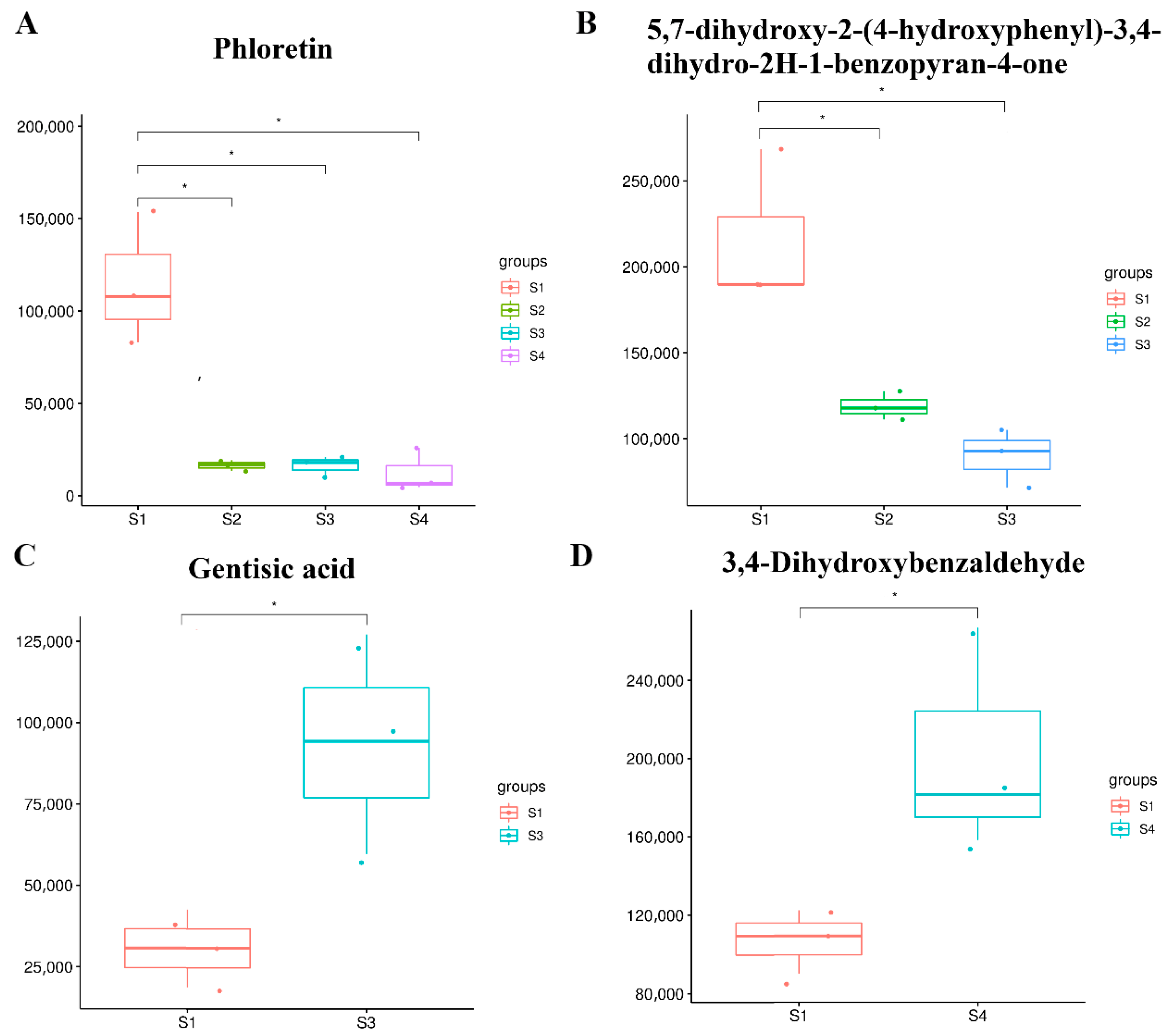
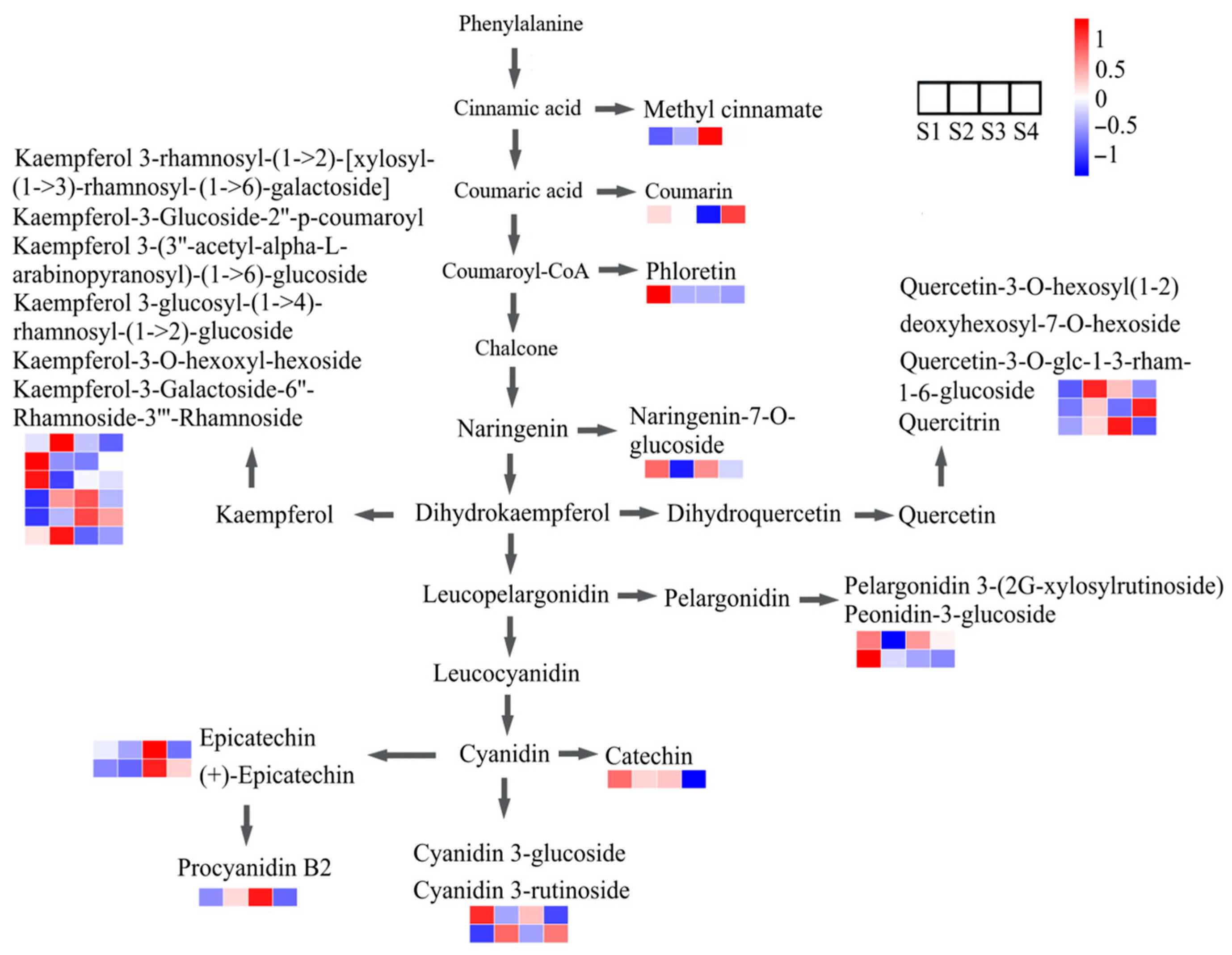
2.6. Identification and Analysis of Flavonoids at Different Seed Maturation Stages
3. Discussion
3.1. General Features of Metabolites during Seed Development of C. Vietnamensis
3.2. Camellia Oil of the Fruit Ripening Period in C. vietnamensis
3.3. Nutritional Components of the Fruit Ripening Period in C. vietnamensis
4. Materials and Methods
4.1. Plant Materials
4.2. Sample Preparation
4.3. UHPLC/Q-TOF-MS Analysis
4.4. Data Preprocessing and Annotation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, J.L.; Yang, Z.J.; Chen, S.P.; El-Kassaby, Y.A.; Chen, H. High throughput sequencing of small RNAs reveals dynamic microRNAs expression of lipid metabolism during Camellia oleifera and C. meiocarpa seed natural drying. BMC Genom. 2017, 18, 546. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.C. Bioactivities of water-soluble polysaccharides from fruit shell of Camellia oleifera Abel: Antitumor and antioxidant activities. Carbohyd. Polym. 2012, 87, 2198–2201. [Google Scholar] [CrossRef]
- Zhu, G.F.; Liu, H.; Xie, Y.C.; Liao, Q.; Lin, Y.W.; Liu, Y.H.; Liu, Y.H.; Xiao, H.W.; Cao, Z.J.; Hu, S.Z. Postharvest Processing and Storage Methods for Camellia oleifera Seeds. Food Rev. Int. 2020, 36, 319–339. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Wu, Y.G.; Ul Haq Muhammad, Z.; Yan, W.P.; Yu, J.; Zhang, J.F.; Yao, G.L.; Hu, X.W. Complementary transcriptome and proteome profiling in the mature seeds of Camellia oleifera from Hainan Island. PLoS ONE 2020, 15, e0226888. [Google Scholar] [CrossRef]
- Ye, Z.C.; Yu, J.; Yan, W.P.; Zhang, J.F.; Yang, D.M.; Yao, G.L.; Liu, Z.J.; Wu, Y.G.; Hou, X.L. Integrative iTRAQ-based proteomic and transcriptomic analysis reveals the accumulation patterns of key metabolites associated with oil quality during seed ripening of Camellia oleifera. Hortic. Res.-Engl. 2021, 8, 157. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.J.; Hu, X.W.; Zhou, K.B. Comparison of the chloroplast genome sequences of 13 oil-tea Camellia samples and identification of an undetermined oil-tea Camellia species from Hainan province. Front. Plant Sci. 2022, 12, 798581. [Google Scholar] [CrossRef]
- Dai, J.N.; Zheng, W.; Yu, J.; Yan, H.Q.; Wang, Y.; Wu, Y.G.; Hu, X.W.; Lai, H.G. cDNA cloning, prokaryotic expression, and functional analysis of squalene synthase (SQS) in Camellia vietnamensis Huang. Protein Express Purif. 2022, 194, 106078. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Shi, Y.; Zhang, Q.; Wang, J.; Ruan, J. Prediction of Chinese green tea ranking by metabolite profiling using ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UPLC–Q-TOF/MS). Food Chem. 2017, 221, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shen, Y.; Ling, T.; Ho, C.-T.; Li, D.; Guo, H.; Xie, Z. Analysis of Differentiated Chemical Components between Zijuan Purple Tea and Yunkang Green Tea by UHPLC-Orbitrap-MS/MS Combined with Chemometrics. Foods 2021, 10, 1070. [Google Scholar] [CrossRef]
- Deng, W.W.; Han, J.Y.; Fan, Y.B.; Tai, Y.L.; Zhu, B.Y.; Lu, M.Q.; Wang, R.J.; Wan, X.C.; Zhang, Z.Z. Uncovering tea-specific secondary metabolism using transcriptomic and metabolomic analyses in grafts of Camellia sinensis and C. oleifera. Tree Genet. Genom. 2018, 14, 23. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhao, X.; Li, X.; Shan, W.; Wang, X.; Wang, S.; Yu, W.; Yang, Z.; Yu, X. Non-targeted metabolomics analysis reveals dynamic changes of volatile and non-volatile metabolites during oolong tea manufacture. Food Res. Int. 2020, 128, 108778. [Google Scholar] [CrossRef]
- Wu, H.L.; Huang, W.J.; Chen, Z.J.; Chen, Z.; Shi, J.F.; Kong, Q.; Sun, S.L.; Jiang, X.H.; Chen, D.; Yan, S.J. GC-MS-based metabolomic study reveals dynamic changes of chemical compositions during black tea processing. Food Res. Int. 2019, 120, 330–338. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, Q.L.; Yang, Q.; Yan, X.X.; Feng, S.X.; Wang, Z.N. Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis. Molecules 2020, 25, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, H.; Xu, J.; Xu, J.D.; Li, Z.L.; Wu, J.; Zou, Y.T.; Liu, L.F.; Li, S.L. UPLC-QTOF-MS/MS-guided isolation and purification of sulfur-containing derivatives from sulfur-fumigated edible herbs, a case study on ginseng. Food Chem. 2018, 246, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, Y.S.; Chang, W.S.; Moon, J.K.; Choung, M.G. Seed maturity differentially mediates metabolic responses in black soybean. Food Chem. 2013, 141, 2052–2059. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.P.; Wu, Z.H.; Peng, Y.; Xie, Y.J. Analyses of seed development of Plukenetia volubilis by joint metabolomics and transcriptomics approaches. Sci. Silvae Sin. 2019, 55, 169–179. [Google Scholar] [CrossRef]
- Yang, C.; Wu, P.; Yao, X.; Sheng, Y.; Zhang, C.; Lin, P.; Wang, K. Integrated Transcriptome and Metabolome Analysis Reveals Key Metabolites Involved in Camellia oleifera Defense against Anthracnose. Int. J. Mol. Sci. 2022, 23, 536. [Google Scholar] [CrossRef]
- He, Y.; Chen, R.; Yang, Y.; Liang, G.; Zhang, H.; Deng, X.; Xi, R. Sugar Metabolism and Transcriptome Analysis Reveal Key Sugar Transporters during Camellia oleifera Fruit Development. Int. J. Mol. Sci. 2022, 23, 822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Chen, X.; Qu, H.M. Synthesis mediated by zirconium, crystal structure and theoretical studies on six-substituted benzene with silalactone structure and butadiene derivative. Chin. J. Struct. Chem. 2018, 37, 905–914. [Google Scholar] [CrossRef]
- Dayan, F.; Howell, J.; Marais, J.; Ferreira, D.; Koivunen, M. Manuka Oil, A Natural Herbicide with Preemergence Activity. Weed Sci. 2011, 59, 464–469. [Google Scholar] [CrossRef]
- Baud, S.; Lepiniec, L. Regulation of de novo fatty acid synthesis in maturing oilseeds of Arabidopsis. Plant Physiol. Biochem. 2009, 47, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.F.; Lu, J.; Zhang, Z.; Ma, L.; Liu, C.X.; Chen, Y.Z. Global transcriptome and correlation analysis reveal cultivar-specific molecular signatures associated with fruit development and fatty acid determination in Camellia oleifera Abel. Int. J. Genom. 2020, 2020, 6162802. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sakurai, T.; Chen, Z.; Furukawa, T.; Gowda, S.G.B.; Wu, Y.; Nouso, K.; Fujii, Y.; Yoshikawa, Y.; Chiba, H.; et al. Analysis of serum lysophosphatidylethanolamine levels in patients with non-alcoholic fatty liver disease by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 245–254. [Google Scholar] [CrossRef]
- Jiang, Y.; Qu, K.; Liu, J.C.; Wen, Y.; Duan, B.H. Metabolomics study on liver of db/db mice treated with curcumin using UPLC-Q-TOF-MS. J. Pharm. Biomed. 2022, 215, 114771. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Yin, T.L.; Chen, Y.; Li, Y.H.; Yin, L.; Ding, J.L.; Yang, J.; Feng, H.-L. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. 2019, 185, 142–149. [Google Scholar] [CrossRef]
- Miao, H.; Chen, H.; Pei, S.W.; Bai, X.; Vaziri, N.D.; Zhao, Y.Y. Plasma lipidomics reveal profound perturbation of glycerophospholipids, fatty acids, and sphingolipids in diet-induced hyperlipidemia. Chem.-Biol. Interact. 2015, 228, 79–87. [Google Scholar] [CrossRef]
- Lin, P.; Wang, K.; Zhou, C.; Xie, Y.; Yao, X.; Yin, H. Seed transcriptomics analysis in Camellia oleifera uncovers genes associated with oil content and fatty acid composition. Int. J. Mol. Sci. 2018, 19, 118. [Google Scholar] [CrossRef]
- Baud, S.; Boutin, J.P.; Miquel, M.; Lepiniec, L.; Rochat, C. An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 2002, 40, 151–160. [Google Scholar] [CrossRef]
- Bvttner, M. The Arabidopsis sugar transporter (AtSTP) family: An update. Plant Biol. 2010, 12, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Periappuram, C.; Steinhauer, L.; Barton, D.L.; Taylor, D.C.; Chatson, B.; Zou, J.T. The plastidic phosphoglucomutase from arabidopsis. A reversible enzyme reaction with an important role in metabolic control. Plant Physiol. 2000, 122, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.B.; Lopez de Biondi, A.C.; Disalvo, E.A. Dehydration of carbonyls and phosphates of phosphatidylcholines determines the lytic action of lysoderivatives. Chem. Phys. Lipids 2003, 122, 153–157. [Google Scholar] [CrossRef]
- Liu, D.M.; Yu, X.; Sun, H.Y.; Zhang, W.; Liu, G.; Zhu, L. Flos lonicerae flavonoids attenuate experimental ulcerative colitis in rats via suppression of NF-κB signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2481–2494. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Li, X.Q.; Jin, S.L.; Zhang, H.Z.; Wang, R.; Pei, L.H.; Zheng, J. The anti-proliferative effect of flavonoid nanoparticles on the human ovarian cancer cell line SK0V3. J. Nanosci. Nanotechnol. 2020, 20, 6040–6046. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Jia, L.Y.; Gao, Y.; Li, B.; Tu, Y.Y. Anti-inflammatory activity of total flavonoids from seeds of Camellia oleifera Abel. Acta Biochim. Biophys. Sin. 2014, 46, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Liu, M.H.; Tan, T.; Yan, A.P.; Guo, L.; Jiang, K.; Tan, C.H.; Wan, Y.Q. Deep eutectic solvents used as extraction solvent for the determination of flavonoids from Camellia oleifera flowers by high-performance liquid chromatography. Phytochem. Anal. 2018, 29, 639–648. [Google Scholar] [CrossRef]
- Han, L.L.; Yuan, Z.H.; Feng, L.J.; Yin, Y. Changes in the composition and contents of pomegranate polyphenols during fruit development. Acta Hortic. 2015, 1089, 53–61. [Google Scholar] [CrossRef]
- Dare, A.P.; Yauk, Y.K.; Tomes, S.; Mcghie, T.K.; Rebstock, R.S.; Rebstock, R.S.; Atkinson, R.G. Silencing a phloretin-specific glycosyltransferase perturbsboth general phenylpropanoid biosynthesis and plant development. Plant J. Cell Mol. Biol. 2017, 91, 237–250. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.X.; Yang, Z.Y.; Rao, J.; Chen, B. Improving antioxidant activity of β-lactoglobulin by nature-inspired conjugation with gentisic acid. J. Agric. Food Chem. 2019, 67, 11741–11751. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.X.; Li, C.; Yang, Z.Y.; Rao, J.J.; Chen, B.C. Design, synthesis and characterization of lysozyme–gentisic acid dual-functional conjugates with antibacterial/antioxidant activities. Food Chem. 2022, 370, 131032. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, N.; Mahmood, R. 3,4-Dihydroxybenzaldehyde attenuates pentachlorophenol-induced cytotoxicity, DNA damage and collapse of mitochondrial membrane potential in isolated human blood cells. Drug Chem. Toxicol. 2022, 45, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Tong, T.T.; Ren, N.; Tu, Y.Y.; Li, B. Saponins from seeds of Genus Camellia: Phytochemistry and bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Pan, Y.G.; Zheng, L.L.; Yang, Y.; Zheng, X.Y.; Ai, B.L.; Xu, Z.M.; Sheng, Z.W. Application of steam explosion in oil extraction of camellia seed (Camellia oleifera Abel.) and evaluation of its physicochemical properties, fatty acid, and antioxidant activities. Food Sci. Nutr. 2019, 7, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Yang, C.-H.; Chang, M.-S.; Ciou, Y.-P.; Huang, Y.-C. Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiong, L.Z.; Luo, J. A Novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Luo, P.; Yin, P.Y.; Zhang, W.J.; Zhou, L.N.; Lu, X.; Lin, X.H.; Xu, G.W. Optimization of large-scale pseudotargeted metabolomics method based on liquid chromatography-mass spectrometry. J. Chromatogr. A 2016, 1437, 127–136. [Google Scholar] [CrossRef]
- Zha, H.B.; Cai, Y.P.; Yin, Y.D.; Wang, Z.Z.; Li, K.; Zhu, Z.J. SWATH to MRM: Development of high-coverage targeted metabolomics method using SWATH technology for biomarker discovery. Anal. Chem. 2018, 90, 4062–4070. [Google Scholar] [CrossRef]
- Kuhl, C.; Tautenhahn, R.; Böttcher, C.; Larson, T.R.; Neumann, S. CAMERA: An integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Zheng, W.; Ye, Z.; Yu, J.; Wu, Y. Comparison of the Main Metabolites in Different Maturation Stages of Camelliavietnamensis Huang Seeds. Molecules 2022, 27, 6817. https://doi.org/10.3390/molecules27206817
Yan H, Zheng W, Ye Z, Yu J, Wu Y. Comparison of the Main Metabolites in Different Maturation Stages of Camelliavietnamensis Huang Seeds. Molecules. 2022; 27(20):6817. https://doi.org/10.3390/molecules27206817
Chicago/Turabian StyleYan, Heqin, Wei Zheng, Zhouchen Ye, Jing Yu, and Yougen Wu. 2022. "Comparison of the Main Metabolites in Different Maturation Stages of Camelliavietnamensis Huang Seeds" Molecules 27, no. 20: 6817. https://doi.org/10.3390/molecules27206817
APA StyleYan, H., Zheng, W., Ye, Z., Yu, J., & Wu, Y. (2022). Comparison of the Main Metabolites in Different Maturation Stages of Camelliavietnamensis Huang Seeds. Molecules, 27(20), 6817. https://doi.org/10.3390/molecules27206817






