Effect of the Application Time of Accentuated Cut Edges (ACE) on Marquette Wine Phenolic Compounds
Abstract
:1. Introduction
2. Results
2.1. Basic Chemical Properties
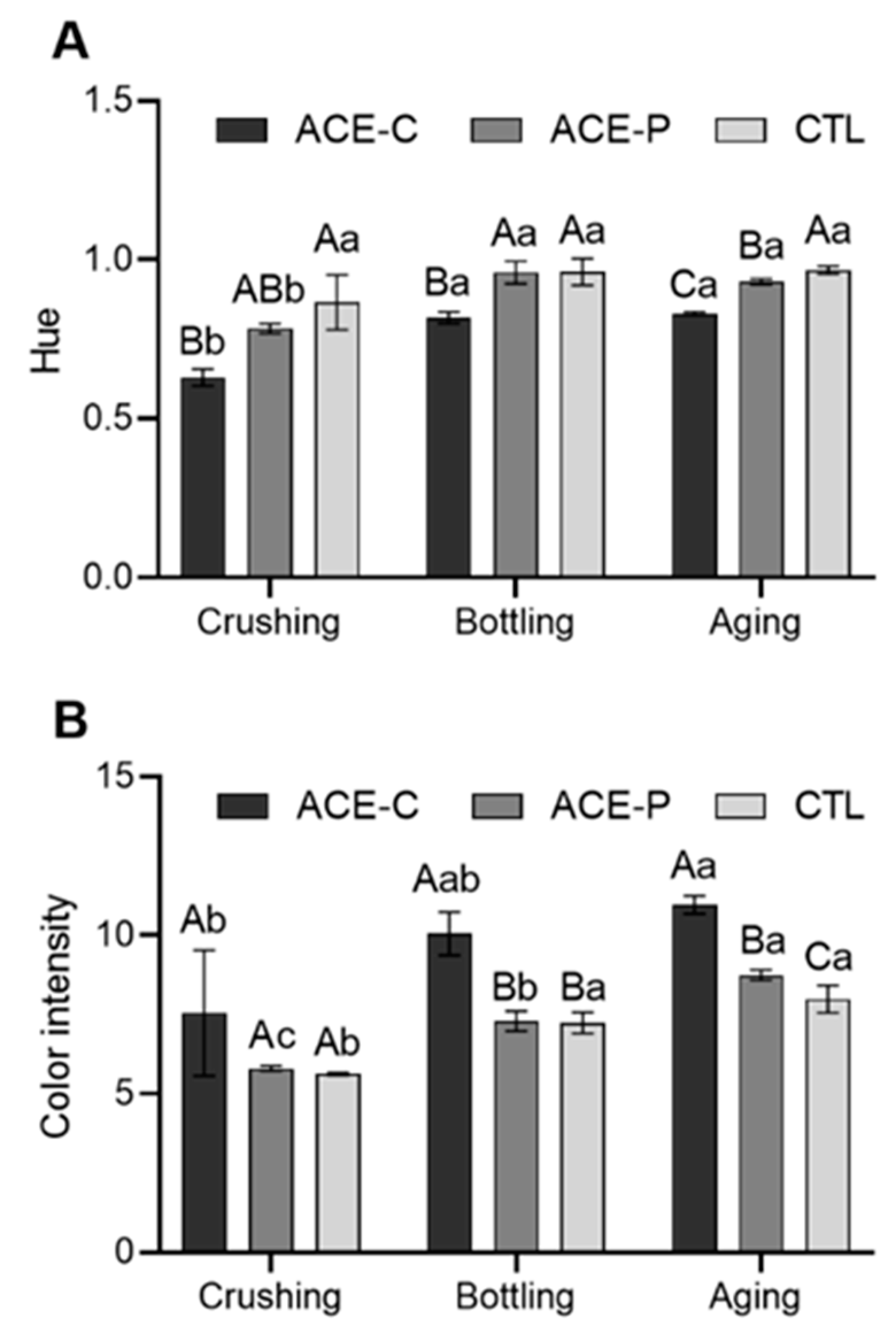
2.2. Color Intensity and Hue
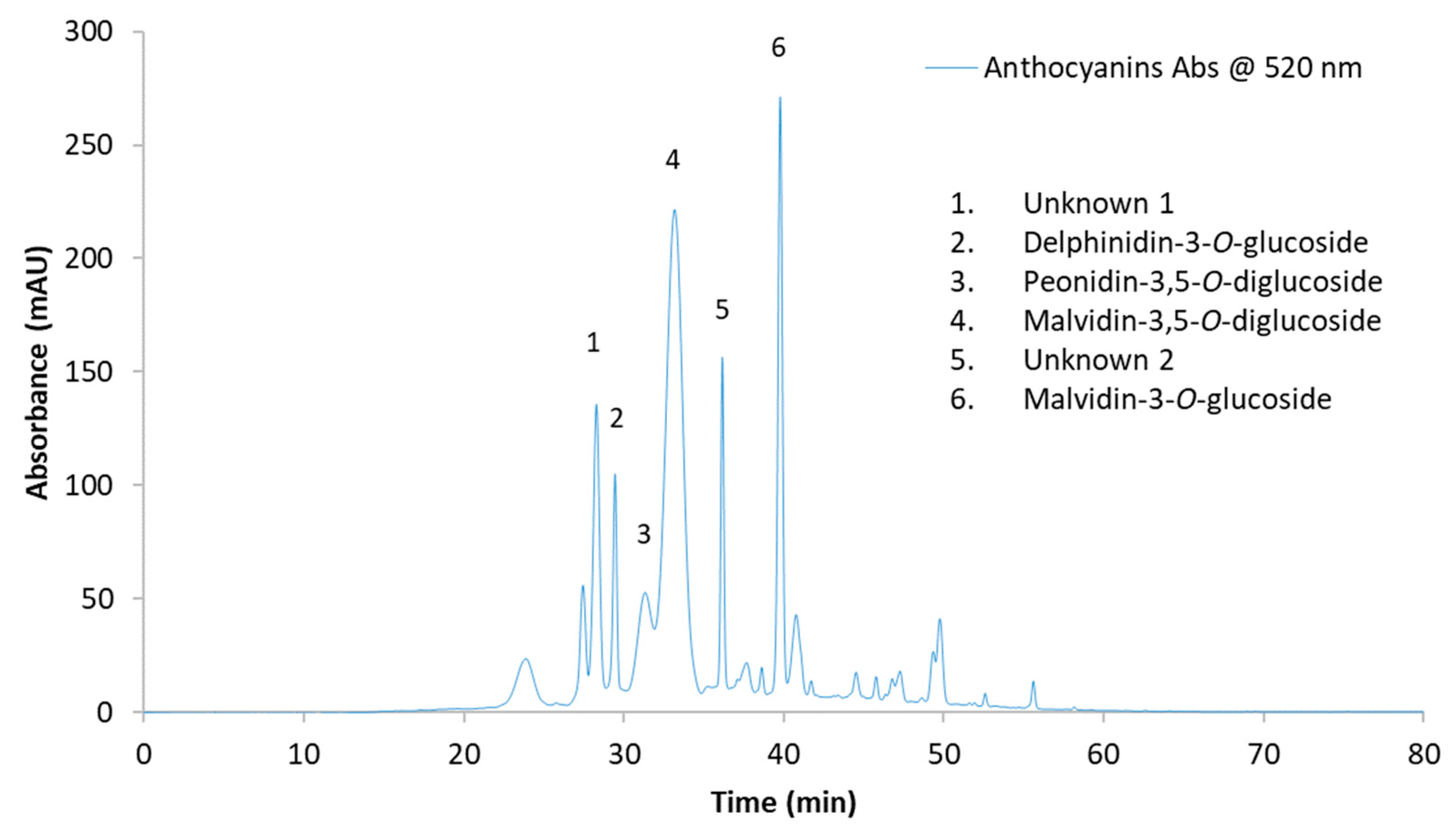
2.3. Monomeric Phenolic Compounds
2.4. Iron-Reactive Phenolic Compounds and Tannins Content
3. Discussion
4. Materials and Methods
4.1. Chemicals and Standards
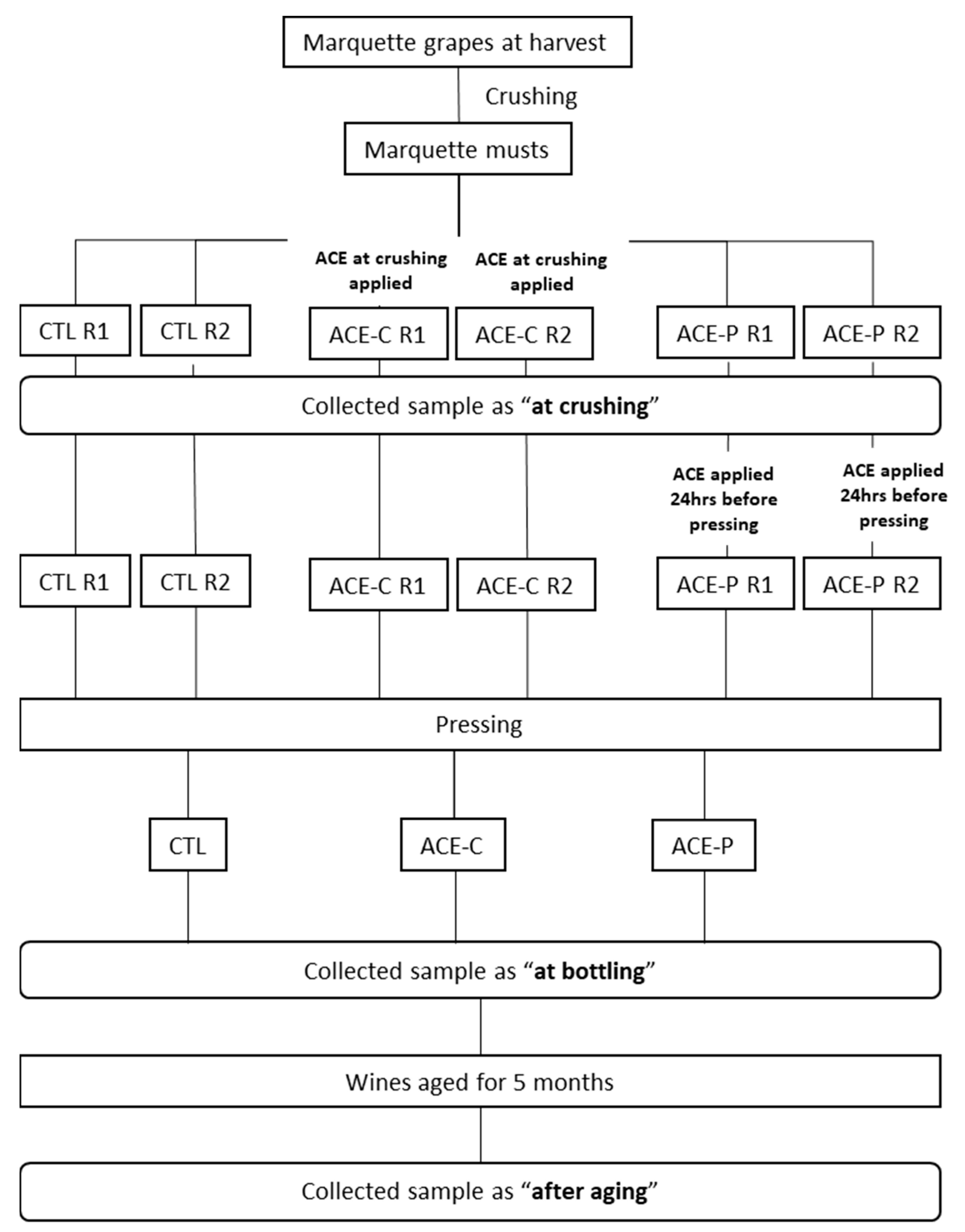
4.2. Winemaking Protocol
4.3. Must and Wine Chemical Properties
4.4. Color Analysis
4.5. Monomeric Phenolic Compound Analysis
4.6. Total Iron-Reactive Phenolic Compounds and Condensed Tannin Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- De Freitas, V.A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. OENO One 2017, 51, 1–15. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar]
- Lingua, M.S.; Fabani, M.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- Burtch, C.E.; Mansfield, A.K.; Manns, D.C. Reaction Kinetics of Monomeric Anthocyanin Conversion to Polymeric Pigments and Their Significance to Color in Interspecific Hybrid Wines. J. Agric. Food Chem. 2017, 65, 6379–6386. [Google Scholar] [CrossRef]
- Rivas-Gonzalo, J.-C.; Bravo-Haro, S.; Santos-Buelga, C. Detection of Compounds Formed through the Reaction of Malvidin 3-Monoglucoside and Catechin in the Presence of Acetaldehyde. J. Agric. Food Chem. 1995, 43, 1444–1449. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roca, J.M.; Gil-Muñoz, R.; Bautista-Ortín, A.B. The Extraction of Anthocyanins and Proanthocyanidins from Grapes to Wine during Fermentative Maceration Is Affected by the Enological Technique. J. Agric. Food Chem. 2011, 59, 5450–5455. [Google Scholar] [CrossRef]
- Kassara, S.; Kennedy, J.A. Relationship between Red Wine Grade and Phenolics. 2. Tannin Composition and Size. J. Agric. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, K.; Provost, C. Fungus resistant grape varieties as a suitable alternative for organic wine production: Benefits, limits, and challenges. Sci. Hortic. 2016, 208, 57–77. [Google Scholar] [CrossRef]
- Manns, D.C.; Lenerz, C.T.M.C.; Mansfield, A.K. Impact of Processing Parameters on the Phenolic Profile of Wines Produced from Hybrid Red Grapes Maréchal Foch, Corot noir, and Marquette. J. Food Sci. 2013, 78, C696–C702. [Google Scholar] [CrossRef] [PubMed]
- Springer, L.F.; Sacks, G.L. Protein-Precipitable Tannin in Wines from Vitis vinifera and Interspecific Hybrid Grapes (Vitis ssp.): Differences in Concentration, Extractability, and Cell Wall Binding. J. Agric. Food Chem. 2014, 62, 7515–7523. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, A.M.; Holt, H.E.; Pearson, W.; Dambergs, R.G.; Close, D.C. Accentuated Cut Edges (ACE): Effects of Skin Fragmentation on the Composition and Sensory Attributes of Pinot noir Wines. Am. J. Enol. Vitic. 2016, 67, 169–178. [Google Scholar] [CrossRef]
- Sparrow, A.M.; Smart, R.E.; Dambergs, R.G.; Close, D.C. Skin Particle Size Affects the Phenolic Attributes of Pinot noir Wine: Proof of Concept. Am. J. Enol. Vitic. 2015, 67, 29–37. [Google Scholar] [CrossRef]
- Kang, W.; Bindon, K.A.; Wang, X.; Muhlack, R.A.; Smith, P.A.; Niimi, J.; Bastian, S.E.P. Chemical and Sensory Impacts of Accentuated Cut Edges (ACE) Grape Must Polyphenol Extraction Technique on Shiraz Wines. Foods 2020, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.; Larsen, R.C.; Hopfer, H.; Heymann, H.; Harbertson, J.F. Influence of Fruit Maturity, Maceration Length, and Ethanol Amount on Chemical and Sensory Properties of Merlot Wines. Am. J. Enol. Vitic. 2013, 64, 437–449. [Google Scholar] [CrossRef]
- Jensen, J.S.; Werge, H.H.M.; Egebo, M.; Meyer, A.S. Effect of Wine Dilution on the Reliability of Tannin Analysis by Protein Precipitation. Am. J. Enol. Vitic. 2008, 59, 103–105. [Google Scholar]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [Green Version]
- González-Neves, G.; Gi, lG.; Barreiro, L. Influence of grape variety on the extraction of anthocyanins during the fermentation on skins. Eur. Food Res. Technol. 2008, 226, 1349–1355. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, A.B.; Suárez, J.A. Adsorption of Anthocyanins by Yeast Cell Walls during the Fermentation of Red Wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.; Gómez-Cordovés, C.; Martín-Álvarez, P. Evolution of red wine anthocyanins during malolactic fermentation, postfermentative treatments and ageing with lees. Food Chem. 2008, 109, 149–158. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M. Interactions between Pectic Compounds and Procyanidins are Influenced by Methylation Degree and Chain Length. Biomacromolecules 2013, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Springer, L.F.; Sherwood, R.W.; Sacks, G.L. Pathogenesis-Related Proteins Limit the Retention of Condensed Tannin Additions to Red Wines. J. Agric. Food Chem. 2016, 64, 1309–1317. [Google Scholar] [CrossRef]
- Bindon, K.A.; Li, S.; Kassara, S.; Smith, P.A. Retention of Proanthocyanidin in Wine-like Solution Is Conferred by a Dynamic Interaction between Soluble and Insoluble Grape Cell Wall Components. J. Agric. Food Chem. 2016, 64, 8406–8419. [Google Scholar] [CrossRef]
- Nicolle, P.; Marcotte, C.; Angers, P.; Pedneault, K. Pomace limits tannin retention in Frontenac wines. Food Chem. 2019, 277, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Ritchey, J.G.; Waterhouse, A.L. A Standard Red Wine: Monomeric Phenolic Analysis of Commercial Cabernet Sauvignon Wines. Am. J. Enol. Vitic. 1999, 50, 91–100. [Google Scholar]
- Watrelot, A.A.; Badet-Murat, M.-L.; Waterhouse, A.L. Oak barrel tannin and toasting temperature: Effects on red wine condensed tannin chemistry. LWT 2018, 91, 330–338. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of Polymeric Pigments in Grape Berry Extracts and Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar]
- Heredia, T.M.; Adams, D.O.; Fields, K.C.; Held, P.G.; Harbertson, J.F. Evaluation of a Comprehensive Red Wine Phenolics Assay Using a Microplate Reader. Am. J. Enol. Vitic. 2006, 4, 497–502. [Google Scholar]

| Time Point | Treatment | °Brix | pH | TA 1 (g/L) | Ethanol (vol %) | Tartaric Acid (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) |
|---|---|---|---|---|---|---|---|---|
| Crushing | ACE-C | 25.75 ± 0.07 A 2 | 3.32 ± 0.02 Ab 3 | 13.31 ± 0.53 Aa | - | 4.94 ± 0.43 Aa | 10.06 ± 0.11 A | - |
| ACE-P | 24.85 ± 0.64 A | 3.33 ± 0.01 Ab | 12.09 ± 1.19 Aa | - | 4.72 ± 0.23 Aa | 10.04 ± 0.31 A | - | |
| CTL | 25.35 ± 0.07 A | 3.31 ± 0.01 Ac | 11.53 ± 1.46 Aa | - | 4.59 ± 0.02 Aa | 10.40 ± 0.16 A | - | |
| Bottling 4 | ACE-C | - | 3.81 ± 0.01 Ca | 8.25 | 14.75 | 3.67 | nd 5 | 3.37 |
| ACE-P | - | 3.91 ± 0.02 Aa | 7.50 | 13.64 | 2.89 | nd | 3.56 | |
| CTL | - | 3.90 ± 0.01 Ba | 7.69 | 13.75 | 2.95 | nd | 3.35 | |
| Aging | ACE-C | - | 3.79 ± 0.01 Ba | 8.23 ± 0.12 Ab | 14.57 ± 0.01 A | 3.61 ± 0.10 Ab | nd | 3.32 ± 0.02 C |
| ACE-P | - | 3.88 ± 0.01 Aa | 7.29 ± 0.31 Bb | 13.52 ± 0.03 C | 3.06 ± 0.10 Cb | nd | 3.35 ± 0.01 B | |
| CTL | - | 3.87 ± 0.01 Ab | 7.57 ± 0.26 Bb | 13.63 ± 0.03 B | 3.27 ± 0.04 Bb | nd | 3.40 ± 0.01 A |
| Time Point | Treatment | Unknown 1 | Delphinidin-3-O-glucoside | Peonidin-3,5-O-diglucoside | Malvidin-3,5-O-diglucoside | Unknown 2 | Malvidin-3-O-glucoside | Total Anthocyanins |
|---|---|---|---|---|---|---|---|---|
| Crushing | ACE-C | nd 3 | nd | 22.30 ± 5.61 A 1 a 2 | 92.32 ± 28.56 Ab | nd | 30.52 ± 16.04 Ab | 145.14 ± 50.22 Ac |
| ACE-P | nd | nd | 16.03 ± 0.96 Ab | 61.28 ± 6.90 Ac | nd | 13.83 ± 4.61 Ac | 91.14 ± 12.47 Ac | |
| CTL | nd | nd | 14.95 ± 1.50 Ab | 54.49 ± 3.64 Ab | nd | nq 4 | 79.32 ± 6.94 Ac | |
| Bottling | ACE-C | 38.69 ± 4.74 Aa | 25.11 ± 2.80 Aa | 29.51 ± 6.54 Aa | 175.27 ± 22.05 Aa | 34.04 ± 1.88 Aa | 56.65 ± 3.45 Aa | 359.27 ± 41.41 Aa |
| ACE-P | 36.76 ± 3.33 Aa | 18.47 ± 1.95 B | 28.55 ± 5.52 Aa | 165.93 ± 18.87 Aa | 24.78 ± 1.26 Ba | 44.83 ± 1.99 Ba | 319.31 ± 32.50 Aa | |
| CTL | 40.16 ± 3.63 Aa | 19.04 ± 1.42 B | 30.09 ± 4.82 Aa | 178.54 ± 22.86 Aa | 27.64 ± 1.43 Ba | 53.74 ± 2.98 Aa | 349.22 ± 37.09 Aa | |
| Aging | ACE-C | 30.32 ± 0.30 Bb | 17.20 ± 0.27 b | 23.47 ± 0.61 Ba | 140.93 ± 1.17 Ba | 24.77 ± 1.18 Ab | 39.95 ± 0.64 Ab | 276.65 ± 3.79 Ab |
| ACE-P | 29.13 ± 0.49 Cb | nq | 23.67 ± 1.56 Bab | 139.24 ± 1.59 Bb | 17.10 ± 0.19 Cb | 31.84 ± 0.67 Bb | 240.97 ± 3.33 Bb | |
| CTL | 32.51 ± 0.40 Ab | nq | 26.28 ± 1.46 Aa | 155.30 ± 2.70 Aa | 19.34 ± 0.82 Bb | 39.18 ± 0.91 Ab | 272.63 ± 5.57 Ab |
| Time Point | Treatment | Gallic Acid | (+)-Catechin | (-)-Epicatechin | Caftaric Acid | Quercetin-3-O-glucoside | Myricetin | Quercetin | Total Phenolics 1 |
|---|---|---|---|---|---|---|---|---|---|
| Crushing | ACE-C | 1.66 ± 2.35 c 2 | 20.44 ± 11.25 A 3b | 8.05 ± 3.94 Ab | 10.39 ± 2.46 Ab | 2.79 ± 0.99 Ab | nd 4 | nd | 43.34 ± 20.99 Ab |
| ACE-P | nd | 14.56 ± 8.96 Ab | 5.42 ± 4.26 Ab | 5.88 ± 2.40 Ab | 2.45 ± 0.37 Ab | nd | nd | 28.31 ± 15.99 Ab | |
| CTL | nd | 7.42 ± 2.33 Ab | 2.44 ± 1.04 Ab | 4.96 ± 2.91 Ab | 1.95 ± 0.40 Ac | nd | nd | 16.77 ± 5.88 Ab | |
| Bottling | ACE-C | 103.29 ± 0.49 Ab | 90.70 ± 0.96 Aa | 36.76 ± 0.51 Aa | 27.44 ± 0.03 Aa | 5.62 ± 0.09 Aa | 2.78 ± 0.00 Ab | nq 5 | 266.59 ± 1.04 Aa |
| ACE-P | 80.10 ± 0.20 Bb | 64.81 ± 1.01 Ba | 27.15 ± 0.14 Ba | 20.56 ± 0.17 Ba | 3.71 ± 0.04 Ca | 2.01 ± 0.02 Cb | nq | 198.35 ± 1.20 Ba | |
| CTL | 77.34 ± 0.45 Cb | 58.11 ± 0.20 Ca | 24.07 ± 0.19 Ca | 20.44 ± 0.00 Ba | 4.29 ± 0.06 Ba | 2.35 ± 0.00 Bb | nq | 186.61 ± 0.00 Ca | |
| Aging | ACE-C | 113.63 ± 1.95 Aa | 84.74 ± 1.16 Aa | 35.29 ± 0.36 Aa | 27.16 ± 0.03 Aa | 4.38 ± 0.02 Aa | 3.71 ± 0.03 Aa | nq—1.79 | 269.73 ± 1.15 Aa |
| ACE-P | 86.71 ± 0.83 Ba | 61.60 ± 0.56 Ba | 26.83 ± 0.38 Ba | 20.63 ± 0.15 Ba | 2.69 ± 0.12 Cb | 2.44 ± 0.02 Ca | nq—1.56 | 201.30 ± 2.54 Ba | |
| CTL | 83.29 ± 1.27 Ca | 57.85 ± 0.80 Ca | 24.93 ± 0.45 Ca | 20.05 ± 0.06 Ca | 3.23 ± 0.14 Bb | 3.05 ± 0.03 Ba | nq—2.17 | 193.45 ± 1.81 Ca |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Savits, J.R.; Watrelot, A.A. Effect of the Application Time of Accentuated Cut Edges (ACE) on Marquette Wine Phenolic Compounds. Molecules 2022, 27, 542. https://doi.org/10.3390/molecules27020542
Cheng Y, Savits JR, Watrelot AA. Effect of the Application Time of Accentuated Cut Edges (ACE) on Marquette Wine Phenolic Compounds. Molecules. 2022; 27(2):542. https://doi.org/10.3390/molecules27020542
Chicago/Turabian StyleCheng, Yiliang, Jennifer Rae Savits, and Aude Annie Watrelot. 2022. "Effect of the Application Time of Accentuated Cut Edges (ACE) on Marquette Wine Phenolic Compounds" Molecules 27, no. 2: 542. https://doi.org/10.3390/molecules27020542
APA StyleCheng, Y., Savits, J. R., & Watrelot, A. A. (2022). Effect of the Application Time of Accentuated Cut Edges (ACE) on Marquette Wine Phenolic Compounds. Molecules, 27(2), 542. https://doi.org/10.3390/molecules27020542






