In Vitro Anti-Inflammatory Activity of Essential Oil and β-Bisabolol Derived from Cotton Gin Trash
Abstract
:1. Introduction
2. Results
2.1. Composition of CGT Oil
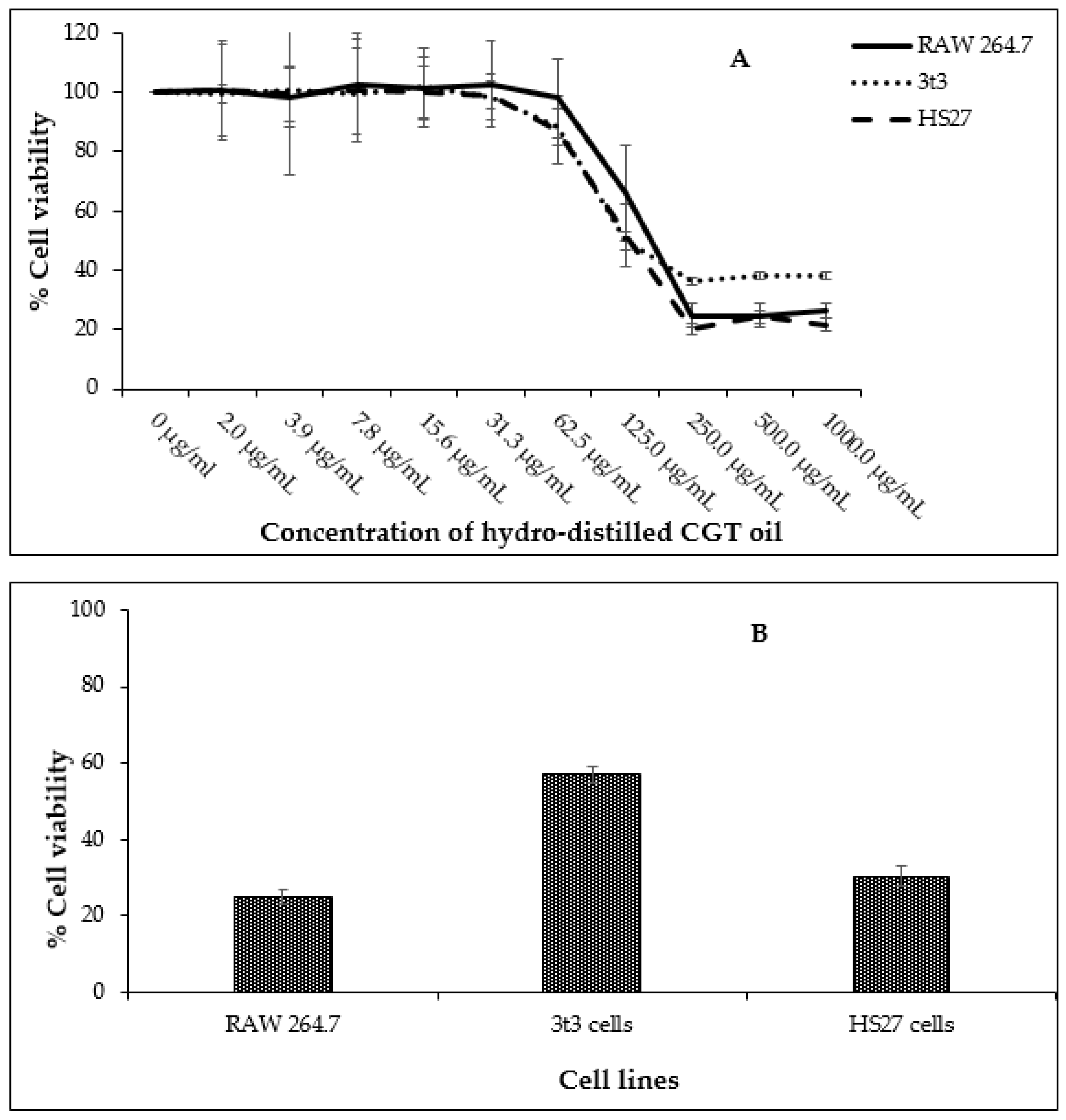
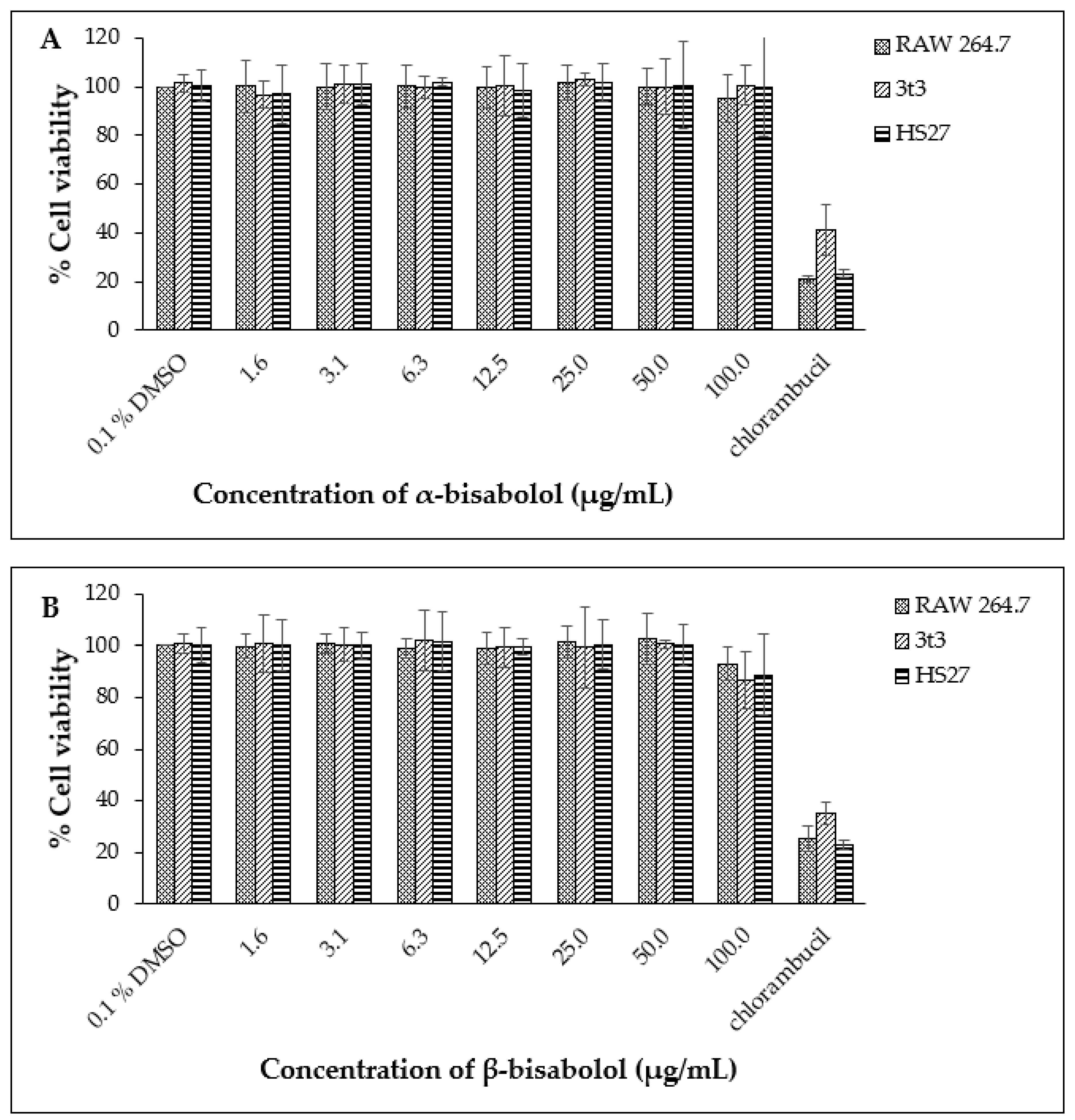
2.2. Effect of CGT Oil and Isolated Compound (β-Bisabolol) on Cell Viability
2.3. Nitric Oxide Inhibition of CGT Oil and β-Bisabolol on RAW 264.7 Cells
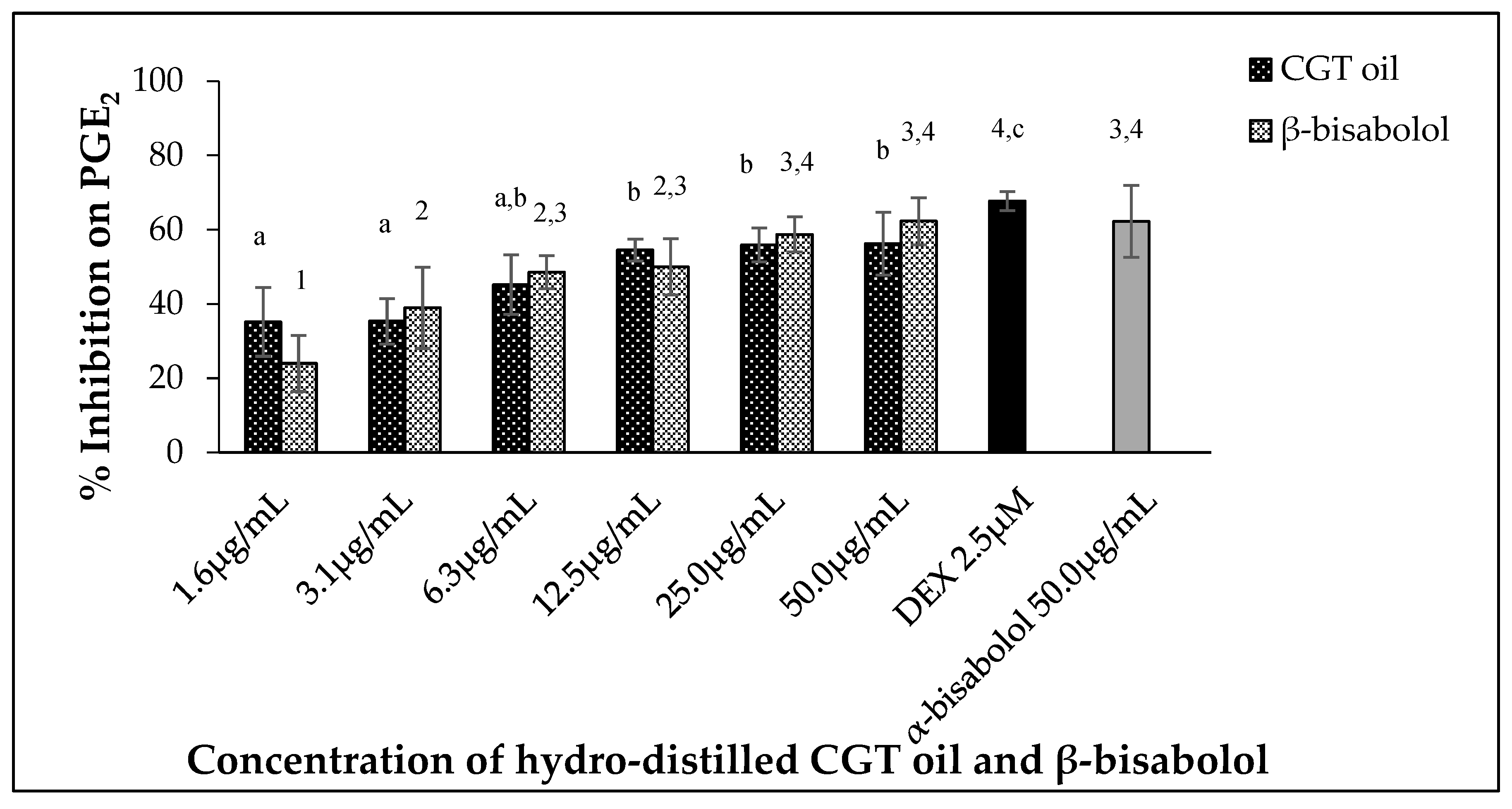
2.4. Inhibition of PGE2 Production by CGT Oil and β-Bisabolol
2.5. CGT Oil and β-Bisabolol Inhibit Pro-Inflammatory Cytokine Production
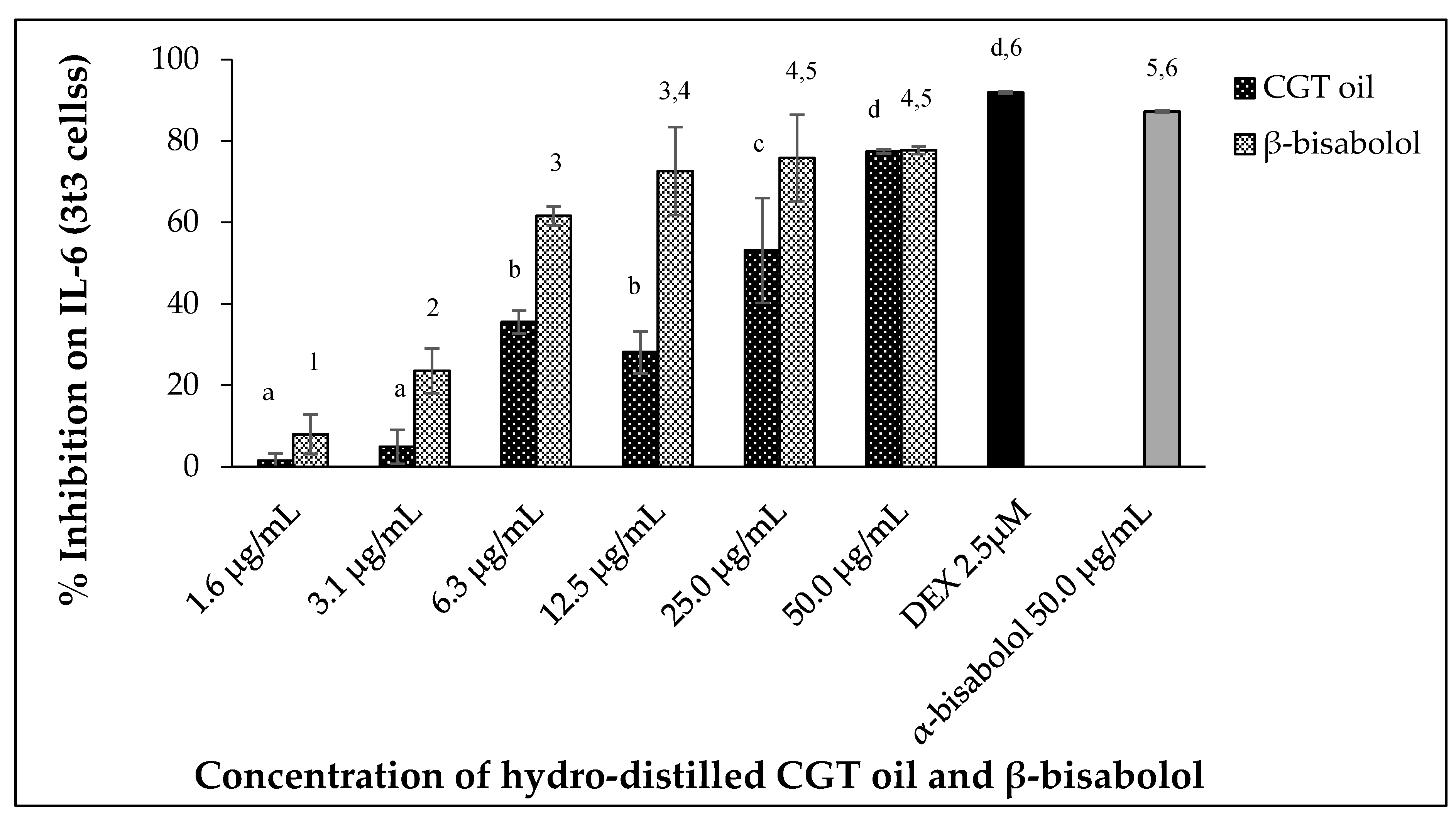
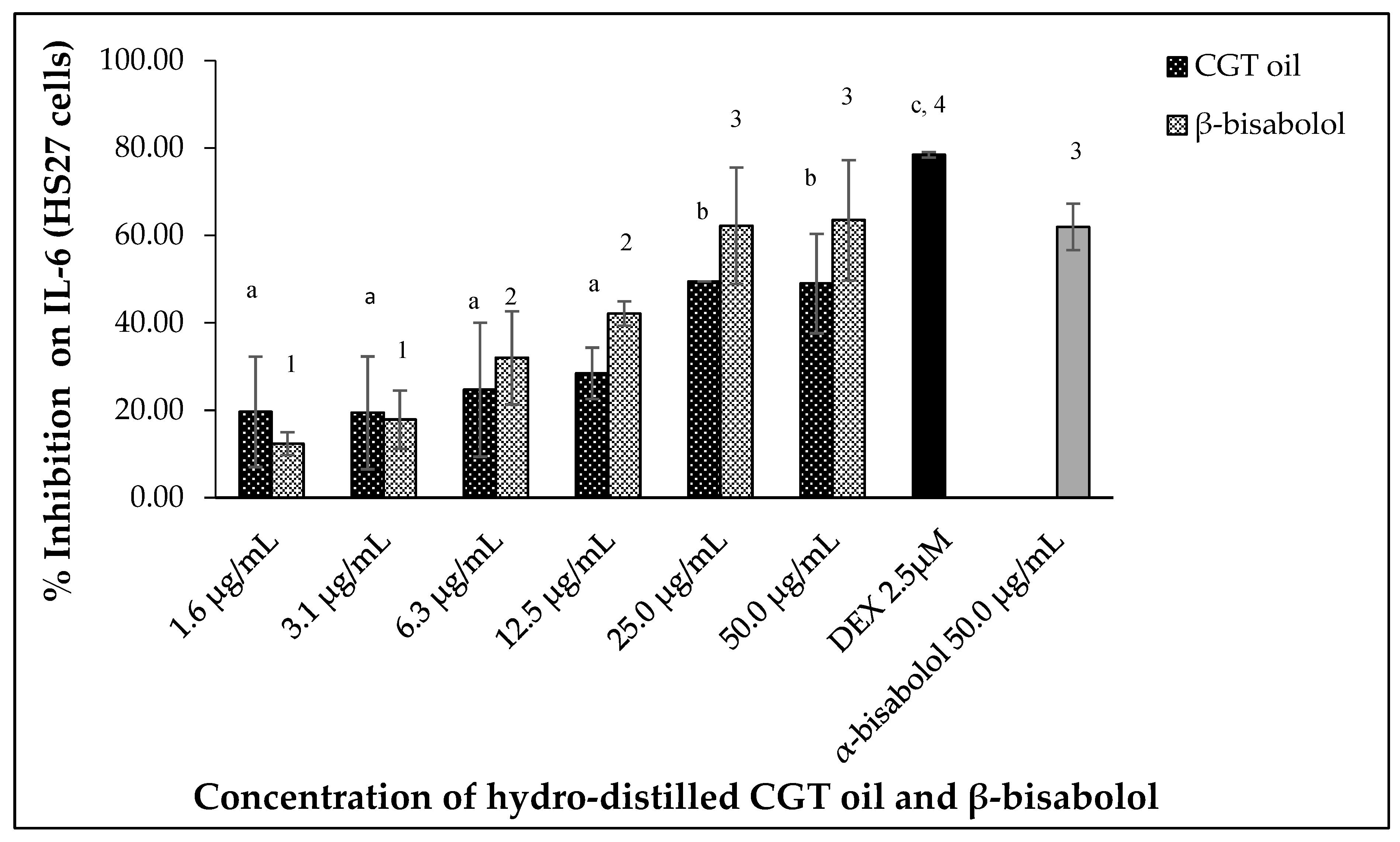
2.5.1. Interleukin 6 (IL-6) Inhibition
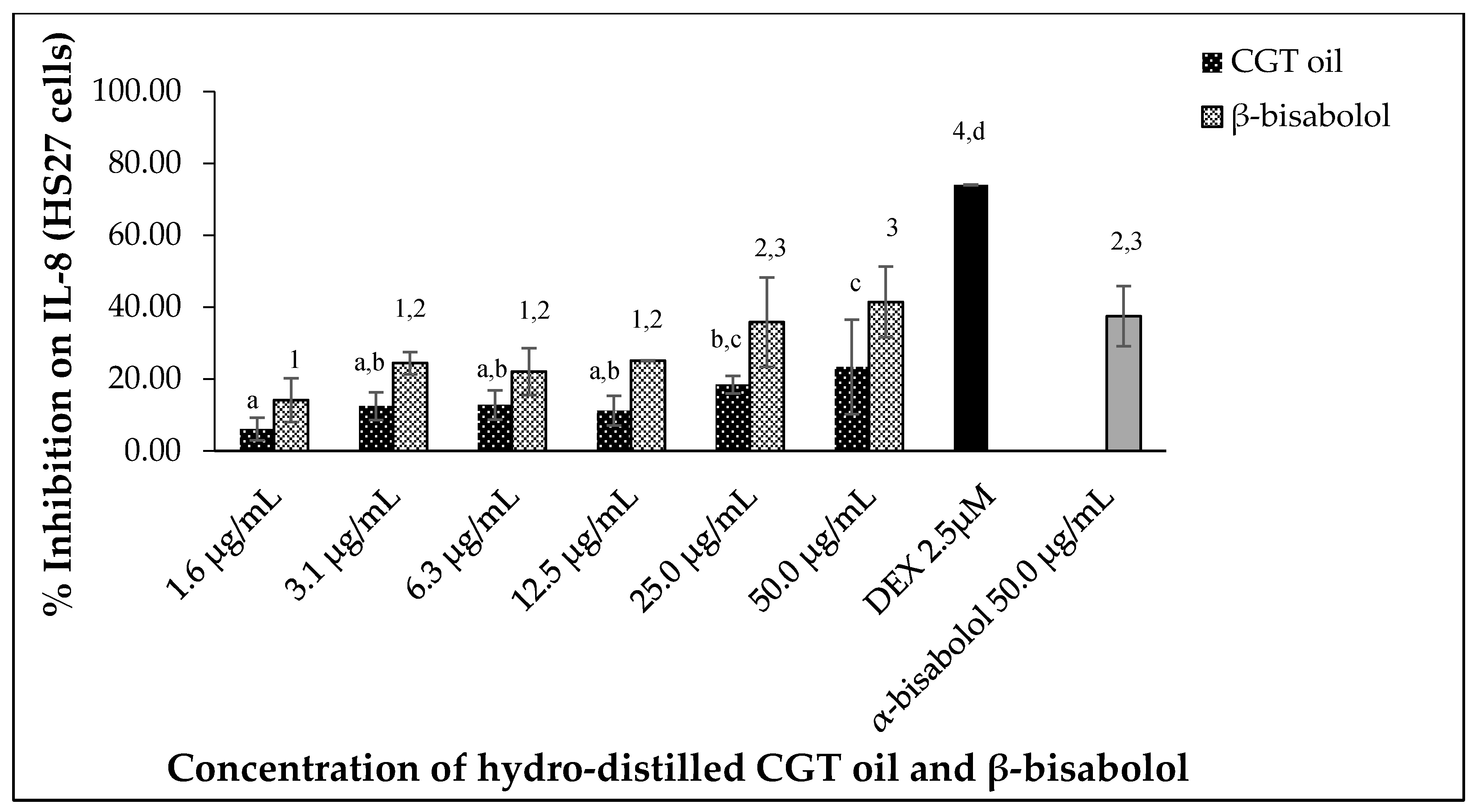
2.5.2. Interleukin 8 (IL-8) Inhibition
2.5.3. Inhibition of TNF-α
2.6. Comparing Effect of β-Bisabolol and α-Bisabolol on Two Inflammation Mediators Produced by RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Extraction of CGT Oil and Chromatographic Analysis
4.2. Cell Lines and Reagents
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. PGE2 Inhibition Assay
4.6. Nitric Oxide Inhibition Assay
4.7. Cytokine Inhibition Assay
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- de las Heras, B.; Sonsoles, H. Molecular Basis of the Anti-Inflammatory Effects of Terpenoids. Inflamm. Allergy-Drug Targets 2009, 8, 28–39. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Zhu, L.; Zeng, D.; Long, W.; Zhu, S.-M. Chemical composition and anti-inflammatory activities of essential oil from Trachydium roylei. J. Food Drug Anal. 2016, 24, 602–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.E.; Kim, O.S.; Yoo, S.-R.; Seo, C.-S.; Kim, Y.; Shin, H.-K.; Jeong, S.-J. Anti-inflammatory effect and action mechanisms of traditional herbal formula Gamisoyo-san in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2016, 16, 219. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Lee, K.; Lee, M.; Ham, I.; Choi, H.-Y. Inhibition of lipopolysaccharide-induced nitric oxide and prostaglandin E2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2012, 12, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maskrey, B.H.; Megson, I.L.; Whitfield, P.D.; Rossi, A.G. Mechanisms of Resolution of Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wang, W.-H.; Chen, S.-H.; Chang, Y.-W.; Hung, L.-C.; Chen, C.-Y.; Chen, Y.-H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. [Google Scholar] [CrossRef] [Green Version]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Grabowska, A.; Chain, B. Nitric oxide up-regulates the release of inflammatory mediators by mouse macrophages. Eur. J. Immunol. 1995, 25, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E(2). J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE(2) in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin- 6. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Barnes, T.C.; Anderson, M.E.; Moots, R.J. The Many Faces of Interleukin-6: The Role of IL-6 in Inflammation, Vasculopathy, and Fibrosis in Systemic Sclerosis. Int. J. Rheumatol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Cuzzocrea, S. TNF-Alpha as a Therapeutic Target in Inflammatory Diseases, Ischemia- Reperfusion Injury and Trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef]
- Popa, C.; Netea, M.G.; van Riel, P.L.C.M.; van der Meer, J.W.M.; Stalenhoef, A.F.H. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskin, D.L. Macrophages and Inflammatory Mediators in Chemical Toxicity: A Battle of Forces. Chem. Res. Toxicol. 2009, 22, 1376–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordana, M.; Sarnstrand, B.; Sime, P.J.; Ramis, I. Immune-inflammatory functions of fibroblasts. Eur. Respir. J. 1994, 7, 2212. [Google Scholar] [CrossRef] [Green Version]
- Warde, N. Cadherin 11: A key mediator of fibroblast inflammation. Nat. Rev. Rheumatol. 2011, 7, 374. [Google Scholar] [CrossRef]
- Williams, I.R. Fibroblasts A2—Delves, Peter J. In Encyclopedia of Immunology, 2nd ed.; Elsevier: Oxford, UK, 1998; pp. 905–909. ISBN 978-0-12-226765-9. [Google Scholar]
- Ariel, A.; Maridonneau-Parini, I.; Rovere-Querini, P.; Levine, J.S.; Mühl, H. Macrophages in inflammation and its resolution. Front. Immunol. 2012, 3, 324. [Google Scholar] [CrossRef] [Green Version]
- Dunster, J.L. The macrophage and its role in inflammation and tissue repair: Mathematical and systems biology approaches. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 87–99. [Google Scholar] [CrossRef]
- Liddiard, K.; Taylor, P.R. Understanding Local Macrophage Phenotypes In Disease: Shape-shifting macrophages. Nat. Med. 2015, 21, 119. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Scull, C.M.; Hays, W.D.; Fischer, T.H. Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J. Inflamm. (Lond.) 2010, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Flavell, S.J.; Hou, T.Z.; Lax, S.; Filer, A.D.; Salmon, M.; Buckley, C.D. Fibroblasts as novel therapeutic targets in chronic inflammation. Br. J. Pharmacol. 2008, 153, S241–S246. [Google Scholar] [CrossRef] [Green Version]
- Scotton, C.J.; Chambers, R.C. Molecular Targets in Pulmonary Fibrosis: The Myofibroblast in Focus. Chest 2007, 132, 1311–1321. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [Green Version]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nighat, S.; Zafar Saeed, S. Naturally Occurring and Synthetic Agents as Potential Anti-Inflammatory and Immunomodulants. Antiinflamm. Antiallergy. Agents Med. Chem. 2012, 11, 3–19. [Google Scholar] [CrossRef]
- Jang, M.; Jeong, S.-W.; Cho, S.K.; Ahn, K.-S.; Kim, B.-K.; Kim, J.-C. Anti-inflammatory effects of 4 medicinal plant extracts in lipopolysaccharide-induced RAW 264.7 cells. Food Sci. Biotechnol. 2013, 22, 213–220. [Google Scholar] [CrossRef]
- Phanse, M.A.; Patil, M.J.; Chaudhari, K.A.P.D.; Patel, B. In-vivo and in-vitro screening of medicinal plants for their anti-inflammatory activity: An overview. J. Appl. Pharm. Sci. 2012, 2, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Takahashi, N.; Hirai, S.; Kawada, T. Various Terpenoids Derived from Herbal and Dietary Plants Function as PPAR Modulators and Regulate Carbohydrate and Lipid Metabolism. PPAR Res. 2010, 2010, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, D.J.; Shenvi, R.A. Synthesis of medicinally relevant terpenes: Reducing the cost and time of drug discovery. Future Med. Chem. 2014, 6, 1127–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef]
- Bhargava, V.V.; Patel, S.C.; Desai, K.S. Importance of terpenoids and essential oils in chemotaxonomic approach. Int. J. Herb. Medcine 2013, 1, 14. [Google Scholar]
- Nóbrega de Almeida, R.; Agra, M.D.F.; Negromonte Souto Maior, F.; De Sousa, D.P. Essential Oils and Their Constituents: Anticonvulsant Activity. Molecules 2011, 16, 2726. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordiaverbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Maurya, A.K.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D.U. alpha-(-)-bisabolol Reduces Pro-inflammatory Cytokine Production and Ameliorates Skin Inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar] [CrossRef]
- Egbuta, M.A.; McIntosh, S.; Waters, D.L.; Vancov, T.; Liu, L. Chemical volatiles present in cotton gin trash: A by-product of cotton processing. PLoS ONE 2019, 14, e0222146. [Google Scholar] [CrossRef] [PubMed]
- Wiygul, G.; Dickens, J.C.; Smith, J.W. Effect of juvenile hormone III and beta-bisabolol on pheromone production in fat bodies from male boll weevils, Anthonomus grandis boheman (Coleoptera: Curculionidae). Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 95, 489–491. [Google Scholar] [CrossRef]
- Chen, W.; Hou, J.; Yin, Y.; Jang, J.; Zheng, Z.; Fan, H.; Zou, G. α-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFκB. Biochem. Pharmacol. 2010, 80, 247–254. [Google Scholar] [CrossRef]
- Uno, M.; Kokuryo, T.; Yokoyama, Y.; Senga, T.; Nagino, M. α-Bisabolol Inhibits Invasiveness and Motility in Pancreatic Cancer Through KISS1R Activation. Anticancer. Res. 2016, 36, 583–589. [Google Scholar]
- Kim, S.; Jung, E.; Kim, J.-H.; Park, Y.-H.; Lee, J.; Park, D. Inhibitory effects of (−)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef]
- Rocha, N.F.M.; Rios, E.R.V.; Carvalho, A.M.R.; Cerqueira, G.S.; de Araújo Lopes, A.; Leal, L.K.A.M.; Dias, M.L.; de Sousa, D.P.; de Sousa, F.C.F. Anti-nociceptive and anti-inflammatory activities of (−)-α-bisabolol in rodents. Naunyn. Schmiedebergs. Arch. Pharmacol. 2011, 384, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Suijun, W.; Zhen, Y.; Ying, G.; Yanfang, W. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 2014, 444, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Blantz, R.C.; Munger, K. Role of Nitric Oxide in Inflammatory Conditions. Nephron 2002, 90, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y.; Chang, H.J.; Lee, S.K.; Kim, H.J.; Hwang, J.K.; Chun, H.S. Amelioration of dextran sulfate sodium-induced colitis in mice by oral administration of β-caryophyllene, a sesquiterpene. Life Sci. 2007, 80, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, N.; Shukla, S.; Srivastava, J.K.; Gupta, S. Chamomile: An anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int. J. Mol. Med. 2010, 26, 935–940. [Google Scholar] [PubMed] [Green Version]
- Pannee, C.; Chandhanee, I.; Wacharee, L. Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 cells. J. Adv. Pharm. Technol. Res. 2014, 5, 164–170. [Google Scholar] [CrossRef]
- An, B.; Kang, J.; Yang, H.; Jung, E.; Kang, H.; Choi, I.; Park, M.S.; Jeung, E. Anti-inflammatory effects of essential oils from Chamaecyparis obtusa via the cyclooxygenase-2 pathway in rats. Mol. Med. Rep. 2013, 8, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, T.G.; Fernandes, A.; Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Nat. Prod. Res. 2009, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Lim, Y.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.-S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bishara, N. Chapter 18-The Use of Biomarkers for Detection of Early- and Late-Onset Neonatal Sepsis A2-Ohls, Robin K. In Hematology, Immunology and Infectious Disease: Neonatology Questions and Controversies, 2nd ed.; Maheshwari, A., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 303–315. ISBN 978-1-4377-2662-6. [Google Scholar]
- Lam, D.K.; Schmidt, B.L. Chapter 10-Molecular Biology of Head and Neck Cancer: Therapeutic Implications A2-Bagheri, Shahrokh C. In Current Therapy in Oral and Maxillofacial Surgery; Bell, R.B., Khan, H.A., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2012; pp. 92–101. ISBN 978-1-4160-2527-6. [Google Scholar]
- Dongari-Bagtzoglou, A.I.; Ebersole, J.L. Production of inflammatory mediators and cytokines by human gingival fibroblasts following bacterial challenge. J. Periodontal Res. 1996, 31, 90–98. [Google Scholar] [CrossRef]
- Bickel, M. The role of interleukin-8 in inflammation and mechaniss of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar]
- Kamatou, G.P.P.; Viljoen, A.M. A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Cavalieri, E.; Mariotto, S.; Fabrizi, C.; de Prati, A.C.; Gottardo, R.; Leone, S.; Berra, L.V.; Lauro, G.M.; Ciampa, A.R.; Suzuki, H. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Biophys. Res. Commun. 2004, 315, 589–594. [Google Scholar] [CrossRef]
- Darra, E.; Abdel-Azeim, S.; Manara, A.; Shoji, K.; Maréchal, J.-D.; Mariotto, S.; Cavalieri, E.; Perbellini, L.; Pizza, C.; Perahia, D.; et al. Insight into the apoptosis-inducing action of α-bisabolol towards malignant tumor cells: Involvement of lipid rafts and Bid. Arch. Biochem. Biophys. 2008, 476, 113–123. [Google Scholar] [CrossRef]
- Dharmendra, K.Y.; Vipin, M.; Jyoti, A.; Anil, K.M.; Dnyaneshwar, U.B.; Chandan, S.C.; Feroz, K.; Sanjog, T.T. Molecular Docking and ADME Studies of Natural Compounds of Agarwood Oil for Topical Anti-Inflammatory Activity. Curr. Comput. Aided. Drug Des. 2013, 9, 360–370. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, F.W.J.; Dantas-Barros, A.M.; Soares, B.M.; Santos, D.D.A.; Resende, M.A.D.; Carvalho, M.A.R.D.; Siqueira, E.P.; Nelson, D.L. The composition and anti-microbial activity of the essential oil from Eremanthus erythropappus (DC) Macleish (Candeia). Int. J. Med. Aromat. Plants 2013, 3, 1–10. [Google Scholar]
- Ahmad, T.; Rudd, D.; Smith, J.; Kotiw, M.; Mouatt, P.; Seymour, L.; Liu, L.; Benkendorff, K. Anti-Inflammatory Activity and Structure-Activity Relationships of Brominated Indoles from a Marine Mollusc. Mar. Drugs 2017, 15, 133. [Google Scholar] [CrossRef]
- Gunawardena, D.; Shanmugam, K.; Low, M.; Bennett, L.; Govindaraghavan, S.; Head, R.; Ooi, L.; Münch, G. Determination of anti-inflammatory activities of standardised preparations of plant- and mushroom-based foods. Eur. J. Nutr. 2014, 53, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, K.-J.; Kim, J.-Y.; Kim, J.-B.; Lee, K.-W.; Jeong, S.-Y.; Park, H.-J.; Jung, H.-J.; Cho, Y.-W.; Yun, K.; Lee, K.-T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-κB inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef]



| Terpenoids | % Composition |
|---|---|
| β-bisabolol | 23.5 |
| γ-bisabolene | 8.7 |
| β-caryophyllene | 6.8 |
| caryophyllene oxide | 4.7 |
| α-humulene | 4.1 |
| α-cuprenene | 4.1 |
| β -ocimene | 3.9 |
| gossonorol | 2.8 |
| β-santalene | 2.0 |
| α-pinene | 1.3 |
| α-copaene | 1.2 |
| myrcene | 1.1 |
| sesquisabinene | 1.1 |
| (E)-nerolidol | 0.8 |
| italicene | 0.7 |
| β-bisabolene | 0.6 |
| 5-hydroxy-cis-calamenene | 0.5 |
| β-pinene | 0.4 |
| epi-β-santalene | 0.3 |
| α-santalene | 0.3 |
| γ-curcumene | 0.3 |
| β-copaene | 0.2 |
| α-curcumene | 0.2 |
| γ-terpineol | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egbuta, M.A.; McIntosh, S.; Waters, D.L.E.; Vancov, T.; Liu, L. In Vitro Anti-Inflammatory Activity of Essential Oil and β-Bisabolol Derived from Cotton Gin Trash. Molecules 2022, 27, 526. https://doi.org/10.3390/molecules27020526
Egbuta MA, McIntosh S, Waters DLE, Vancov T, Liu L. In Vitro Anti-Inflammatory Activity of Essential Oil and β-Bisabolol Derived from Cotton Gin Trash. Molecules. 2022; 27(2):526. https://doi.org/10.3390/molecules27020526
Chicago/Turabian StyleEgbuta, Mary A., Shane McIntosh, Daniel L. E. Waters, Tony Vancov, and Lei Liu. 2022. "In Vitro Anti-Inflammatory Activity of Essential Oil and β-Bisabolol Derived from Cotton Gin Trash" Molecules 27, no. 2: 526. https://doi.org/10.3390/molecules27020526
APA StyleEgbuta, M. A., McIntosh, S., Waters, D. L. E., Vancov, T., & Liu, L. (2022). In Vitro Anti-Inflammatory Activity of Essential Oil and β-Bisabolol Derived from Cotton Gin Trash. Molecules, 27(2), 526. https://doi.org/10.3390/molecules27020526








