Design and Evaluation of a Lactate Microbiosensor: Toward Multianalyte Monitoring of Neurometabolic Markers In Vivo in the Brain
Abstract
1. Introduction
2. Results and Discussion
2.1. Platinization of Carbon Fiber Microelectrodes
2.2. Electrochemical Behavior of CFM/Pt Microelectrodes
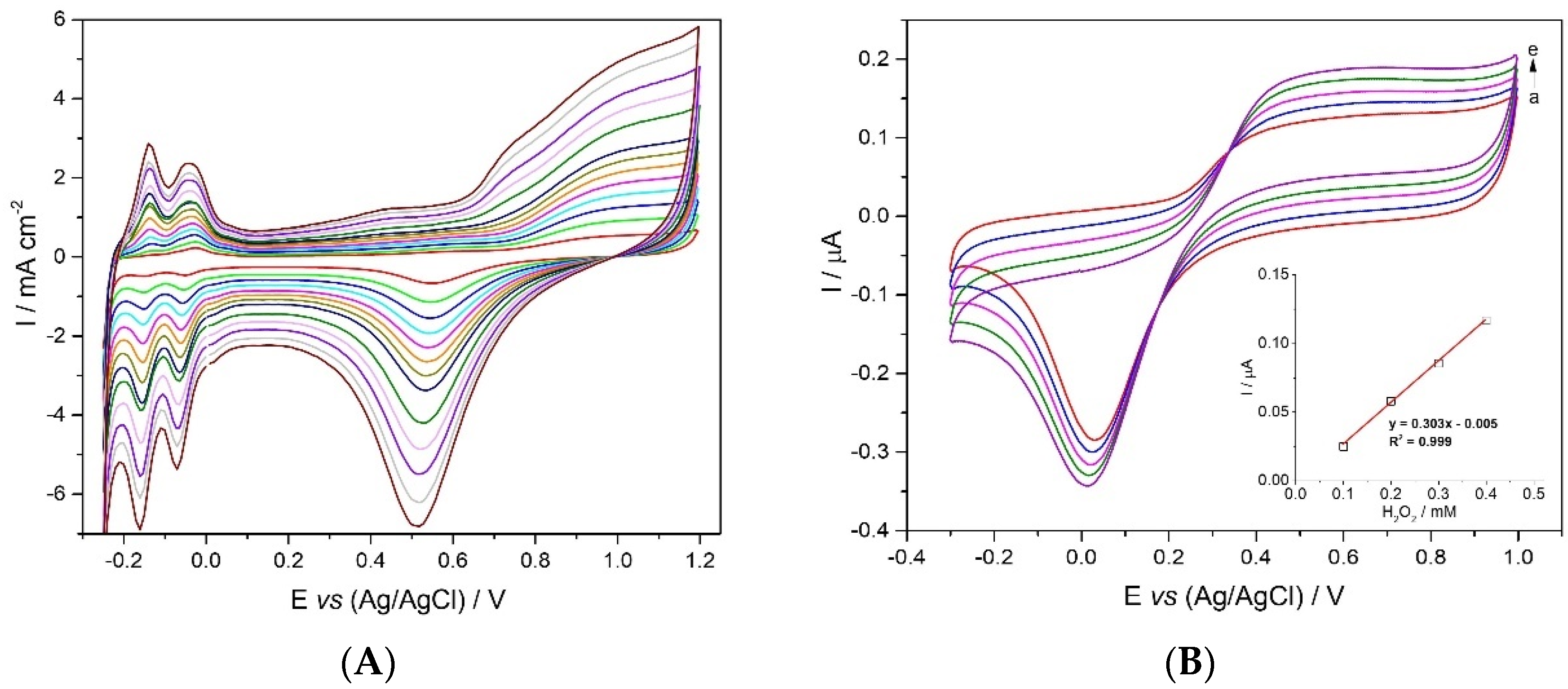
2.2.1. Acidic Electrolyte
2.2.2. Cyclic Voltammetry of Hydrogen Peroxide
2.3. CFM/Pt-Based Lactate Microbiosensors
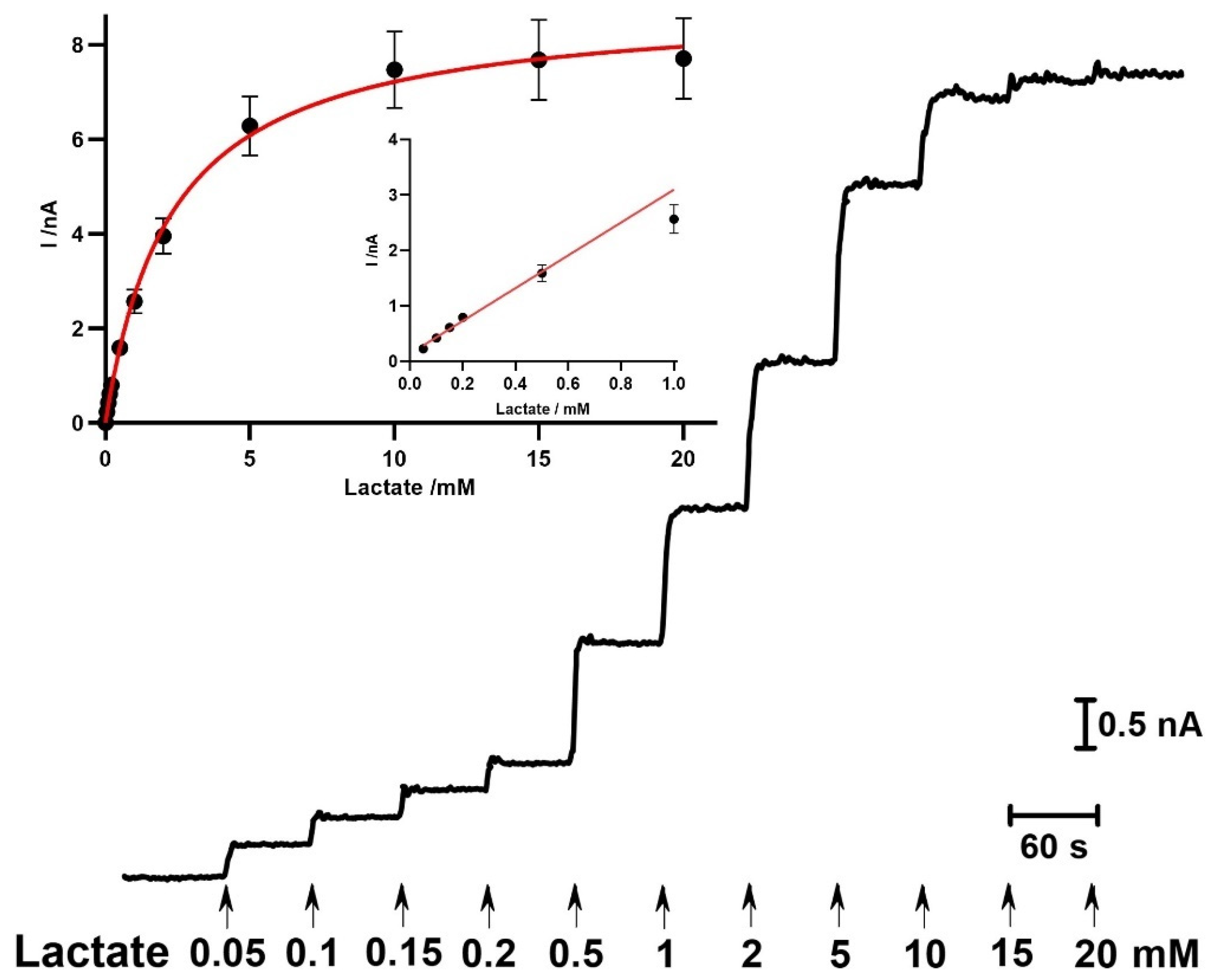
2.3.1. Enzyme Kinetics and Analytical Performance
2.3.2. Response Time
2.3.3. Oxygen Dependency
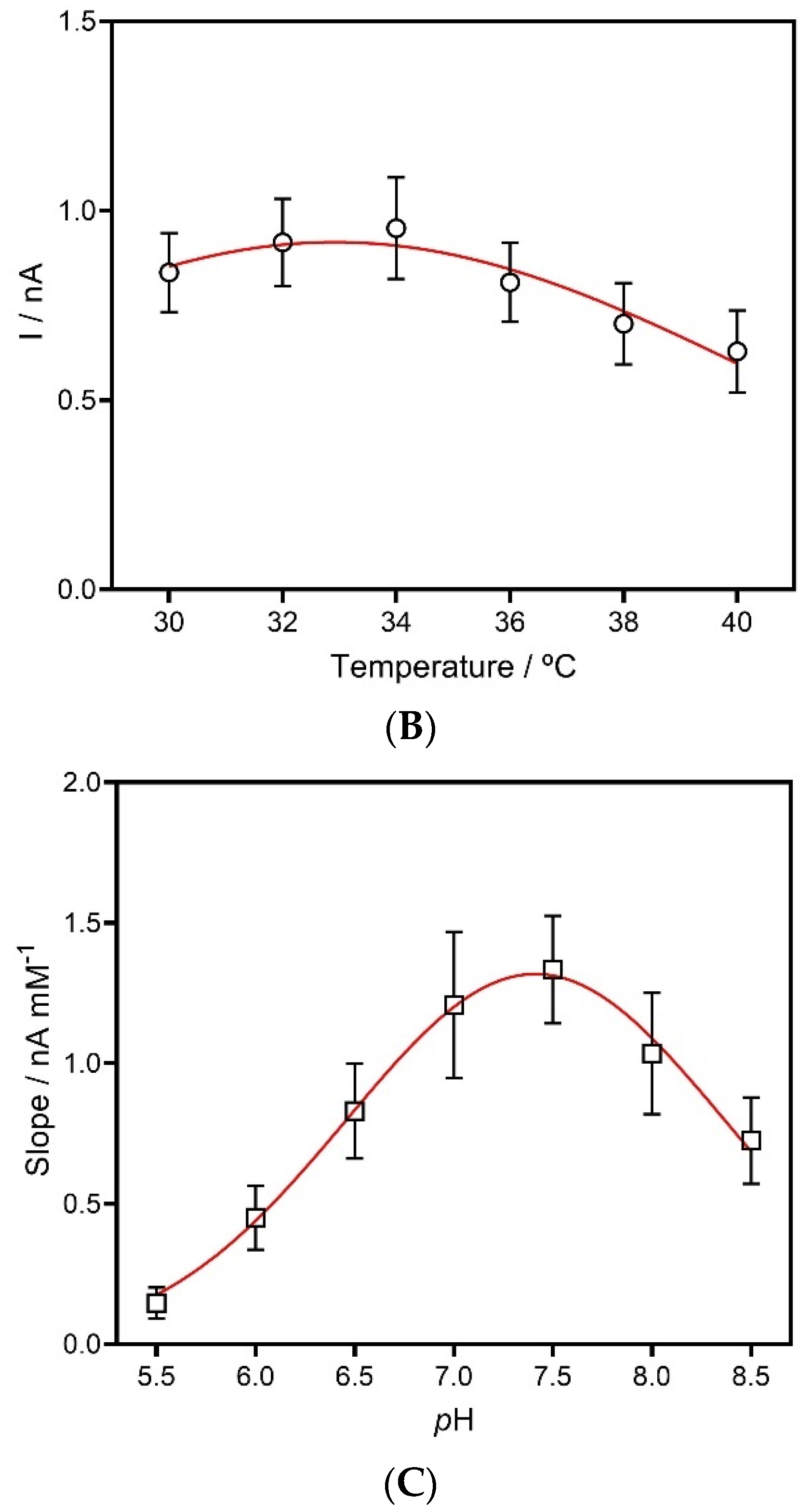
2.3.4. Effect of Temperature and pH
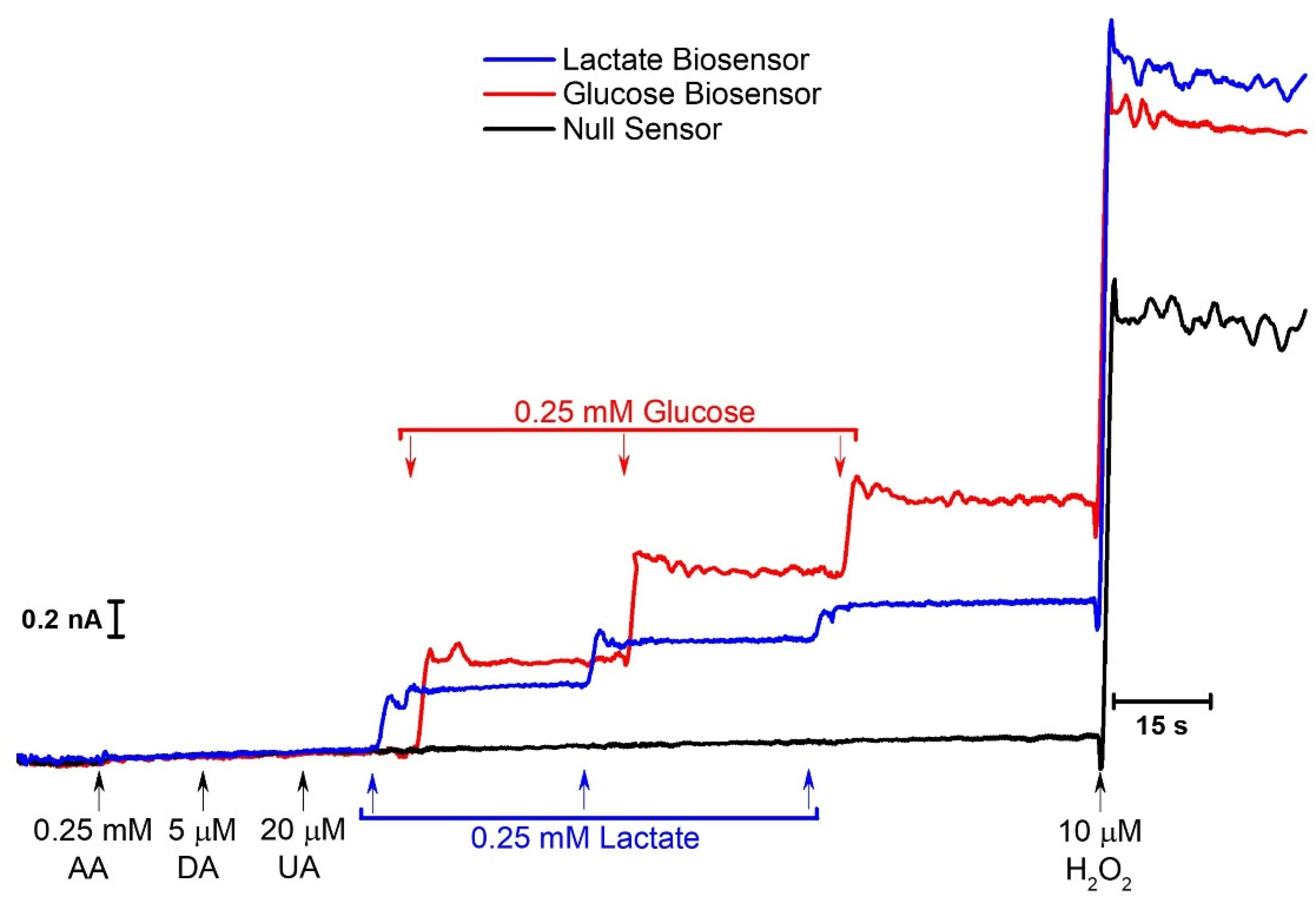
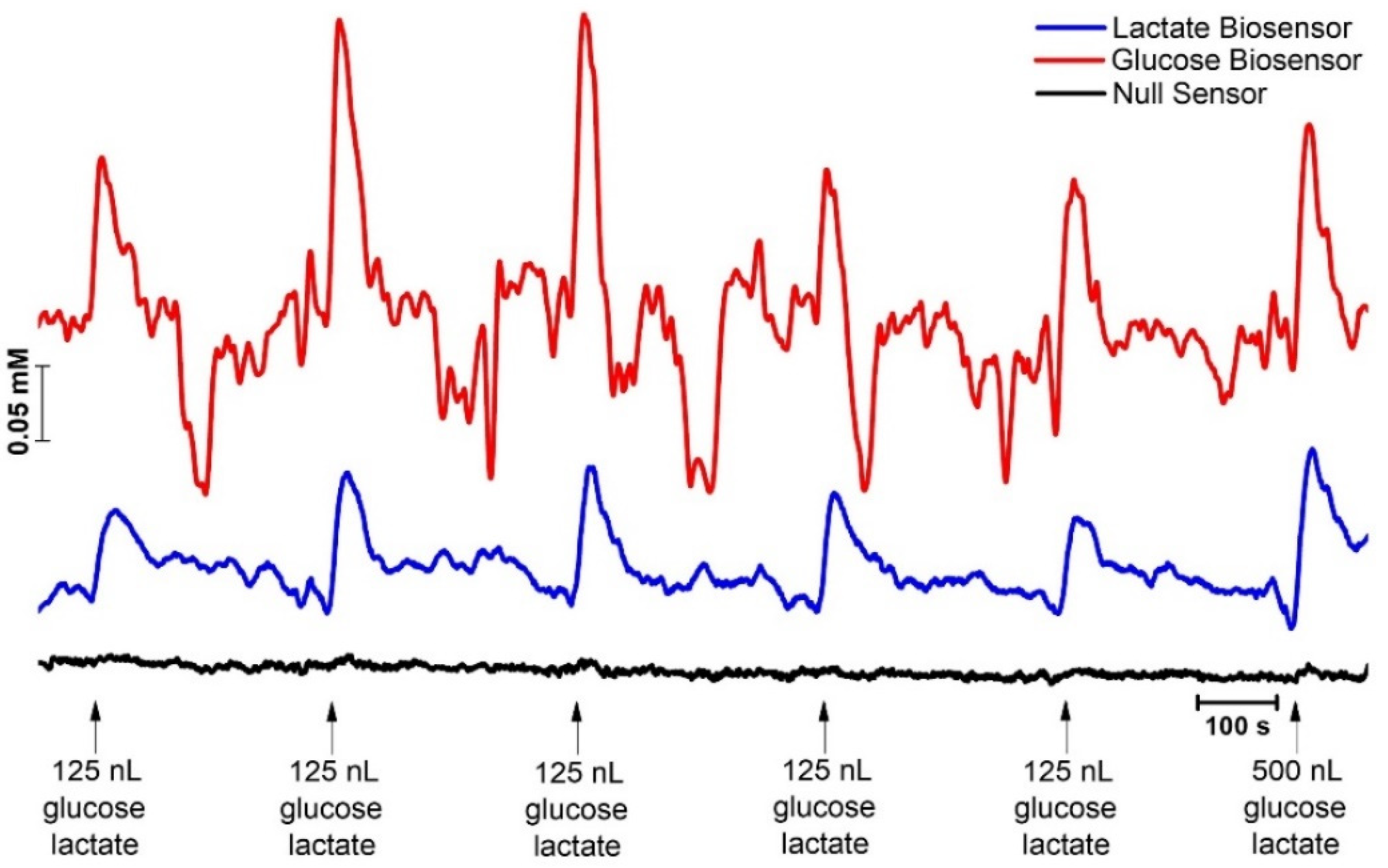
2.4. Concurrent Measurements of Lactate and Glucose
2.4.1. Microbiosensor Dual Calibration
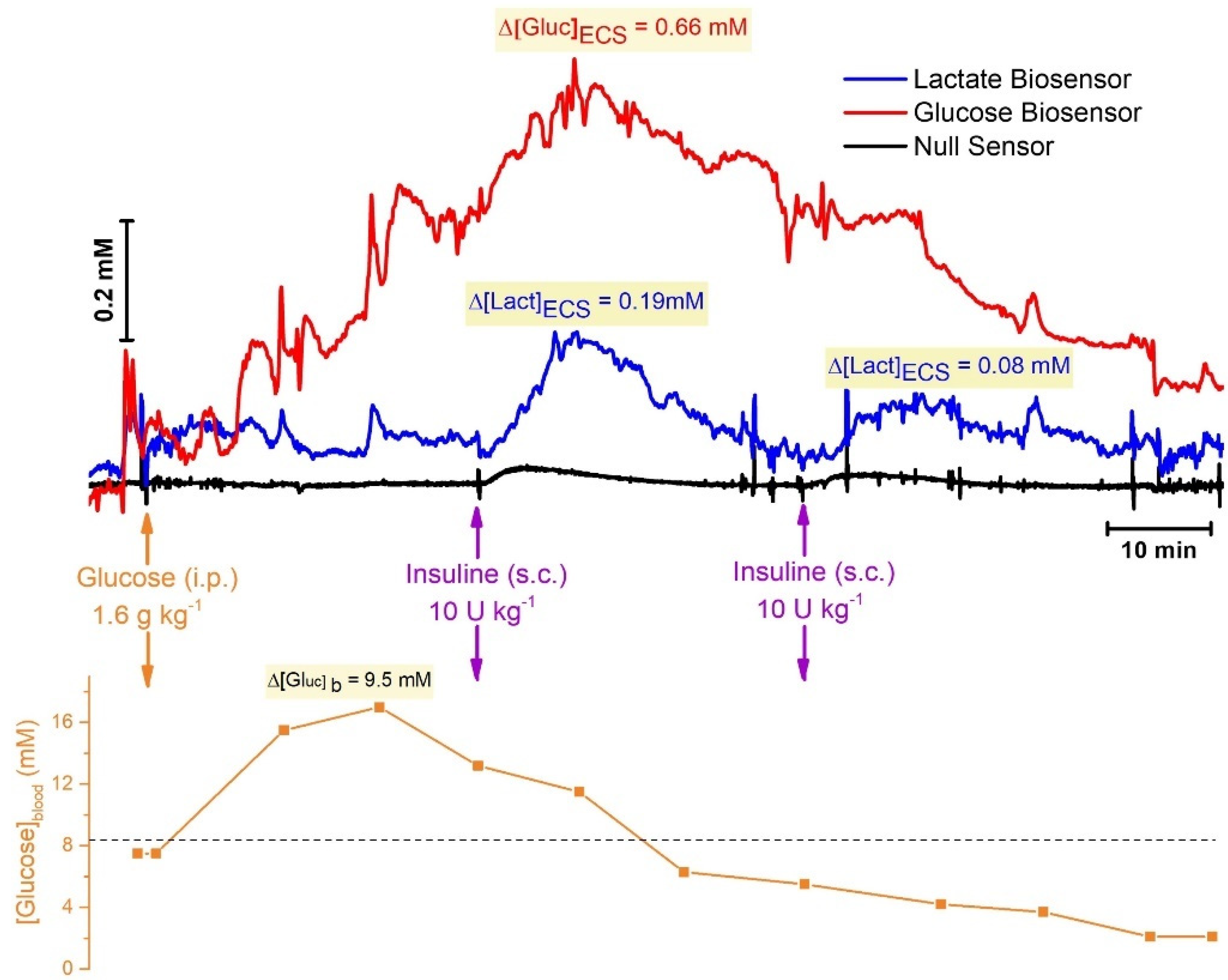
2.4.2. In Vivo Monitoring of Neurometabolic Markers in the Rat Brain
3. Materials and Methods
3.1. Chemicals and Solutions
3.2. Electrochemical Instrumentation
3.3. Fabrication of Carbon Fiber Microelectrodes
3.4. Carbon Fiber Microelectrode Platinization
3.5. Immobilization of Lactate Oxidase
3.6. In Vitro Evaluation and Characterization
3.7. Surgical Procedures and In Vivo Experiments
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. Rev. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021, 33, 1546–1564. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain lactate metabolism: The discoveries and the controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef]
- Wu, F.; Yu, P.; Mao, L. Analytical and Quantitative in Vivo Monitoring of Brain Neurochemistry by Electrochemical and Imaging Approaches. ACS Omega 2018, 3, 13267–13274. [Google Scholar] [CrossRef]
- Ganesana, M.; Lee, S.T.; Wang, Y.; Venton, B.J. Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem. 2017, 89, 314–341. [Google Scholar] [CrossRef]
- Soldà, A.; Valenti, G.; Marcaccio, M.; Giorgio, M.; Pelicci, P.G.; Paolucci, F.; Rapino, S. Glucose and Lactate Miniaturized Biosensors for SECM-Based High-Spatial Resolution Analysis: A Comparative Study. ACS Sensors 2017, 2, 1310–1318. [Google Scholar] [CrossRef]
- Sardesai, N.P.; Ganesana, M.; Karimi, A.; Leiter, J.C.; Andreescu, S. Platinum-doped ceria based biosensor for in vitro and in vivo monitoring of lactate during hypoxia. Anal. Chem. 2015, 87, 2996–3003. [Google Scholar] [CrossRef]
- Jobst, G.; Moser, I.; Varahram, M.; Svasek, P.; Aschauer, E.; Trajanoski, Z.; Wach, P.; Kotanko, P.; Skrabal, F.; Urban, G. Thin-Film Microbiosensors for Glucose-Lactate Monitoring. Anal. Chem. 1996, 68, 3173–3179. [Google Scholar] [CrossRef]
- Lama, R.D.; Charlson, K.; Anantharam, A.; Hashemi, P. Ultrafast detection and quantification of brain signaling molecules with carbon fiber microelectrodes. Anal. Chem. 2012, 84, 8096–8101. [Google Scholar] [CrossRef]
- Huffman, M.L.; Venton, B.J. Carbon-fiber microelectrodes for in vivo applications. Analyst. 2009, 134, 18–24. [Google Scholar] [CrossRef]
- Hermans, A.; Seipel, A.T.; Miller, C.E.; Wightman, R.M. Carbon-fiber microelectrodes modified with 4-sulfobenzene have increased sensitivity and selectivity for catecholamines. Langmuir 2006, 22, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring rapid chemical communication in the brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar] [CrossRef] [PubMed]
- Kita, J.M.; Wightman, R.M. Microelectrodes for studying neurobiology. Curr. Opin. Chem. Biol. 2008, 12, 491–496. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.D.; Rocchitta, G.; McMahon, C.P.; Serra, P.A.; Lowry, J.P. Designing sensitive and selective polymer/enzyme composite biosensors for brain monitoring in vivo. TrAC Trends Anal. Chem. 2008, 27, 78–88. [Google Scholar] [CrossRef]
- Chatard, C.; Sabac, A.; Moreno-Velasquez, L.; Meiller, A.; Marinesco, S. Minimally Invasive Microelectrode Biosensors Based on Platinized Carbon Fibers for in Vivo Brain Monitoring. ACS Cent. Sci. 2018, 4, 1751–1760. [Google Scholar] [CrossRef]
- Booth, M.A.; Gowers, S.A.N.; Hersey, M.; Samper, I.C.; Park, S.; Anikeeva, P.; Hashemi, P.; Stevens, M.M.; Boutelle, M.G. Fiber-Based Electrochemical Biosensors for Monitoring pH and Transient Neurometabolic Lactate. Anal. Chem. 2021, 93, 6646–6655. [Google Scholar] [CrossRef]
- Gerhardt, G.A.; Hoffman, A.F. Effects of recording media composition on the responses of Nafion-coated carbon fiber microelectrodes measured using high-speed chronoamperometry. J. Neurosci. Methods 2001, 109, 13–21. [Google Scholar] [CrossRef]
- Harley, C.C.; Annibaldi, V.; Yu, T.; Breslin, C.B. The selective electrochemical sensing of dopamine at a polypyrrole film doped with an anionic β−cyclodextrin. J. Electroanal Chem. 2019, 855, 113614. [Google Scholar] [CrossRef]
- Rothwell, S.A.; McMahon, C.P.; O’Neill, R.D. Effects of polymerization potential on the permselectivity of poly(o-phenylenediamine) coatings deposited on Pt-Ir electrodes for biosensor applications. Electrochim. Acta. 2010, 55, 1051–1060. [Google Scholar] [CrossRef]
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, P.G. Glucose Fast-Response Amperometric Sensor Based on Glucose Oxidase Immobilized in an Electropolymerized Poly(o-phenylenediamine) Film. Anal. Chem. 1990, 62, 2735–2740. [Google Scholar] [CrossRef]
- McMahon, C.P.; Killoran, S.J.; Kirwan, S.M.; O’Neill, R.D. The selectivity of electrosynthesised polymer membranes depends on the electrode dimensions: Implications for biosensor applications. Chem. Commun. 2004, 18, 2128–2130. [Google Scholar] [CrossRef] [PubMed]
- Killoran, S.J.; O’Neill, R.D. Characterization of permselective coatings electrosynthesized on Pt-Ir from the three phenylenediamine isomers for biosensor applications. Electrochim. Acta 2008, 53, 7303–7312. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Caetano, M.; Ledo, A.; Barbosa, R.M. Platinized carbon fiber-based glucose microbiosensor designed for metabolic studies in brain slices. Bioelectrochemistry 2019, 130, 107325. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, S.; McNeil, C.J.; Lowry, J.P.; McMahon, C.P.; O’Neill, R.D. Highly selective and stable microdisc biosensors for l-glutamate monitoring. Sens. Actuators B Chem. 2013, 178, 606–614. [Google Scholar] [CrossRef]
- Naylor, E.; Aillon, D.V.; Barrett, B.S.; Wilson, G.S.; Johnson, D.A.; Johnson, D.A.; Harmon, H.P.; Gabbert, S.; Petillo, P.A. Lactate as a biomarker for sleep. Sleep. 2012, 35, 1209–1222. [Google Scholar] [CrossRef]
- Chen, D.; Tao, Q.; Liao, L.W.; Liu, S.X.; Chen, Y.X.; Ye, S. Determining the Active Surface Area for Various Platinum Electrodes. Electrocatalysis 2011, 2, 207–219. [Google Scholar] [CrossRef]
- Daubinger, P.; Kieninger, J.; Unmüssig, T.; Urban, G.A. Electrochemical characteristics of nanostructured platinum electrodes-A cyclic voltammetry study. Phys. Chem. Chem. Phys. 2014, 16, 8392–8399. [Google Scholar] [CrossRef]
- Yadav, A.P. Stability of Platinum in Sulfuric Acid Solution Studied by Electrochemical Quartz Crystal Microbalance. J. Nepal Chem. Soc. 2012, 29, 24–27. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. International Union of Pure and Applied Chemistry Physical Chemistry Division Commission on Electrochemistry: Real Surface Area Measurements in Electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Zhan, D.; Velmurugan, J.; Mirkin, M.V. Adsorption/desorption of hydrogen on Pt nanoelectrodes: Evidence of surface diffusion and spillover. J. Am. Chem. Soc. 2009, 131, 14756–14760. [Google Scholar] [CrossRef] [PubMed]
- Katsounaros, I.; Schneider, W.B.; Meier, J.C.; Benedikt, U.; Biedermann, P.U.; Auer, A.A.; Mayrhofer, K.J.J. Hydrogen peroxide electrochemistry on platinum: Towards understanding the oxygen reduction reaction mechanism. Phys. Chem. Chem. Phys. 2012, 14, 7384–7391. [Google Scholar] [CrossRef]
- Wilson, G.S.; Gifford, R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 2005, 20, 2388–2403. [Google Scholar] [CrossRef] [PubMed]
- Schuvailo, O.M.; Soldatkin, O.O.; Lefebvre, A.; Cespuglio, R.; Soldatkin, A.P. Highly selective microbiosensors for in vivo measurement of glucose, lactate and glutamate. Anal. Chim. Acta. 2006, 573–574, 110–116. [Google Scholar] [CrossRef]
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Non-linearization of modified Michaelis-Menten kinetics. J. Comput. Theor. Nanosci. 2014, 11, 2596–2602. [Google Scholar] [CrossRef]
- Sandstrom, M.I.; Rebec, G.V. Extracellular ascorbate modulates glutamate dynamics: Role of behavioral activation. BMC Neurosci. 2007, 8, 32. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Santos, R.M.; Laranjinha, J.; Barbosa, R.M. Real Time In Vivo Measurement of Ascorbate in the Brain Using Carbon Nanotube-Modified Microelectrodes. Electroanalysis 2013, 25, 1757–1763. [Google Scholar] [CrossRef]
- Day, B.K.; Pomerleau, F.; Burmeister, J.J.; Huettl, P.; Gerhardt, G.A. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J. Neurochem. 2006, 96, 1626–1635. [Google Scholar] [CrossRef]
- Barbosa, R.M.; Lourenço, C.F.; Santos, R.M.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Laranjinha, J. In Vivo Real-Time Measurement of Nitric Oxide in Anesthetized Rat Brain. Methods Enzymol. 2008, 441, 351–367. [Google Scholar]
- Shram, N.F.; Netchiporouk, L.I.; Martelet, C.; Jaffrezic-Renault, N.; Bonnet, C.; Cespuglio, R. In Vivo Voltammetric Detection of Rat Brain Lactate with Carbon Fiber Microelectrodes Coated with Lactate Oxidase. Anal Chem. 1998, 70, 2618–2622. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; O’Neill, R.D.; Roche, R.; González-Mora, J.L. Biosensors based on prussian blue modified carbon fibers electrodes for monitoring lactate in the extracellular space of brain tissue. Int. J. Electrochem. Sci. 2012, 7, 5910–5926. [Google Scholar]
- Smith, S.K.; Gosrani, S.P.; Lee, C.A.; McCarty, G.S.; Sombers, L.A. Carbon-Fiber Microbiosensor for Monitoring Rapid Lactate Fluctuations in Brain Tissue Using Fast-Scan Cyclic Voltammetry. Anal. Chem. 2018, 90, 12994–12999. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Ledo, A.; Gerhardt, G.A.; Laranjinha, J.; Barbosa, R.M. Neurometabolic and electrophysiological changes during cortical spreading depolarization: Multimodal approach based on a lactate-glucose dual microbiosensor arrays. Sci. Rep. 2017, 7, 6764. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. L-lactate measures in brain tissue with ceramic-based multisite microelectrodes. Biosens. Bioelectron. 2005, 20, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.A.; de Vries, M.G.; Ngabi, W.; Oomen, P.E.; Cremers, T.I.F.H.; Westerink, B.H.C. In vivo continuous and simultaneous monitoring of brain energy substrates with a multiplex amperometric enzyme-based biosensor device. Biosens. Bioelectron. 2015, 67, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Secchi, O.; Alvau, M.D.; Farina, D.; Bazzu, G.; Calia, G.; Migheli, R.; Desole, M.S.; O’Neill, R.D.; Serra, P.A. Simultaneous telemetric monitoring of brain glucose and lactate and motion in freely moving rats. Anal. Chem. 2013, 85, 10282–10288. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.P.; O’Neill, R.D. Polymer-enzyme composite biosensor with high glutamate sensitivity and low oxygen dependence. Anal. Chem. 2005, 77, 1196–1199. [Google Scholar] [CrossRef]
- Nair, P.K.; Buerk, D.G.; Halsey, J.H. Comparisons of oxygen metabolism and tissue po2in cortex and hippocampus of gerbil brain. Stroke 1987, 18, 616–622. [Google Scholar] [CrossRef]
- Michiels, C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef]
- Murr, R.; Berger, S.; Schürer, L.; Peter, K.; Baethmann, A. A novel, remote-controlled suspension device for brain tissue PO2 measurements with multiwire surface electrodes. Pflügers Arch. Eur. J. Physiol. 1994, 426, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Mrozek, S.; Vardon, F.; Geeraerts, T. Brain temperature: Physiology and pathophysiology after brain injury. Anesthesiol. Res. Pract. 2012, 2012, 989487. [Google Scholar] [CrossRef]
- Chesler, M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Coman, D.; Trubel, H.K.; Rycyna, R.E.; Hyder, F. Brain temperature and pH measured by 1H chemical shift imaging of a thulium agent. NMR Biomed. 2009, 22, 229–239. [Google Scholar] [CrossRef]
- Murphy, L.J. Reduction of Interference Response at a Hydrogen Peroxide Detecting Electrode Using Electropolymerized Films of Substituted Naphthalenes. Anal. Chem. 1998, 70, 2928–2935. [Google Scholar] [CrossRef]
- Vasylieva, N.; Barnych, B.; Meiller, A.; Maucler, C.; Pollegioni, L.; Lin, J.S.; Barbier, D.; Marinesco, S. Covalent enzyme immobilization by poly(ethylene glycol) diglycidyl ether (PEGDE) for microelectrode biosensor preparation. Biosens. Bioelectron. 2011, 26, 3993–4000. [Google Scholar] [CrossRef]
- Gramsbergen, J.B.; Leegsma-Vogt, G.; Venema, K.; Noraberg, J.; Korf, J. Quantitative on-line monitoring of hippocampus glucose and lactate metabolism in organotypic cultures using biosensor technology. J. Neurochem. 2003, 85, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Blood Brain Barrier Permeability to Lactate. Cereb Blood Flow Intracran. Press. 1971, 6, 49–55. [Google Scholar] [CrossRef]
- Netchiporouk, L.I. In vivo brain glucose measurements: Differential normal pulse voltammetry with enzyme-modified carbon fiber microelectrodes. Anal. Chem. 1996, 68, 4358–4364. [Google Scholar] [CrossRef]
- Fillenz, M.; Lowry, J.P. The relation between local cerebral blood flow and extracellular glucose concentration in rat striatum. Exp. Physiol. 1998, 83, 233–238. [Google Scholar] [CrossRef]
- Vasylieva, N.; Marinesco, S.; Barbier, D.; Sabac, A. Silicon/SU8 multi-electrode micro-needle for in vivo neurochemical monitoring. Biosens. Bioelectron. 2015, 72, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Song, Y.; Shi, W.; Lin, N.; Jiang, T.; Cai, X. A high sensitivity MEA probe for measuring real time rat brain glucose flux. Biosens. Bioelectron. 2014, 55, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Ledo, A.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Microelectrode array biosensor for high-resolution measurements of extracellular glucose in the brain. Sens. Actuators B Chem. 2016, 237, 298–307. [Google Scholar] [CrossRef]
- Roche, R.; Salazar, P.; Martín, M.; Marcano, F.; González-Mora, J.L. Simultaneous measurements of glucose, oxyhemoglobin and deoxyhemoglobin in exposed rat cortex. J. Neurosci. Methods. 2011, 202, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.; Lourenço, C.F.; Laranjinha, J.; Brett, C.M.A.; Gerhardt, G.A.; Barbosa, R.M. Ceramic-Based Multisite Platinum Microelectrode Arrays: Morphological Characteristics and Electrochemical Performance for Extracellular Oxygen Measurements in Brain Tissue. Anal. Chem. 2017, 89, 1674–1683. [Google Scholar] [CrossRef]
- Herrero-Mendez, A.; Almeida, A.; Fernández, E.; Maestre, C.; Moncada, S.; Bolaños, J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009, 11, 747–752. [Google Scholar] [CrossRef]
- Ledo, A.; Barbosa, R.; Cadenas, E.; Laranjinha, J. Dynamic and Interacting Profiles of •NO and O2 in Rat Hippocampal Slices. Free Radic. Biol. Med. 2010, 48, 1044–1050. [Google Scholar] [CrossRef][Green Version]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Santos, R.M.; Lourenço, C.F.; Piedade, A.P.; Andrews, R.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Laranjinha, J.; Barbosa, R.M. A comparative study of carbon fiber-based microelectrodes for the measurement of nitric oxide in brain tissue. Biosens. Bioelectron. 2008, 24, 704–709. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Ledo, A.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Simultaneous measurements of ascorbate and glutamate in vivo in the rat brain using carbon fiber nanocomposite sensors and microbiosensor arrays. Bioelectrochemistry 2018, 121, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2007. [Google Scholar]

| Type (N) | Km,app(L) (mM) | Sensitivity (nA mM−1) | Imax (nA) | LOD (µM) | %BE |
|---|---|---|---|---|---|
| CFM/Pt-LOx(0.1%) (8) | 1.5 ± 0.1 | 4.5 ± 0.5 | 7.5 ± 0.9 | 10.2 ± 1.2 | 2.5 ± 0.1 |
| CFM/Pt-LOx(0.1%)/PU (8) | 2.5 ± 0.3 | 3.2 ± 0.6 | 9.3 ± 1.7 | 18.9 ± 2.4 | 4.2 ± 0.5 |
| CFM/Pt-LOx(0.5%)/PU (6) | 2.2 ± 0.3 | 10.8 ± 1.2 | 26.3 ± 3.9 | 10.0 ± 1.8 | 8.7 ± 3.0 |
| CFM/Pt/Nafion®-LOx(0.5%)/PU (9) | 2.3 ± 0.2 | 3.9 ± 0.4 | 8.9 ± 1.1 | 11.1 ± 1.5 | 1.1 ± 0.3 |
| Electrode type | Km,app (mM) | Imax (nA) | Sensitivity (nA mM−1) | L. Range (mM) | LOD (µM) | Ref. |
|---|---|---|---|---|---|---|
| CFM/Pt/Nafion®-LOx(0.5%)/PU | 2.3 | 8.9 | 3.9 | 0.05–0.5 | 11.1 ± 1.5 | Present Study |
| CFM/LOx/CA | - | - | 9.15 (nA M−1 cm−2) | 0.1–2.0 | - | [42] |
| CFM/PB/LOx/PoPD | 0.44 | 2.88 | 2.68 | 0.0056–0.6 | 5.6 | [43] |
| CFM/PB/LOx/PoPD/Nafion® | 0.97 | 1.57 | 0.77 | 0.0168–1.2 | 16.8 | |
| CFM/Chit-LOx | - | - | 22 | 0–1 | 7.0 | [44] |
| CFM/Ru/LOx/Nafion®-PU | 0.48 | 54 | - | 0–1.75 | 2 | [36] |
| CFM/Ti-Pt/LOx/PU | 2.60 | - | 0.20 nA (nA µM−1 mm−2) | - | 1.16 | [18] |
| MEA/LOx-PU | 22 | - | 3.0 (µA µM−1 cm−2) | 0.05–5 | 10.6 ± 2.2 | [45] |
| MEA/Nafion®/LOx/PU | - | - | 0.089 | 2–20 | 78 ± 13 | [46] |
| Ptwire/PmPD/LOx(0.2)/PAN | 2.26 | 544.5 | 241.14 | 0.02–1.13 | 0.86 ± 0.21 | [47] |
| Ptwire/Ir/p-OPD/LOx/PU | 8.7 | 63.6 | 4.16 | 0–10 | - | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, E.; Ledo, A.; Barbosa, R.M. Design and Evaluation of a Lactate Microbiosensor: Toward Multianalyte Monitoring of Neurometabolic Markers In Vivo in the Brain. Molecules 2022, 27, 514. https://doi.org/10.3390/molecules27020514
Fernandes E, Ledo A, Barbosa RM. Design and Evaluation of a Lactate Microbiosensor: Toward Multianalyte Monitoring of Neurometabolic Markers In Vivo in the Brain. Molecules. 2022; 27(2):514. https://doi.org/10.3390/molecules27020514
Chicago/Turabian StyleFernandes, Eliana, Ana Ledo, and Rui M. Barbosa. 2022. "Design and Evaluation of a Lactate Microbiosensor: Toward Multianalyte Monitoring of Neurometabolic Markers In Vivo in the Brain" Molecules 27, no. 2: 514. https://doi.org/10.3390/molecules27020514
APA StyleFernandes, E., Ledo, A., & Barbosa, R. M. (2022). Design and Evaluation of a Lactate Microbiosensor: Toward Multianalyte Monitoring of Neurometabolic Markers In Vivo in the Brain. Molecules, 27(2), 514. https://doi.org/10.3390/molecules27020514






