Abstract
Paper-based analytical devices (PADs), including lateral flow assays (LFAs), dipstick assays and microfluidic PADs (μPADs), have a great impact on the healthcare realm and environmental monitoring. This is especially evident in developing countries because PADs-based point-of-care testing (POCT) enables to rapidly determine various (bio)chemical analytes in a miniaturized, cost-effective and user-friendly manner. Low sensitivity and poor specificity are the main bottlenecks associated with PADs, which limit the entry of PADs into the real-life applications. The application of nanomaterials in PADs is showing great improvement in their detection performance in terms of sensitivity, selectivity and accuracy since the nanomaterials have unique physicochemical properties. In this review, the research progress on the nanomaterial-based PADs is summarized by highlighting representative recent publications. We mainly focus on the detection principles, the sensing mechanisms of how they work and applications in disease diagnosis, environmental monitoring and food safety management. In addition, the limitations and challenges associated with the development of nanomaterial-based PADs are discussed, and further directions in this research field are proposed.
1. Introduction
As an easily accessible and cheap material made from cellulose (the most abundant polymer on earth) or nitrocellulose, paper offers many advantages for development of biosensing platforms, in particular point-of-care-testing (POCT) devices [1,2,3,4,5,6,7]. For instance, various in situ analyses can be achieved by the paper-based analytical devices (PADs) because many recognition probes, such as ligands, antibodies and aptamers, can be easily immobilized within the (nitro)cellulose matrix [8,9,10,11,12,13,14,15,16,17,18,19,20]. Because of the controlled porosity and capillary forces of the nitrocellulose/cellulose network, the fluidics can be efficiently transported via capillary flow. In addition, the PADs are compatible with different detection systems, including naked eye and simple optical or electrical readers, which meets the needs in developing countries [21,22,23,24,25]. In the past decades, PADs, including lateral flow assays (LFAs), dipstick assays and microfluidic PADs (μPADs), have been well developed and shown a great impact on the clinical diagnosis, environmental monitoring and food safety management [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. For example, one type of PAD, the lateral flow immunoassay (LFIA), is commercially available and is extensively used as a powerful diagnostic platform for the rapid detection of antibodies or antigens at a low cost [34,35,36]. The LFIA has dominated the market of rapid diagnostic testing since the lateral flow immunochromatographic strip was first developed for screening the supernatants of hybridomas in 1982 through antigen–antibody interaction on paper to produce a color change visible to the naked eye [34,35,36,37].
The PADs detection methods include optical techniques (colorimetry, fluorescence and surface enhancement Raman scattering (SERS), etc.) and electrochemical (EC) methods (amperometry, potentiometry, voltammetry, electrochemical impedance spectroscopy (EIS), electrochemiluminescence (ECL) and photoelectrochemistry (PEC)) [21,22,23,24,25]. Among these detection methods, colorimetry offers simplicity and convenience and, until 2009, had been one of the main detection methods in PADs. The biggest advantage of paper-based colorimetric devices is that the presence of a specific analyte can be distinguished easily with the naked eye without expensive and complex instruments through the change of color. However, the colorimetric method is limited to qualitative yes/no detections and/or semi-quantitative analysis because it has several inherent disadvantages, such as narrow dynamic range, poor sensitivity and being easily interfered with by environmental light and biased by users’ subjectivity. The EC method was first used as a PAD detection technique in 2009 by Dungachi et al. [38]. Generally, the analytical performances (especially sensitivity) of electrochemical paper-based analytical devices (also known as ePADs) are better than those of paper-based colorimetric analytical devices [18,23,24,25,28,31]. Unfortunately, the analytical performance (in particular, selectivity) of ePADs can be decreased significantly in complex matrices. Currently, the EC detection and optical detection are accounted for as the two main detection methods of PADs (as shown in Figure 1).
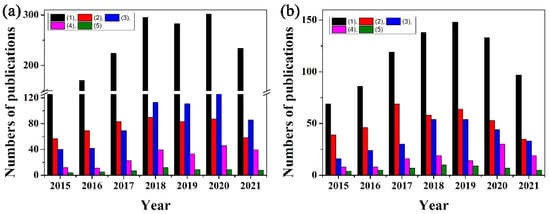
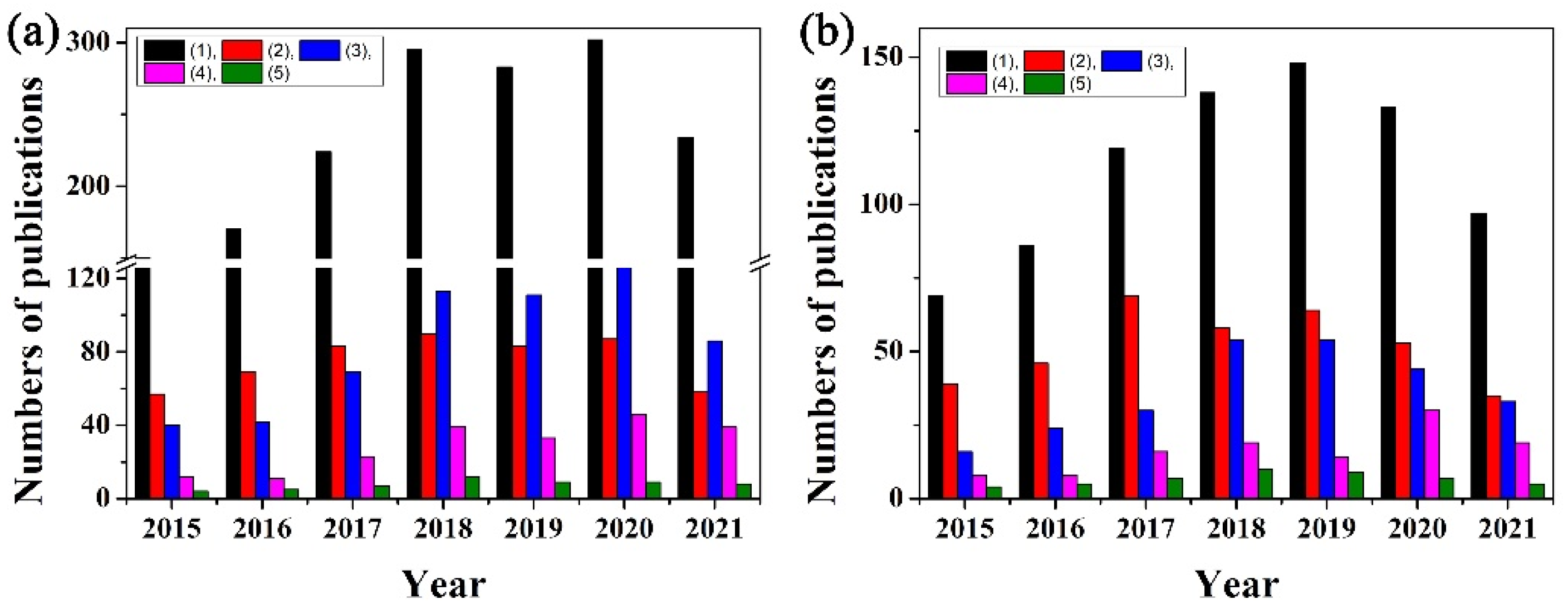
Figure 1.
The annual publications numbers from 1 January 2015 to 30 October 2021 on the keywords: (a) (1) paper-based analytical devices and paper-based analytical devices plus (2) electrochemical, (3) colorimetric, (4) fluorescence, (5) Raman; and (b) (1) paper-based analytical devices plus nanoparticle/nanoparticles, and paper-based analytical devices plus nanoparticle or nanomaterial plus (2) electrochemical, (3) colorimetric, (4) fluorescence, (5) Raman. The data were obtained from Web of ScienceTM.
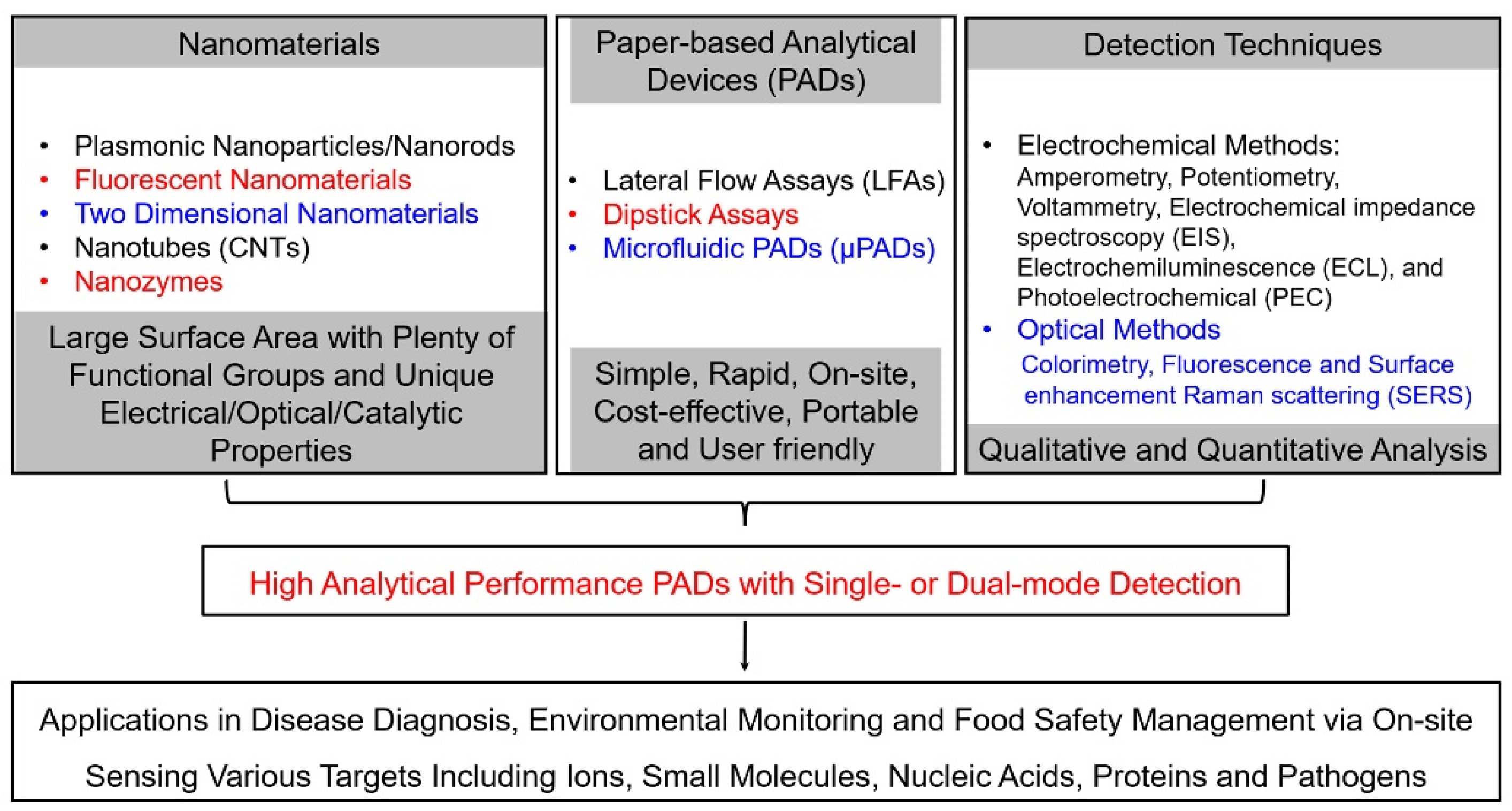
To resolve these limitations, nanomaterials were utilized to produce selective and sensitive detection signals on PADs because nanomaterials and their composites exhibit unique physical and chemical properties (as shown in Figure 2) [26,27,28,29,30,31,32,33,34,35]. For instance, nanoparticles, including gold nanoparticles (AuNPs) and magnetic nanoparticles (MNPs), have been extensively used as signal indicators for paper-based colorimetric analytical devices [32,33,34]. Several AuNPs labeled lateral-flow test-strip (LFTS) immunosensors, such as human chorionic gonadotropin (HCG) and Hepatitis B surface antigen (HBsAg) colloidal gold immunoassay strips, have been clinically approved for rapid testing. Transition metal dichalcogenides (TMDs) and carbon nanomaterials, such as carbon nanotubes and graphenes, can accelerate electron transfer and increase actual electrode area when they are used as functional materials on electrode surfaces, resulting in the enhancement of the sensitivities of ePADs [18,24,31]. Fluorescent nanoparticles, such as quantum dots (QDs), carbon nanodots (CDs) and upconversion nanoparticles (UCNPs), offer new opportunities to update the assaying performance of paper-based fluorescent analytical devices in the responding time, sensitivity and selectivity because they have unique optical properties, such as tunable fluorescence color, high quantum yields, wide excitation wavelength, narrow emission band and excellent optical stability [33]. Moreover, nanomaterials have large amounts of surface-active sites as well as high surface-to-volume ratios, which support diverse functionalization with high densities of recognition units. The phenomenon further improves the detection performances of PADs. Therefore, the integration of nanomaterials with PADs enables strong quantitative capabilities of PADs and expands their applicable fields.
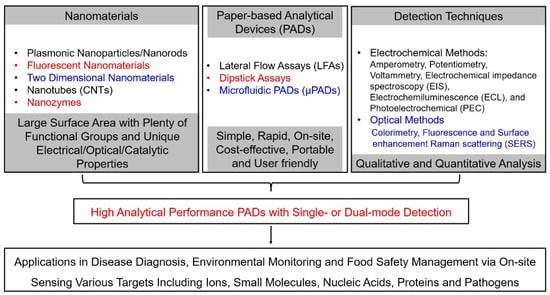
Figure 2.
Outline of nanomaterial-based paper-based analytical devices.
Currently, great efforts are being made for improving the detection performance of PADs by using advanced materials, such as nanomaterials and their composites [33,34,35,36]. The latest reviews have extensively summarized the specific characteristics of PADs. For instance, the fabrication of nanomaterial-based colorimetric and fluorescent PADs was reviewed by Patel et al. [33]. The signal amplification strategies of nanoparticle-based lateral flow testing strips (LFTSs) have been discussed by Shirshahi and Liu [34], and Díaz-González and de la Escosura-Muñiz [35], respectively. The engineering strategies for enhancing the performance of ePAD were reviewed by Baharfar et al. [39]. Based on the detection targets, the applications of nanomaterial-based PADs have also been reviewed by several groups [27,28,36]. The purpose of this review is to introduce readers to a general overview of the recent developments regarding nanomaterial-based PADs in terms of the detection modes and their representative applications.
2. Nanomaterial-Enhanced Paper-Based Analytical Device
2.1. Electrochemical Paper-Based Analytical Devices
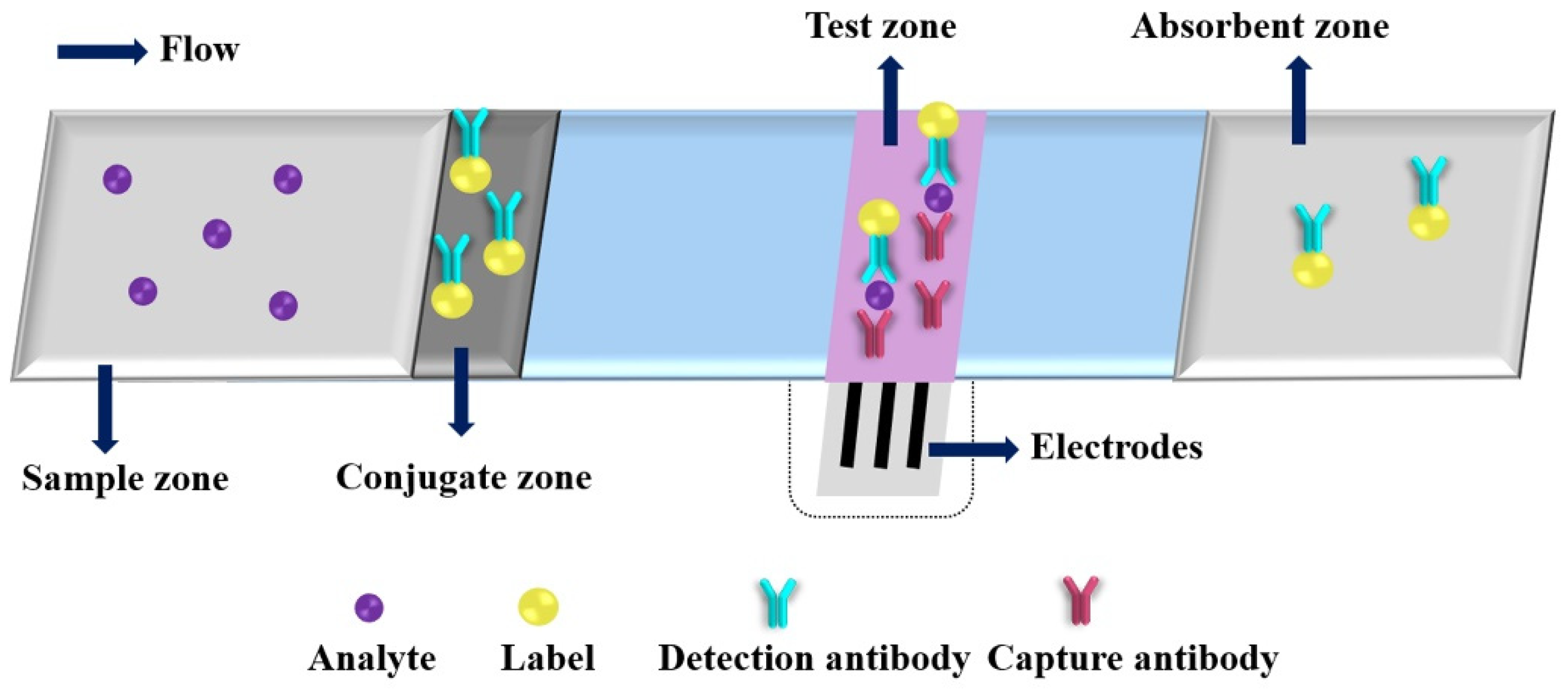
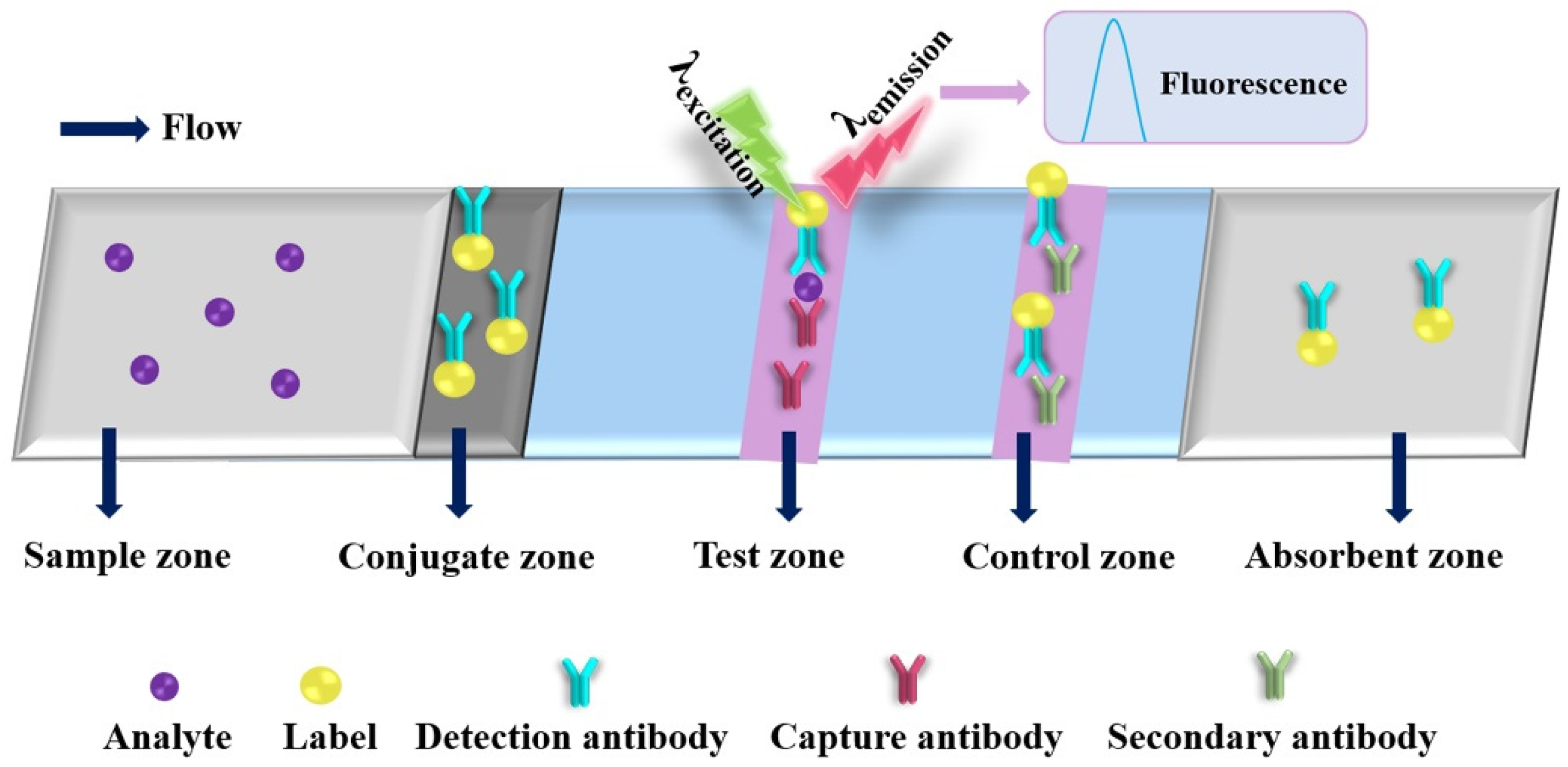
Due to its desirable features, such as high sensitivity, rapid response and easy miniaturization, the EC detection has gradually become one of the most commonly used detection principles for PADs. A typical immunoassay of ePAD is shown in Figure 3. After adding a drop of analyte solution on the sample zone, the analyte will bind to the detection antibody at the conjugate zone and then bind to the capture antibody at the test zone; the excess conjugates are migrated to the absorbent zone under driving by capillary action. Three electrodes (working electrode, counter electrode and reference electrode) are needed for EC analysis, and the concentration of the target analyte can be correlated to the EC response intensity of the electroactive species. The electroactive species are produced by labels catalyzed EC substrates. Dungachi et al. fabricated the first paper-based ePAD for the simultaneous determination of glucose and lactate in real samples by photolithography and screen-printing technology in 2009 [38]. Various nanomaterials and their nanocomposites have been demonstrated as powerful EC transducers and efficient electroactive label carriers in the design strategy of ePADs, which offers great improvements of the analytical performance of ePADs through increasing EC signal (e.g., current) production and decreasing background noise (i.e., enhancing signal-to-noise ratio (S/N)) (as shown in in Table 1) [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].
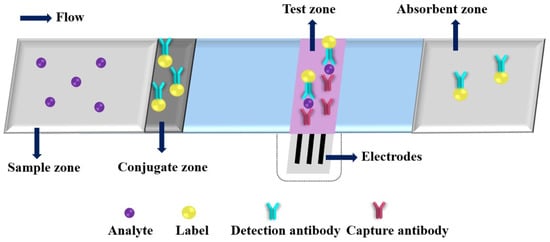
Figure 3.
An illustration of a typical electrochemical paper-based analytical device.

Table 1.
The typical nanomaterial-enhanced ePADs for sensing various analytes.
Various nanoparticles, such as noble metal nanoparticles, metallic oxide nanoparticles and silica nanoparticles (SiNPs), have been extensively used to fabricate and/or modify the working electrodes of ePADs for achieving good detection performance through different methods, including directly dispersing nanoparticles in the printing ink and in situ growth, electrogeneration and drop-casting of nanoparticles on the screen-printed carbon electrodes (SPCE) [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. For example, Pavithra et al. developed an ePAD for immunosensing carcinoembryonic antigen (CEA) by using AuNP working electrode, which was fabricated by directly screening printed self-made AuNP ink on the Whatman® grade 1 chromatography paper [47]. The as-developed ePAD exhibited a linear range of 1.0 ng mL−1 to 100.0 ng mL−1 with a limit of detection (LOD) of 0.33 ng mL−1, The ePAD was used successfully to analyze CEA in the diluted human serum samples, demonstrating that the ePAD has good practicability. Zheng et al. developed an ePAD for ultrasensitive detection of CEA and prostate-specific antigen (PSA) by using cyclodextrin functionalized AuNPs (CD@AuNPs) and AuNPs modified paper working electrode (PWE) [40]. The CD@AuNPs exhibited mimicking properties of both glucose oxidase (GOX) and horseradish peroxidase (HRP) simultaneously, which can efficiently electrocatalytically reduce H2O2. The AuNPs modified PWE was constructed by in situ growth of AuNPs on the surfaces of cellulose fibers of paper. Taking advantage of the high conductivity of AuNP modified PWE and good catalytic activity of CD@AuNPs, the as-developed ePAD exhibited wide linear ranges (0.005 to 100 ng mL−1 (CEA) and 0.002 to 40 ng mL−1 (PSA)), low LODs (0.002 ng mL−1 (CEA) 0.001 ng mL−1 (PSA) and high stability (retaining 90% of the initial responses after stored at 4 °C for 15 days). The ePAD was used to detect CEA and PSA in spiked human serum samples, and satisfactory recoveries (in the range of 100.4% to 109.2% for CEA and in the range of 100.9% to 114.0% for PSA) were obtained, indicating that the ePAD has a great potential application of detecting CEA and PSA in clinical samples. Pinyorospathum et al. developed an ePAD for detection of C-reactive protein (CRP) through electrodeposited AuNPs on SPCE, followed by the self-assembly of PMPC-SH on AuNP surface [41]. In the presence of CRP and Ca2+, the current of ePAD was decreased by increasing the concentration of CRP, while [Fe(CN)6]3−/4− was used as the electrochemical probe. The as-developed ePAD exhibited a linear range of 5 to 5000 ng·mL−1 with an LOD of 1.6 ng·mL−1, which was applied successfully to detect CRP in the certified human serum samples. De França developed an ePAD for detection of dopamine through drop-casting CdSe/CdS magic-sized QDs (MSQDs) on the graphite working electrode (5 mm in diameter), which was drawn on chromatography paper by 6B grade pencil [52]. After being decorated by 10 μg MSQDs, the peak current was enhanced ca. 46% in comparison with that of bare electrode, besides a decrease in the charge transfer resistance and increase in the electroactive area of the sensor. The as-developed ePAD exhibited excellent analytical performance, including low LOD (96 nmol L−1), good stability (within a period of 7 days without major variations of peak current), repeatability (ca. 2.85% relative standard deviation (RSD) and reproducibility (ca. 7.2% RSD). The ePAD was employed successfully for sensing dopamine in human blood serum samples with recovery rates between 95.2% and 102.6%. Because of their high catalytic activity, nanomaterials can also be used as active probe for developed ECL PAD [54,55,56]. For instance, Huang et al. developed an auto-cleaning ECL PAD for detection of Ni2+ and Hg2+ through using superior peroxidase-like activity of cubic Cu2O-Au nanoparticles and a large specific surface area and excellent conductivity of silver nanoparticles (AgNPs) [55]. The cubic Cu2O-Au nanoparticles can catalyze H2O2 to generate reactive oxygen species, promoting the luminescence of N-(4-Aminobutyl)-N-ethylisoluminol (ABEI)). The as-developed ECL PAD exhibited wide linear ranges (10 nmol L−1 to 0.2 mmol L−1 (Ni2+) and 10 pmol L−1 to 1 μmol L−1 (Hg2+)) and low LODs (3.1 nmol L−1 (Ni2+) and 3.8 pmol L−1 (Hg2+)), which was used successfully to detect Ni2+ and Hg2+ in the spiked lake water.
As transduction materials, carbon nanomaterials, including single-walled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs) and different types of graphene materials, have been getting great attention in the ePAD fabrication because they have high surface area, excellent electrical conductivity and rich surface-chemical properties [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. For instance, Valentine et al. found that the device-to-device reproducibility and current intensity of ePAD can be efficiently improved through formation of MWCNT network in the porous structures of paper [58]. Tran et al. developed an ePAD for non-enzymatic detection of glucose through the deposition of the SWCNT layer on wax-printed nitrocellulose (NC) membrane [59]. The SWCNT electrode exhibits high conductivity with an average resistivity of less than 100 Ω/sq and excellent mechanical property. After modification of the SWCNT electrode by AuNPs, the as-developed ePAD exhibited excellent glucose detection performance, including good reproducibility (RSD < 8%) and high sensitivity (240 μA/mM cm2), which was used successfully to determine glucose in Coke. Pungjunun et al. developed an origami-based ePAD for sensing NO and NO2 (as NOX) by using a screen-printed graphene electrode modified with copper nanoparticles (CuNP/SPGE) [64]. Because of the good catalytic property of CuNP towards the EC conversion of NOX and excellent conductivity of graphene, the as-developed ePAD exhibited high selectivity, low LODs (0.23 vppm and 0.03 vppm with exposure times of 25 min and 1 h, respectively), good reproducibility (RSD < 5.1%) and long lifetime (>30 days). The ePAD was applied to detect NOX in air and exhaust gases from cars, and satisfactory results were obtained. Cai group has been developed a series of ePADs for detection of various biomarkers, including CEA and neuronspecific enolase (NSE), by using amino functional graphene (NG)-Thionin (THI)-AuNPs nanocomposites modified SPCEs [66,67]. Integration of SiNPs modified paper microzones with reduced graphene (RG) modified SPCE, Scala-Benuzzi et al. developed an ePAD for the quantitative determination of ethinylestradiol (EE2) in water samples through capturing EE2 by the immobilized anti-EE2 specific antibodies on the paper microzones, subsequently releasing the adsorbed EE2 by adding a diluted solution of sulfuric acid and detecting the desorbed EE2 by Osteryoung square wave voltammetry (OSWV) [69]. The as-developed ePAD exhibited excellent analytical performance, including wide linear range (0.5 to 120 ng L−1), low LOD (0.1 ng L−1) good recovery values (from 97% to 104%) and good reproducibility (RSD < 4.9%). There are no significant differences were found between the results of ePAD and the results of spectrophotometric immunoassay, when two methods were used for the quantification of EE2 in river water samples and spiked water samples.
The unique features of metal-organic frameworks (MOFs), including high porosity, tunable framework structures, large surface areas and multiple functionalities, make them extremely attractive for improving the detection performance of biosensors [78,79]. Recently, MOFs and their nanocomposites have been used for developing ePAD with excellent detection performance [75,76,77]. Wei et al. fabricated a cobalt-MOF (Co-MOF) modified carbon cloth/paper (CC/Paper) hybrid button-based PAD (Co-MOF/CC PAD) for nonenzymatic quantitative EC detection of glucose through in situ growth of Co-MOF on CC [75]. As a typical nanozyme, the environment tolerance of Co-MOFs is much better than that of natural enzyme, which can increase significantly the stability of ePAD. Densely grown Co-MOF on CC can maximize its catalytic sites, resulting in high sensitivity of ePAD. The Co-MOF/CC PAD exhibits linear range from 0.8 mmol L−1 to 16 mmol L−1 with an LOD of 0.15 mmol L−1 and maintains at a stable detection performance in 60 days, and then gradually decreased to about 60% after 120 days. The ePAD was used successfully to determine glucose in multiple body fluids, including serum, urine and saliva. Lu et al. developed an ePAD-based DNA hybridization for detection of human immunodeficiency virus (HIV) DNA by using the nickel MOF (Ni-MOF) composite/AuNPs/CNTs/polyvinyl alcohol (Ni–Au composite/CNT/PVA) paper electrode as working electrode and methylene blue (MB) as a redox indicator [77]. The CNT/PVA were deposited on the cellulose membrane by vacuum filtration, and Ni–Au composites were loaded on CNT/PVA film by the drop-casting method. The Ni–Au composite/CNT/PVA film electrode has large specific surface area and conjugated π-electron system, which makes a higher loading of the single-stranded DNA probe than that of CNT/PVA film electrode. The phenomenon improves the sensitivity for detecting target DNA. The ePAD exhibited excellent sensing performance with a wide linear range of 10 nmol L−1 to 1 μmol L−1, a low LOD of 0.13 nmol L−1, good selectivity against one-base mismatch DNA sequences and excellent stability after 20 days of storage. The target HIV DNA was detected successfully even in complex serum samples by the as-developed ePAD.
2.2. Colorimetric Paper-Based Analytical Devices
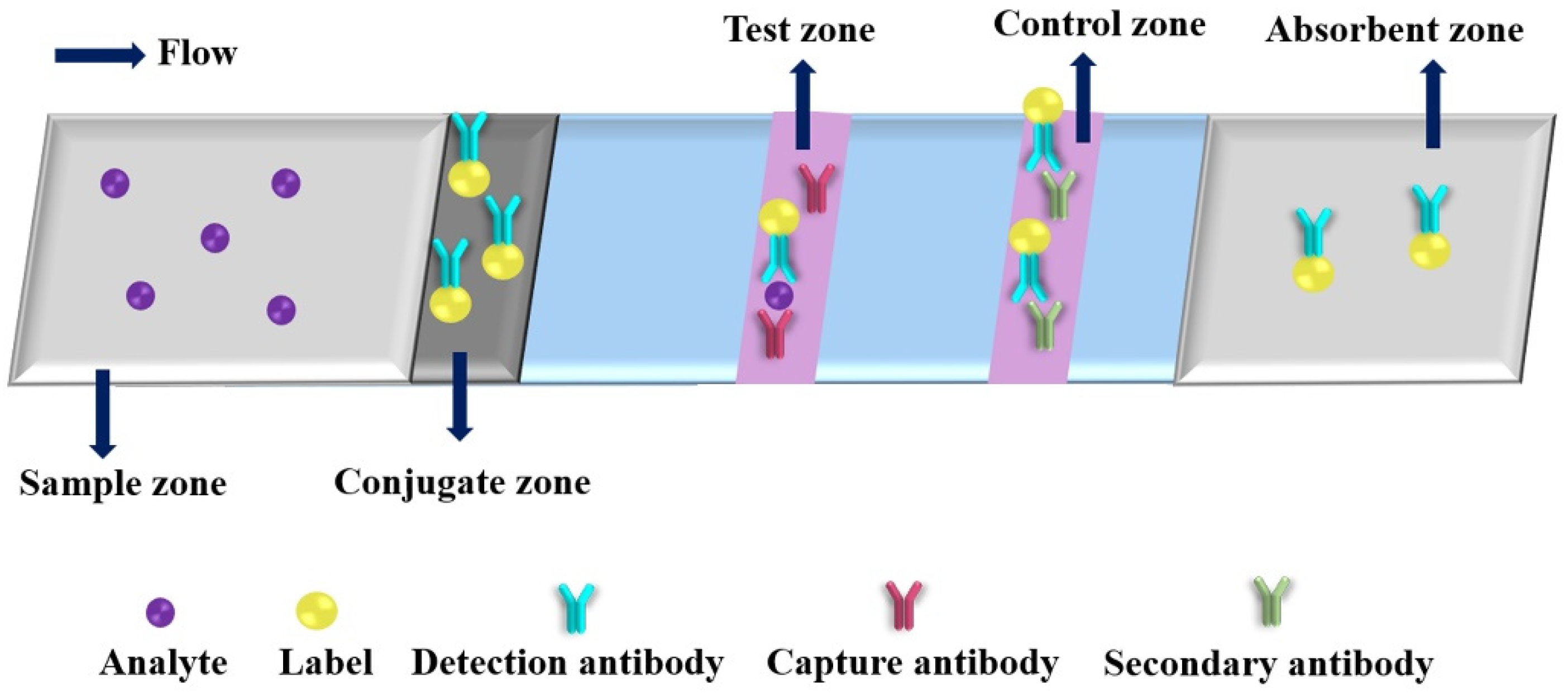
Colorimetric detection is the most common method in PADs. Figure 4 shows a typical schematic of colorimetric test strip. Generally, the test strip includes five functional zones: sample, conjugate, test, control and absorbent zones. Under the analyte solution migration, conjugates will capture the analyte with detection antibody at conjugate zone, and then the conjugates and analyte bind to the capture antibody at test zone, the excess conjugates are migrated to the control zone conjugate with secondary antibody. After finishing the reaction, the ImageJ is usually used for the collecting colorimetric signals of the test and control lines. Up to now, various kinds of nanomaterials are used as colorimetric labels. The nanomaterial-based colorimetric PADs have been extensively used for detection of various targets (as shown in Table 2) [43,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]. Plasmonic nanoparticles, such as AuNPs and AgNPs, are extremely useful indicators for the fabrication of colorimetric PADs because of their strong local surface plasmon resonance (SPR) bands [80]. Moreover, multiple, simultaneous tests can be rapidly performed with low sample consumption by incorporating these surface-modified AuNPs into a PADs that can be read using just a smartphone. For example, Díaz-Amaya et al. developed a μPAD for multiplexed aptamer-based detection of analytical targets through a salt-induced aggregation of single strand DNA (ssDNA) functionalized polyethyleneimine (PEI) encapsulation of gold-decorated polystyrene (PS) core particles (ssDNA-PEI-Au-PS) [85]. The net positive charge of the PEI layer avoids the direct interaction of metallic ions and ssDNAs with the AuNPs, which provides ideal conditions for controlled induction of aggregation of AuNPs on the test zones of μPAD, resulting in high sensitivity, selectivity and reproducibility (RSD = 5.69%). Using a smartphone as detector, the analytical performance of as-proposed μPAD was demonstrated by multiplexed detection of Hg2+ and As3+ with low LODs (1 μg mL−1) and high specificity (p > 0.05) versus different interferent ions (Ca2+, Fe2+, Mg2+ and Pb2+). The authors provided a universal idea for fabricating μPAD with the capability of multiplex and quantitative colorimetric detection. Monisha et al. reported a PAD with AgNPs for on-site determination of Hg2+ from environmental water samples by inkjet-printing polyvinyl pyrrolidine (PVP) stabilized AgNPs on Whatman® grade 1 chromatography paper [96]. In the presence of Hg2+, the color of AgNP is changed from yellow to colorless because of the oxidation of AgNPs into Ag+ ions on the paper substrate. The as-proposed PAD exhibited the linear range from 40 to 1200 ng mL−1 with LOD of 10 ng mL−1, and was used successfully for quantitative detection of Hg2+ in different types of water samples collected from river, tube well, pond, coal mines and industrial waste. This approach could be used to determine Hg2+ in other real samples, such as biological and vegetable samples. Recently, Mettakoonpitak et al. developed an uncomplicated, affordable and environmentally friendly method for fabrication of μPAD by screen-printing biodegradable polycaprolactone (PCL) as high-resolution hydrophobic barriers [98]. The proposed method can produce as narrow as 510 ± 40 μm hydrophilic channel and 490 ± 30 μm hydrophobic edge, respectively. The as-developed method was used successfully to fabricate μPADs for detection of Cr3+ and Cl− with high selectivity. For Cr3+ analysis, the μPADs achieved a linear range of 50.0 to 1000.0 ng mL−1 with an LOD of 15.0 ng mL−1, when AgNPs were used as the colorimetric probe. Integration of nanoparticle as color indicator and PCL screen-printing could provide a simple and environmentally friendly method for fabricating μPADs with high analytical performance, which are ultimately utilized in wide-ranging applications.
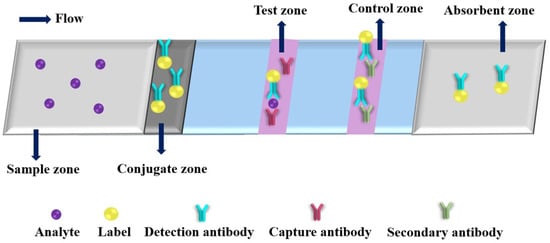
Figure 4.
An illustration of a typical colorimetric paper-based analytical device.

Table 2.
The typical nanomaterial-enhanced colorimetric PADs for sensing various analytes.
In addition, the AuNPs can also serve as carriers for the simultaneous immobilization of different biomolecules (e.g., antibodies, DNA and aptamers) with an abundant number of biorecognition elements or optical/electrochemical tags on their surfaces to provide more binding sites or signal amplification of analyte for a single recognition reaction. Furthermore, different antibodies can be easily introduced to the AuNP surface via electrostatic interactions to provide highly specific recognition sites for biomolecular sensing, resulting in simplify the PAD fabrication procedure. Huang et al. reported a highly sensitive colorimetric PAD for detection of PSA by using AuNPs labeled with biotinylated poly(adenine) ssDNA sequences and streptavidin-HRP for enzymatic signal enhancement [87]. The as-proposed PAD was able to detect as low as 10 pg mL−1 PSA in a test that could be completed in as little as 15 min.
Comparison with natural enzymes, nanomaterials with enzyme-like characteristics (i.e., nanozymes), such as magnetic nanoparticles, noble metal nanoparticles, MOFs, heterojunctions, etc., exhibit several advantages, including easy production with large-scale, low cost and high stability in harsh environments. These unique properties endow them with attractive applications in the fabrication of PADs with high analytical performance [104,111,112]. Zhang et al. developed a ready-to-use PAD for the determination of H2O2 by simply immobilization of mesoporous carbon-dispersed palladium nanoparticles (Pd NPs/meso-C) and the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate onto a common chromatography paper [104]. Taking the advantage of large surface area of the meso-C support and the good dispersity of PdNPs, the PdNPs/meso-C show excellent catalytic performance to trigger the chromogenic reaction of colorless TMB to blue TMBox mediated by H2O2. The as-developed PAD exhibited a linear range of 5 to 300 mol L−1, and can be used to determine H2O2 in complex matrices, such as milk. Kitchawengkul et al. developed a laminated three-dimensional (3D)-μPAD for colorimetric determination of total cholesterol (TC) in human blood by using the peroxidase-like activity of nitrogen-doped CDs (N-CDs) [108]. The 3D-μPAD with a 6 mm circular detection zone was fabricated by a simple wax screen-printing technique, which consisted of four layers laminated together vertically. The 3D-μPAD exhibited a linear range of 0.05 to 10 mmol L−1 with an LOD of 0.014 mmol L−1. In particular, TC in human blood could be determined by the naked eye within 10 min by simple comparison with a color chart. Overall, the as-proposed 3D-μPAD serves as a simple, low cost, rapid, sensitive and selective alternative for detection of TC in whole blood samples that is friendly to unskilled end users. Cui et al. fabricated an origami PAD (oPAD) assisted by Pd decorated Cu/Co co-doped CeO2 (CuCo-CeO2-Pd) nanospheres, for dual-mode electrochemical/visual detection of amyloid-β (Aβ) peptide with high sensitivity [48]. In this case, the CuCo-CeO2-Pd nanospheres were introduced as an enhanced “signal transducer layer”, which act as an outstanding catalyst for catalyzing glucose to produce H2O2 for DPV signal readout and further 3,3′,5,5′-tetramethylbenzidine (TMB) oxidation for colorimetric analysis. The oPAD exhibited linear ranges from 1.0 pmol L−1 to 100 nmol L−1 (EC detection) and 10 pmol L−1 to 100 nmol L−1 (visual detection) with LODs of 0.05 pmol L−1 (EC detection) and 0.5 pmol L−1 (visual detection), respectively. The oPAD was used successfully to analysis Aβ peptide in artificial cerebrospinal fluid (aCSF) and serum samples. Al Lawati et al. developed a PAD for the colorimetric/fluorometric monitoring of glucose by co-immobilizing two-dimensional cobalt-terephthalate MOF nanosheets (2D CoMOFs) and GOX on chromatography paper [112]. Due to its highly porous and extraordinarily stable structures, the 2D CoMOF increased significantly the stability and performance of GOX, and also acted as a catalyst to accelerate the reaction of H2O2 produced by the enzymatic oxidation of glucose with o-phenylenediamine (OPD) serving as a peroxidase substrate, resulting in a yellow-brown color change and a high fluorescence emission. The as-developed PAD showed high analytical performance for the quantification of glucose including high accuracy, wide linear range (50 mol L−1 to 15 mmol L−1) and low LODs (16.3 (colorimetric detection) and 3.2 mmol L−1 (fluorometric detection)), and was used successfully to determine glucose in blood samples from healthy and diabetic volunteers.
2.3. Fluorometric Paper-Based Analytical Devices
Recently, the nanomaterial-based fluorometric PADs are increasingly being developed for sensing various targets (as shown in Table 3) [113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161]. The schematic diagram of the working principle of the fluorometric PAD is shown in Figure 5. The reaction process of the analyte on the test strip is common with that of the colorimetric test strip. After the reaction is finished, the fluorometric signals of emission wavelengths at the test and control zones are recorded by fluorometric spectrophotometer under irradiating with excitation light. Fluorescent nanomaterials, including metal nanoclusters (NCs), CDs, QDs, UCNPs and MOFs, have unique properties, such as wide excitation wavelength, narrow emission band, tunable fluorescence color, highly optical stability and good surface-modified flexibility. For instance, Ungor et al. developed a fluorescent PAD with red-emitting (λemission = 645 nm, d = 1.5 ± 0.3 nm) fluorescent gold nanoclusters (AuNCs) for rapid detection of L-kynurenine (Kyn) with an LOD of 5 μmol L−1, which is in good accordance with the toxic Kyn concentration of liquor and serum for several cancers (vulvar, ovarian cancer and leukemia) [116]. Lert-itthiporn et al. reported a fluorescent PAD for membraneless gas-separation with subsequent determination of iodate (IO3−) by fabrication of two circular reservoirs (donor reservoir and the acceptor reservoir) in a folded chromatography paper [117]. The IO3− is reduced to free iodine (I) by iodide (I−) in the donor reservoir, while the gold core of the bovine serum albumin-stabilized gold NCs (BSA-AuNCs) in the acceptor reservoir is etched by diffused I from the donor reservoir, which results in the quenching of the red emission of BSA-AuNCs in the acceptor reservoir. After folding, the donor reservoir and acceptor reservoir were mounted together through a two-sided mounting tape to allow membraneless gas-separation of free I from the donor reservoir to diffuse into the acceptor reservoir. Under the ultraviolet (UV) light (365 nm) irradiation, the PAD exhibited a linear range from 0.005 to 0.1 mmol L−1, an LOD of 0.01 mmol L−1, high accuracy (mean recovery: 95.1 (±4.6) %) and high precision (RSD < 3%), which was applied successfully for the measurement of IO3− in iodized salts and fish sauces without prior sample pre-treatment. Yin et al. developed a fluorescent/colorimetric dual-model PAD based on the quenching effect of graphitic carbon nitride on palladium nanoclusters (PdNCs) for detection of miRNA let-7a [119]. In this case, the PdNCs not only was used as a fluorescence probe but also could catalyze a chromogenic reaction for the generation of color change. Combined with nucleic acid cycle signal amplification, the fluorescent/colorimetric dual-model PAD exhibited linear ranges of 50 pmol L−1 to 1 mol L−1 (colorimetry) and 10 fmol L−1 to 1 nmol L−1 with LODs 16 pmol L−1 (colorimetry) and 3 fmol L−1 (fluorescence), respectively. In addition, the fluorescent/colorimetric PAD has excellent stability (about 90% of the fluorescent response remaining after 6 weeks) and good reproducibility (both of intra-assay and inter-assay RSDs were less than 5.5%). The experimental results demonstrate that the fluorescent/colorimetric dual-model PAD can be used for on-site detection of miRNAs with good analytical performance.

Table 3.
The typical nanomaterial-enhanced fluorescent PADs for sensing various analytes.
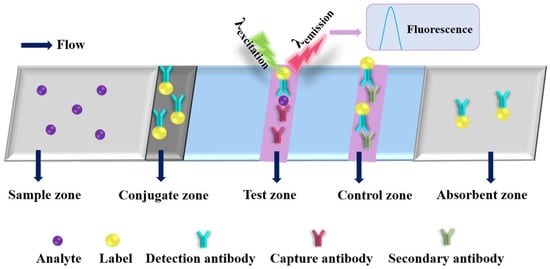
Figure 5.
Schematic diagram of a typical fluorometric paper-based analytical device.
QDs are generally good donors for the fabrication of FRET sensing platforms since the fluorescence of QDs is easily quenched by many substances. Liu et al. reported a fluorescent PAD for detection of Ag+ and AgNPs by inkjet-printing CdTe QDs on Whatman® grade 3030–861 chromatography paper because Ag+ enables to quench the fluorescence of CdTe QDs via a cation exchange reaction between Ag+ and the CdTe QDs [121]. Under optimized conditions, the as-proposed PAD exhibited high analytical performance of Ag+ or AgNPs (pretreated by HNO3), including high selectivity, low LOD (0.05 μg mL−1) and good accuracy (4.5% and 2.2% RSDs for 1 μg mL−1 and 7 μg mL−1 of Ag+, respectively). In addition, the practicality of the fluorescent PAD was demonstrated by detecting Ag+ and AgNPs in river water and 12 commercial products, including four textiles, three gynecologicallotions, one surgical dressing and four baby products. Zhou et al. developed a 3D rotary fluorescent PAD for multiplexed detection of Cd2+ and Pb2+ by transferring the liquid phase of ZnSe QDs@ion imprinted polymers to solid glass fiber paper [132]. Under optimized experiment conditions, the as-proposed 3D rotary fluorescent PAD exhibited a linear range from 1 to 70 ng mL−1 with an LOD of 0.245 ng mL−1 for Cd2+, and a linear range from 1 to 60 ng mL−1 with an LOD of 0.335 ng mL−1 for Pb2+, respectively. Qu et al. developed a fluorescent PAD based on polythiophene (CP)-CdTe QD conjugates for detection of acetylcholinesterase (AChE) by turning on the fluorescence of the CP-CdTe QD conjugates via the interaction between CP and thiocholine produced by ATCh hydrolysis and aggregation induced emission enhancement (AIEE) [125]. Under optimized conditions, the as-developed fluorescent PAD exhibited a low LOD of 0.14 U L−1. Zhang et al. developed a fluorescent PAD for the ratiometric fluorescence determination of 2,4-dichlorophenoxyacetic acid through fluorescence resonance energy transfer (FRET) of nitrobenzoxadiazole (NBD) and CdTe QDs [122]. Under optimized conditions, the as-developed fluorescent PAD exhibited a linear range of 0.56 to 80 μmol L−1 with an LOD of 90 nmol L−1. The PAD was applied successfully for detection of 2,4-dichlorophenoxyacetic acid in spiked soybean sprouts and lake water samples with high recovery rates ranging from 86.2% to 109.5% and the RSD less than 4.19%.
As a new class of fluorescent nanomaterials, CDs (also known as carbon QDs and carbon dots) have been used to fabricate fluorescent PADs because of their superior merits, such as easy synthesis, biocompatibility, environmental friendliness, fluorescence tunability and stable luminescent emission [141,142,143,144,145,146,147,148,149,150,151,152]. Wang et al. developed an instrument-free fluorescent PAD for detection of Pb2+ by directly inject-printing dual-emission CDs (blue CDs and red CDs) in A4 paper [144]. The blue fluorescence was quenched by Pb2+, while the red fluorescence was kept unchanged. The as-developed fluorescent PAD can detect as low as 2.89 nmol L−1 Pb2+ under a 365 nm UV lamp irradiation. The fluorescent PAD was used successfully to determine Pb2+ in real samples, including tape water and lake water. Tian et al. developed a fluorescent PAD for detection of F− in water by immobilizing the Ca2+, CDs and hexametaphosphate capped AuNPs on the cellulose chromatography paper [149]. Under a 365 nm UV lamp irradiation, the fluorescence color of the PAD changed from orange to blue through the aggregation induced FRET mechanism when various concentrations of F− (0, 100, 200, 300 and 400 mol L−1) were applied. Li et al. developed a fluorescent PAD based on hybrid polydimethylsiloxane (PDMS)/paper for detection of folic acid (FA) by using CDs as fluorophores, which were immobilized on the cellulose paper by Schiff base chemistry [143]. Under optimized conditions, the as-developed fluorescent PAD exhibited a wide range of 1 to 300 μmol L−1 with an LOD of 0.28 μmol L−1. The feasibility of the fluorescent PAD was further verified by detection of FA in orange juice and urine samples with satisfactory results. Liang et al. developed a flower-like AgNPs (FLS)-enhanced fluorescent/visual bimodal PAD for detection of multiple miRNAs [151]. In this case, the ssDNA functionalized CDs (DNA1-N-CDs) were immobilized on the FLS layer, which was in situ grown on the surfaces of cellulose fibers of chromatography paper. The fluorescences of DNA1-N-CDs were quenched by ssDNA functionalized CeO2 NPs (DNA2-CeO2) through DNA hybridization. In the presence of miRNA, the fluorescent intensities of DNA1-N-CDs were recovered and strengthened by FLS. The disengaged DNA2-CeO2 could result in color change after adding H2O2, leading to the real-time visual detection of miRNA. The as-developed FLS-enhanced fluorescent PAD exhibited linear ranges of 0.1 fmol L−1 to 1 nmol L−1 and 0.2 fmol L−1 to 2 nmol L−1 for miRNA210 and miRNA21 with LODs of 0.03 fmol L−1 for miRNA210 and 0.06 fmol L−1 for miRNA21, respectively. The practicability of the FLS-enhanced fluorescence PAD was demonstrated by successful detection of miRNA210 in different cell lysates.
Because of NIR-excitation and the visible light emission nature of UCNPs, the fluorescent PADs using UCNPs as the label can avoid the interference of autofluorescence and scattering light from biological samples and paper substrates, resulting in an improvement in the detection accuracy of the PAD [153,154,155]. Recently, He et al. developed a UCNP-based fluorescent PAD for detection of total immunoglobulin E (IgE) in human serum through resonance energy transfer between UCNPs and organic dye tetramethylrhodamine (TAMRA) [155]. The UCNP-based fluorescent PAD exhibited a linear range of 0.5 to 50 IU mL−1 with an LOD of 0.13 IU mL−1. The practicability of the UCNP-based fluorescent PAD was demonstrated by the determination of IgE in 20 human serum samples. The results of the UCNP-based PAD were well consistent with those of the commercial ELISA kit. The RSDs (n = 3) of the PAD varied from 2.7% to 19.7%. The results suggested that the UCNP-based fluorescent PAD could be used as a POCT device for individual diagnostic and real-time detection.
Recently, a MOFs-based fluorescent PAD has been developed for the detection of various targets [157,158,159,160,161]. Lv et al. developed a fluorescent PAD for detection of CEA through wet NH3-triggered structural change of NH2-MIL-125(Ti) impregnated on paper [157]. The NH2-MIL-125(Ti)-based PAD exhibited a linear range of 0.1 ng mL−1 to 200 ng mL−1 with an LOD of 0.041 ng mL−1. Yue et al. developed a portable smartphone-assisted ratiometric fluorescent PAD for detection of malachite green (MG) by using fluorescent Al-MOF nanosheet and rhodamine B (RhB) as fluorescent probes [161]. The as-developed fluorescent PAD exhibited a wide linear range of 0.5 to 200 μg mL−1, a low LOD of 1.6 μg mL−1, satisfactory recoveries (in the range of 81.90% to 108.00% and low RSD (in the range of 1.00% to 4.69%). The practicability of the fluorescent PAD was verified by detection of MG in spiked fish tissues. The as-obtained results were in good agreement with those obtained by high performance liquid chromatography (HPLC).
2.4. Paper-Based Surface-Enhanced Raman Spectroscopic Analytical Devices
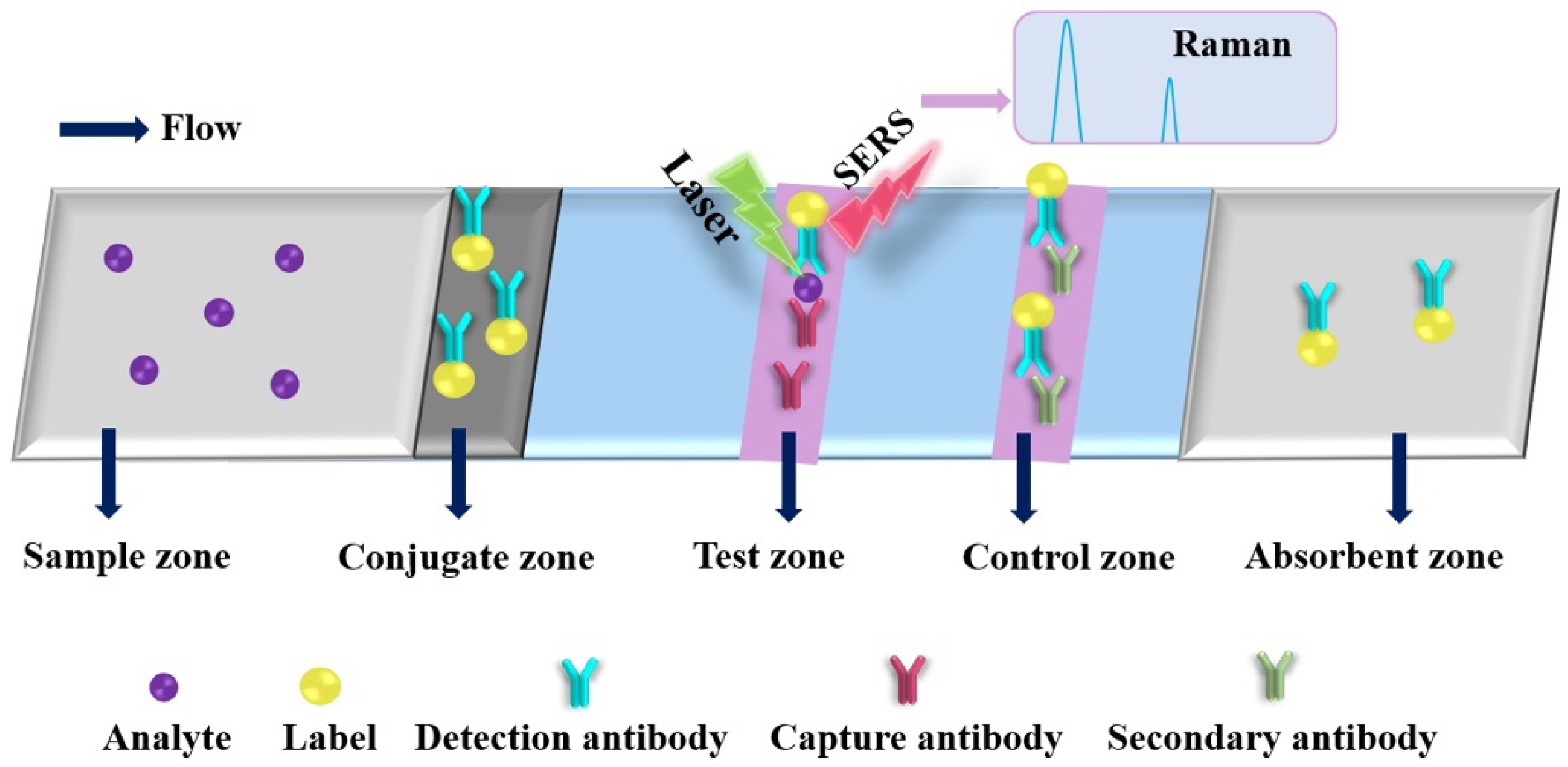
The basic principle of SERS is that the signal of the analyte is strongly amplified through LSPR phenomena (i.e., electromagnetic hot spots) generated by light when it interacts with labels (plasmonic metal nanoparticles), such as gold nanorods (GNRs) and AgNPs, as shown in Figure 6. The PAD-based SERS substrates have gained considerable attention since they enable on-site label-free detection of a wide variety of analytes and provide “fingerprint” signatures of analytes (as shown in Table 4) [134,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176]. Saha and Jana developed a PAD-based SERS assay for the detection of proteins by mixing plasmonic nanomaterials (silver coated AuNPs (Ag@AuNPs)) and analyte in the mobile phase, where the analyte induced Ag@Au nanoparticles form controlled aggregates and generate electromagnetic hot spots inside the microfluidic channel, resulting in a strong SERS signal [173]. The as-developed PAD-based SERS assay exhibited high reproducibility and sensitivity, which can be used to detect 1 fmol L−1 concanavalin A within 3 min. Qi et al. developed an oPAD for miRNA detection through modification of DNA-encoded Raman-active anisotropic AgNPs in the hydrophilic channels [171]. In the presence of analyte, the Raman signals on DNA-encoded AgNPs were amplified through a target-dependent, sequence-specific DNA hybridization assembly. The simple and low-cost oPAD is generic and applicable to various miRNAs, which holds promising applications in point-of-care diagnostics because it can be used to detect as low as 1 pmol L−1 within 15 min. Wu et al. developed a PAD for detection of acrylamide (AAm) in food products by using the strawberry-like SiO2/Ag nanocomposites (SANC) immersed chromatography paper [172]. Under the optimized conditions, the as-developed PAD SERS assay exhibited a wide linear response from 0.1 nmol L−1 to 50 μmol L−1 with a low LOD of 0.02 nmol L−1 and good recoveries of 80.5% to 105.6% for practical samples, including cookies, chips and bread. In addition, the total assaying time of the PAD was less than 10 min. The result indicated that the PAD SERS assay could be a promising strategy in food analysis and verification. Li et al. developed a colorimetric/SERS dual-mode PAD for sensing SO2 by immobilization of 4-mercaptopyridine (Mpy)-modified GNRs-reduced graphene oxide (rGO) hybrids (rGO/MPy-GNRs), anhydrous methanol and starch-iodine complex into cellulose-based chromatography paper through a vacuum filtration method [174]. The PAD can be used not only as a naked-eye indicator of SO2 changed from blue to colorless but also as a highly sensitive SERS substrate because of the SO2-triggered conversion of Mpy to pyridine methyl sulfate on the GNRs. The PAD-based SERS method exhibited a wide linear range from 1 μmol L−1 to 2000 μmol L−1 with a low LOD of 1 μmol L−1. The colorimetric/SERS method was employed for the detection of SO2 in wine, and the as-obtained results matched well with those obtained from the traditional Monier-Williams method. In addition, the color intensities and profiles of the SERS spectra of the colorimetric/SERS dual-mode PAD after 10 weeks are very similar to those of freshly prepared PADs, indicating excellent stability of the colorimetric/SERS dual-mode PAD. This study provides a new strategy for designing of paper-based sensing platform for a wide range of on-site testing applications. Moreover, taking advantage of different nanomaterials, Xia et al. developed a smart PAD (named as vapor generation (paper-based thin-film microextraction system) capable of both sensitive on-site fluorescence detection and accurate SERS quantification of volatile benzaldehyde (BA) by utilizing stimuli-responsive core@shell GNR@QD-embedded MOF structures [125]. The fluorescence emission of carboxyl-capped QDs was completely quenched by amino-modified GNRs via electrostatic interaction. In the presence of BA, the GNRs-QD assemblies was dissociated through the Schiff base reactions between the amine group of 4-mercaptonoaniline and the aldehyde moiety of BA, resulting in the increase in the fluorescence and Raman signal of the hybrid systems. In addition, gaseous BA molecules can be efficiently and selectively concentrated on the GNR surface through the “cavity-diffusion” effect of porous MOF shells, allowing the discrimination of BA in exhaled breath rapidly and precisely even at the sub-ng mL−1 level with excellent specificity against other volatile organic compounds. The as-developed fluorescent/SERS dual-mode PAD was used successfully to accurately discriminate lung cancer from controls.
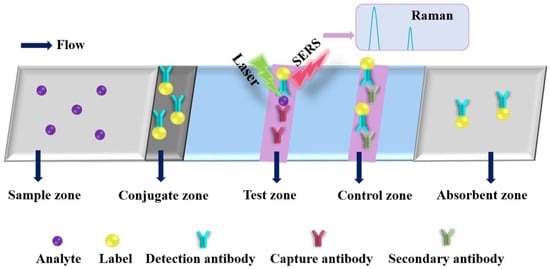
Figure 6.
Schematic diagram of a typical paper-based surface-enhanced Raman spectroscopic analytical device.

Table 4.
The typical nanomaterial-based SERS PADs for sensing various analytes.
2.5. Comparison of the Detection Methods of Paper-Based Analytical Devices
The above introduced detection methods of PADs indicate that their detection performances have been greatly enhanced with the great advance of nanofabrication science. For the convenience of the readers, the merits and drawbacks of the four major PAD detection methods (EC, colorimetric, fluorometric, SERS PADs) are compared and summarized in Table 5.

Table 5.
The comparison of four representative detection methods of PADs.
3. Conclusions and Perspective
The PADs have gained remarkable consideration as simple, low-cost and powerful POCT platforms since the first commercialized lateral flow immunoassay (LFIA) was used for a home pregnancy test. Although PADs have achieved great success in the rapid testing area, some parameters on the analytical performance of PADs need to be further improved to meet the specific needs of different detection fields. Integrating nanomaterials/nanotechnology into PADs can help improve their analytical performance, including sensitivity, selectivity, reproducibility, stability and multiplexed analysis. For instance, the sensitivity of ePADs can be increased significantly when carbon nanomaterials with high conductivity and high specific surface area are used as electrode materials or electrode modifiers. The AuNPs are commonly used color indicators of colorimetric PADs. The sensitivity of colorimetric PADs will be further increased when AuNPs are conjugated with other catalytic NPs, such as CeO2 NPs, and/or enlarged through the metallic atoms (such as Au or Ag) surface deposition strategy. In comparison with organic dyes, the fluorescent nanomaterials, including QDs, CDs and metallic NCs, have wide excitation wavelength, high fluorescence brightness and strong resistance to photobleaching, resulting in the excellent stability, reliability and accuracy of PADs. Because the upconverted phosphorescence of UCNP can efficiently avoid the interference of biological autofluorescence, the reliability and accuracy of PADs can also be improved by using UCNP as a fluorescence probe. Nanozymes such as CeO2 NPs and MOFs have higher robustness than natural enzymes. Therefore, the PADs exhibit high stability and sensitivity, while the nanozymes are used for signal amplification in the PAD fabrication. Meanwhile, the nanomaterials have surface-functionalized flexibility for creating multiple reactive/recognizable sites with analytes to achieve multiplexed analysis. In addition, the nanomaterial-based PADs are compatible with dual-mode detection, such as colorimetry–fluorescence and EC-optical detection, which enables the reliability and accuracy of the PADs.
The nanomaterial-based PADs have managed great achievements for sensing various targets, including ions, small molecules, nucleic acids, proteins and pathogens, in the bench research. However, there are limited examples that have transformed from proof-of-principle analytical devices to commercial products. More efforts should be made continuously to develop novel strategies for increasing the longevity, robustness and reliability in the real-time monitoring, which are largely dependent on the synthesis of advanced nanomaterials, introduction of a new sensing mechanism and multiple detection modes, development of a reliable microfabricating methodology/standard and simplifying the detection procedures. The next generation of PADs will be simple, cost-effective and multiplexed and be able to provide on-site qualitative/quantitative analysis by the naked eye and/or portable equipment, such as smartphones and wearable electronic devices.
Author Contributions
Writing—original draft preparation, R.P., Q.Z., X.M. and Z.W.; writing review and editing, R.P., Q.Z., J.W., X.M. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 21775145), and Science and Technology Developing Foundation of Jilin Province (Grant No. 20210204049YY) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China and Science and Technology Developing Foundation of Jilin Province for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication Techniques for Microfluidic Paper-Based Analytical Devices and Their Applications for Biological Testing: A Review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef]
- Ahmed, S.; Bui, M.N.; Abbas, A. Paper-Based Chemical and Biological Sensors: Engineering Aspects. Biosens. Bioelectron. 2016, 77, 249–263. [Google Scholar] [CrossRef]
- Liu, S.; Su, W.; Ding, X. A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors 2016, 16, 2086. [Google Scholar] [CrossRef]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper Based Diagnostics for Personalized Health Care: Emerging Technologies and Commercial Aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef]
- Wang, P.; Kricka, L.J. Current and Emerging Trends in Point-of-Care Technology and Strategies for Clinical Validation and Implementation. Clin. Chem. 2018, 64, 1439–1452. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, L. Progress in Paper-Based Colorimetric Sensor Array. Chin. J. Anal. Chem. 2020, 48, 1448–1457. [Google Scholar] [CrossRef]
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of Heavy Metal by Paper-Based Microfluidics. Biosens. Bioelectron. 2016, 83, 256–266. [Google Scholar] [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Paper-Based Analytical Devices for Environmental Analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef]
- Kaushik, A.; Tiwari, S.; Jayant, R.D.; Marty, A.; Nair, M. Towards Detection and Diagnosis of Ebola Virus Disease at Point-of-Care. Biosens. Bioelectron. 2016, 75, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Bhat, M.P.; Patil, P.; Uthappa, U.T.; Jung, H.-Y.; Altalhi, T.; Kumeria, T.; Aminabhavi, T.M.; Pai, R.K.; Kurkuri, M.D. Paper-Based Microfluidic Analytical Devices for Colorimetric Detection of Toxic Ions: A Review. Trends Anal. Chem. 2017, 93, 212–227. [Google Scholar] [CrossRef]
- Altundemir, S.; Uguz, A.K.; Ulgen, K. A Review on Wax Printed Microfluidic Paper-Based Devices for International Health. Biomicrofluidics 2017, 11, 041501. [Google Scholar] [CrossRef]
- Campbell, J.M.; Balhoff, J.B.; Landwehr, G.M.; Rahman, S.M.; Vaithiyanathan, M.; Melvin, A.T. Microfluidic and Paper-Based Devices for Disease Detection and Diagnostic Research. Int. J. Mol. Sci. 2018, 19, 2731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, M.Z.; Li, S.; Wang, S.; Lu, X. Detecting Chemical Hazards in Foods Using Microfluidic Paper-Based Analytical Devices (µPADs): The Real-World Application. Micromachines 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Lai, Q.; Ju, Q.; Li, L.; Yu, H.-D.; Huang, W. Paper-Based Fluorogenic Devices for in Vitro Diagnostics. Biosens. Bioelectron. 2018, 102, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.M.; White, I.M. A Critical Review of Flexible and Porous SERS Sensors for Analytical Chemistry at the Point-of-Sample. Anal. Chim. Acta 2019, 1060, 17–29. [Google Scholar] [CrossRef]
- Ming, T.; Luo, J.P.; Liu, J.T.; Sun, S.; Xing, Y.; Wang, H.; Xiao, G.H.; Deng, Y.; Cheng, Y.; Yang, Z.G.; et al. Paper-Based Microfluidic Aptasensors. Biosens. Bioelectron. 2020, 170, 112649. [Google Scholar] [CrossRef]
- Ding, R.Y.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy Metals Detection with Paper-Based Electrochemical Sensors. Anal. Chem. 2021, 93, 1880–1888. [Google Scholar] [CrossRef]
- Alahmad, W.; Sahragard, A.; Varanusupakul, P. Online and Offline Preconcentration Techniques on Paper-Based Analytical Devices for Ultrasensitive Chemical and Biochemical Analysis: A Review. Biosens. Bioelectron. 2021, 194, 113574. [Google Scholar] [CrossRef]
- Ozer, T.; Henry, C.S. Paper-Based Analytical Devices for Virus Detection: Recent Strategies for Current and Future Pandemics. Trends Anal. Chem. 2021, 144, 116424. [Google Scholar] [CrossRef]
- Fu, L.-M.; Wang, Y.-N. Detection Methods and Applications of Microfluidic Paper-Based Analytical Devices. Trends Anal. Chem. 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Morbioli, G.G.; Mazzu-Nascimento, T.; Stockton, A.M.; Carrilho, E. Technical Aspects and Challenges of Colorimetric Detection with Microfluidic Paper-Based Analytical Devices (mPADs)—A Review. Anal. Chim. Acta 2017, 970, 1–22. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, J.; Ngoc Le, H.T.; Santhosh, M.; Kadam, A.N.; Cho, S. Recent Advances in Microfluidic Paper-Based Electrochemiluminescence Analytical Devices for Point-of-Care Testing Applications. Biosens. Bioelectron. 2019, 126, 68–81. [Google Scholar] [CrossRef]
- Solhi, E.; Hasanzadeh, M.; Babaie, P. Electrochemical Paper-Based Analytical Devices (ePADs) toward Biosensing: Recent Advances and Challenges in Bioanalysis. Anal. Methods 2020, 12, 1398–1414. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhar, B.R. Paper-Based Platforms for Microbial Electrochemical Cell-Based Biosensors: A Review. Biosens. Bioelectron. 2021, 192, 113485. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-Based Lateral Flow Biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Devi, R.V.; Doble, M.; Verma, R.S. Nanomaterials for Early Detection of Cancer Biomarker with Special Emphasis on Gold Nanoparticles in Immunoassays/Sensors. Biosens. Bioelectron. 2015, 68, 688–698. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical Biosensors based on Nanomodified Screen-Printed Electrodes: Recent Applications in Clinical Analysis. Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, M.; Kong, N.; Liu, J. Lab-on-Paper Micro- and Nano-Analytical Devices: Fabrication, Modification, Detection and Emerging Applications. Microchim. Acta 2016, 183, 1521–1542. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Zhang, Y.; Lan, F.; Yan, M.; Yu, J. Nanomaterials-Modified Cellulose Paper as a Platform for Biosensing Applications. Nanoscale 2017, 9, 4366–4382. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Selvakumar, B.; Kathiravan, A. Sensory Materials for Microfluidic Paper Based Analytical Devices—A Review. Talanta 2021, 235, 122733. [Google Scholar] [CrossRef]
- Patel, S.; Jamunkar, R.; Monisha, D.S.; Patle, T.K.; Kant, T.; Dewangan, K.; Shrivas, K. Recent Development in Nanomaterials Fabricated Paper-Based Colorimetric and Fluorescent Sensors: A Review. Trends Environ. Anal. Chem. 2021, 31, e00136. [Google Scholar] [CrossRef]
- Shirshahi, V.; Liu, G. Enhancing the Analytical Performance of Paper Lateral Flow Assays: From Chemistry to Engineering. Trends Anal. Chem. 2021, 136, 116200. [Google Scholar] [CrossRef]
- Díaz-González, M.; Escosura-Muñiz, A. Strip Modification and Alternative Architectures for Signal Amplification in Nanoparticle-Based Lateral Flow Assays. Anal. Bioanal. Chem. 2021, 413, 4111–4117. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care Diagnostics for Infectious Diseases: From Methods to Devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Hawkes, R.; Niday, E.; Gordon, J. A Dot-Immunobinding Assay for Monoclonal and Other Antibodies. Anal. Biochem. 1982, 119, 142–147. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Baharfar, M.; Rahbar, M.; Tajik, M.; Liu, G.Z. Engineering Strategies for Enhancing the Performance of Electrochemical Paper-Based Analytical Devices. Biosens. Bioelectron. 2020, 167, 112506. [Google Scholar] [CrossRef]
- Zheng, X.; Li, L.; Cui, K.; Zhang, Y.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive Enzyme-Free Biosensor by Coupling Cyclodextrin Functionalized Au Nanoparticles and High-Performance Au-Paper Electrode. ACS Appl. Mater. Interfaces 2018, 10, 3333–3340. [Google Scholar] [CrossRef]
- Pinyorospathum, C.; Chaiyo, S.; Sae-ung, P.; Hoven, V.; Damsongsang, P.; Siangproh, W.; Chailapakul, O. Disposable Paper-Based Electrochemical Sensor using Thiol-Terminated Poly(2-Methacryloyloxyethyl Phosphorylcholine) for the Label-Free Detection of C-Reactive Protein. Microchim. Acta 2019, 186, 472. [Google Scholar] [CrossRef]
- Tian, T.; Liu, H.; Li, L.; Yu, J.; Ge, S.; Song, X.; Yan, M. Paper-Based Biosensor for Noninvasive Detection of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer Patients. Sens. Actuators B Chem. 2017, 251, 440–445. [Google Scholar] [CrossRef]
- Cinti, S.; Cinotti, G.; Parolo, C.; Nguyen, E.P.; Caratelli, V.; Moscone, D.; Arduini, F.; Merkoci, A. Experimental Comparison in Sensing Breast Cancer Mutations by Signal ON and Signal OFF Paper-Based Electroanalytical Strips. Anal. Chem. 2020, 92, 1674–1679. [Google Scholar] [CrossRef]
- Eksin, E.; Torul, H.; Yarali, E.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Paper-Based Electrode Assemble for Impedimetric Detection of miRNA. Talanta 2021, 225, 122043. [Google Scholar] [CrossRef]
- Ortone, V.; Matino, L.; Santoro, F.; Cinti, S. Merging Office/Filter Paper-Based Tools for Pre-Concentring and Detecting Heavy Metals in Drinking Water. Chem. Commun. 2021, 57, 7100–7103. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.; Fung, C.M.; Lloyd, J.S.; Deganello, D.; Smith, N.A.; Teng, K.S. Direct Patterning of Gold Nanoparticles using Flexographic Printing for Biosensing Applications. Nanoscale Res. Lett. 2015, 10, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavithra, M.; Muruganand, S.; Parthiban, C. Development of Novel Paper Based Electrochemical Immunosensor with Self-Made Gold Nanoparticle Ink and Quinone Derivate for Highly Sensitive Carcinoembryonic Antigen. Sens. Actuators B Chem. 2018, 257, 496–503. [Google Scholar] [CrossRef]
- Cui, K.; Zhou, C.; Zhang, B.; Zhang, L.; Liu, Y.; Hao, S.; Tang, X.; Huang, Y.; Yu, J. Enhanced Catalytic Activity Induced by the Nanostructuring Effect in Pd Decoration onto Doped Ceria Enabling an Origami Paper Analytical Device for High Performance of Amyloid-β Bioassay. ACS Appl. Mater. Interfaces 2021, 13, 33937–33947. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Zhang, Y.; Xu, C.; Zhang, L.; Cheng, X.; Liu, H.; Chen, X.; Yu, J. Editable TiO2 Nanomaterial-Modified Paper in Situ for Highly Efficient Detection of Carcinoembryonic Antigen by Photoelectrochemical Method. ACS Appl. Mater. Interfaces 2018, 10, 14594–14601. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, L.; Gao, C.; Zhu, P.; Yu, J. Microfluidic Paper-Based Photoelectrochemical Sensing Platform with Electron-Transfer Tunneling Distance Regulation Strategy for Thrombin Detection. Biosens. Bioelectron. 2019, 133, 1–7. [Google Scholar] [CrossRef]
- De França, C.C.L.; Meneses, D.; Silva, A.C.A.; Dantas, N.O.; De Abreu, F.C.; Petroni, J.M.; Lucca, B.G. Development of Novel Paper-Based Electrochemical Device Modified with CdSe/CdS Magic-Sized Quantum Dots and Application for the Sensing of Dopamine. Electrochim. Acta 2021, 367, 137486. [Google Scholar] [CrossRef]
- Mohan, J.M.; Amreen, K.; Kulkarni, M.B.; Javed, A.; Dubey, S.K.; Goel, S. Optimized Ink Jetted Paper Device for Electroanalytical Detection of Picric Acid. Colloid. Surface. B. 2021, 208, 112056. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sitanurak, J.; Sroysee, W.; Sodanat, S.; Chairam, S.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A. Highly Sensitive and Selective Electrochemical Paper-Based Device using a Graphite Screen-Printed Electrode Modified with Molecularly Imprinted Polymers Coated Fe3O4@Au@SiO2 for Serotonin Determination. Anal. Chim. Acta 2019, 1077, 255–265. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, N.; Luo, X.; Ding, S. Patchy Gold Coated Fe3O4 Nanospheres with Enhanced Catalytic Activity Applied for Paper-Based Bipolar Electrode-Electrochemiluminescence Aptasensors. Biosens. Bioelectron. 2018, 114, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Li, L.; Zhang, Y.; Zhang, L.N.; Ge, S.G.; Yu, J.H. Auto-Cleaning Paper-Based Electrochemiluminescence Biosensor Coupled with Binary Catalysis of Cubic Cu2O-Au and Polyethyleneimine for Quantification of Ni2+ and Hg2+. Biosens. Bioelectron. 2019, 126, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, L.; Zhang, S.; Zhao, P.; Ge, S.; Yu, J. Paper-Based Electrochemiluminescence Determination of Streptavidin Using Reticular DNA-Functionalized PtCu Nanoframes and Analyte-Triggered DNA Walker. Microchim. Acta 2020, 187, 530. [Google Scholar] [CrossRef] [PubMed]
- Pokpas, K.; Jahed, N.; McDonald, E.; Bezuidenhout, P.; Smith, S.; Land, K.; Iwuoha, E. Graphene-AuNP Enhanced Inkjet-Printed Silver Nanoparticle Paper Electrodes for the Detection of Nickel(II)-Dimethylglyoxime [Ni(dmgH2)] Complexes by Adsorptive Cathodic Stripping Voltammetry (AdCSV). Electroanalysis 2020, 32, 3017–3031. [Google Scholar] [CrossRef]
- Valentine, C.J.; Takagishi, K.; Umezu, S.; Daly, R.; De Volder, M. Paper-Based Electrochemical Sensors using Paper as a Scaffold to Create Porous Carbon Nanotube Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 30680–30685. [Google Scholar] [CrossRef]
- Tran, V.; Ko, E.; Geng, Y.; Kim, M.; Jin, G.; Son, S.; Hur, W.; Seong, G. Micro-Patterning of Single-Walled Carbon Nanotubes and Its Surface Modification with Gold Nanoparticles for Electrochemical Paper-Based Non-Enzymatic Glucose Sensor. J. Electroanal. Chem. 2018, 826, 29–37. [Google Scholar] [CrossRef]
- Jampasa, S.; Pummoree, J.; Siangproh, W.; Khongchareonporn, N.; Ngamrojanavanich, N.; Chailapakul, O.; Chaiyo, S. “Signal-On” Electrochemical Biosensor based on a Competitive Immunoassay Format for the Sensitive Determination of Oxytetracycline. Sens. Actuators B Chem. 2020, 320, 128389. [Google Scholar] [CrossRef]
- Srisomwat, C.; Teengam, P.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Pop-up Paper Electrochemical Device for Label-Free Hepatitis B Virus DNA Detection. Sens. Actuators B Chem. 2020, 316, 128077. [Google Scholar] [CrossRef]
- Pungjunun, K.; Yakoh, A.; Chaiyo, S.; Praphairaksit, N.; Siangproh, W.; Kalcher, K.; Chailapakul, O. Laser Engraved Microapillary Pump Paper-Based Microfluidic Device for Colorimetric and Electrochemical Detection of Salivary Thiocyanate. Microchim. Acta 2021, 188, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cheng, Z.; Chen, Q.; Qu, L.; Miao, X.; Feng, Q. Construction of a Paper-Based Electrochemical Biosensing Platform for Rapid and Accurate Detection of Adenosine Triphosphate (ATP). Sens. Actuators B Chem. 2018, 256, 931–937. [Google Scholar] [CrossRef]
- Pungjunun, K.; Chaiyo, S.; Praphairaksit, N.; Siangproh, W.; Ortner, A.; Kalcher, K.; Chailapaku, O.; Mehmeti, E. Electrochemical Detection of NOx Gas Based on Disposable Paper-Based Analytical Device using a Copper Nanoparticles-Modified Screen-Printed Graphene Electrode. Biosens. Bioelectron. 2019, 143, 111606. [Google Scholar] [CrossRef]
- Prasad, K.; Cao, X.; Gao, N.; Jin, Q.; Sanjay, S.; Henao-Pabon, G.; Li, X. A Low-Cost Nanomaterial-Based Electrochemical Immunosensor on Paper for High-Sensitivity Early Detection of Pancreatic Cancer. Sens. Actuators B Chem. 2020, 305, 127516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Luo, J.; Liu, J.; Wang, L.; Fan, Y.; Yan, S.; Yang, Y.; Cai, X. A Novel Label-Free Microfluidic Paper-Based Immunosensor for Highly Sensitive Electrochemical Detection of Carcinoembryonic Antigen. Biosen. Bioelectron. 2016, 83, 319–326. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-Free Microfluidic Paper-Based Electrochemical Aptasensor for Ultrasensitive and Simultaneous Multiplexed Detection of Cancer Biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Ortega, F.; Regiart, M.; Rodríguez-Martínez, A.; Miguel-Pérez, D.; Serrano, M.; Lorente, J.; Tortella, G.; Rubilar, O.; Sapag, K.; Bertotti, M.; et al. Sandwich-Type Electrochemical Paper-Based Immunosensor for Claudin 7 and CD81 Dual Determination on Extracellular Vesicles from Breast Cancer Patients. Anal. Chem. 2021, 93, 1143–1153. [Google Scholar] [CrossRef]
- Scala-Benuzzi, M.L.; Raba, J.; Soler-Illia, G.J.A.A.; Schneider, R.J.; Messina, G.A. Novel Electrochemical Paper-Based Immunocapture Assay for the Quantitative Determination of Ethinylestradiol in Water Samples. Anal. Chem. 2018, 90, 4104–4111. [Google Scholar] [CrossRef] [Green Version]
- Cincotto, F.H.; Fava, E.L.; Moraes, F.C.; Fatibello-Filho, O.; Faria, R.C. A New Disposable Microfluidic Electrochemical Paper-Based Device for the Simultaneous Determination of Clinical Biomarkers. Talanta 2019, 195, 62–68. [Google Scholar] [CrossRef]
- Boonkaew, S.; Jang, I.; Noviana, E.; Siangproh, W.; Chailapakul, O.; Henry, C. Electrochemical Paper-Based Analytical Device for Multiplexed, Point-of-Care Detection of Cardiovascular Disease Biomarkers. Sens. Actuators B Chem. 2021, 330, 129336. [Google Scholar] [CrossRef]
- Dave, K.; Pachauri, N.; Dinda, A.; Solanki, P. RGO Modified Mediator Free Paper for Electrochemical Biosensing Platform. Appl. Surf. Sci. 2019, 463, 587–595. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Luo, J.; Xiong, Y.; Ming, T.; Liu, J.; Ma, Y.; Yan, S.; Yang, Y.; Yang, Z.; et al. Low Sample Volume Origami-Paper-Based Graphene-Modified Aptasensors for Label-Free Electrochemical Detection of Cancer Biomarker-EGFR. Microsyst. Nanoeng. 2020, 6, 32. [Google Scholar] [CrossRef]
- Ming, T.; Cheng, Y.; Xing, Y.; Luo, J.; Mao, G.; Liu, J.; Sun, S.; Kong, F.; Jin, H.; Cai, X. Electrochemical Microfluidic Paper-Based Aptasensor Platform Based on a Biotin-Streptavidin System for Label-Free Detection of Biomarkers. ACS Appl. Mater. Interfaces 2021, 13, 46317–46324. [Google Scholar] [CrossRef]
- Wei, X.; Guo, J.; Lian, H.; Sun, X.; Liu, B. Cobalt Metal-Organic Framework Modified Carbon Cloth/Paper Hybrid Electrochemical Button-Sensor for Nonenzymatic Glucose Diagnostics. Sens. Actuators B Chem. 2021, 329, 129205. [Google Scholar] [CrossRef]
- Zhou, C.; Cui, K.; Liu, Y.; Hao, S.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive Microfluidic Paper-Based Electrochemical/Visual Analytical Device via Signal Amplification of Pd@Hollow Zn/Co Core–Shell ZIF67/ZIF8 Nanoparticles for Prostate-Specific Antigen Detection. Anal. Chem. 2021, 93, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Su, T.; Shang, Z.; Jin, D.; Shu, Y.; Xu, Q.; Hu, X. Flexible Paper-Based Ni-MOF Composite/AuNPs/CNTs Film Electrode for HIV DNA Detection. Biosens. Bioelectron. 2021, 184, 113229. [Google Scholar] [CrossRef]
- Ma, C.; Cao, Y.; Gou, X.; Zhu, J.-J. Recent Progress in Electrochemiluminescence Sensing and Imaging. Anal. Chem. 2020, 92, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [Green Version]
- Choleva, T.G.; Kappi, F.A.; Giokas, D.L.; Vlessidis, A.G. Paper-Based Assay of Antioxidant Activity Using Analyte-Mediated on-Paper Nucleation of Gold Nanoparticles as Colorimetric Probes. Anal. Chim. Acta 2015, 860, 61–69. [Google Scholar] [CrossRef]
- Alba-Patiño, A.; Russell, S.M.; Rica, R. Origami-Enabled Signal Amplification for Paper-Based Colorimetric Biosensors. Sens. Actuators B Chem. 2018, 273, 951–954. [Google Scholar] [CrossRef]
- Xie, L.; Zi, X.; Zeng, H.; Sun, J.; Xu, L.; Chen, S. Low-Cost Fabrication of a Paper-Based Microfluidic using a Folded Pattern Paper. Anal. Chim. Acta 2019, 1053, 131–138. [Google Scholar] [CrossRef]
- Liang, P.; Yu, H.; Guntupalli, B.; Xiao, Y. Paper-Based Device for Rapid Visualization of NADH Based on Dissolution of Gold Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 15023–15030. [Google Scholar] [CrossRef]
- Liu, C.; Miao, Y.; Zhang, X.; Zhang, S.; Zhao, X. Colorimetric Determination of Cysteine by a Paper-Based Assay System using Aspartic Acid Modified Gold Nanoparticles. Microchim. Acta 2020, 187, 362. [Google Scholar] [CrossRef]
- Díaz-Amaya, S.; Zhao, M.; Allebach, J.P.; Chiu, G.T.-C.; Stanciu, L.A. Ionic Strength Influences on Biofunctional Au-Decorated Microparticles for Enhanced Performance in Multiplexed Colorimetric Sensors. ACS Appl. Mater. Interfaces 2020, 12, 32397–32409. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-T.; Huang, C.-Y.; Chen, C.-A.; Shen, S.-W.; Wang, M.-C.; Cheng, C.-M.; Chen, C.-F. Diagnosis of Tuberculosis Using Colorimetric Gold Nanoparticles on a Paper-Based Analytical Device. ACS Sens. 2017, 2, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Lin, H.-T.; Chen, T.-H.; Chen, C.-A.; Chang, H.-T.; Chen, C.-F. Signal Amplified Gold Nanoparticles for Cancer Diagnosis on Paper-Based Analytical Devices. ACS Sens. 2018, 3, 174–182. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Alvarez-Diduk, R.; Parolo, C.; Torné-Morató, H.; Müller, A.; Merkoçi, A. Paper-Based Electrophoretic Bioassay: Biosensing in Whole Blood Operating via Smartphone. Anal. Chem. 2021, 93, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Devadhasan, J.P.; Gu, J.; Chen, P.; Smith, S.; Thomas, B.; Gates-Hollingsworth, M.; Hau, D.; Pandit, S.; AuCoin, D.; Zenhausern, F. Critical Comparison between Large and Mini Vertical Flow Immunoassay Platforms for Yersinia Pestis Detection. Anal. Chem. 2021, 93, 9337–9344. [Google Scholar] [CrossRef]
- Lei, K.F.; Huang, C.-H.; Kuo, R.-L.; Chang, C.-K.; Chen, K.-F.; Tsao, K.-C.; Tsang, N.-M. Paper-Based Enzyme-Free Immunoassay for Rapid Detection and Subtyping of Influenza A H1N1 and H3N2 Viruses. Anal. Chim. Acta 2015, 883, 37–44. [Google Scholar] [CrossRef]
- Xu, F.Q.; Zhu, Q.Y.; Zhang, H.; Wang, Z.X. Preparation of Colloidal Gold-Labeled Immunochromatographic Strips for Detection of Soluble Interleukin-2 Receptors in Real Samples. Chinese J. Anal. Chem. 2021, 49, 973–981. [Google Scholar]
- Pang, R.Z.; Zhu, Q.Y.; Wei, J.; Wang, Y.Q.; Xu, F.Q.; Meng, X.Y.; Wang, Z.X. Development of a Gold-Nanorod-Based Lateral Flow Immunoassay for a Fast and Dual-Modal Detection of C-Reactive Protein in Clinical Plasma Samples. RSC Adv. 2021, 11, 28388–28394. [Google Scholar] [CrossRef]
- Li, F.; Guo, L.; Hu, Y.; Li, Z.; Liu, J.; He, J.; Cui, H. Multiplexed Chemiluminescence Determination of Three Acute Myocardial Infarction Biomarkers Based on Microfluidic Paper-Based Immunodevice Dual Amplified by Multifunctionalized Gold Nanoparticles. Talanta 2020, 207, 120346. [Google Scholar] [CrossRef]
- Ying, B.; Park, S.; Chen, L.; Dong, X.; Young, E.W.K.; Liu, X. NanoPADs and NanoFACEs: An Optically Transparent Nanopaper-Based Device for Biomedical Applications. Lab Chip 2020, 20, 3322–3333. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Hsu, C.-H.; Lu, B.-J.; Lin, P.-Y.; Ho, M.-L. Determination of Nitrite Ions in Environment Analysis with a Paper-Based Microfluidic Device. Dalton Trans. 2018, 47, 14799–14807. [Google Scholar] [CrossRef]
- Monisha; Shrivas, K.; Kant, T.; Patel, S.; Devi, R.; Dahariya, N.S.; Pervez, S.; Deb, M.K.; Rai, M.K.; Rai, J. Inkjet-Printed Paper-Based Colorimetric Sensor Coupled with Smartphone for Determination of Mercury (Hg2+). J. Hazard. Mater. 2021, 414, 125440. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Giordano, G.F.; Soares, C.; De Oliveira, J.; Mendes, R.; Piazzetta, M.; Gobbi, A.; Cardoso, M. Optical Paper-Based Sensor for Ascorbic Acid Quantification using Silver Nanoparticles. Talanta 2015, 141, 188–194. [Google Scholar] [CrossRef]
- Mettakoonpitak, J.; Khongsoun, K.; Wongwan, N.; Kaewbutdee, S.; Siripinyanond, A.; Kuharuk, A.; Henry, C.S. Simple Biodegradable Plastic Screen-Printing for Microfluidic Paper-Based Analytical Devices. Sens. Actuators B Chem. 2021, 331, 129463. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Aprian, A.; Meileza, N.; Hitsmi, M.; Elvia, R.; Rahmidar, L.; Khaydarov, R. Smartphone Coupled with a Paper-Based Colorimetric Device for Sensitive and Portable Mercury Ion Sensing. Chemosensors 2019, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Mavaei, M.; Chahardoli, A.; Fattahi, A.; Khoshroo, A. A Simple Method for Developing a Hand-Drawn Paper-Based Sensor for Mercury; Using Green Synthesized Silver Nanoparticles and Smartphone as a Hand-Held-Device for Colorimetric Assay. Glob. Chall. 2021, 5, 2000099. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Patel, M.; Thakur, S.; Shankar, R. Food Safety Monitoring of the Pesticide Phenthoate using a Smartphone-Assisted Paperbased Sensor with Bimetallic Cu@Ag Core–Shell Nanoparticles. Lab Chip 2020, 20, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M. Paper Based Colorimetric Detection of miRNA-21 using Ag/Pt Nanoclusters. Spectrochim. Acta A 2020, 227, 117529. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, W.; Li, B.; Yang, C.J.; Jin, Y. Multichannel Paper Chip-Based Gas Pressure Bioassay for Simultaneous Detection of Multiple MicroRNAs. ACS Appl. Mater. Interfaces 2021, 13, 15008–15016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niua, X.; Li, X.; He, Y.; Song, H.; Peng, Y.; Pan, J.; Qiu, F.; Zhao, H.; Lan, M. A Smartphone-Integrated Ready-to-Use Paper-Based Sensor with Mesoporous Carbon-Dispersed Pd Nanoparticles as a Highly Active Peroxidase Mimic for H2O2 Detection. Sens. Actuators B Chem. 2018, 265, 412–420. [Google Scholar] [CrossRef]
- Feng, L.-X.; Tang, C.; Han, X.-X.; Zhang, H.-C.; Guo, F.-N.; Yang, T.; Wang, J.-H. Simultaneous and Sensitive Detection of Multiple Small Biological Molecules by Microfluidic Paper-Based Analytical Device Integrated with Zinc Oxide Nanorods. Talanta 2021, 232, 122499. [Google Scholar] [CrossRef]
- Jamil, S.; Nasir, M.; Ali, Y.; Nadeem, S.; Rashid, S.; Javed, M.Y.; Hayat, A. Cr2O3−TiO2-Modified Filter Paper-Based Portable Nanosensors for Optical and Colorimetric Detection of Hydrogen Peroxide. ACS Omega 2021, 6, 23368–23377. [Google Scholar] [CrossRef]
- Sawetwong, P.; Chairam, S.; Jarujamrus, P.; Amatatongchai, M. Enhanced Selectivity and Sensitivity for Colorimetric Determination of Glyphosate using Mn–ZnS Quantum Dot Embedded Molecularly Imprinted Polymers Combined with a 3D-Microfluidic Paper-Based Analytical Device. Talanta 2021, 225, 122077. [Google Scholar] [CrossRef]
- Kitchawengkul, N.; Prakobkij, A.; Anutrasakda, W.; Yodsin, N.; Jungsuttiwong, S.; Chunta, S.; Amatatongchai, M.; Jarujamrus, P. Mimicking Peroxidase-Like Activity of Nitrogen-Doped Carbon Dots (N-CDs) Coupled with a Laminated Three-Dimensional Microfluidic Paper-Based Analytical Device (Laminated 3D-μPAD) for Smart Sensing of Total Cholesterol from Whole Blood. Anal. Chem. 2021, 93, 6989–6999. [Google Scholar] [CrossRef]
- Liu, C.; Luo, Y.; Wen, H.; Qi, Y.; Shi, G.; Deng, J.; Zhou, T. Red-to-Blue Paper-Based Colorimetric Sensor Integrated with Smartphone for Point-of-Use Analysis of Cerebral AChE upon Cd2+ Exposure. Nanoscale 2021, 13, 1283–1290. [Google Scholar] [CrossRef]
- Huy, B.; Nghia, N.; Lee, Y.-I. Highly Sensitive Colorimetric Paper-Based Analytical Device for the Determination of Tetracycline using Green Fluorescent Carbon Nitride Nanoparticles. Microchem. J. 2020, 158, 105151. [Google Scholar] [CrossRef]
- Pimentel, E.S.; Brito-Pereira, R.; Marques-Almeida, T.; Ribeiro, C.; Vaz, F.; Lanceros-Mendez, S.; Cardoso, V.F. Tailoring Electrospun Poly(L-lactic acid) Nanofibers as Substrates for Microfluidic Applications. ACS Appl. Mater. Interfaces 2020, 12, 60–69. [Google Scholar] [CrossRef]
- Lawati, H.; Hassanzadeh, J. Dual-Function 2D Cobalt Metal-Organic Framework Embedded on Paper as a Point-of-Care Diagnostic Device: Application for the Quantification of Glucose. Anal. Chim. Acta 2020, 1139, 15–26. [Google Scholar] [CrossRef]
- Marín-Barroso, E.; Moreira, C.M.; Messina, G.A.; Bertolino, F.A.; Alderete, M.; Soler-Illia, G.J.A.A.; Raba, J.; Pereira, S.V. Paper Based Analytical Device Modified with Nanoporous Material for the Fluorescent Sensing of Gliadin Content in Different Food Samples. Microchem. J. 2018, 142, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhang, Q.; Zhang, Z.; Ding, X.; Jiang, J.; Zhang, W.; Li, P. Rapid, On-Site and Quantitative Paper-Based Immunoassay Platform for Concurrent Determination of Pesticide Residues and Mycotoxins. Anal. Chim. Acta 2019, 1078, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Kong, F.; Zhao, C.-Q.; Ding, S.-N. Ratiometric Fluorescent Nanosensors for Ultra-Sensitive Detection of Mercury Ions Based on AuNCs/MOFs. Analyst 2019, 144, 2523–2530. [Google Scholar] [CrossRef]
- Ungor, D.; Horváth, K.; Dékány, I.; Csapó, E. Red-Emitting Gold Nanoclusters for Rapid Fluorescence Sensing of Tryptophan Metabolites. Sens. Actuators B Chem. 2019, 288, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Lert-itthiporn, A.; Srikritsadawong, P.; Choengchan, N. Foldable Paper-Based Analytical Device for Membraneless Gas-Separation and Determination of Iodate Based on Fluorescence Quenching of Gold Nanoclusters. Talanta 2021, 221, 121574. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Capón, N.; Calle, I.; Lavilla, I.; Bendicho, C. Fluorescent Poly(vinylpyrrolidone)-Supported Copper Nanoclusters in Miniaturized Analytical Systems for Iodine Sensing. Sens. Actuators B Chem. 2019, 299, 126979. [Google Scholar] [CrossRef]
- Yin, X.; Liang, L.; Zhao, P.; Lan, F.; Zhang, L.; Ge, S.; Yu, J. Double Signal Amplification Based on Palladium Nanoclusters and Nucleic Acid Cycles on a lPAD for Dual-Model Detection of MicroRNAs. J. Mater. Chem. B 2018, 6, 5795–5801. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Song, Z.; Jiang, Y.; Zhang, X.; Wang, L.; Zhao, H.; Cui, Y.; Gu, F.; Wang, Y.; Zheng, G. A Three-Dimensional Pinwheel-Shaped Paper-Based Microfluidic Analytical Device for Fluorescence Detection of Multiple Heavy Metals in Coastal Waters by Rational Device Design. Anal. Bioanal. Chem. 2021, 413, 3299–3313. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, Y.; Xiong, J.; Wu, L.; Hou, X.; Xu, K.; Zheng, C. Disposable Paper-Based Analytical Device for Visual Speciation Analysis of Ag(I) and Silver Nanoparticles (AgNPs). Anal. Chem. 2019, 91, 3359–3366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, X.; Li, B.; Zhao, J.; Qi, J.; Hao, G.; Rong, J.; Yang, X. Fluorescence Detection of 2,4-Dichlorophenoxyacetic Acid by Ratiometric Fluorescence Imaging on Paper-Based Microfluidic Chips. Analyst 2020, 145, 963–974. [Google Scholar] [CrossRef]
- Thepmanee, O.; Prapainop, K.; Noppha, O.; Rattanawimanwong, N.; Siangproh, W.; Chailapakul, O.; Songsrirote, K. A Simple Paper-Based Approach for Arsenic Determination in Water using Hydride Generation Coupled with Mercaptosuccinic-Acid Capped CdTe Quantum Dots. Anal. Methods 2020, 12, 2718–2726. [Google Scholar] [CrossRef]
- Kuang, Y.; Wang, X.; Tian, X.; Yang, C.; Li, Y.; Nie, Y. Silica-Embedded CdTe Quantum Dots Functionalized with Rhodamine Derivative for Instant Visual Detection of Ferric Ions in Aqueous Media. J. Photoch. Photobiol. A 2019, 372, 140–146. [Google Scholar] [CrossRef]
- Ou, Q.; Tawfik, S.M.; Zhang, X.; Lee, Y.-I. Novel “Turn On–Off” Paper Sensor Based on Nonionic Conjugated Polythiophene-Coated CdTe QDs for Efficient Visual Detection of Cholinesterase Activity. Analyst 2020, 145, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, Y.; Zhang, L.; Liu, W. An Inkjet Printing Paper-Based Immunodevice for Fluorescence Determination of Immunoglobulin G. Anal. Methods 2019, 11, 3452–3459. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Wang, Q.; Tang, D.; Lin, Y. Distance-Dependent Visual Fluorescence Immunoassay on CdTe Quantum Dot–Impregnated Paper through Silver Ion-Exchange Reaction. Microchim. Acta 2020, 187, 563. [Google Scholar] [CrossRef]
- Moreira, C.M.; Mar’ın-Barroso, E.; Pereira, S.V.; Raba, J.; Messina, G.A.; Bertolino, F.A. A Nanostructured Paper-Based Device for Phenylalanine Neonatal Screening by LED-Induced Fluorescence. Anal. Methods 2020, 12, 1624–1630. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Yeh, Y.-C. Rapid Synthesis of Microwave-Assisted Zinc Oxide Nanorods on a Paper-Based Analytical Device for Fluorometric Detection of L-Dopa. Colloids Surf. B 2021, 207, 111995. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Liu, W.; Chu, W.; Chen, Y. Paper-Based Laser Induced Fluorescence Immunodevice Combining with CdTe Embedded Silica Nanoparticles Signal Enhancement Strategy. Sens. Actuators B Chem. 2017, 242, 87–94. [Google Scholar] [CrossRef]
- Qiu, Z.; Shu, J.; Tang, D. Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper. Anal. Chem. 2017, 89, 5152–5160. [Google Scholar] [CrossRef]
- Zhou, J.; Li, B.; Qi, A.; Shi, Y.; Qi, J.; Xu, H.; Chen, L. ZnSe Quantum Dot Based Ion Imprinting Technology for Fluorescence Detecting Cadmium and Lead Ions on a Three-Dimensional Rotary Paper-Based Microfluidic Chip. Sens. Actuators B Chem. 2020, 305, 127462. [Google Scholar] [CrossRef]
- Noor, M.O.; Hrovat, D.; Moazami-Goudarzi, M.; Espie, G.S.; Krull, U.J. Ratiometric Fluorescence Transduction by Hybridization after Isothermal Amplification for Determination of Zeptomole Quantities of Oligonucleotide Biomarkers with a Paper-Based Platform and Camera-Based Detection. Anal. Chim. Acta 2015, 885, 156–165. [Google Scholar] [CrossRef]
- Xia, Z.; Li, D.; Deng, W. Identification and Detection of Volatile Aldehydes as Lung Cancer Biomarkers by Vapor Generation Combined with Paper-Based Thin Film Microextraction. Anal. Chem. 2021, 93, 4924–4931. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Lan, F.; Li, L.; Su, M.; Ge, S.; Yu, J.; Liu, H.; Yan, M. Fluorescence “Turn-On” Determination of H2O2 using Multilayer Porous SiO2/NGQDs and PdAu Mimetics Enzymatic/Oxidative Cleavage of Single-Stranded DNA. Biosens. Bioelectron. 2016, 82, 204–211. [Google Scholar] [CrossRef]
- Wang, X.; Hou, J.; Lan, S.; Shen, C.; Huo, D.; Ji, Z.; Ma, Y.; Luo, H.; Zhang, S.; He, Q.; et al. MoS2 QDs-Based Sensor for Measurement of Fluazinam with Triple Signal Output. Anal. Chim. Acta 2020, 1108, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Apilux, W.; Siangproh, N.; Insin, O.C.; Prachayasittikule, V. Paper-Based Thioglycolic Acid (TGA)-Capped CdTe QD Device for Rapid Screening of Organophosphorus and Carbamate Insecticides. Anal. Methods 2017, 9, 519–527. [Google Scholar] [CrossRef]
- El-Shaheny, R.; Yoshida, S.; Fuchigami, T. Graphene Quantum Dots as a Nanoprobe for Analysis of O- and P-nitrophenols in Environmental Water Adopting Conventional Fluorometry and Smartphone Image Processing-Assisted Paper-Based Analytical Device. Microchem. J. 2020, 158, 105241. [Google Scholar] [CrossRef]
- Shah, K.G.; Singh, V.; Kauffman, P.C.; Abe, K.; Yager, P. Mobile Phone Ratiometric Imaging Enables Highly Sensitive Fluorescence Lateral Flow Immunoassays without External Optical Filters. Anal. Chem. 2018, 90, 6967–6974. [Google Scholar] [CrossRef]
- Liang, L.; Su, M.; Li, L.; Lan, F.; Yang, G.; Ge, S.; Yu, J.; Song, X. Aptamer-Based Fluorescent and Visual Biosensor for Multiplexed Monitoring of Cancer Cells in Microfluidic Paper-Based Analytical Devices. Sens. Actuators B Chem. 2016, 229, 347–354. [Google Scholar] [CrossRef]
- Ye, J.; Geng, Y.; Cao, F.; Sun, D.; Xu, S.; Chang, J.; Xu, W.; Chen, Q. A Smartphone-Assisted Paper-Based Analytical Device for Fluorescence Assay of Hg2+. Chem. Res. Chin. Univ. 2019, 35, 972–977. [Google Scholar] [CrossRef]
- Ninwong, B.; Sangkaew, P.; Hapa, P.; Ratnarathorn, N.; Menger, R.F.; Henry, C.S.; Dungchai, W. Sensitive Distance-Based Paper-Based Quantification of Mercury Ions Using Carbon Nanodots and Heating-Based Preconcentration. RSC Adv. 2020, 10, 9884–9893. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, X.; Miao, C.; Li, R.; Ji, Y. Fluorescent Paper–Based Sensor Based on Carbon Dots for Detection of Folic Acid. Anal. Bioanal. Chem. 2020, 412, 2805–2813. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Chu, S.; Liu, B.; Zhang, Q.; Zou, L.; Yu, S.; Jiang, C. Semiquantitative Visual Detection of Lead Ions with a Smartphone via a Colorimetric Paper-Based Analytical Device. Anal. Chem. 2019, 91, 9292–9299. [Google Scholar] [CrossRef]
- Hu, J.; Yang, X.; Peng, Q.; Wang, F.; Zhu, Y.; Hu, X.; Zheng, B.; Du, J.; Xiao, D. A Highly Sensitive Visual Sensor for Tetracycline in Food Samples by a Double-Signal Response Fluorescent Nanohybrid. Food Control 2020, 108, 106832. [Google Scholar] [CrossRef]
- Yehia, A.M.; Farag, M.A.; Tantawy, M.A. A Novel Trimodal System on a Paper-Based Microfluidic Device for Onsite Detection of the Date Rape Drug “Ketamine”. Anal. Chim. Acta 2020, 1104, 95–104. [Google Scholar] [CrossRef]
- Ortiz-Gomez, I.; Ortega-Muñoz, M.; Marín-Sánchez, A.; de Orbe-Payá, I.; Hernandez-Mateo, F.; Capitan-Vallvey, L.; Santoyo-Gonzalez, F.; Salinas-Castillo, A. A Vinyl Sulfone Clicked Carbon Dot-Engineered Microfluidic Paper-Based Analytical Device for Fluorometric Determination of Biothiols. Microchim. Acta 2020, 187, 421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Zhuo, S.; Ji, Y.; Li, R. A Carbon Dots Functionalized Paper Coupled with AgNPs Composites Platform: Application as a Sensor for Hydrogen Peroxide Detection Based on Surface Plasmon-Enhanced Energy Transfer. New J. Chem. 2021, 45, 6025–6032. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Li, Y.; Yang, C.; Lu, L.; Nie, Y. Sensitive Determination of Hardness and Fluoride in Ground Water by a Hybrid Nanosensor Based on Aggregation Induced FRET On and Off Mechanism. Sens. Actuators B Chem. 2018, 262, 522–530. [Google Scholar] [CrossRef]
- Ortiz-Gomez, I.; Toral-Lopez, V.; Romero, F.J.; De Orbe-Paya, I.; García, A.; Rodríguez, N.; Capitan-Vallvey, L.; Morales, D.; Salinas-Castillo, A. In Situ Synthesis of Fluorescent Silicon Nanodots for Determination of Total Carbohydrates in a Paper Microfluidic Device Combined with Laser Prepared Graphene Heater. Sens. Actuators B Chem. 2021, 332, 129506. [Google Scholar] [CrossRef]
- Liang, L.; Lan, F.; Yin, X.; Ge, S.; Yu, J.; Yan, M. Metal-Enhanced Fluorescence/Visual Bimodal Platform for Multiplexed Ultrasensitive Detection of MicroRNA with Reusable Paper Analytical Devices. Biosens. Bioelectron. 2017, 95, 181–188. [Google Scholar] [CrossRef]
- El-Shaheny, R.; Al-Khateeb, L.A.; El-Maghrabey, M. Dual-Excitation In-Lab-Made Device Based on a Handy UV Lamp and GQDs-Modified PADs for Simultaneous Determination of Acetaminophen and Its Endocrine Disrupting Impurity 4-Nitrophenol. Sens. Actuators B Chem. 2021, 348, 130657. [Google Scholar] [CrossRef]
- Wang, F.M.; Li, W.; Wang, J.S.; Ren, J.S.; Qu, X.G. Detection of Telomerase on Upconversion Nanoparticle Modified Cellulose Paper. Chem. Commun. 2015, 51, 11630–11633. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Y.H.; Chen, Y.; Xiong, Q.R.; Wang, Y.H.; Duan, H.W.; Lai, W.H. Improving the Performance of Upconversion Nanoprobe-Based Lateral Flow Immunoassays by Supramolecular Self-Assembly Core/Shell Strategies. Sens. Actuators B Chem. 2020, 318, 128233. [Google Scholar] [CrossRef]
- He, M.; Shang, N.; Zhu, Q.; Xu, J. Paper-Based Upconversion Fluorescence Aptasensor for the Quantitative Detection of Immunoglobulin E in Human Serum. Anal. Chim. Acta 2021, 1143, 93–100. [Google Scholar] [CrossRef]
- Na, M.; Zhang, S.; Liu, J.; Ma, S.; Han, Y.; Wang, Y.; He, Y.; Chen, H.; Chen, X. Determination of Pathogenic Bacteria―Bacillus Anthrax Spores in Environmental Samples by Ratiometric Fluorescence and Test Paper Based on Dual-Emission Fluorescent Silicon Nanoparticles. J. Hazard. Mater. 2020, 386, 121956. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Tang, Y.; Zhang, K.; Tang, D. Wet NH3-Triggered NH2-MIL-125(Ti) Structural Switch for Visible Fluorescence Immunoassay Impregnated on Paper. Anal. Chem. 2018, 90, 14121–14125. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zheng, Q.; Wang, H.; Liu, C.; Huang, X.; Xiao, Y. Double-Color Lanthanide Metal–Organic Framework Based Logic Device and Visual Ratiometric Fluorescence Water Microsensor for Solid Pharmaceuticals. Anal. Chem. 2020, 92, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; He, C.; Zheng, Q.; Feng, L.; Xiong, L.; Xiao, Y. Dual Eu-MOFs Based Logic Device and Ratiometric Fluorescence Paper Microchip for Visual H2O2 Assay. J. Mater. Chem. C 2020, 8, 3562–3570. [Google Scholar] [CrossRef]
- Zhang, D.; Du, P.; Chen, J.; Guo, H.; Lu, X. Pyrazolate-Based Porphyrinic Metal-Organic Frameworks as Catechol Oxidase Mimic Enzyme for Fluorescent and Colorimetric Dual-Mode Detection of Dopamine with High Sensitivity and Specificity. Sens. Actuators B Chem. 2021, 341, 130000. [Google Scholar] [CrossRef]
- Yue, X.; Li, Y.; Xu, S.; Li, J.; Li, M.; Jiang, L.; Jie, M.; Bai, Y. A Portable Smartphone-Assisted Ratiometric Fluorescence Sensor for Intelligent and Visual Detection of Malachite Green. Food Chem. 2022, 371, 131164. [Google Scholar] [CrossRef]
- Abbas, A.; Brimer, A.; Slocik, J.M.; Tian, L.; Naik, R.R.; Singamaneni, S. Multifunctional Analytical Platform on a Paper Strip: Separation, Preconcentration, and Subattomolar Detection. Anal. Chem. 2013, 85, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Torul, H.; Çiftçi, H.; Çetin, D.; Suludere, Z.; Boyacı, I.; Tamer, U. Paper Membrane-Based SERS Platform for the Determination of Glucose in Blood Samples. Anal. Bioanal. Chem. 2015, 407, 8243–8251. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic Diatomite Analytical Devices for Illicit Drug Sensing with ppb-Level Sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Choi, S.; Moon, S.; Lee, S.; Kim, W.; Kim, S.; Kim, S.; Shin, J.-H.; Park, Y.-G.; Jin, K.-H.; Kim, T. A Recyclable CNC-Milled Microfluidic Platform for Colorimetric Assays and Label-Free Aged-Related Macular Degeneration Detection. Sens. Actuators B Chem. 2019, 290, 484–492. [Google Scholar] [CrossRef]
- Zheng, T.; Gao, Z.; Luo, Y.; Liu, X.; Zhao, W.; Lin, B. Manual-Slide-Engaged Paper Chip for Parallel SERS-Immunoassay Measurement of Clenbuterol from Swine Hair. Electrophoresis 2016, 37, 418–424. [Google Scholar] [CrossRef]
- Huang, Z.; Siddhanta, S.; Zhang, C.; Kickler, T.; Zheng, G.; Barman, I. Painting and Heating: A Nonconventional, Scalable Route to Sensitive Biomolecular Analysis with Plasmon-Enhanced Spectroscopy. J. Raman Spectrosc. 2017, 48, 1365–1374. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Hassan, M.; Zuo, M.; Wei, W.; Wu, X.; Ouyang, Q.; Chen, Q. Rapid Detection of Chloramphenicol in Food Using SERS Flexible Sensor Coupled Artificial Intelligent Tools. Food Control 2021, 128, 108186. [Google Scholar] [CrossRef]
- Tegegne, W.; Su, W.; Beyene, A.; Huang, W.; Tsai, M.; Hwang, B. Flexible Hydrophobic Filter Paper-Based SERS Substrate Using Silver Nanocubes for Sensitive and Rapid Detection of Adenine. Microchem. J. 2021, 168, 106349. [Google Scholar] [CrossRef]
- Duan, H.; Deng, W.; Gan, Z.; Li, D.; Li, D. SERS-Based Chip for Discrimination of Formaldehyde and Acetaldehyde in Aqueous Solution using Silver Reduction. Microchim. Acta 2019, 186, 175. [Google Scholar] [CrossRef]
- Qi, L.; Xiao, M.; Wang, X.; Wang, C.; Wang, L.; Song, S.; Qu, X.; Li, L.; Shi, J.; Pei, H. DNA-Encoded Raman-Active Anisotropic Nanoparticles for microRNA Detection. Anal. Chem. 2017, 89, 9850–9856. [Google Scholar] [CrossRef]
- Li, D.; Duan, H.; Ma, Y.; Deng, W. Headspace-Sampling Paper-Based Analytical Device for Colorimetric/Surface-Enhanced Raman Scattering Dual Sensing of Sulfur Dioxide in Wine. Anal. Chem. 2018, 90, 5719–5727. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Jana, N.R. Paper-Based Microfluidic Approach for Surface-Enhanced Raman Spectroscopy and Highly Reproducible Detection of Proteins beyond Picomolar Concentration. ACS Appl. Mater. Interfaces 2015, 7, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, W.; Liu, C.; Foda, M.; Zhu, Y. Strawberry-Like SiO2/Ag Nanocomposites Immersed Filter Paper as SERS Substrate for Acrylamide Detection. Food Chem. 2020, 328, 127106. [Google Scholar] [CrossRef]
- Chen, M.; Yang, H.; Rong, L.; Chen, X. A Gas-Diffusion Microfluidic Paper-Based Analytical Device (μPAD) Coupled with Portable Surface-Enhanced Raman Scattering (SERS): Facile Determination of Sulphite in Wines. Analyst 2016, 141, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Hou, X.; Gao, Y.; Fan, X.; Qiu, T. Inkjet-Printed Paper-Based Semiconducting Substrates for Surface-Enhanced Raman Spectroscopy. Nanotechnology 2020, 31, 055502. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).