Production of COx-Free Hydrogen and Few-Layer Graphene Nanoplatelets by Catalytic Decomposition of Methane over Ni-Lignin-Derived Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ni-Lignin Composites
2.3. Pretreatment of Ni-Lignin Composites
2.4. Catalytic Carbonization of Ni-Lignin Composites to Graphene-Encapsulated Nickel Nanoparticles (GENNs, Labelled as Ni@G)
2.5. Temperature-Programmed Catalytic Decomposition of Methane (TPCDM)
2.6. Catalytic Testing for Methane Decomposition
2.7. Characterization
3. Results and Discussion
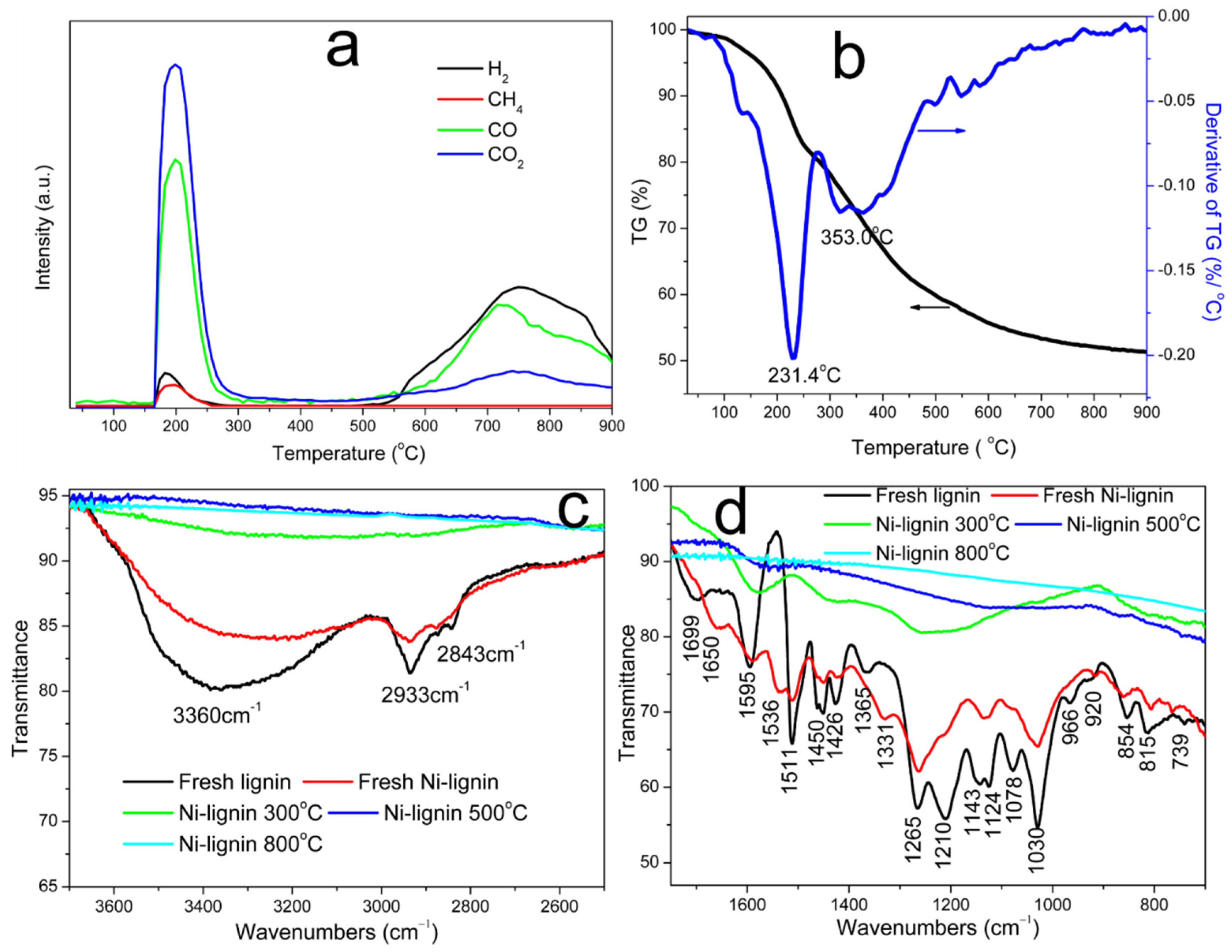
3.1. Catalytic Carbonization of Ni-Lignin Composite to Few-Layer Graphene Encapsulated Nickel Nanoparticles (Ni@G)
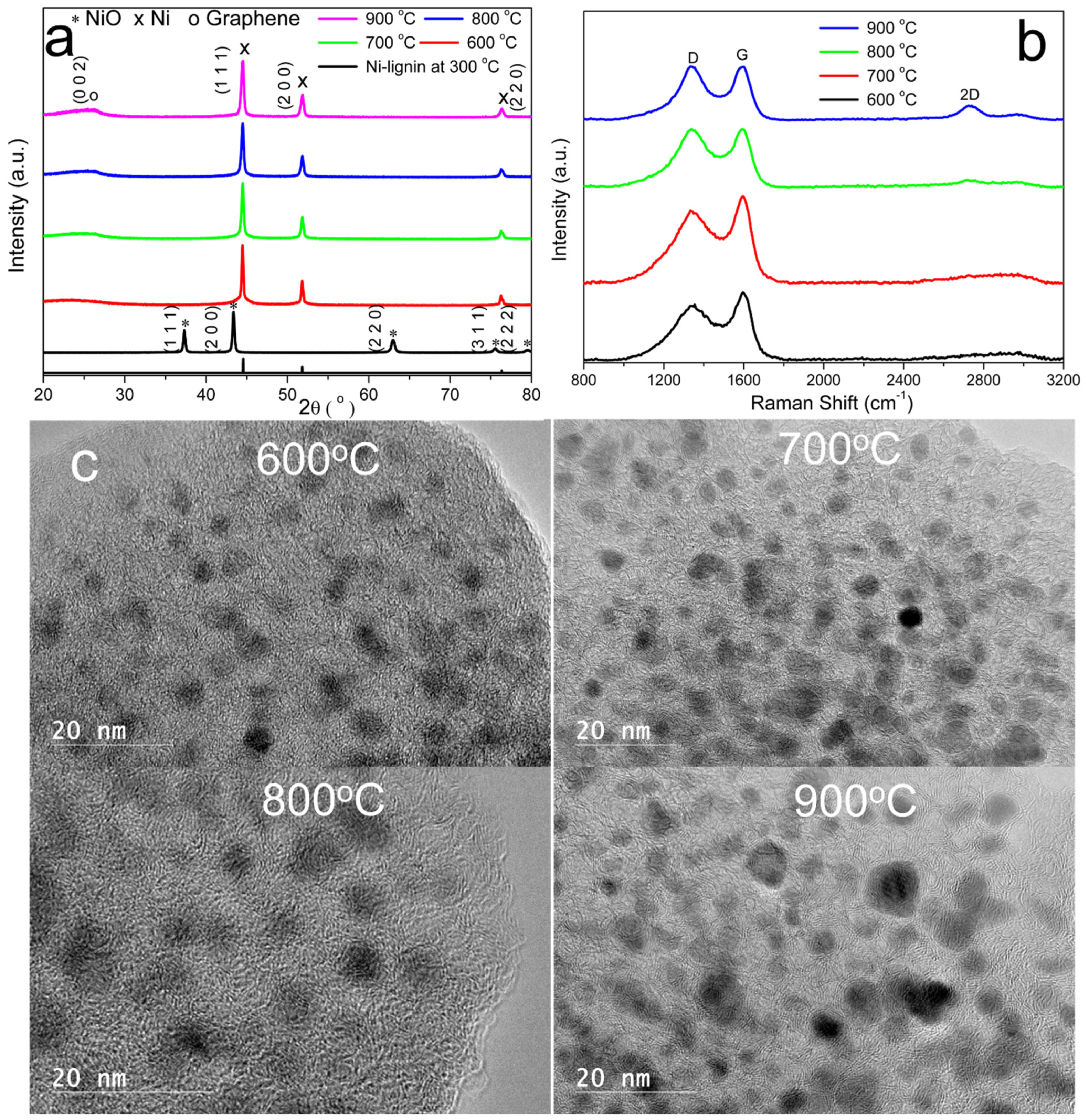
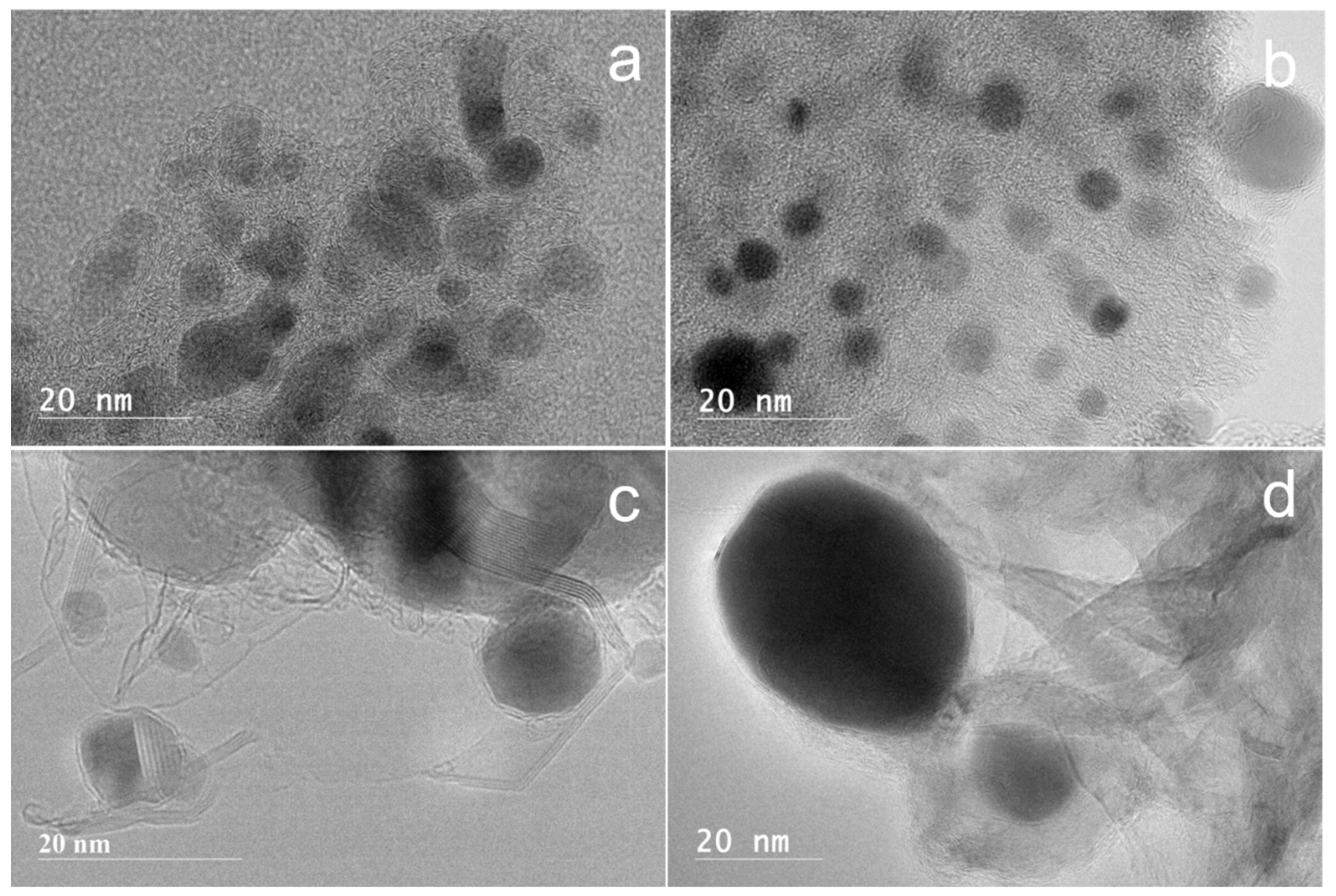
3.2. Characterization of Ni@G Samples
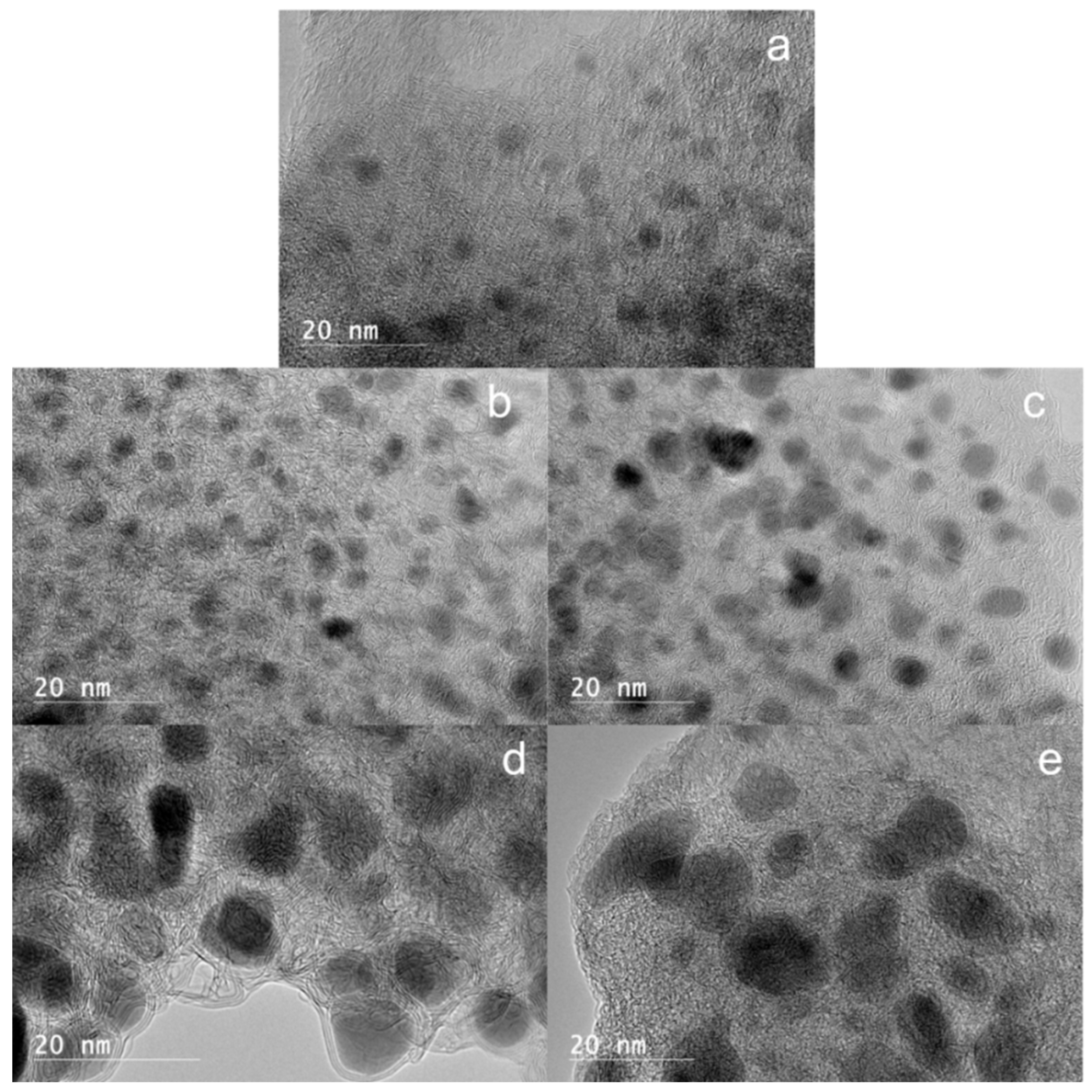
High-Resolution Transmission Electron Microscopy
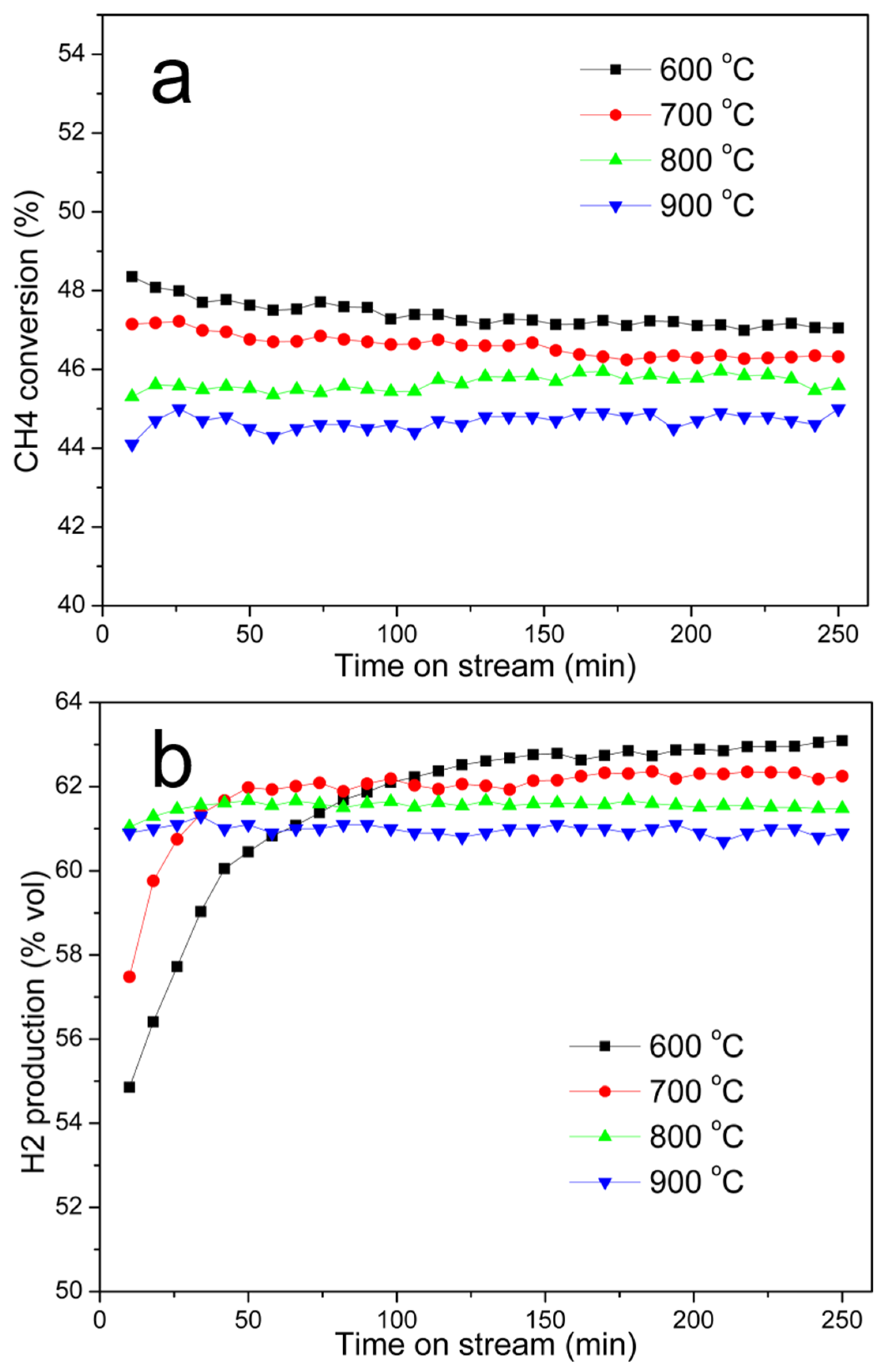
3.3. Catalytic Decomposition of Methane over Ni@G Samples
3.3.1. Temperature-Programmed Catalytic Decomposition of Methane (TPCDM) over Ni@G Nanoparticles
Effect of Carbonization Temperature
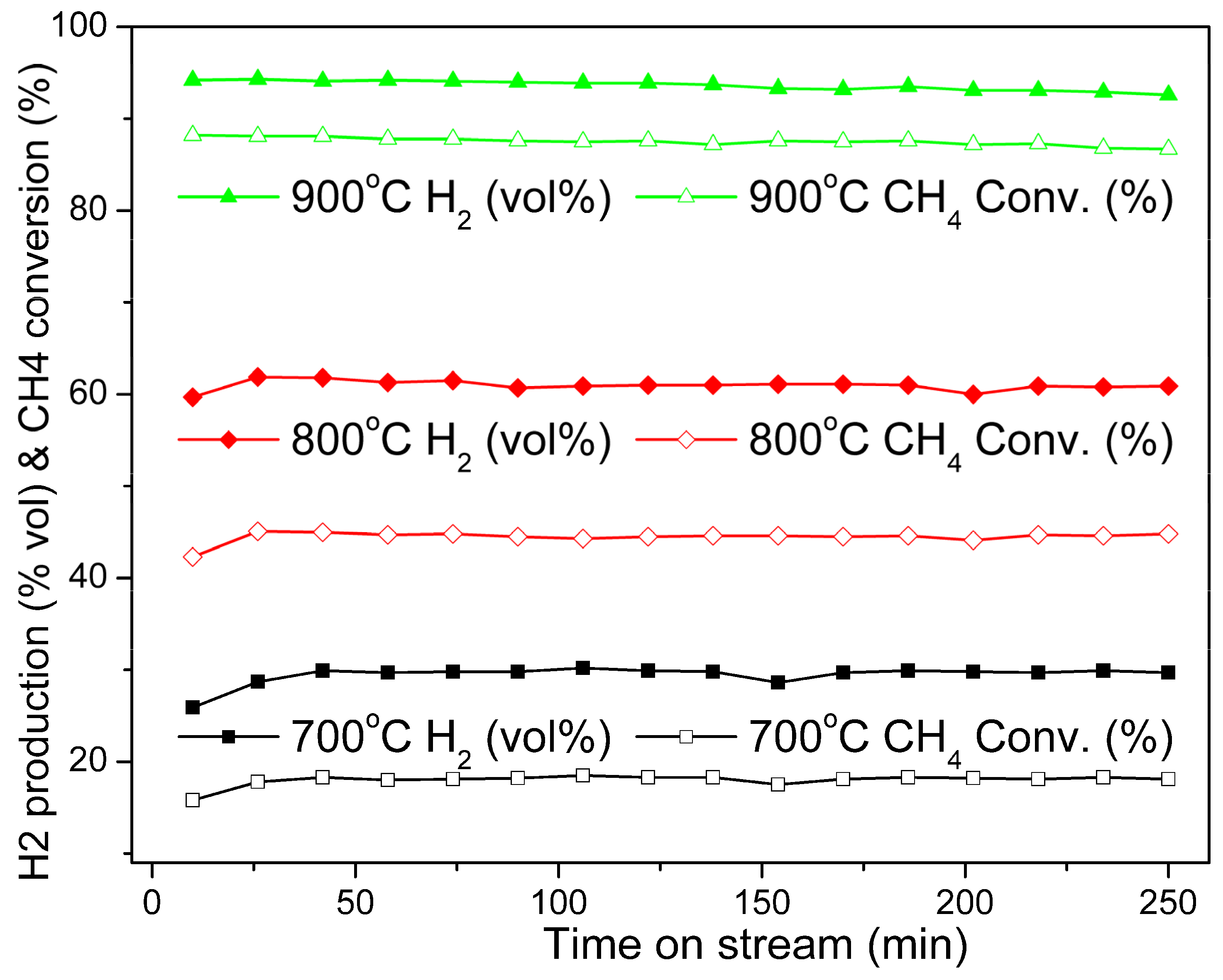
Effect of the Reaction Temperature
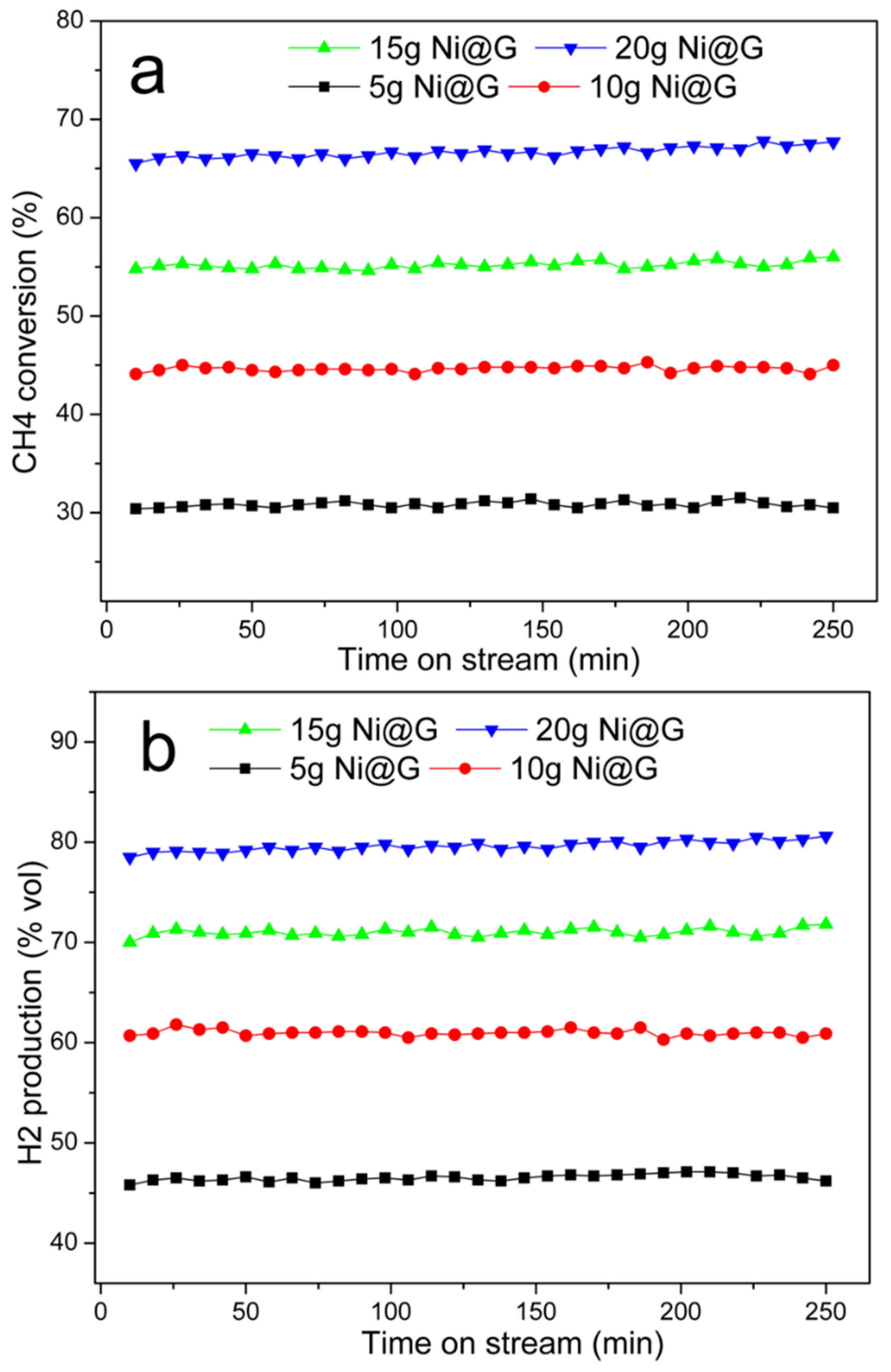
Effect of the Amount of Catalyst
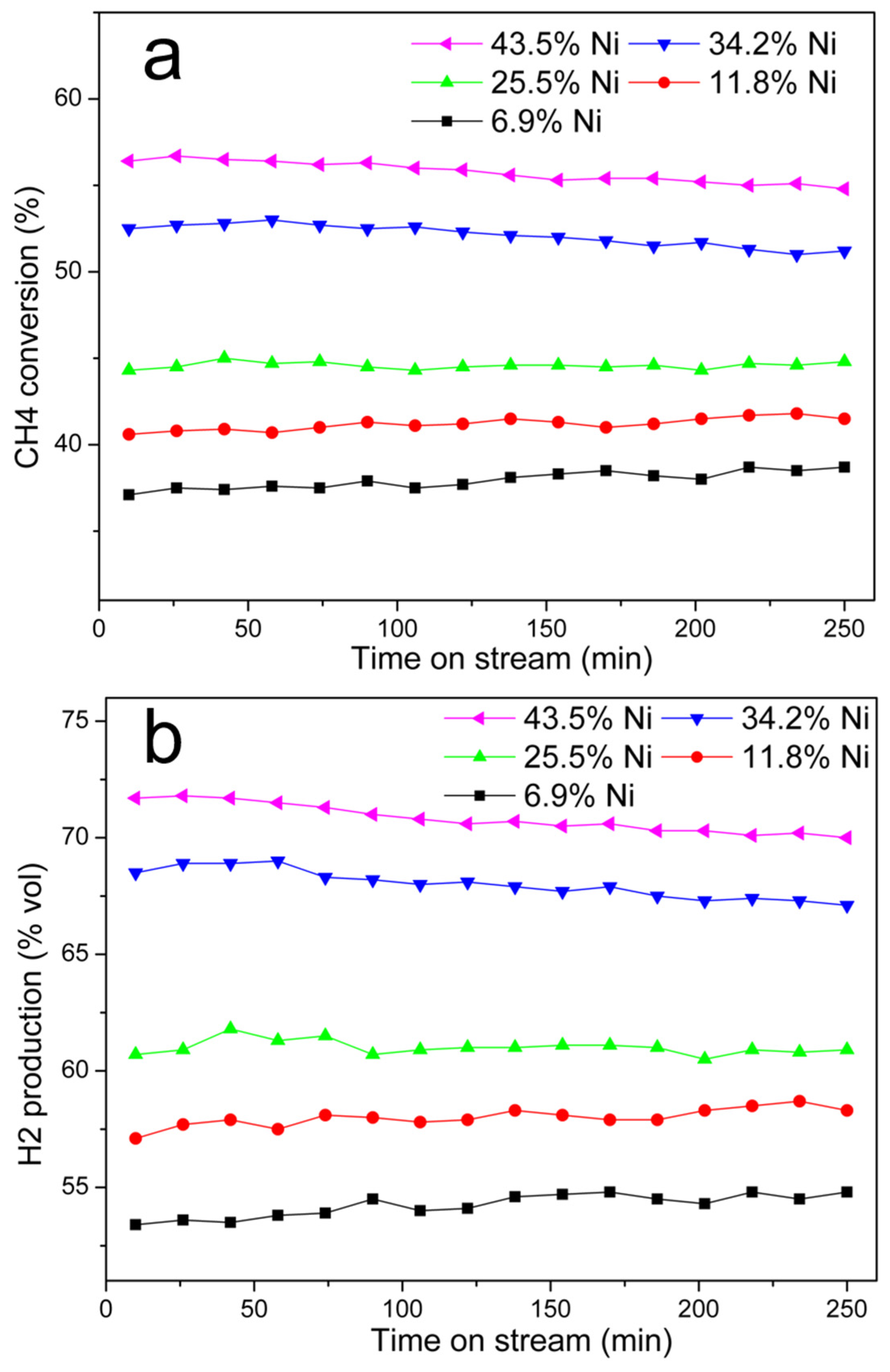
Effect of Ni@G Nickel Content
Stability of the Catalyst
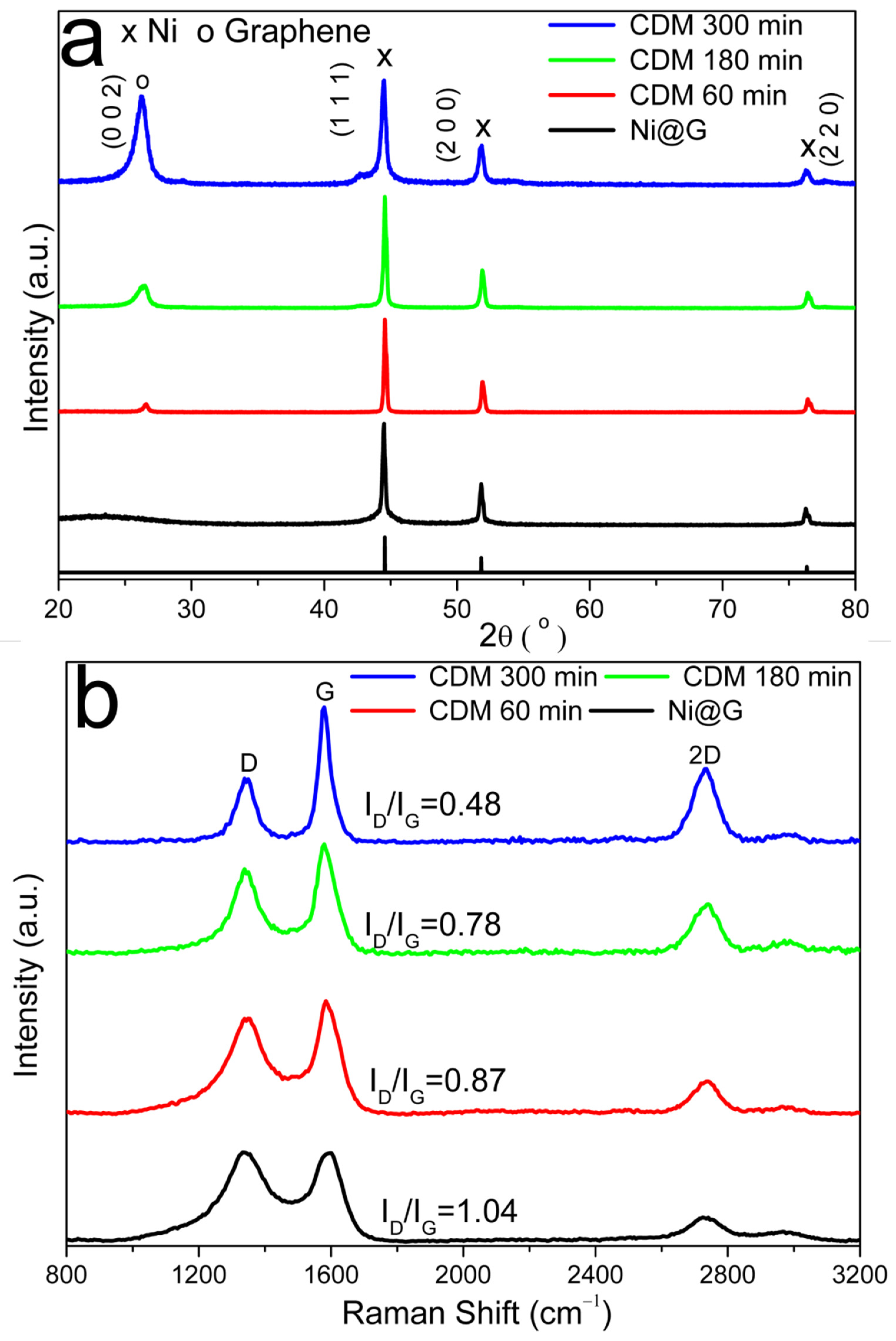
3.4. Sample Characterization after CDM Reaction
Possible Decomposition Mechanism of Methane over Ni@G
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ren, X.; Wang, Y.; Liu, A.; Zhang, Z.; Lv, Q.; Liu, B. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Knaebel, K.S.; Hill, F.B. Pressure swing adsorption: Development of an equilibrium theory for gas separations. Chem. Eng. Sci. 1985, 40, 2351–2360. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- UAshik, P.M.; Daud, W.M.A.W.; Abbas, H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane—A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef] [Green Version]
- Muradov, N.; Smith, F.; T-Raissi, A. Catalytic activity of carbons for methane decomposition reaction. Catal. Today 2005, 102–103, 225–233. [Google Scholar] [CrossRef]
- Srilatha, K.; Bhagawan, D.; Shiva Kumar, S.; Himabindu, V. Sustainable fuel production by thermocatalytic decomposition of methane—A review. S. Afr. J. Chem. Eng. 2017, 24, 156–167. [Google Scholar] [CrossRef]
- Dipu, A.L. Methane decomposition into CO-free hydrogen over a Ni-based catalyst: An overview. Int. J. Energy Res. 2021, 45, 9858–9877. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Timmiati, S.N.; Lim, K.L.; Yaakob, Z.; Kamaruddin, N.H.N.; Teh, L.P. Recent developments in methane decomposition over heterogeneous catalysts: An overview. Mater. Renew. Sustain. Energy 2020, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Poursorkhabi, V.; Abdelwahab, M.A.; Misra, M.; Khalil, H.; Gharabaghi, B.; Mohanty, A.K. Processing, Carbonization, and Characterization of Lignin Based Electrospun Carbon Fibers: A Review. Front. Energy Res. 2020, 8, 208. [Google Scholar] [CrossRef]
- Yan, Q.; Arango, R.; Li, J.; Cai, Z. Fabrication and characterization of carbon foams using 100% Kraft lignin. Mater. Des. 2021, 201, 109460–109470. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, X.; Hassan, E.B.; Wang, C.; Zhang, J.; Cai, Z. Catalytic graphitization of kraft lignin to graphene-based structures with four different transitional metals. J. Nanopart. Res. 2018, 20, 223. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Porous nanoplate-like tungsten trioxide/reduced graphene oxide catalyst for sonocatalytic degradation and photocatalytic hydrogen production. Surf. Interfaces 2021, 24, 101075. [Google Scholar] [CrossRef]
- Sait Izgi, M.; Ece, M.S.; Kazici, H.C.; Sahin, O.; Onat, E. Hydrogen production by using Ru nanoparticle decorated with Fe3O4@SiO2–NH2 core-shell microspheres. Int. J. Hydrogen Energy 2020, 45, 30415–30430. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, S.; Yang, Y. Insight into Structural Evolution, Active Site and Stability of Heterogeneous Electrocatalysts. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Yan, Q.; Boardman, C.R.; Cai, Z. Thermal stability of metal-lignin composites prepared by coprecipitation method. Thermochim. Acta 2020, 690, 178659. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. Synthetic Bio-Graphene Based Nanomaterials through Different Iron Catalysts. Nanomaterials 2018, 8, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudasaka, M.; Tasaka, K.; Kikuchi, R.; Ohki, Y.; Yoshimura, S.; Ota, E. Influence of chemical bond of carbon on Ni catalyzed graphitization. J. Appl. Phys. 1997, 81, 7623–7629. [Google Scholar] [CrossRef]
- Antonino, L.D.; Gouveia, J.R.; de Sousa Júnior, R.R.; Garcia, G.E.S.; Gobbo, L.C.; Tavares, L.B.; dos Santos, D.J. Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules 2021, 26, 2131. [Google Scholar] [CrossRef]
- Laurichesse, S.; Averous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Yan, Q.; Cai, Z. Effect of Solvents on Fe–Lignin Precursors for Production Graphene-Based Nanostructures. Molecules 2020, 25, 2167. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; El-Naas, M.H.; Kumar, D.; Kumar, A. Catalytic Methane Decomposition to Carbon Nanostructures and COx-Free Hydrogen: A Mini-Review. Nanomaterials 2021, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Msheik, M.; Rodat, S.; Abanades, S. Methane Cracking for Hydrogen Production: A Review of Catalytic and Molten Media Pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Yan, Q.; Ketelboeter, T.; Cai, Z. A Study of the Key Factors on Production of Graphene Materials from Fe-Lignin Nanocomposites through a Molecular Cracking and Welding (MCW) Method. Molecules 2022, 27, 154. [Google Scholar] [CrossRef]
- Tian, W.; Li, W.; Yu, W.; Liu, X. A Review on Lattice Defects in Graphene: Types, Generation, Effects and Regulation. Micromachines 2017, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.P.; Botas, J.A.; Guil-Lopez, R. H2 production from methane pyrolysis over commercial carbon catalysts: Kinetic and deactivation study. Int. J. Hydrogen Energy 2009, 34, 4488–4494. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. Mass production of graphene materials from solid carbon sources using a molecular cracking and welding method. J. Mater. Chem. A 2019, 7, 13978–13985. [Google Scholar] [CrossRef] [Green Version]



| Ni-Lignin Precursor | Carbonization Temperature (°C) | Ni Content in Ni@G (%) a | Ni Particle Size (nm) b | Surface Area (m2/g) c |
|---|---|---|---|---|
| 10% Ni-lignin | 600 | 22.8 | 5.7 | 79.1 |
| 10% Ni-lignin | 700 | 23.6 | 8.5 | 92.6 |
| 10% Ni-lignin | 800 | 24.9 | 9.4 | 108.2 |
| 10% Ni-lignin | 900 | 25.5 | 10.2 | 117.5 |
| 2.5% Ni-lignin | 900 | 6.9 | 4.9 | 57.4 |
| 5% Ni-lignin | 900 | 11.8 | 7.3 | 83.7 |
| 15% Ni-lignin | 900 | 34.2 | 27.6 | 91.6 |
| 20% Ni-lignin | 900 | 43.5 | 32.5 | 73.5 |
| Nickel Content in Ni@G (%) | Ni@G Used (g) | Reaction Temperature (°C) | Reaction Time (min) | Ni Particle Size in the Product (nm) | Surface Area (m2/g) | Cl/Ni Ratio (g/g) | Cm/Ni Ratio (g/g) | Ct/Ni Ratio (g/g) |
|---|---|---|---|---|---|---|---|---|
| 22.8 | 10 | 800 | 250 | 7.9 | 69.3 | - | - | 5.82 |
| 23.6 | 10 | 800 | 250 | 10.7 | 75.1 | - | - | 5.63 |
| 24.9 | 10 | 800 | 250 | 17.5 | 81.9 | - | - | 5.35 |
| 25.5 | 10 | 800 | 250 | 27.2 | 87.2 | 2.92 | 4.73 | 5.29 |
| 25.5 | 10 | 700 | 250 | 15.8 | 101.3 | 2.92 | 0.95 | 3.87 |
| 25.5 | 10 | 900 | 250 | 29.6 | 75.3 | 2.92 | 4.46 | 7.39 |
| 25.5 | 5 | 900 | 250 | - | - | 2.92 | 3.26 | 6.18 |
| 25.5 | 10 | 800 | 250 | - | - | 2.92 | 2.36 | 5.29 |
| 25.5 | 15 | 800 | 250 | - | - | 2.92 | 1.93 | 4.85 |
| 25.5 | 20 | 800 | 250 | - | - | 2.92 | 1.73 | 4.65 |
| 6.9 | 10 | 800 | 250 | 7.5 | 53.7 | 13.49 | 6.79 | 20.29 |
| 11.8 | 10 | 800 | 250 | 12.7 | 62.5 | 7.47 | 4.54 | 12.01 |
| 34.2 | 10 | 800 | 250 | 35.8 | 73.8 | 1.92 | 1.96 | 3.88 |
| 43.5 | 10 | 800 | 250 | 47.1 | 58.2 | 1.30 | 1.66 | 2.96 |
| 25.5 | 10 | 800 | 30 | 13.6 | - | 2.92 | 0.30 | 3.22 |
| 25.5 | 10 | 800 | 60 | 16.7 | - | 2.92 | 0.59 | 3.51 |
| 25.5 | 10 | 800 | 120 | 19.3 | - | 2.92 | 1.15 | 4.08 |
| 25.5 | 10 | 800 | 180 | 25.2 | - | 2.92 | 1.70 | 4.62 |
| 25.5 | 10 | 800 | 300 | 30.5 | - | 2.92 | 2.80 | 5.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Ketelboeter, T.; Cai, Z. Production of COx-Free Hydrogen and Few-Layer Graphene Nanoplatelets by Catalytic Decomposition of Methane over Ni-Lignin-Derived Nanoparticles. Molecules 2022, 27, 503. https://doi.org/10.3390/molecules27020503
Yan Q, Ketelboeter T, Cai Z. Production of COx-Free Hydrogen and Few-Layer Graphene Nanoplatelets by Catalytic Decomposition of Methane over Ni-Lignin-Derived Nanoparticles. Molecules. 2022; 27(2):503. https://doi.org/10.3390/molecules27020503
Chicago/Turabian StyleYan, Qiangu, Timothy Ketelboeter, and Zhiyong Cai. 2022. "Production of COx-Free Hydrogen and Few-Layer Graphene Nanoplatelets by Catalytic Decomposition of Methane over Ni-Lignin-Derived Nanoparticles" Molecules 27, no. 2: 503. https://doi.org/10.3390/molecules27020503
APA StyleYan, Q., Ketelboeter, T., & Cai, Z. (2022). Production of COx-Free Hydrogen and Few-Layer Graphene Nanoplatelets by Catalytic Decomposition of Methane over Ni-Lignin-Derived Nanoparticles. Molecules, 27(2), 503. https://doi.org/10.3390/molecules27020503








