1. Introduction
At present, Ginkgo biloba extract (EGb) can be regarded as a typical representative of traditional Chinese medicine (TCM), which displays multi-component, multi-target, and multi-activity characteristics [
1]. The Schwabe company has given much attention to the various pharmacological effects of EGb [
2]. The biological activities of Ginkgo biloba extracts produced by different production processes are different. Although various chemical components of EGb are integrated, there are complementary and synergistic effects between several members. However, one or several active ingredients in traditional Chinese medicine cannot represent the overall efficacy. In addition, some enterprises at home and abroad pursue high ketone esters, such as the Shanghai Xingling Company, which produces raw materials for the new Class II ginkgo ketone esters: flavonoid glycosides ≥ 44% and lactones ≥ 6%, which is far from the “24 + 6” standard of EGb761
3. The high ketone ester would change the content of the ingredients. In other words, 24% of flavonoids and 6% of lactones are the main active ingredients of Ginkgo biloba extract. Is it necessary to add the other 70% of the ingredients? Whether there is a synergistic effect between the effective factors deserves further study [
3].
During cellular metabolism, reactive oxygen species (ROS) are produced, leading to cellular damage and disease. It mainly includes superoxide anion, NO radicals, hydroxyl radicals, and hydrogen peroxide. It has been found that the occurrence and development of many diseases are closely related to the oxidation of oxygen free radicals, for example, cardiovascular diseases, neurodegenerative diseases, atherosclerosis, tumors, etc. [
4,
5,
6,
7]. Ginkgo biloba extract can prevent and inhibit the toxicity of oxygen free radicals and reduce lipid-induced peroxidative damage. In EGb, ginkgo flavonoids, proanthocyanidins, and organic acids have a large number of reduced hydroxyl functional groups, which can play an antioxidant role by scavenging oxygen free radicals and regulating the activity of superoxide dismutase and catalase.
According to various animal model studies, EGb exhibited obvious antioxidant functions. EGb plays a role mainly directly inactivating various oxygen free radicals, increasing the activity of antioxidant enzymes such as glutathione peroxidase (GSH-Px), and inhibiting lipid peroxidation [
8], and has strong biological effects such as antioxidation, scavenging free radicals activity [
9]. Wang et al. [
10] reported that the study of EGb exhibited the effects of increasing superoxide dismutase (SOD) activity and decreasing the apoptosis rate of diabetic vascular cells in a dose-dependent manner. Meanwhile, the animal model study revealed that EGb can reduce oxidative stress and significantly increase antioxidant capacity in diabetic rat hearts [
11]. Ischemia-reperfusion injury is the most obvious damage to the antioxidant system of the animal organisms, and the application of antioxidants is very important to reduce oxidative damage. The protective effect of EGb on ischemia-reperfusion injury of myocardial and neural tissues has been demonstrated, the protective mechanism of which is that EGb can directly inactivate various oxygen radicals, inhibit lipid peroxidation reactions, and increase antioxidant enzymes such as SOD and GSH-Px [
12,
13]. Besides, Wang et al. [
14] found that EGb exhibited antioxidant and radical scavenging effects in an animal model of acute liver injury, involving reducing the production of free radicals and increasing GSH-Px and SOD activities. Xiao et al. [
15] found that EGb had significant protective effects against oxidative stress injury in melanocytes in a dose-dependent manner.
Ginkgo biloba extract, as a kind of traditional Chinese medicine with multi-component, has four typical components: ginkgo flavone (GF), ginkgolide (G), procyanidins (OPC), and organic acids (OA). Its antioxidant activity has been associated with the complex network regulatory mechanism. In this study, to investigate the antioxidant activity of each component and the synergistic antioxidant effects between different components, we would attempt to compare the antioxidant activity of each component based on two classical anti-oxidation tests firstly. Secondly, the combinations of various components in different proportions will be explored, and the best match between different components could be revealed. In addition, The study of network pharmacology can be employed for predicting new targets, establishing molecular target-disease network relationships, and analyzing their antioxidant mechanisms more effectively [
16,
17,
18]. The results from the network pharmacology study will also be used for explaining the antioxidant activity of EGb components, particularly GF, G, OPC, and OA.
4. Experimental Materials and Methods
4.1. Experimental Reagents and Instruments
The solvents (methanol and ethanol) were purchased from Nanjing Jianghua Glass Instrument Co., Ltd. (Nanjing, China). The DPPH (2, 2-Di(4-tert-octyl phenyl)-1-picrylhydrazyl, free radical) was purchased from Sigma. The ABTS (2’-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) reagent was purchased from Shanghai Biyuntian Biotechnology Co., Ltd. (Shanghai, China). All the instruments and equipment were listed as follows: HH-4 warm water bath (Guohua Electric Co., Ltd., Nanjing, China); IKARV05 basic rotary evaporator (IKA group, Germany); KH3200B ultrasonic cleaner (Kunshan Hechuang Ultrasonic Instrument Co., Ltd., Suzhou, China); SBH-III circulating water multi-purpose vacuum pump (Zhengzhou Great Wall Technology Industry and Trade Co., Ltd., Zhengzhou, China); WH861 vortex mixer (Taicang science and education equipment factory); BS124s Electronic balance (Beijing saiduolis Instrument System Co., Ltd., Beijing, China); Allegra x-22r desktop high-speed centrifuge (Beckman Kurt Co., Ltd., Brea, CA, USA); QB-9006 constant temperature microporous plate fast oscillator (Shanghai Shupei Experimental Equipment Co., Ltd., Shanghai, China); direct-plate electrophoresis instrument and film transfer instrument (Bio Rad Co., Ltd., Hercules, CA, USA); TY-80R Decoloring shaker (Nanjing Kai Ji Biotechnology Co., Ltd., Nanjing, China); UVP gel imaging system (Beckman Kurt Co., Ltd., Brea, CA, USA); vertical flat electrophoresis apparatus (Bio-Rad, USA); and UPLCLTQ-Orbitrap XL (Thermo Co., Ltd., Hercules, CA, USA).
4.2. Preparation and HPLC Analysis of the Typical Efficacy Components of EGb
A mixture of 20 g of dried EGB was taken into diluted ethanol (70%, volume ratio, 200 mL), stirred, and cooled to 12 °C. The filtrate was extracted three times with 9:1 ethyl acetate-hexane, and each time with 1/3 of the filtrate volume. The organic phase was washed twice with water, and each time with 1/5 of the organic phase volume. The solid mass of activated carbon (4×) was added, stirred, and adsorbed for 1 h, and subsequently, it was filtered, separated, and washed with a small amount of ethyl acetate. The ginkgo lactone sample can be prepared by concentrating filtrate and washing liquid under reduced pressure. After extraction, a small amount of dissolved organic phase was removed. The organic phase was washed with water after three extraction times with 1/3 volume of n-butanol. The organic solvent was completely removed by vacuum concentration. The residue was a sample of ginkgo flavonoids. Besides, proanthocyanidins [
25] and organic acids were prepared from DESs [
26,
27,
28,
29]. The components were weighed and incubated at a concentration of 1 mg/mL and then diluted to a concentration of 20–50 μg/mL of the substance to be measured. The four functional components were sampled simultaneously to prepare HPLC or HPLC-ELSD test samples.
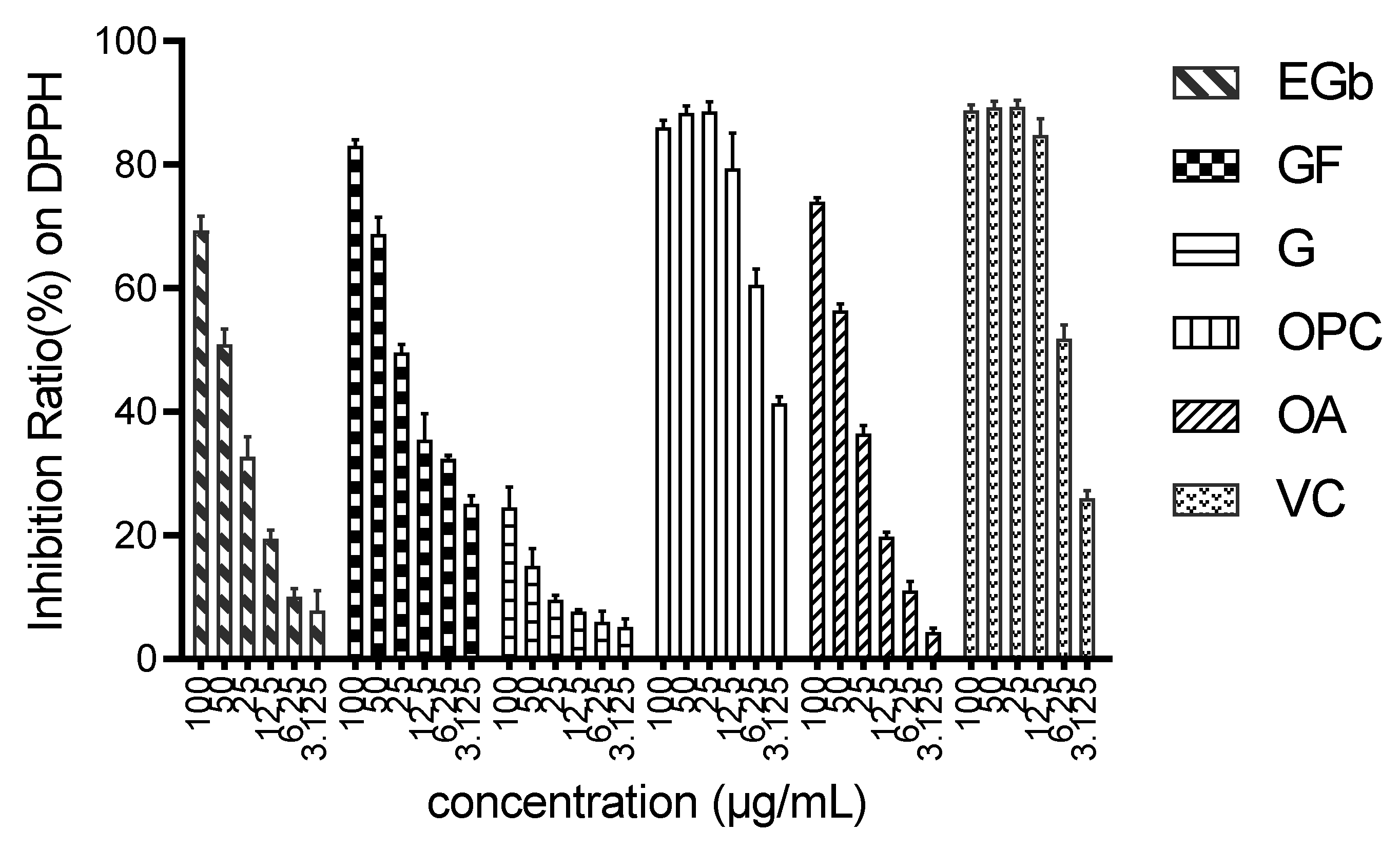
4.3. Determination of DPPH Clearance Rate
Then, 1.927 mg of DPPH were accurately weighed and then dissolved in 10 mL of methanol at a concentration of 0.5 mmol/mL and stored at 0–4 °C away from light. The sample to be tested was obtained by EGb gradient dilution, and 100 μL of the sample were put into the enzyme standard plate. Subsequently, 100 μL of DPPH solution were added, and the reaction was carried out in the dark at room temperature for 30 min, and finally, the absorbance at OD517 nm was measured. The A2 represented a normal group that did not contain the sample to be tested, and the A0 represented the blank group without DPPH. The clearance rate was calculated according to the following formula. Each sample has three parallel samples.
where:
A0 is the blank absorbance of the determination solution
A1 is the absorbance after adding the sample to be measured
A2 is the absorbance without a sample to be tested
4.4. Determination of Total Antioxidant Capacity by ABTS Method
The total antioxidant capacity was detected by the ABTS method according to the instructions of the ABTS kit. The ABTS mother liquor was prepared according to the number of samples to be tested. The ABTS solution and oxidant solution were evenly mixed at room temperature and stored in the dark for 12–16 h. The ABTS mother liquor was diluted with 80% ethanol before use. The sample of certain concentration was mixed with 2 mL of methanol, centrifuged, and diluted gradiently to obtain the sample to be tested. Next, 200 μL of ABTS working solution were added into each hole of the enzyme label plate, and then 10 μL of the sample to be tested were added. After being incubated in the dark at room temperature for 5 min, the absorbance A1 at 734 nm wavelength was determined. The calculation method of the ABTS+·scavenging rate is the same as that of DPPH radical scavenging.
4.5. Isoradiometric Analysis
The four typical components of EGb were prepared as 10 mg/mL mother liquor, and the fixed ratio (volume ratio) of them was 9:1, 7:3, 1:1, 3:7, and 1:9. Then, 18 μL of Ginkgo flavone and 2 μL of ginkgolide were added to 200 μL volume to obtain the concentration of 100 μg/mL GF and G (volume ratio of 9:1). The DPPH scavenging rate and ABTS clearance rate of the abovementioned mixed samples with different proportions were determined. The IC
50 value was calculated by GraphPad 9.0 software. The IC
50 value of drug A was used as ordinate, and the IC
50 value of drug B was used as abscissa. The IC
50 value of compound A was ordinate and the IC
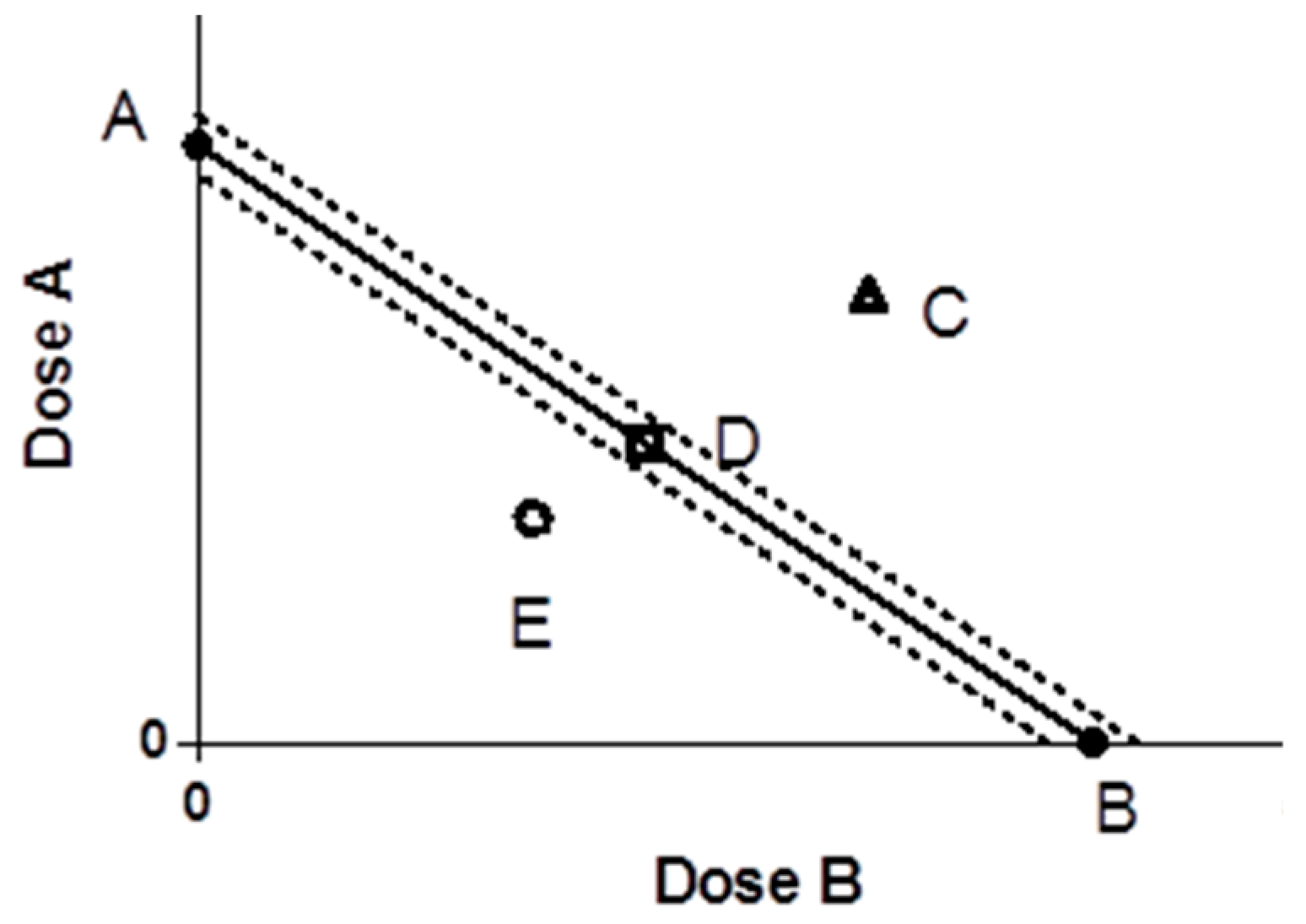
50 of compound B was abscissa. Coordinate position points were determined and three functions were judged. Evaluation of the degree of synergistic or antagonistic action is often expressed by interaction coefficient (γ) (
Figure 6) [
30].
A: The dose of drug A when used alone; B: the dose of drug B when used alone; the line segment connecting the two points of AB is the contour line; the dotted line is the 95% confidence line. C is synergistic, E is antagonistic, and D is additive.
IC
50Amix and IC
50Bmix were the IC
50 values of A and B antioxidants in the compound group; IC
50A and IC
50B were the IC
50 values of A and B antioxidants alone. If γ = 1, it indicates that the interaction is additive; if γ < 1, it indicates that the interaction is synergistic, and the more obvious the value of γ, the stronger the synergistic effect; if γ > 1, it indicates that the interaction is antagonistic [
31].
4.6. Data Collection and Structure Processing of EGb Small Molecular Compounds
Using the TCM information database TCMSP (
http://ibts.hkbu.edu.hk/LSP/tcmsp.php, The last visit was on 11 October 2020) and National Center for Biotechnology Information (NCBI) database (
https://www.ncbi.nlm.nih.gov/, The last visit was on 11 October 2020.), 465 compounds with different structures were collected after optimization analysis. At the same time, the corresponding compounds were downloaded from the PubChem database and saved in SDF format. By importing these molecules into Maestro 10.1, using the living preparation module for small molecule processing, selecting the opls 2005 force field default settings for conformational optimization, and then importing the prepared ligand molecules into canvas in Schrodinger 2015 software 2.3, the module calculates the related molecular descriptors (relative molecular weight, number of rotatable bonds, number of hydrogen bond acceptors, number of hydrogen bond donors, number of molecular rings, number of molecular aromatic rings, molecular volume, molecular surface area, molecular polar surface area, lipid water partition coefficient). According to Lipinski’s “five principles of drug-like” [
32], the main parameters are relative molecular weight <500, the number of hydrogen bond donors <5, the number of hydrogen bond receptors <10, the partition coefficient of lipid and water <5, and the number of rotatable bonds not more than 10.
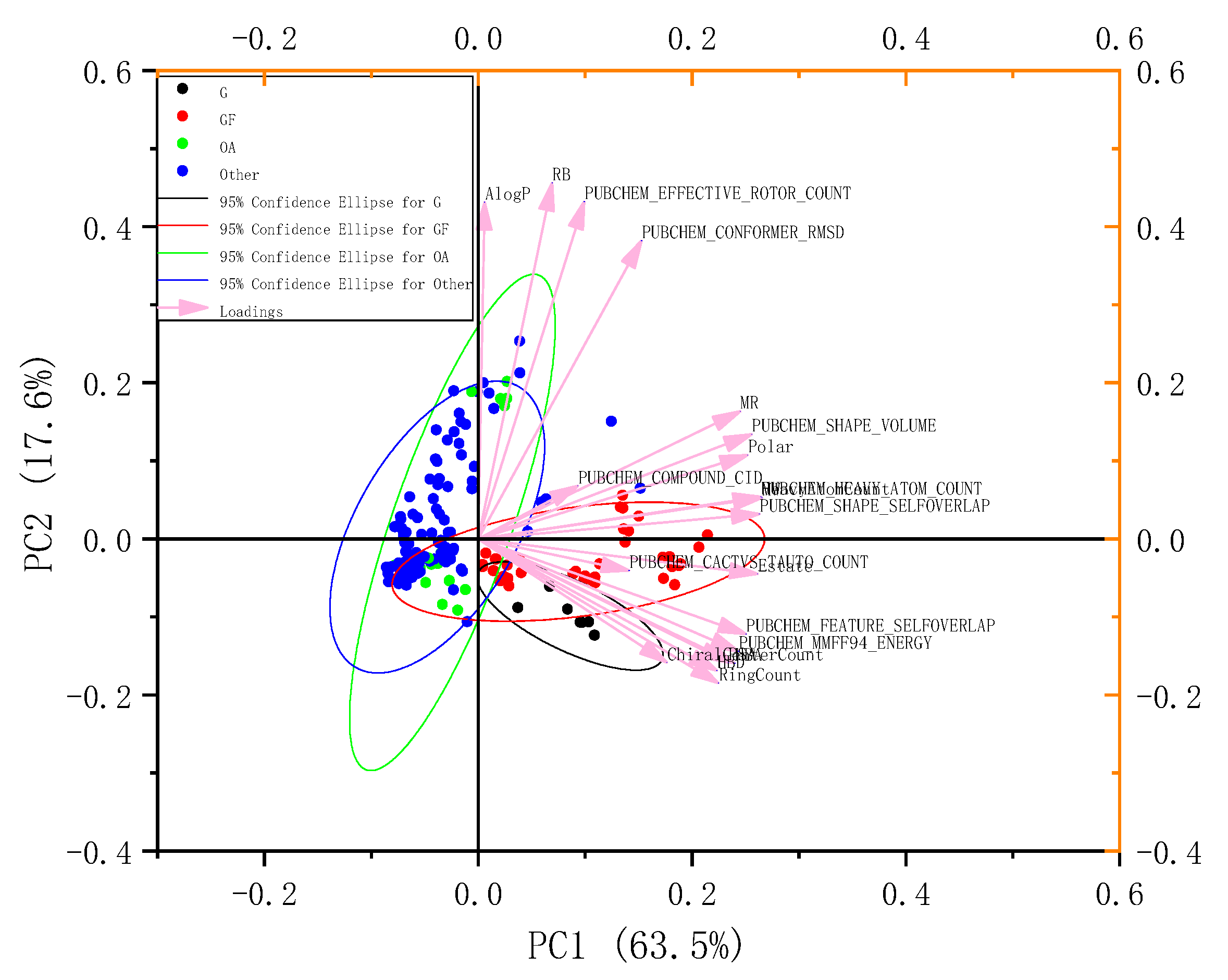
4.7. Principal Component Analysis of EGb Molecular Data Set
Import the EGb molecular data set into canvas 2.3, calculate the corresponding molecular descriptors, and select 22 of the 60 important molecular descriptors as Alogp, Chiralcentercount, Chiral-center-count, Estate, HBAS, HBD, Heavy atom count, MR, MW, PSA, Cactvs-Tauto-Count, Compound-CID, Conformer-RMSD, Effective-rotor-count, Feature-Selfoverlap, Heavy-Atom-Count, Polar, RB, MMFF94-Energy, Shape-Selfoverlap, SHAPE. The EGb compounds were divided into four categories: Ginkgo flavone, ginkgolide, organic acid, and other components. The PCA plug-in of Origin 8.0 was employed for analysis, and the results were transformed into a 2D and 3D chemical spatial map of Ginkgo biloba extract molecules.
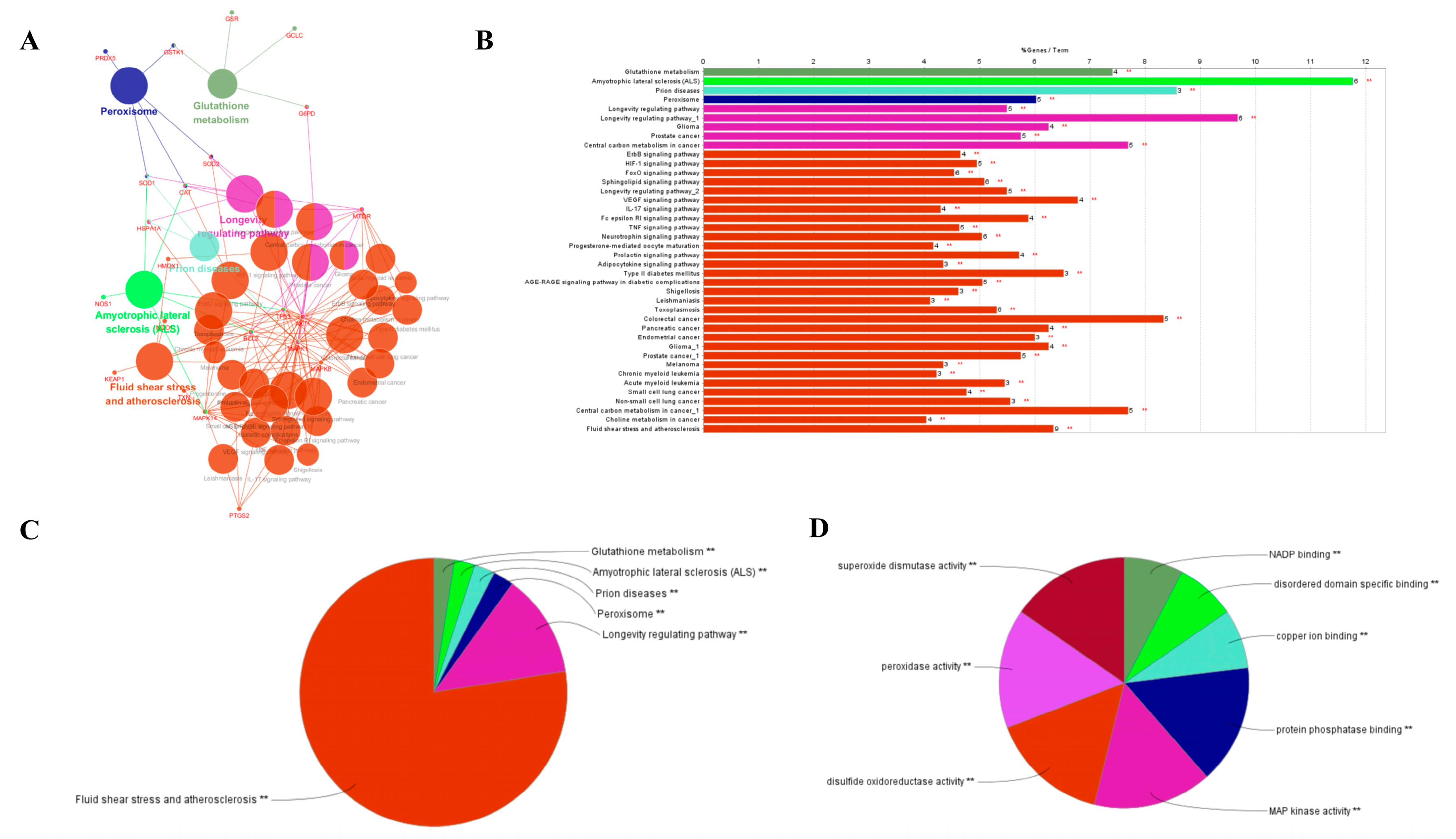
4.8. Screening of Antioxidant Related Targets
The target proteins of anti-oxidation, anti-inflammatory and anti-tumor approved by the FDA were selected from the Drugbank database [
33], TTD database [
34], and COOLGEN database, and the representative targets of anti-oxidation were selected based on the existing research results.
4.9. Molecular Docking Process
The 193 molecules reported in EGb and the existing inhibitor molecules in the anti-inflammatory target protein crystal were introduced into Maestro 10.1 and prepared by the living preparation module. The current inhibitor molecules in anti-inflammatory target protein crystal do not perform energy minimization but only deal with hydrogenation, distribution elements, and bond types. The human receptor protein structure was downloaded from the RSCB protein database and imported into the protein preparation module of Maestro 10.1. The target proteins were modified, dehydrated, and hydrogenated under the default parameters, especially the eutectic water molecules around the ligand molecules in the protein crystal (<5 × 10−10 m), and the ligand molecules themselves were removed from the docking lattice, and the tautomeric structure and ionic residues were standardized and optimized in the pH neutral state. Finally, 34 kinds of receptor target proteins and 193 kinds of ligand compounds optimized by the software were introduced into Glide 6.6 software for molecular docking simulation.
4.10. Docking Process
Standard precision (SP) was set for docking 193 EGb molecules, and the conformations of the existing inhibitors in the protein crystal structure were not searched. The sampling process was set to “none (score in place only)”, and the scoring operation was only performed. The active site is defined by the location of the existing inhibitors in the protein crystal. The lattice box of the active site is set as: x = 2 × 10−9 m, y = 2 × 10−9 m, z = 2 × 10−9 m, and others are default settings. Monte Carlo (MC) algorithm was used to search for the reasonable conformation of the docking compounds in the active site area, and the docking results were screened. The target protein was butted with the existing ligands in the protein crystal. The docking fraction was used as the reference value; secondly, the docking fraction was better than the reference value, and the results were sorted.
4.11. Construction of Molecular Target Protein Interaction Network
A molecular target network of EGb small molecules interacting with anti-inflammatory receptor target proteins was constructed by selecting the top 1000 pairs of molecules and target proteins and importing their information into the software of Cytoscape 2.8.1. Nodes represent EGb ligand molecule and anti-inflammatory receptor target protein, and the relationship between EGb ligand molecule and receptor target protein is defined by an edge. Network analyzer, a plug-in in the software, analyzes the characteristic parameters (network degree, network density, medium number, and shortest path, etc.) [
35,
36], predicts the potential active molecular groups and potential target protein groups in EGb components, and lists the top 10 receptor target proteins and EGb functional molecular information.
4.12. Gene Pathway and Function Analysis
The gene function of the anti-inflammatory target was analyzed by molecular biological function in Cluego, a plug-in of Cytoscape 2.8.1 software. The metabolic pathway of the active target was enriched by Kyoto gene and genome Encyclopedia (KEGG). The species is Homo sapiens, ontology reference set, the kappa score threshold is 0.4, p-value ≤ 0.05, and the rest are set as default parameters. The node represents the target and metabolic pathway in the generated network, while the edge represents the interaction between the target and route.
4.13. Statistical Analysis Method
In this section, GraphPad Prism 5 software (Version 5.01) was used to analyze the data of antioxidant, anti-inflammatory factors, tumor cell proliferation inhibition rate, gray analysis, etc. The data were expressed as mean ± standard deviation, and one-way ANOVA was used between groups, p < 0.05 was the difference, with statistical significance.