Aliphatic Anion Exchange Ionomers with Long Spacers and No Ether Links by Ziegler–Natta Polymerization: Properties and Alkaline Stability
Abstract
:1. Introduction
2. Results and Discussion
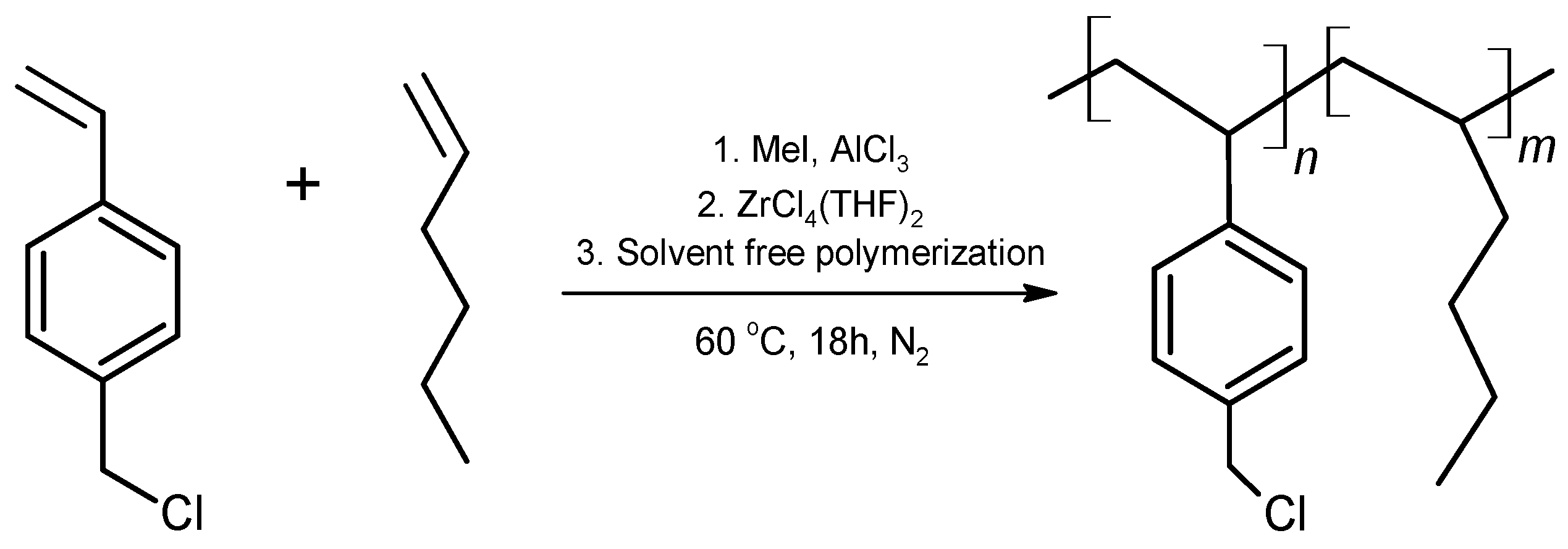
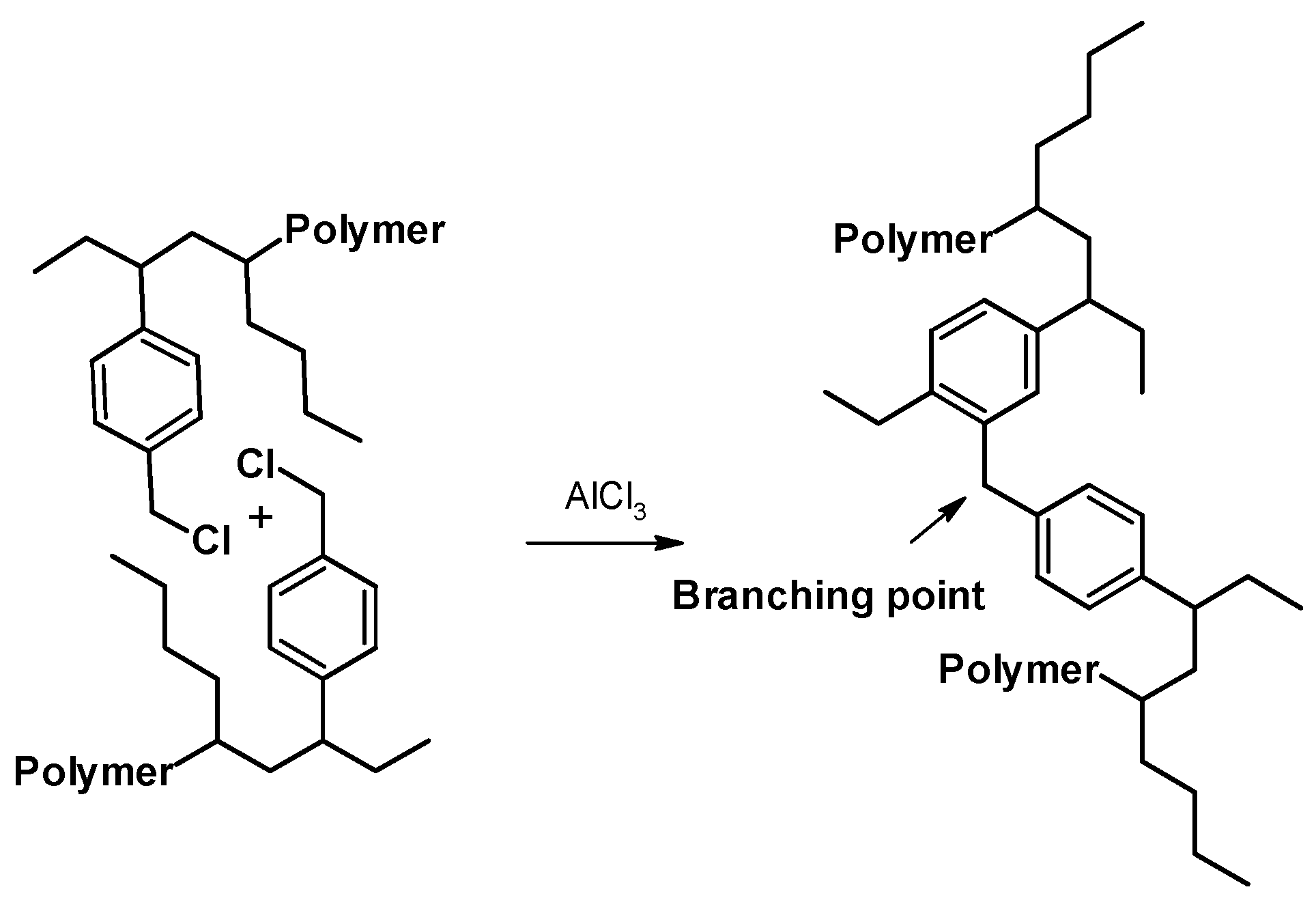
2.1. Synthesis and Characterization of the Copolymer Poly(VBCl-co-hexene)
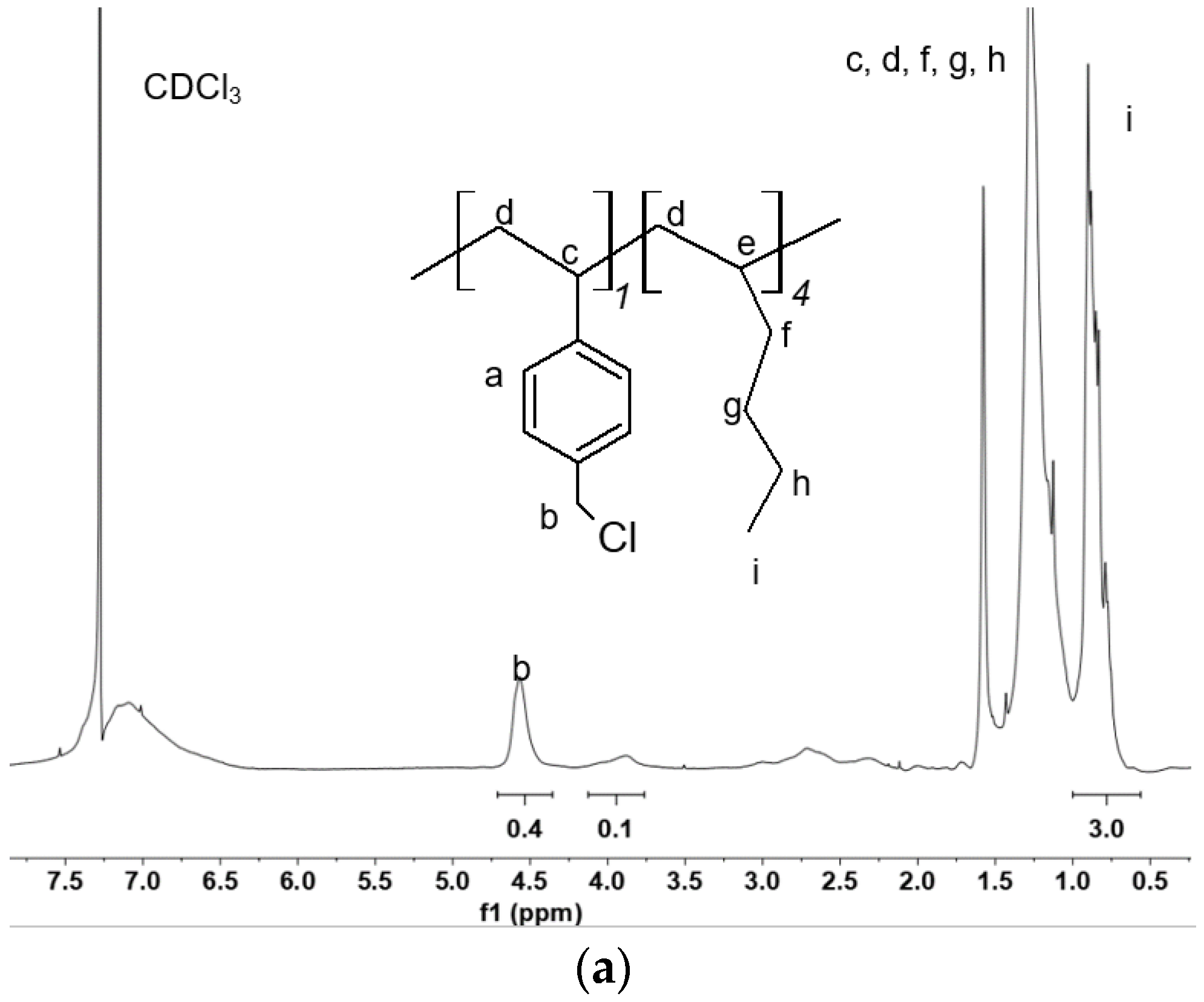
2.2. 1H-NMR Analysis
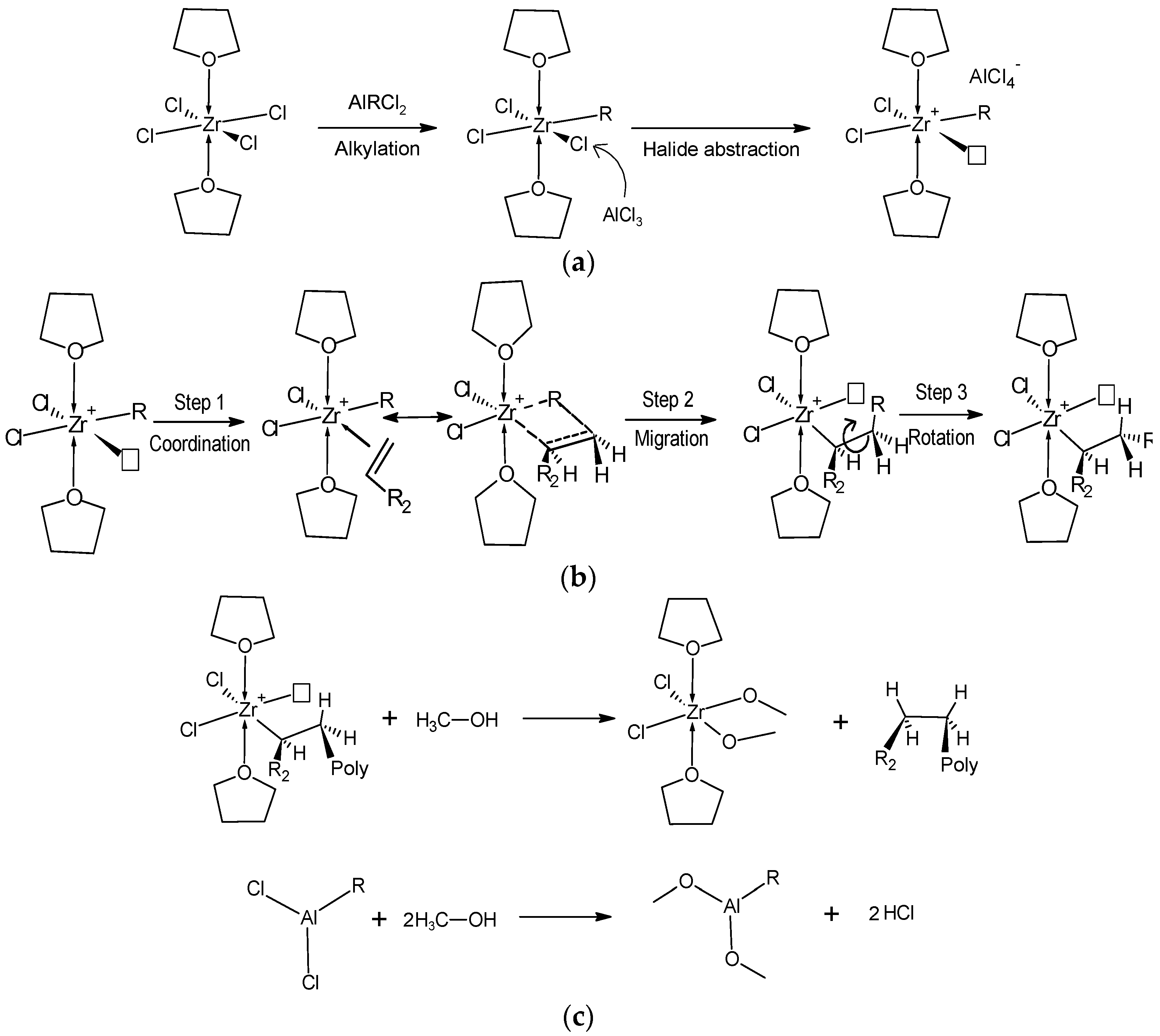
2.3. Catalytic Activity of ZNp
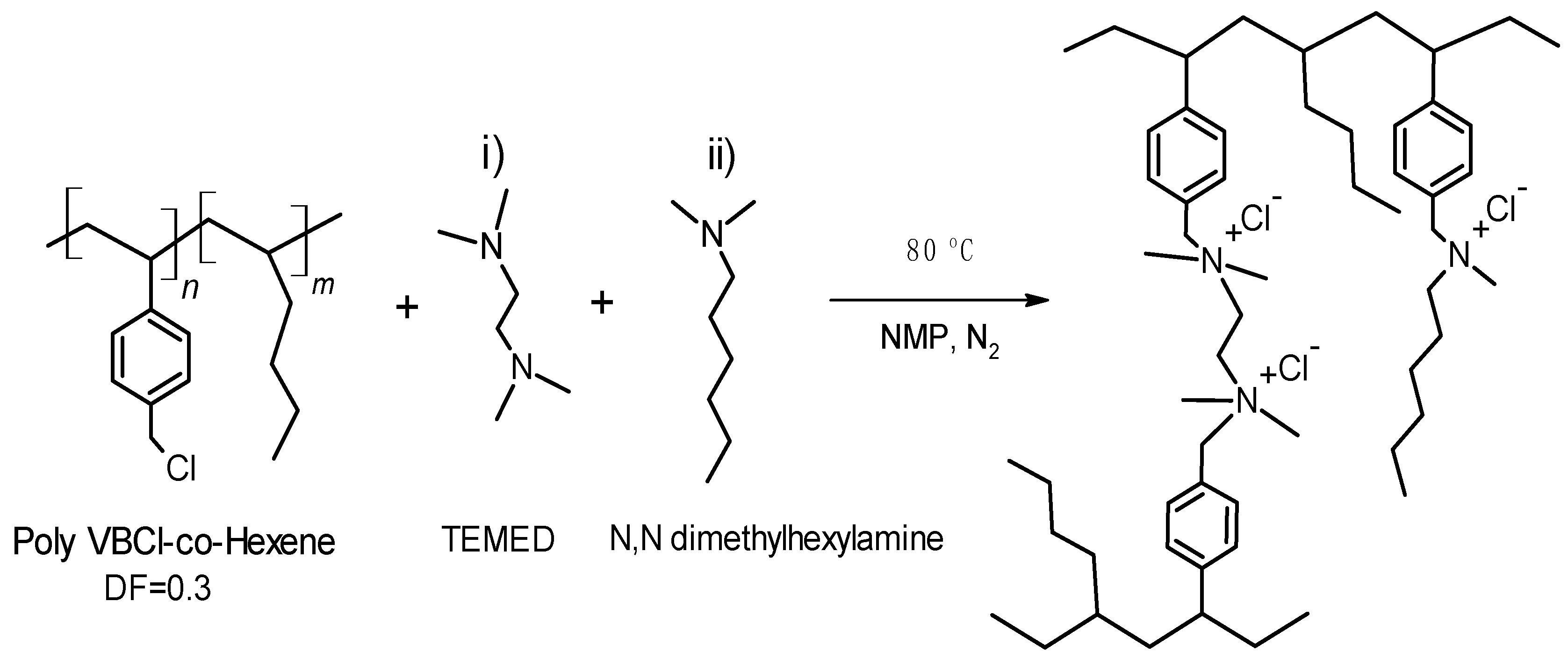
2.4. Synthesis of the Ionomer Based on the Copolymer Poly(VBCl-co-hexene)
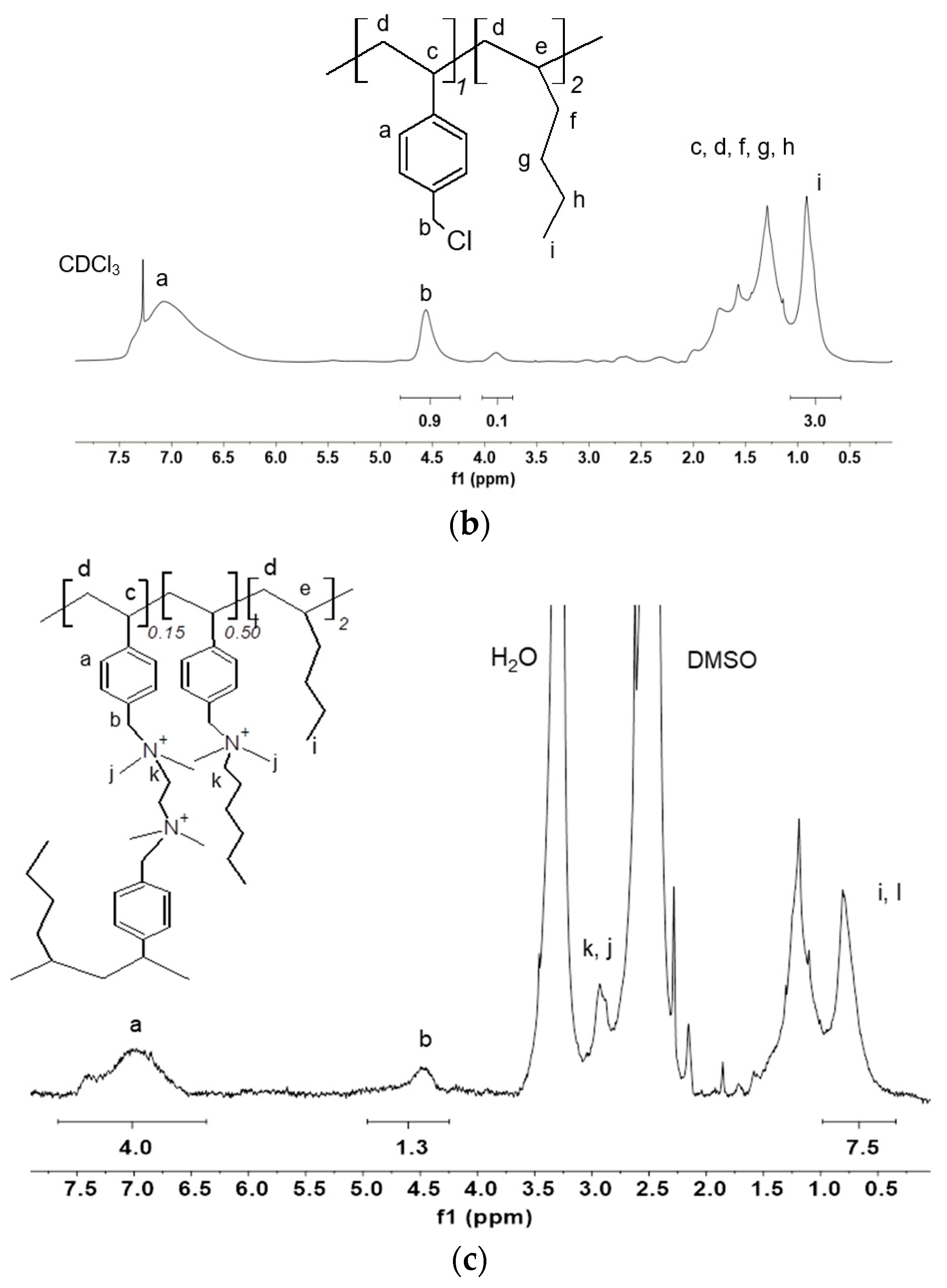
2.5. 1H-NMR Analysis of the Crosslinked Ionomer
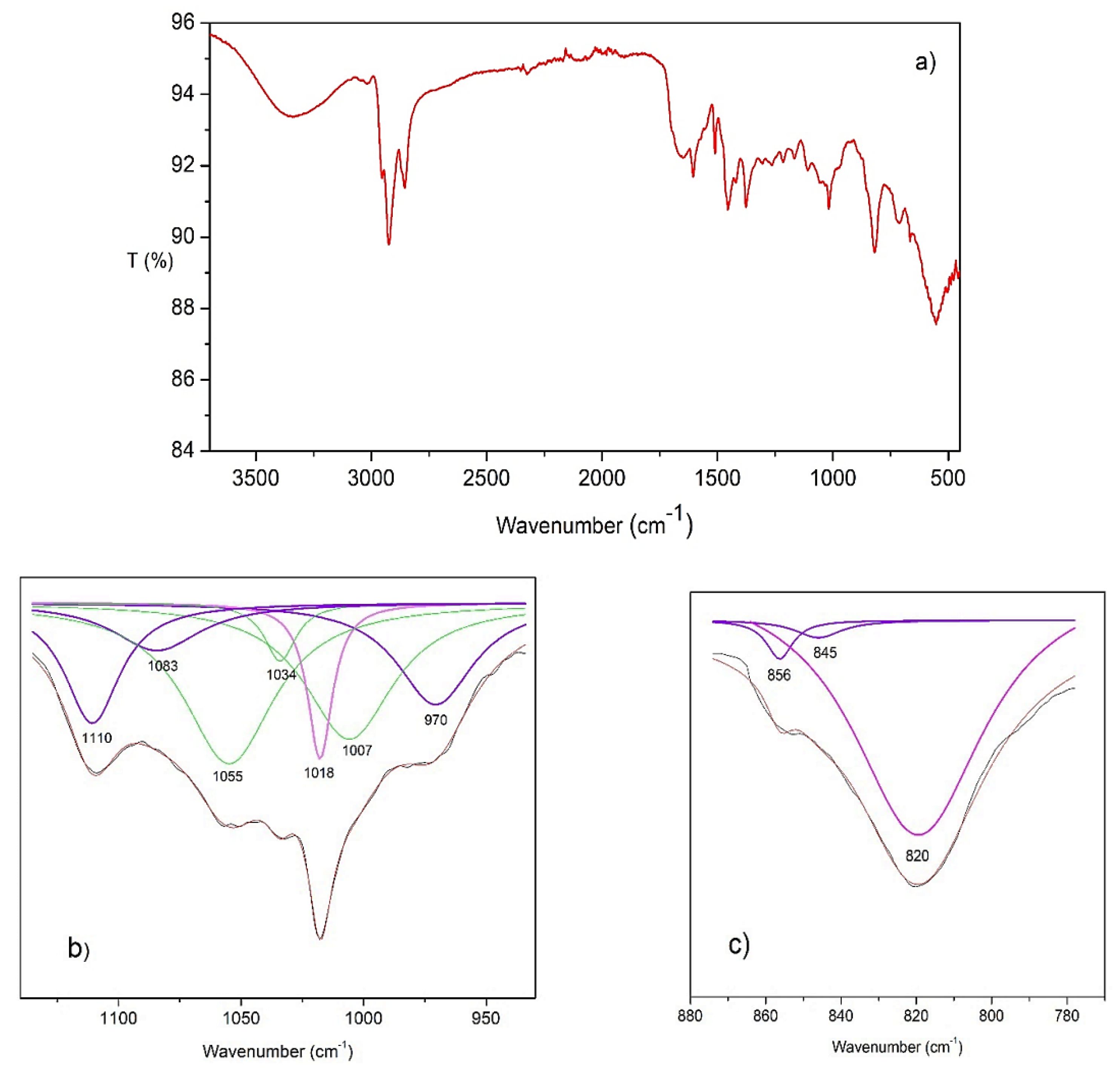
2.6. Infrared Spectra Analysis
2.7. Stability Test of the Crosslinked Ionomer
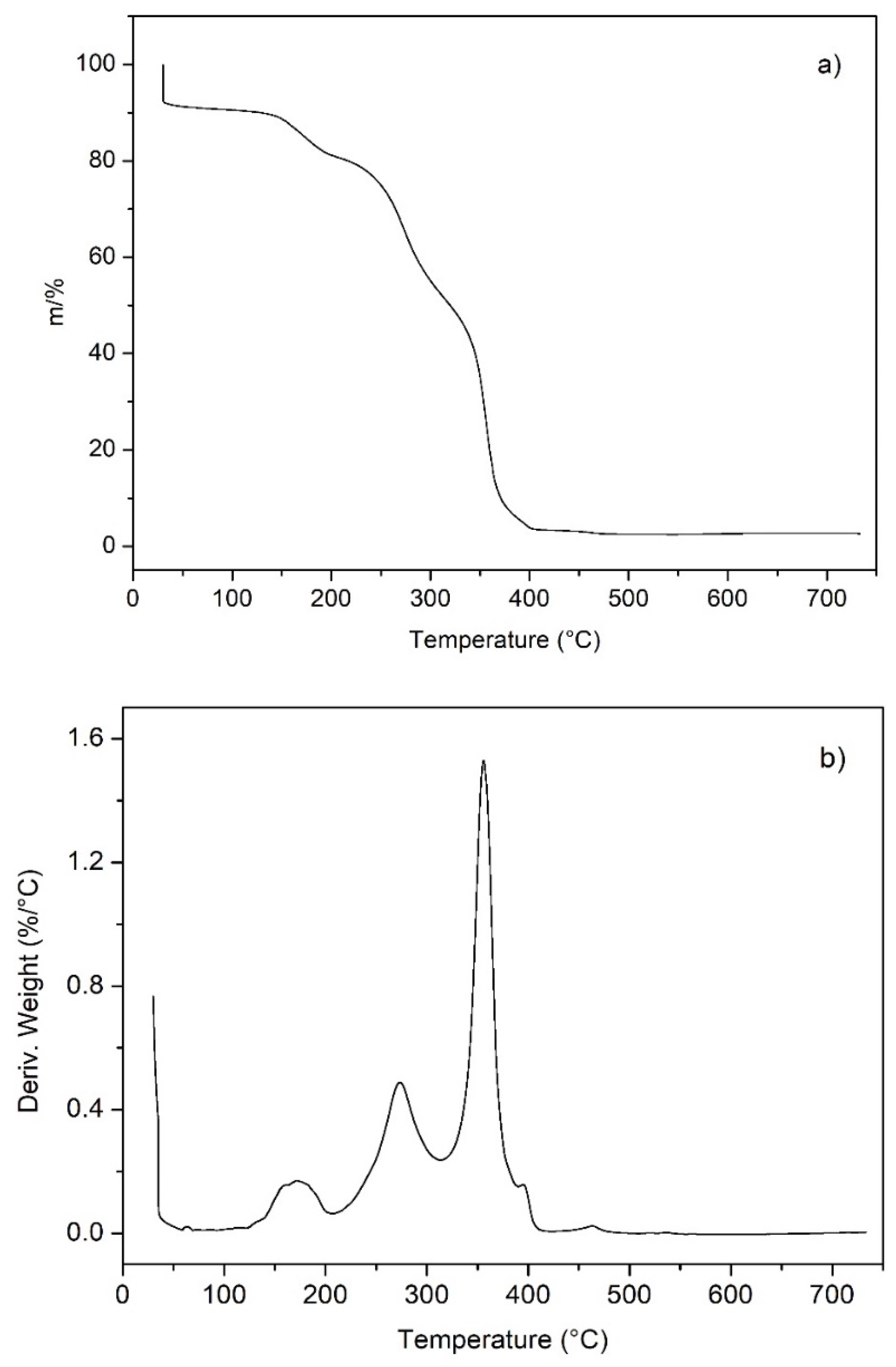
2.8. Thermogravimetry of the Crosslinked Ionomer
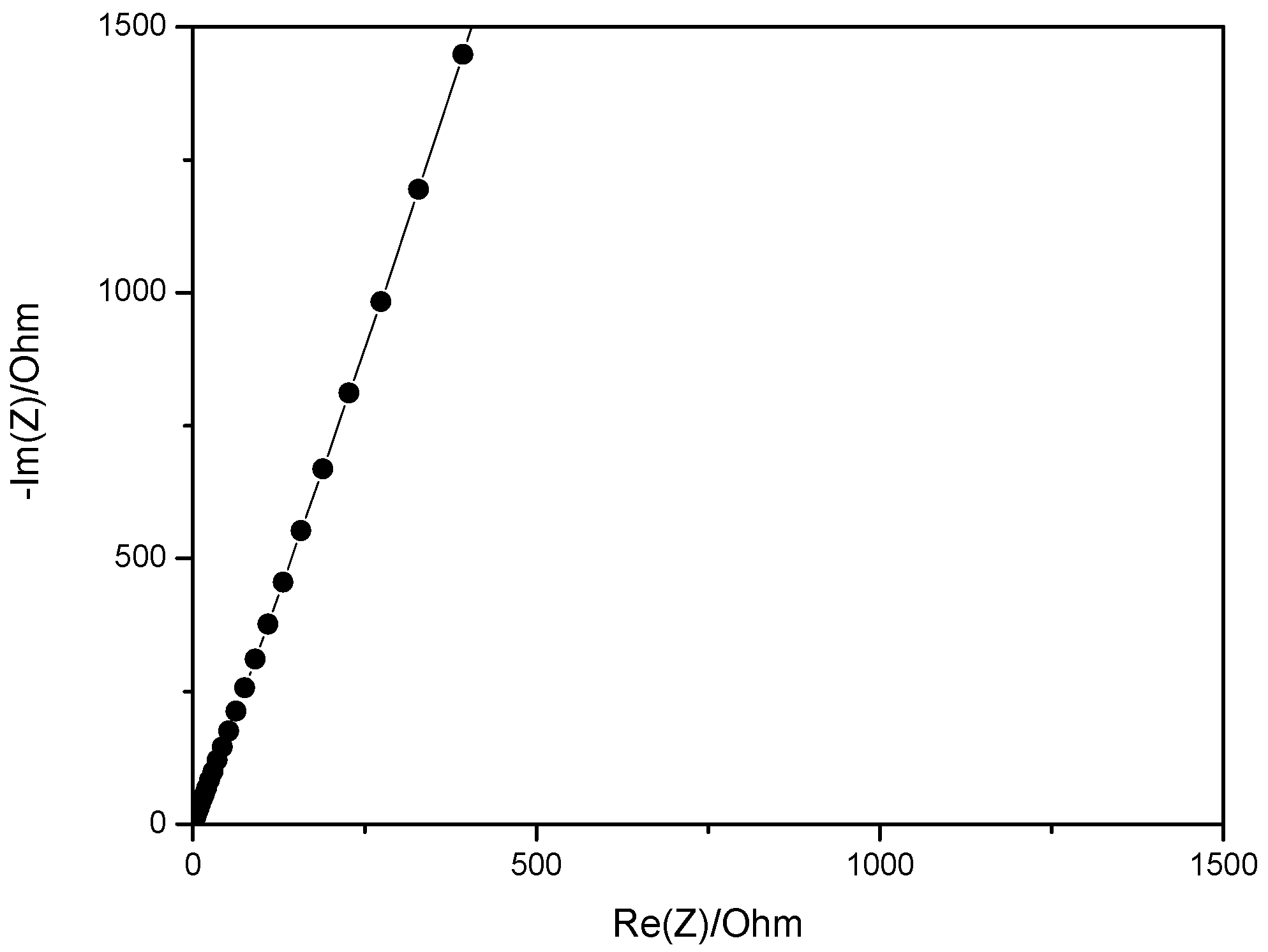
2.9. Water Uptake and Ionic Conductivity of Blend Membrane with PVA
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Poly(VBCl-co-hexene)
3.2.1. Synthesis of the Cocatalyst
3.2.2. Catalyst Activation
3.2.3. Random Copolymerization
3.3. Crosslinking of the Copolymer with TEMED
3.4. Ionomer Quaternization
3.5. Membrane Fabrication
3.6. Stability Test
3.7. Determination of Ion Exchange Capacity (IEC) by Back-Titration
3.8. NMR Spectroscopy
3.9. FTIR Spectroscopy
3.10. Thermogravimetry
3.11. Ionic Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Varcoe, J.R.; Slade, R.C.T. Prospects for alkaline anion-exchange membranes in low temperature fuel cells. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion Exchange Membranes: Current Status and Moving Forward. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 101345. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cell—Fundamentals and Applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q.H. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.F.; An, L.; Zhao, T.S.; Tang, Z.K. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Mai, Z.S.; Zhang, H.M.; Zhang, H.Z.; Xu, W.X.; Wei, W.P.; Na, H.; Li, X.F. Anion-Conductive Membranes with Ultralow Vanadium Permeability and Excellent Performance in Vanadium Flow Batteries. Chemsuschem 2013, 6, 328–335. [Google Scholar] [CrossRef]
- Chu, X.M.; Liu, L.; Huang, Y.D.; Guiver, M.D.; Li, N.W. Practical implementation of bis-six-membered N-cyclic quaternary ammonium cations in advanced anion exchange membranes for fuel cells: Synthesis and durability. J. Membr. Sci. 2019, 578, 239–250. [Google Scholar] [CrossRef]
- Becerra-Arciniegas, R.A.; Narducci, R.; Ercolani, G.; Antonaroli, S.; Sgreccia, E.; Pasquini, L.; Knauth, P.; Di Vona, M.L. Alkaline stability of model anion exchange membranes based on poly(phenylene oxide) (PPO) with grafted quaternary ammonium groups: Influence of the functionalization route. Polymer 2019, 185, 121931. [Google Scholar] [CrossRef]
- Bauer, B.; Strathmann, H.; Effenberger, F. Anion-exchange membranes with improved alkaline stability. Desalination 1990, 79, 125–144. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. Chemsuschem 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Li, C.P.; Wang, J.C.; Li, L.; Wei, Z.D. A general strategy to enhance the alkaline stability of anion exchange membranes. J. Mater. Chem. A 2017, 5, 6318–6327. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.H.; Zhang, F.X. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Pandey, T.P.; Sarode, H.N.; Yang, Y.T.; Yang, Y.; Vezzu, K.; Di Noto, V.; Seifert, S.; Knauss, D.M.; Liberatore, M.W.; Herring, A.M. A Highly Hydroxide Conductive, Chemically Stable Anion Exchange Membrane, Poly(2,6 dimethyl 1,4 phenylene oxide)-b-Poly(vinyl benzyl trimethyl ammonium), for Electrochemical Applications. J. Electrochem. Soc. 2016, 163, H513–H520. [Google Scholar] [CrossRef]
- Narducci, R.; Ercolani, G.; Becerra-Arciniegas, R.A.; Pasquini, L.; Knauth, P.; Di Vona, M.L. “Intrinsic” Anion Exchange Polymers through the Dissociation of Strong Basic Groups: PPO with Grafted Bicyclic Guanidines. Membranes 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.L.; Wang, Y.G.; An, L.A.; Guiver, M.D.; Li, N.W. Highly stable anion exchange membranes based on quaternized polypropylene. J. Mater. Chem. A 2015, 3, 12284–12296. [Google Scholar] [CrossRef]
- Lin, C.X.; Wang, X.Q.; Hu, E.N.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Quaternized triblock polymer anion exchange membranes with enhanced alkaline stability. J. Membr. Sci. 2017, 541, 358–366. [Google Scholar]
- Dang, H.S.; Jannasch, P. Exploring Different Cationic Alkyl Side Chain Designs for Enhanced Alkaline Stability and Hydroxide Ion Conductivity of Anion-Exchange Membranes. Macromolecules 2015, 48, 5742–5751. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, X.D.; Hickner, M.A. Exploring backbone-cation alkyl spacers for multi-cation side chain anion exchange membranes. J. Power Sources 2018, 375, 433–441. [Google Scholar] [CrossRef]
- Long, H.; Kim, K.; Pivovar, B.S. Hydroxide Degradation Pathways for Substituted Trimethylammonium Cations: A DFT Study. J. Phys. Chem. C 2012, 116, 9419–9426. [Google Scholar] [CrossRef]
- Becerra-Arciniegas, R.A.; Narducci, R.; Ercolani, G.; Sgreccia, E.; Pasquini, L.; Di Vona, M.L.; Knauth, P. Model Long Side-Chain PPO-Based Anion Exchange Ionomers: Properties and Alkaline Stability. J. Phys. Chem. C 2020, 124, 1309–1316. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Narducci, R.; Pasquini, L.; Pelzer, K.; Knauth, P. Anion-conducting ionomers: Study of type of functionalizing amine and macromolecular cross-linking. Int. J. Hydrogen Energy 2014, 39, 14039–14049. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Casciola, M.; Donnadio, A.; Nocchetti, M.; Pasquini, L.; Narducci, R.; Knauth, P. Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 3197–3205. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Ciotta, E.; Ferrari, I.V.; Narducci, R.; Pasquini, L.; Varone, A.; Richetta, M.; Antonaroli, S.; Braglia, M.; Knauth, P.; et al. Layered Double Hydroxides Containing an Ionic Liquid: Ionic Conductivity and Use in Composite Anion Exchange Membranes. Chemelectrochem 2018, 5, 2781–2788. [Google Scholar] [CrossRef]
- Pasquini, L.; Becerra-Arciniegas, R.A.; Narducci, R.; Sgreccia, E.; Gressel, V.; Di Vona, M.L.; Knauth, P. Properties and Alkaline Stability of Composite Anion Conducting Ionomers Based on Poly(phenylene oxide) Grafted with DABCO and Mg/Al Lamellar Double Hydroxide. Chemelectrochem 2020, 7, 2917–2924. [Google Scholar] [CrossRef]
- Narducci, R.; Sgreccia, E.; Di Vona, M.L.; Knauth, P. Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review. Polymers 2021, 13, 3887. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Kim, Y.S.; Bae, C. Robust Hydroxide Ion Conducting Poly(biphenyl alkylene)s for Alkaline Fuel Cell Membranes. ACS Macro Lett. 2015, 4, 814–818. [Google Scholar] [CrossRef]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. Poly(arylene alkylene)s with pendant N-spirocyclic quaternary ammonium cations for anion exchange membranes. J. Mater. Chem. A 2018, 6, 16537–16547. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.J.; Lu, C.R.; Li, Y.X.; Long, C.; Li, Z.M.; Zhu, H. Tunable multi-cations-crosslinked poly(arylene piperidinium)-based alkaline membranes with high ion conductivity and durability. J. Membr. Sci. 2019, 588, 117120. [Google Scholar] [CrossRef]
- Long, C.A.; Wang, Z.H.; Zhu, H. High chemical stability anion exchange membrane based on poly(aryl piperidinium): Effect of monomer configuration on membrane properties. Int. J. Hydrogen Energy 2021, 46, 18524–18533. [Google Scholar] [CrossRef]
- Zhou, X.X.; Wu, L.X.; Zhang, G.X.; Li, R.Y.; Hu, X.; Chang, X.W.; Shen, Y.H.; Liu, L.; Li, N.W. Rational design of comb-shaped poly(arylene indole piperidinium) to enhance hydroxide ion transport for H2/O2 fuel cell. J. Membr. Sci. 2021, 631, 119335. [Google Scholar] [CrossRef]
- Shirbakht, S.; Mirmohammadi, S.A.; Didehban, K.; Sadjadi, S.; Bahri-Laleh, N. Effects of monomer length on alpha-olefins polymerization using a conventional Ziegler-Natta catalyst. Adv. Polym. Technol. 2018, 37, 2588–2596. [Google Scholar] [CrossRef]
- Talapatra, S.; Rao, P.V.C.; Ravindranathan, M. Copolymerization of propylene and styrene with Ziegler-Natta catalyst systems: Thermal and spectroscopic characterization of the products. Eur. Polym. J. 1999, 35, 1073–1078. [Google Scholar] [CrossRef]
- Soga, K.; Uozumi, T.; Yanagihara, H.; Siono, T. Polymerization of styrene with heterogeneous Ziegler-Natta catalysts. Makromol. Chem. Rapid Commun. 1990, 11, 229–234. [Google Scholar] [CrossRef]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The Influence of Ziegler-Natta and Metallocene Catalysts on Polyolefin Structure, Properties, and Processing Ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Gambarotta, S. Vanadium-based Ziegler-Natta: Challenges, promises, problems. Coord. Chem. Rev. 2003, 237, 229–243. [Google Scholar] [CrossRef]
- Claverie, J.R.; Schaper, F. Ziegler-Natta catalysis: 50 years after the Nobel Prize. Mrs Bull. 2013, 38, 213–218. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, X.D.; Peng, X.; Zimudzi, T.J.; Saikia, N.; Kwasny, M.T.; Song, S.F.; Kushner, D.I.; Fu, Z.S.; Tew, G.N.; et al. Poly(olefin)-Based Anion Exchange Membranes Prepared Using Ziegler-Natta Polymerization. Macromolecules 2019, 52, 4030–4041. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Ul Hassan, N.; Mustain, W.E.; Kohl, P.A. Poly(norbornene) anion conductive membranes: Homopolymer, block copolymer and random copolymer properties and performance. J. Mater. Chem. A 2020, 8, 17568–17578. [Google Scholar] [CrossRef]
- Chen, W.T.; Mandal, M.; Huang, G.; Wu, X.M.; He, G.H.; Kohl, P.A. Highly Conducting Anion-Exchange Membranes Based on Cross-Linked Poly(norbornene): Ring Opening Metathesis Polymerization. ACS Appl. Energy Mater. 2019, 2, 2458–2468. [Google Scholar] [CrossRef]
- Proto, A.; Capacchione, C.; Motta, O.; De Carlo, F. ZrCl4 as catalyst for olefins and styrene polymerization: Effect of ethereal donors on the activity and stereospecificity. Macromolecules 2003, 36, 5942–5946. [Google Scholar] [CrossRef]
- Niyomthai, T.; Jongsomjit, B.; Praserthdam, P. Impact of AlCl3 and FeCl2 Addition on Catalytic Behaviors of TiCl4/MgCl2/THF Catalysts for Ethylene Polymerization and Ethylene/l-Hexene Copolymerization. Bull. Chem. React. Eng. Catal. 2018, 13, 393–404. [Google Scholar] [CrossRef]
- Bradley, D.C.; Abd-el Halim, F.M.; Wardlaw, W. The chloride ethoxides of zirconium. J. Chem. Soc. 1950, 676, 3450–3454. [Google Scholar] [CrossRef]
- Soto, M.; Hiller, M.; Oschkinat, H.; Koschek, K. Multifunctional Benzoxazines Feature Low Polymerization Temperature and Diverse Polymer Structures. Polymers 2016, 8, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vengatesan, S.; Vasudevan, D.; Radhakrishnan, S. Time- and temperature-resolved in-situ NMR studies on simultaneous quaternization/cross-linking of poly(vinylbenzyl chloride) polymer with hexamine. Colloid Polym. Sci. 2015, 293, 3439–3448. [Google Scholar] [CrossRef]
- Szczegot, K.; Flisak, Z.; Sibelska, I.; Dawidowska-Marynowicz, B. Transition metal chlorides complexes with tetrahydrofuran MtCl(4)(THF)(2) used as precursors of ethylene polymerization catalysts. Polimery 2002, 47, 85–89. [Google Scholar] [CrossRef]
- Proto, A.; Luciano, E.; Capacchione, C.; Motta, O. ZrCl4(THF)2/methylaluminoxane as the catalyst for the syndiotactic polymerization of styrene. Macromol. Rapid Commun. 2002, 23, 183–186. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, C.Q.; Mecking, S.; Jian, Z.B. Ultrahigh Branching of Main-Chain-Functionalized Polyethylenes by Inverted Insertion Selectivity. Angew. Chem. Int. Ed. 2020, 59, 14296–14302. [Google Scholar] [CrossRef]
- Alhan, H.B.E.; Jones, G.R.; Harth, E. Branching Regulation in Olefin Polymerization via Lewis Acid Triggered Isomerization of Monomers. Angew. Chem. Int. Ed. 2020, 59, 4743–4749. [Google Scholar] [CrossRef]
- Ertem, S.P.; Caire, B.R.; Tsai, T.H.; Zeng, D.; Vandiver, M.A.; Kusoglu, A.; Seifert, S.; Hayward, R.C.; Weber, A.Z.; Herring, A.M.; et al. Ion Transport Properties of Mechanically Stable Symmetric ABCBA Pentablock Copolymers with Quaternary Ammonium Functionalized Midblock. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 612–622. [Google Scholar] [CrossRef]
- Lin, B.C.; Xu, F.; Su, Y.; Zhu, Z.J.; Ren, Y.R.; Ding, J.N.; Yuan, N.Y. Facile Preparation of Anion-Exchange Membrane Based on Polystyrene-b-polybutadiene-b-polystyrene for the Application of Alkaline Fuel Cells. Ind. Eng. Chem. Res. 2019, 58, 22299–22305. [Google Scholar] [CrossRef]
- Cao, Y.C.; Wang, X.; Mamlouk, M.; Scott, K. Preparation of alkaline anion exchange polymer membrane from methylated melamine grafted poly(vinylbenzyl chloride) and its fuel cell performance. J. Mater. Chem. 2011, 21, 12910–12916. [Google Scholar] [CrossRef]
- Wang, C.; Mo, B.M.; He, Z.F.; Shao, Q.; Pan, D.; Wujick, E.; Guo, J.; Xie, X.L.; Xie, X.F.; Guo, Z.H. Crosslinked norbornene copolymer anion exchange membrane for fuel cells. J. Membr. Sci. 2018, 556, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.W.; He, X.H.; Huang, S.M.; Zhang, F.; Guo, Y.; Wen, Y.F.; Wu, B.; Chen, D.F. Novel self-cross-linked multi-imidazolium cations long flexible side chains triblock copolymer anion exchange membrane based on ROMP-type polybenzonorbornadiene. Int. J. Hydrogen Energy 2020, 45, 19676–19690. [Google Scholar] [CrossRef]
- Ponce-Gonzalez, J.; Whelligan, D.K.; Wang, L.Q.; Bance-Soualhi, R.; Wang, Y.; Peng, Y.Q.; Peng, H.Q.; Apperley, D.C.; Sarode, H.N.; Pandey, T.P.; et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 2016, 9, 3724–3735. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.S.; Wu, F.G.; Zhou, Y.; Zheng, Y.Z.; Yu, Z.W. Selective recognition induced nanostructures in a cucurbit 7 uril-based host-guest system: Micelles, nanorods and nanosheets. Phys. Chem. Chem. Phys. 2012, 14, 8506–8510. [Google Scholar] [CrossRef] [PubMed]
- Nhung, L.T.T.; Kim, I.Y.; Yoon, Y.S. Quaternized Chitosan-Based Anion Exchange Membrane Composited with Quaternized Poly(vinylbenzyl chloride)/Polysulfone Blend. Polymers 2020, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Vengatesan, S.; Santhi, S.; Jeevanantham, S.; Sozhan, G. Quaternized poly (styrene-co-vinylbenzyl chloride) anion exchange membranes for alkaline water electrolysers. J. Power Sources 2015, 284, 361–368. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, X.; Wu, C.M.; Han, X.Z.; Xu, C. Anion exchange membranes with excellent monovalent anion perm-selectivity for electrodialysis applications. Chem. Eng. Res. Des. 2020, 158, 24–32. [Google Scholar] [CrossRef]
- Braglia, M.; Ferrari, I.V.; Djenizian, T.; Kaciulis, S.; Soltani, P.; Di Vona, M.L.; Knauth, P. Bottom-Up Electrochemical Deposition of Poly(styrene sulfonate) on Nanoarchitectured Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 22902–22910. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.F.; Xuan, Y.M.; Li, Q.A. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Yan, Y.B.; Wang, D.F.; He, S.Y.; Ren, H.G.; Xu, Y.J. Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications. E-Polym. 2019, 19, 511–518. [Google Scholar] [CrossRef]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Karimi, M. Study on unsaturated structure and tacticity of poly1-hexene and new copolymer of 1-hexene/5-hexene-1-ol prepared by metallocene catalyst. J. Organomet. Chem. 2016, 819, 103–108. [Google Scholar] [CrossRef]
- Ruiz-Orta, C.; Fernandez-Blazquez, J.P.; Pereira, E.J.; Alamo, R.G. Time-resolved FTIR spectroscopic study of the evolution of helical structure during isothermal crystallization of propylene 1-hexene copolymers. Identification of regularity bands associated with the trigonal polymorph. Polymer 2011, 52, 2856–2868. [Google Scholar] [CrossRef]
- Nunez, S.A.; Hickner, M.A. Quantitative H-1 NMR Analysis of Chemical Stabilities in Anion-Exchange Membranes. ACS Macro Lett. 2013, 2, 49–52. [Google Scholar] [CrossRef]
- Miyanishi, S.; Yamaguchi, T. Ether cleavage-triggered degradation of benzyl alkylammonium cations for polyethersulfone anion exchange membranes. Phys. Chem. Chem. Phys. 2016, 18, 12009–12023. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.S. Quaternized aryl ether-free polyaromatics for alkaline membrane fuel cells: Synthesis, properties, and performance—A topical review. J. Mater. Chem. A 2018, 6, 15456–15477. [Google Scholar] [CrossRef]
- Yadav, V.; Rathod, N.H.; Kulshrestha, V. Series-Connected Tetracation Partially Cross-Linked Anion Exchange Membranes: Insight towards Consequences of Alkyl Spacer Length. ACS Appl. Polym. Mater. 2021, 3, 3307–3320. [Google Scholar] [CrossRef]
- Li, N.W.; Leng, Y.J.; Hickner, M.A.; Wang, C.Y. Highly Stable, Anion Conductive, Comb-Shaped Copolymers for Alkaline Fuel Cells. J. Am. Chem. Soc. 2013, 135, 10124–10133. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Dong, J.H.; Cao, X.R.; Ren, X.R.; Hao, Z.; Yang, J.S. Diamine crossklinked anion exchange membranes based on poly(vinyl benzyl methylpyrrolidinium). Polymer 2021, 212, 123156. [Google Scholar] [CrossRef]
- Nouri-Ahangarani, F.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Karbalaie, M. Synthesis of highly isotactic poly 1-hexene using Fe-doped Mg(OEt)2/TiCl4/ED Ziegler-Natta catalytic system. Des. Monomers Polym. 2016, 19, 394–405. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Zhao, T.S.; Li, Y.S. Synthesis and characterization of crosslinked poly (vinyl alcohol)/layered double hydroxide composite polymer membranes for alkaline direct ethanol cells. Int. J. Hydrogen Energy 2012, 37, 18425–18432. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Dutta, K. Poly(vinyl alcohol)-based membranes for fuel cell and water treatment applications: A review on recent advancements. Polym. Adv. Technol. 2021, 32, 4175–4203. [Google Scholar] [CrossRef]
- Yang, J.M.; Wang, S.A. Preparation of graphene-based poly(vinyl alcohol)/chitosan nanocomposites membrane for alkaline solid electrolytes membrane. J. Membr. Sci. 2015, 477, 49–57. [Google Scholar] [CrossRef]
| Monomer Ratio: Hexene:VBCl | Reaction Yield (%) | Polymerization Activity (a) | Number of Branches/1000 C (b) |
|---|---|---|---|
| 4:1 | 43 | 0.021 | 6.1 |
| 2:1 | 77 | 0.049 | 4.7 |
| Temperature (°C) | Conductivity (mS/cm) |
|---|---|
| 25 | 29.5 |
| 60 | 32.2 |
| 80 | 34.8 |
| 25 (After cooling down) | 27.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra-Arciniegas, R.A.; Narducci, R.; Ercolani, G.; Pasquini, L.; Knauth, P.; Di Vona, M.L. Aliphatic Anion Exchange Ionomers with Long Spacers and No Ether Links by Ziegler–Natta Polymerization: Properties and Alkaline Stability. Molecules 2022, 27, 395. https://doi.org/10.3390/molecules27020395
Becerra-Arciniegas RA, Narducci R, Ercolani G, Pasquini L, Knauth P, Di Vona ML. Aliphatic Anion Exchange Ionomers with Long Spacers and No Ether Links by Ziegler–Natta Polymerization: Properties and Alkaline Stability. Molecules. 2022; 27(2):395. https://doi.org/10.3390/molecules27020395
Chicago/Turabian StyleBecerra-Arciniegas, Raul Andres, Riccardo Narducci, Gianfranco Ercolani, Luca Pasquini, Philippe Knauth, and Maria Luisa Di Vona. 2022. "Aliphatic Anion Exchange Ionomers with Long Spacers and No Ether Links by Ziegler–Natta Polymerization: Properties and Alkaline Stability" Molecules 27, no. 2: 395. https://doi.org/10.3390/molecules27020395
APA StyleBecerra-Arciniegas, R. A., Narducci, R., Ercolani, G., Pasquini, L., Knauth, P., & Di Vona, M. L. (2022). Aliphatic Anion Exchange Ionomers with Long Spacers and No Ether Links by Ziegler–Natta Polymerization: Properties and Alkaline Stability. Molecules, 27(2), 395. https://doi.org/10.3390/molecules27020395










