A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quality Control
2.2. Pharmacokinetic Evaluation
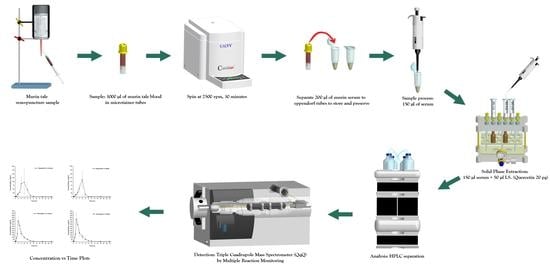
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Tablet Manufacturing
3.3. Equipment
3.4. LC-MS/MS Quality Control
3.5. Animals
3.6. Sample Preparation
3.7. Pharmacokinetic Evaluation
3.8. Software and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ahirwar, R.; Mondal, P.R. Prevalence of obesity in India: A systematic review. Diabetes Metab. Syndr. 2019, 13, 318–321. [Google Scholar] [CrossRef]
- Schmidt, A.M. Diabetes Mellitus and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Sönnichsen, A.; Trampisch, U.S.; Rieckert, A.; Piccoliori, G.; Vögele, A.; Flamm, M.; Johansson, T.; Esmail, A.; Reeves, D.; Löffler, C.; et al. Polypharmacy in chronic diseases-Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support (PRIMA-eDS): Study protocol for a randomized controlled trial. Trials 2016, 17, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Recillas, A.; González-Rivero, N.A.; Barrea-Canto, V.; Ibarra-Barajas, M.; Estrada-Soto, S.; Ortiz-Andrade, R. Vasorelaxant and antihypertensive activities of citroflavonoids (Hesperidin/Naringenin mixture): Potential prophylactic of cardiovascular endothelial dysfunction. Pharmacogn. Mag. 2019, 15, 84–91. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.; Araujo-León, J.A.; Sánchez-Recillas, A.; Navarrete-Vazquez, G.; González-Sánchez, A.A.; Hidalgo-Figueroa, S.; Alonso-Castro, Á.J.; Aranda-González, I.; Hernández-Núñez, E.; Coral-Martínez, T.I.; et al. Toxicological Screening of Four Bioactive Citroflavonoids: In Vitro, In Vivo, and In Silico Approaches. Molecules 2020, 25, 5959. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Pal, S.; Mohamed, R.; Singh, P.; Chattopadhyay, S.; Porwal, K.; Sanyal, S.; Gayen, J.R.; Chattopadhyay, N. A nutraceutical composition containing diosmin and hesperidin has osteogenic and anti-resorptive effects and expands the anabolic window of teriparatide. Biomed. Pharmacother. 2019, 118, 10920. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, M.R. Pharmacological targets of drugs employed in chronic venous and lymphatic insufficiency. Int. Angiol. 2002, 21, 33–39. [Google Scholar]
- Araujo-León, J.A.; Ortiz-Andrade, R.; Vera-Sánchez, R.A.; Oney-Montalvo, J.E.; Coral-Martínez, T.I.; Cantillo-Ciau, Z. Development and Optimization of a High Sensitivity LC-MS/MS Method for the Determination of Hesperidin and Naringenin in Rat Plasma: Pharmacokinetic Approach. Molecules 2020, 25, 4241. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Herrador, M.A. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. Trac.-Trend. Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory II. Analytical method validation and quality assurance. Trac.-Trend. Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Deng, Z.; Wu, W.; Liu, J.; Wang, H. Development of a LC-MS/MS Method for the Quantification of Myricitrin: Application to a Pharmacokinetic Study in Rats. Rev. Bras. Farmacogn. 2021, 31, 102–106. [Google Scholar] [CrossRef]
- Rofiee, M.S.; Yusof, M.I.M.; Kek, T.L.; Salleh, M.Z. A Pharmacokinetic Study by LC-MS/MS to Quantify Isoquercetin and Astragalin in Rat Serum After Oral Administration of a Combined Extract of Moringa oleifera and Centella asiatica. Rev. Bras. Farmacogn. 2020, 30, 804–809. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Shao, S.; Lin, W.; Huang, D.; Pan, R.; Xia, Y. Pharmacokinetic Studies of Multiple Active Components in Rat Plasma Using LC-MS/MS after Oral Administration of Shaoyao-Gancao-Fuzi Decoction. Rev. Bras. Farmacogn. 2020, 30, 810–817. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Chen, D.; Fang, T.; Li, H.; Su, W. LC/MS/MS quantitation assay for pharmacokinetics of naringenin and double peaks phenomenon in rats plasma. Int. J. Pharm. 2006, 307, 292–299. [Google Scholar] [CrossRef]
- Srirangam, R.; Hippalgaonkar, K.; Majumdar, S. Intravitreal kinetics of hesperidin, hesperetin, and hesperidin G: Effect of dose and physicochemical properties. J. Pharm. Sci. 2012, 101, 1631–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wang, S.; Jia, X.; Bajimaya, S.; Lin, H.; Tam, V.H.; Hu, M. Disposition of flavonoids via recycling: Comparison of intestinal versus hepatic disposition. Drug Metab. Dispos. 2005, 33, 1777–1784. [Google Scholar] [CrossRef] [Green Version]
- Jambhekar, S.; Breen, P. Basic Pharmacokinetics, 2nd ed.; Pharmaceutical Press: Padstow, Cornwall, 2009. [Google Scholar]
- Escudero, B.; Calani, L.; Fernández, M.S. Absorption, metabolism, and excretion of fermented orange juice (poly)phenols in rats. Biofactors 2014, 40, 327–335. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004, 38, 771–785. [Google Scholar] [CrossRef]
- Zeng, X.; Yao, H.; Zheng, Y. Tissue distribution of naringin and derived metabolites in rats after a single oral administration. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1136, 121846. [Google Scholar] [CrossRef]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxid. Med. Cell. Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingili, R.; Vemulapalli, S.; Mullapudi, S.; Nuthakki, S.; Pendyala, S.; Naveenbabu, K. Pharmacokinetic interaction study between flavanones (hesperetin, naringenin) and rasagiline mesylate in wistar rats. Drug Dev. Ind. Pharm. 2016, 42, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Peng, W.; Yang, C. Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front. Pharmacol. 2020, 11, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, L.L.; Pandey, R.P.; Jung, N.; Jung, H.J.; Kim, E.H.; Sohng, J.K. Hydroxylation of diverse flavonoids by CYP450 BM3 variants: Biosynthesis of eriodictyol from naringenin in whole cells and its biological activities. Microbial. Cell. Factories 2016, 15, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintieri, L.; Bortolozzo, S.; Stragliotto, S.; Moro, S.; Pavanetto, M.; Nassi, A.; Palatini, P.; Floreani, M. Flavonoids diosmetin and hesperetin are potent inhibitors of cytochrome P450 2C9-mediated drug metabolism in vitro. Drug Metab. Pharmacokinet. 2010, 25, 466–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameter | Mean ± SD | |||
|---|---|---|---|---|
| Hesperidin in Mix-160 | Hesperidin in Tablet | Naringenin in Mix-160 | Naringenin in Tablet | |
| kabs | 0.39 ± 0.02 | 0.45 ± 0.04 | 0.79 ± 0.06 | 0.64 ± 0.03 |
| ke | 0.24 ± 0.05 | 0.25 ± 0.03 | 0.40 ± 0.02 | 0.41 ± 0.02 |
| T ½ elim (h) | 7.71 ± 1.55 | 8.67 ± 1.15 | 4.67 ± 0.61 | 4.02 ± 0.65 |
| Tmax (h) | 4.66 ± 1.15 | 5.33 ± 1.15 | 2.00 ± 00 | 2.00 ± 00 |
| Cmax (ng/mL) | 120.00 ± 72.46 | 121.352 ± 43.70 | 472.308 ± 92.18 | 515.937 ± 142.78 |
| AUC 0→24 (ng/mL·h) | 516.10 ± 146.10 | 410.80 ± 85.58 | 1538.14 ± 124.40 | 1648.57 ± 181.30 |
| AUC0→∞ (ng/mL·h) | 522.12 ± 147.27 | 413.79 ± 86.14 | 1543.64 ± 191.9 | 1653.14 ± 181.70 |
| Vd/F (L/kg) | 745.17 ± 558.31 | 732.78 ± 200.98 | 151.93 ± 29.2 | 141.79 ± 44.25 |
| CL/F (L/kg·h) | 157.96 ± 78.62 | 173.14 ± 38.44 | 60.20 ± 7.32 | 56.614 ± 11.24 |
| MRT (h) | 6.51 ± 1.08 | 6.22219 ± 0.4776 | 4.38102 ± 0.134 | 4.32512 ± 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-León, J.A.; Ortiz-Andrade, R.; Hernández-Baltazar, E.; Hernández-Núñez, E.; Rivera-Leyva, J.C.; Yáñez-Pérez, V.; Vazquez-Garcia, P.; Cicero-Sarmiento, C.G.; Sánchez-Salgado, J.C.; Segura-Campos, M.R. A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture. Molecules 2022, 27, 391. https://doi.org/10.3390/molecules27020391
Araujo-León JA, Ortiz-Andrade R, Hernández-Baltazar E, Hernández-Núñez E, Rivera-Leyva JC, Yáñez-Pérez V, Vazquez-Garcia P, Cicero-Sarmiento CG, Sánchez-Salgado JC, Segura-Campos MR. A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture. Molecules. 2022; 27(2):391. https://doi.org/10.3390/molecules27020391
Chicago/Turabian StyleAraujo-León, Jesús Alfredo, Rolffy Ortiz-Andrade, Efrén Hernández-Baltazar, Emanuel Hernández-Núñez, Julio César Rivera-Leyva, Víctor Yáñez-Pérez, Priscila Vazquez-Garcia, Carla Georgina Cicero-Sarmiento, Juan Carlos Sánchez-Salgado, and Maira Rubí Segura-Campos. 2022. "A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture" Molecules 27, no. 2: 391. https://doi.org/10.3390/molecules27020391
APA StyleAraujo-León, J. A., Ortiz-Andrade, R., Hernández-Baltazar, E., Hernández-Núñez, E., Rivera-Leyva, J. C., Yáñez-Pérez, V., Vazquez-Garcia, P., Cicero-Sarmiento, C. G., Sánchez-Salgado, J. C., & Segura-Campos, M. R. (2022). A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture. Molecules, 27(2), 391. https://doi.org/10.3390/molecules27020391