NIRS and Aquaphotomics Trace Robusta-to-Arabica Ratio in Liquid Coffee Blends
Abstract
:1. Introduction
2. Results and discussion
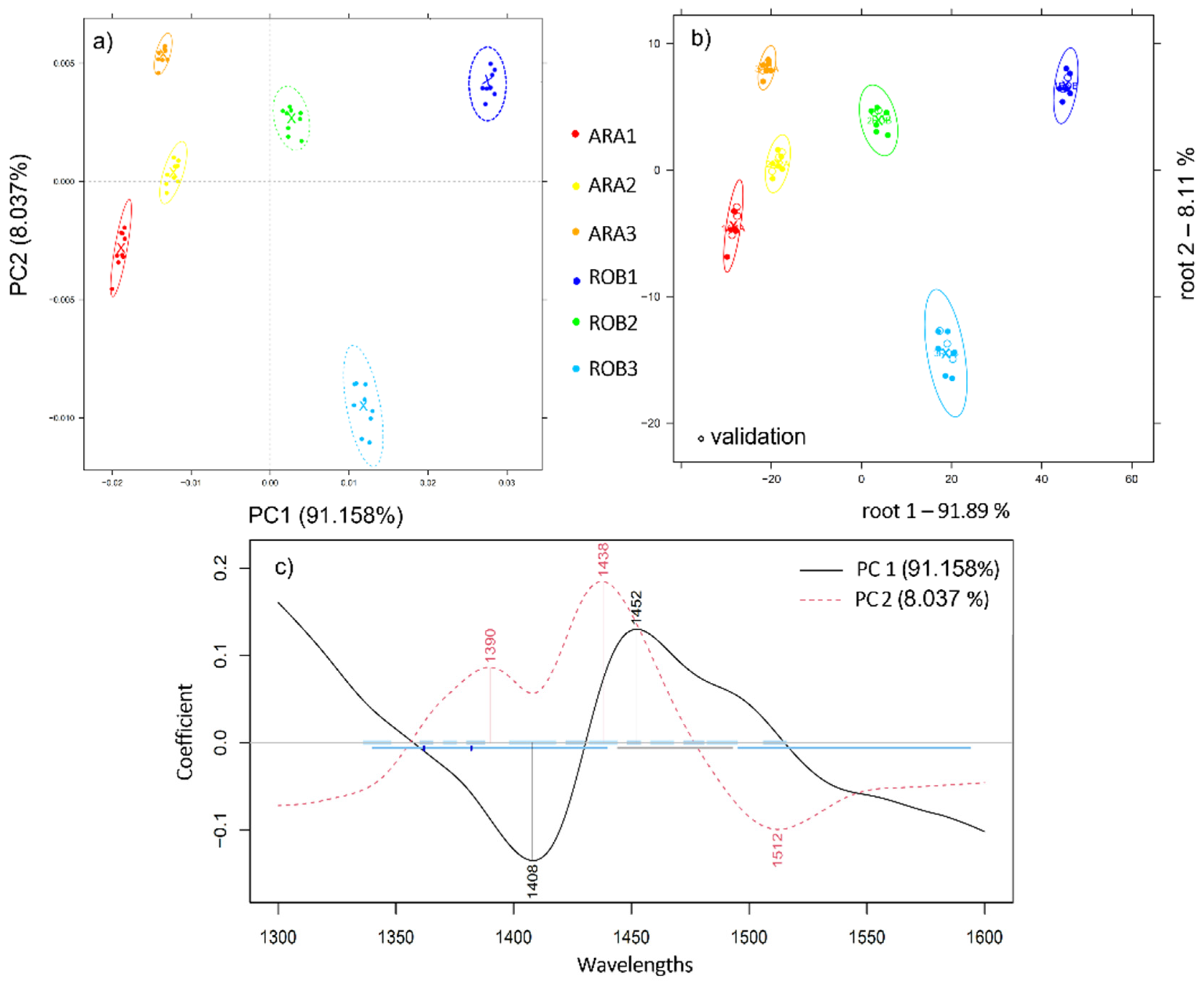
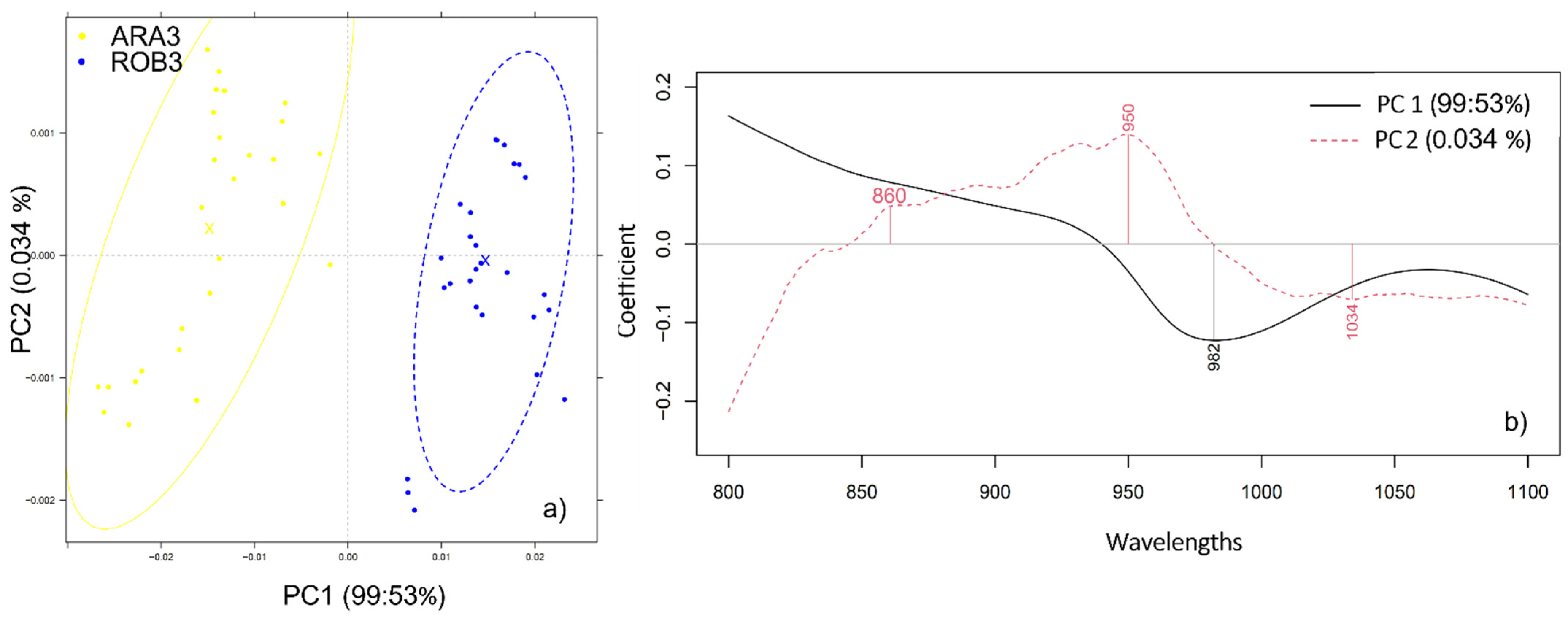
2.1. Varietal Discrimination of Pure Ground Coffee
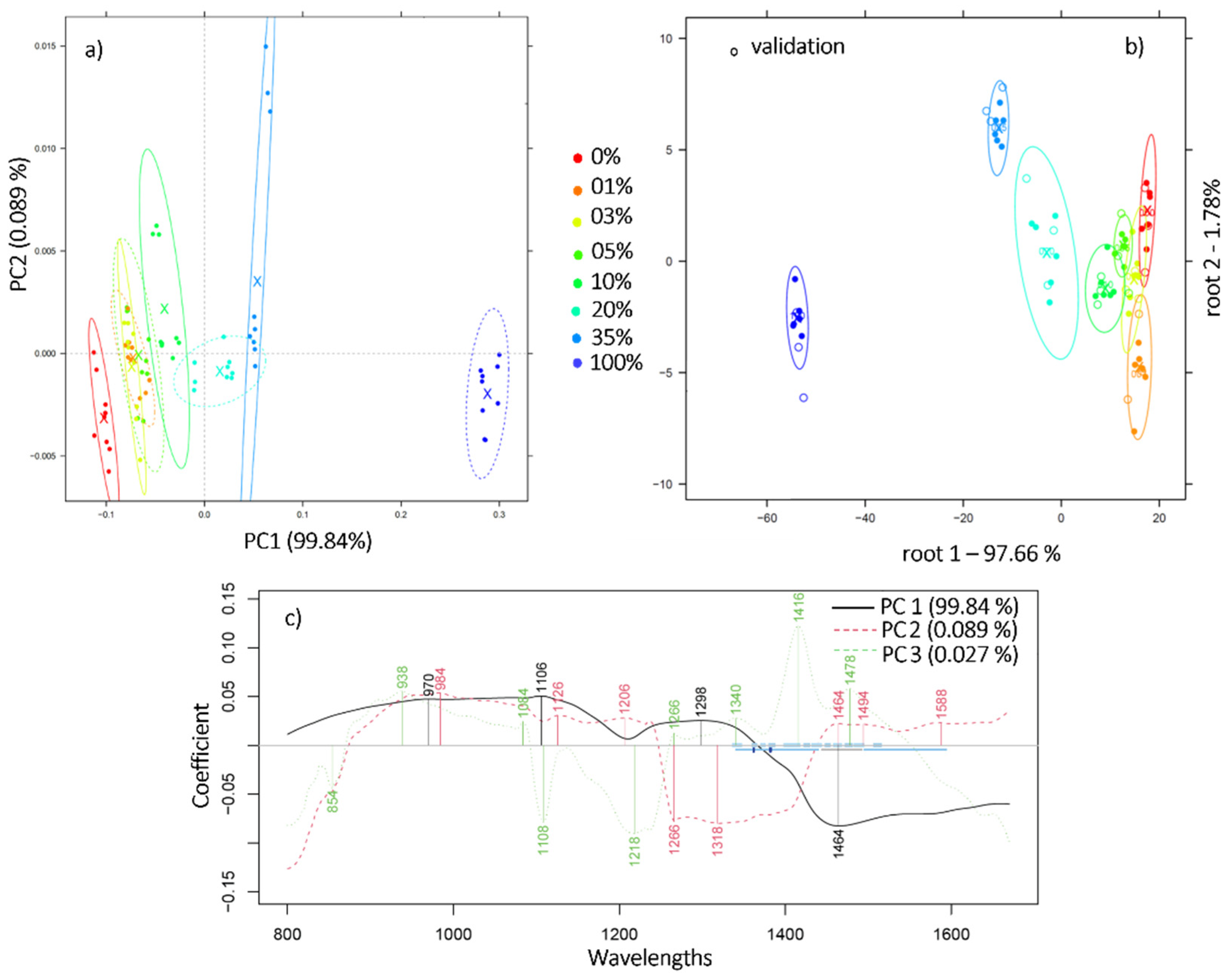
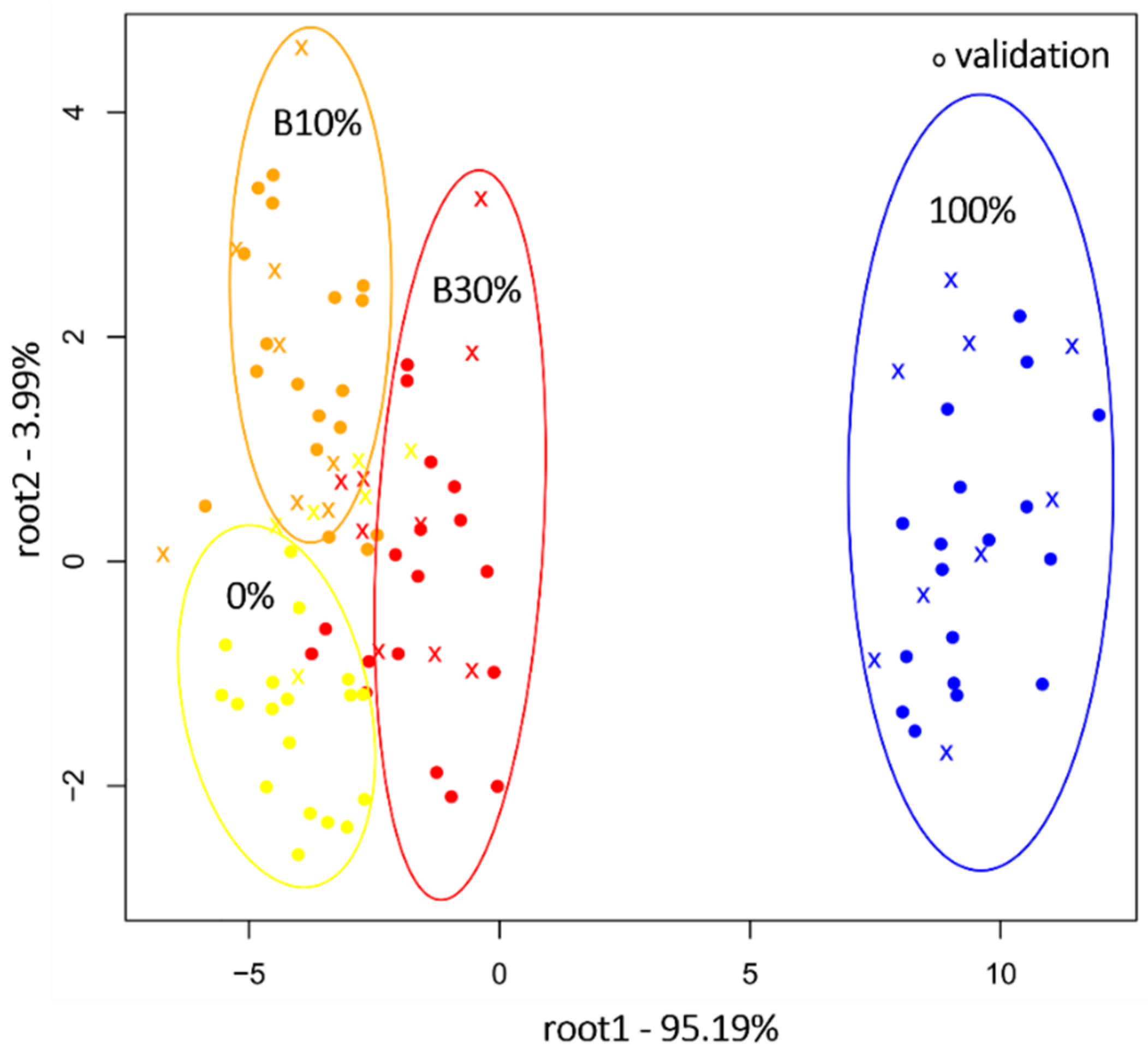
2.2. Near Infrared Analysis of Ground Coffee Mixtures
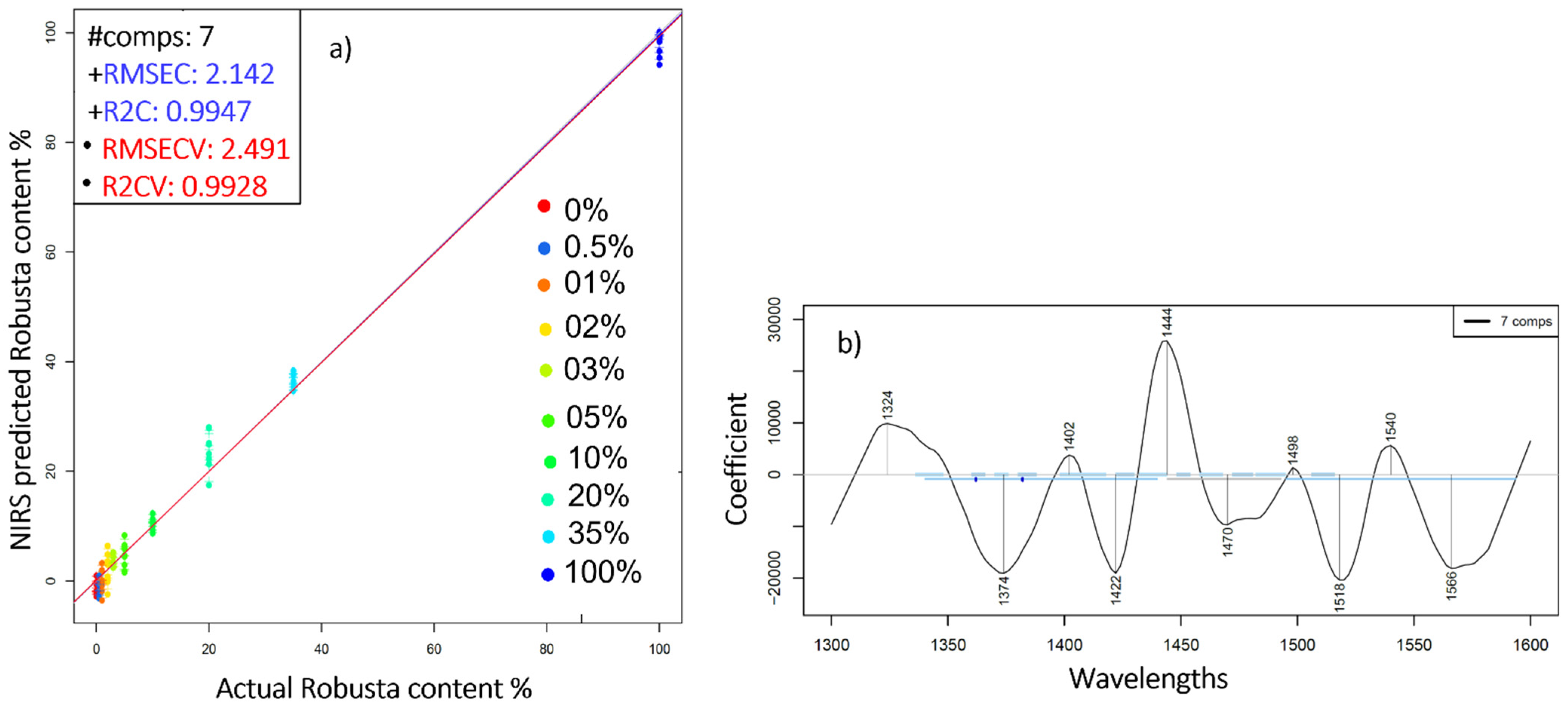
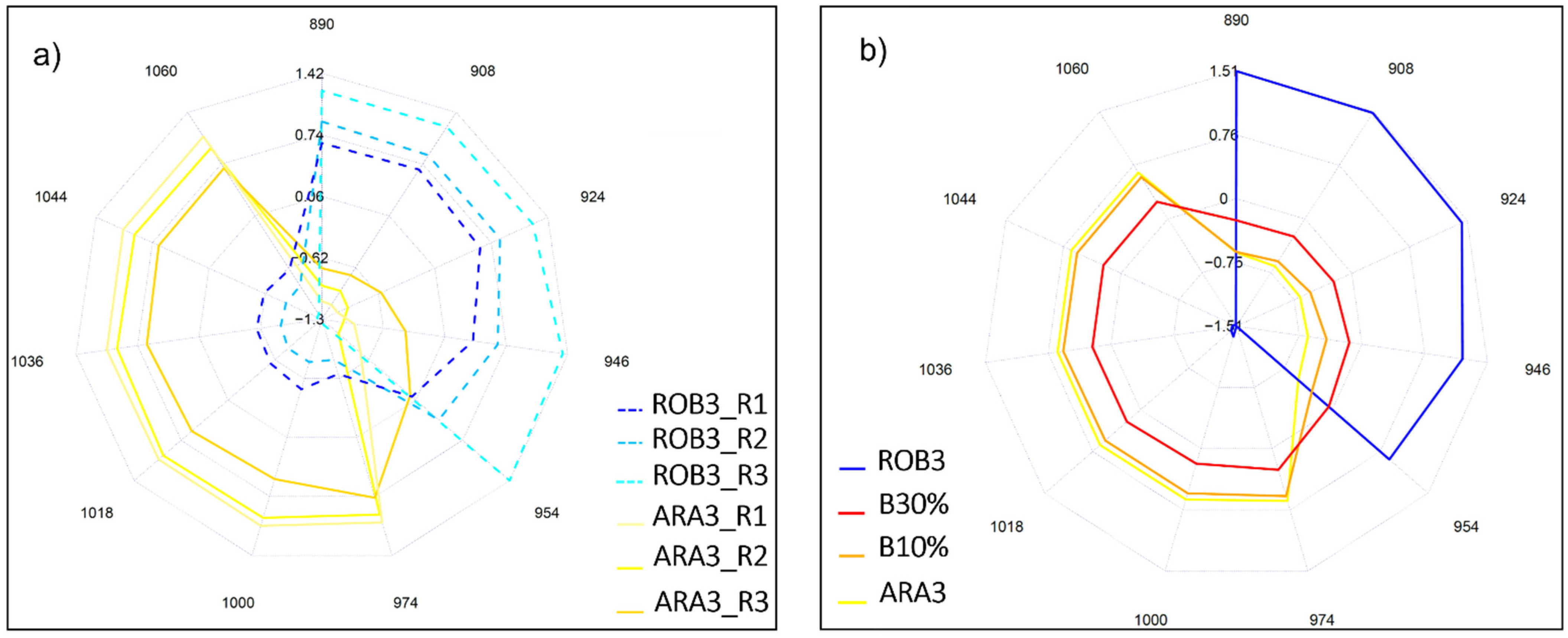
2.3. Near Infrared Analysis of Pure Liquid Coffee Extracts
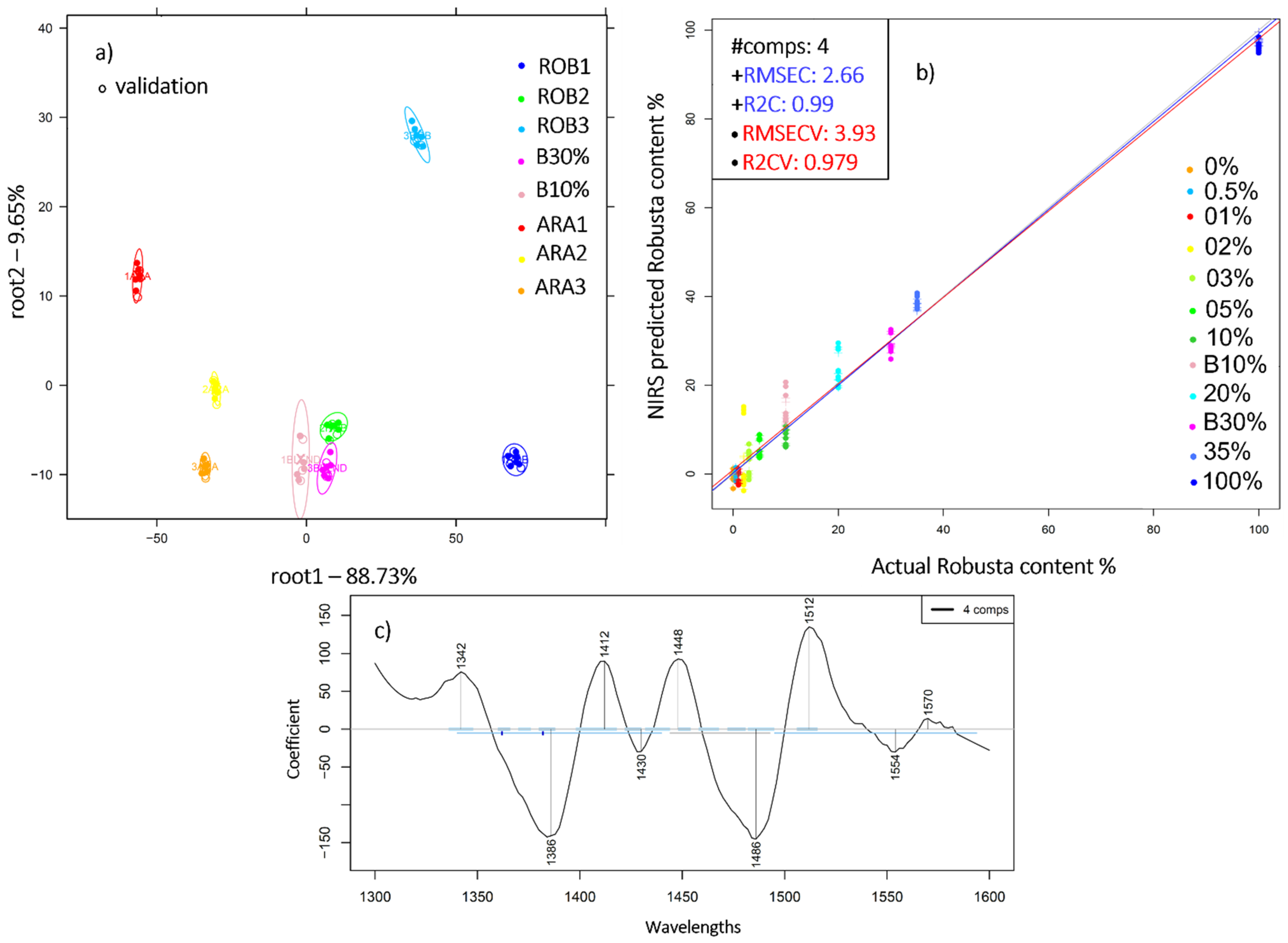
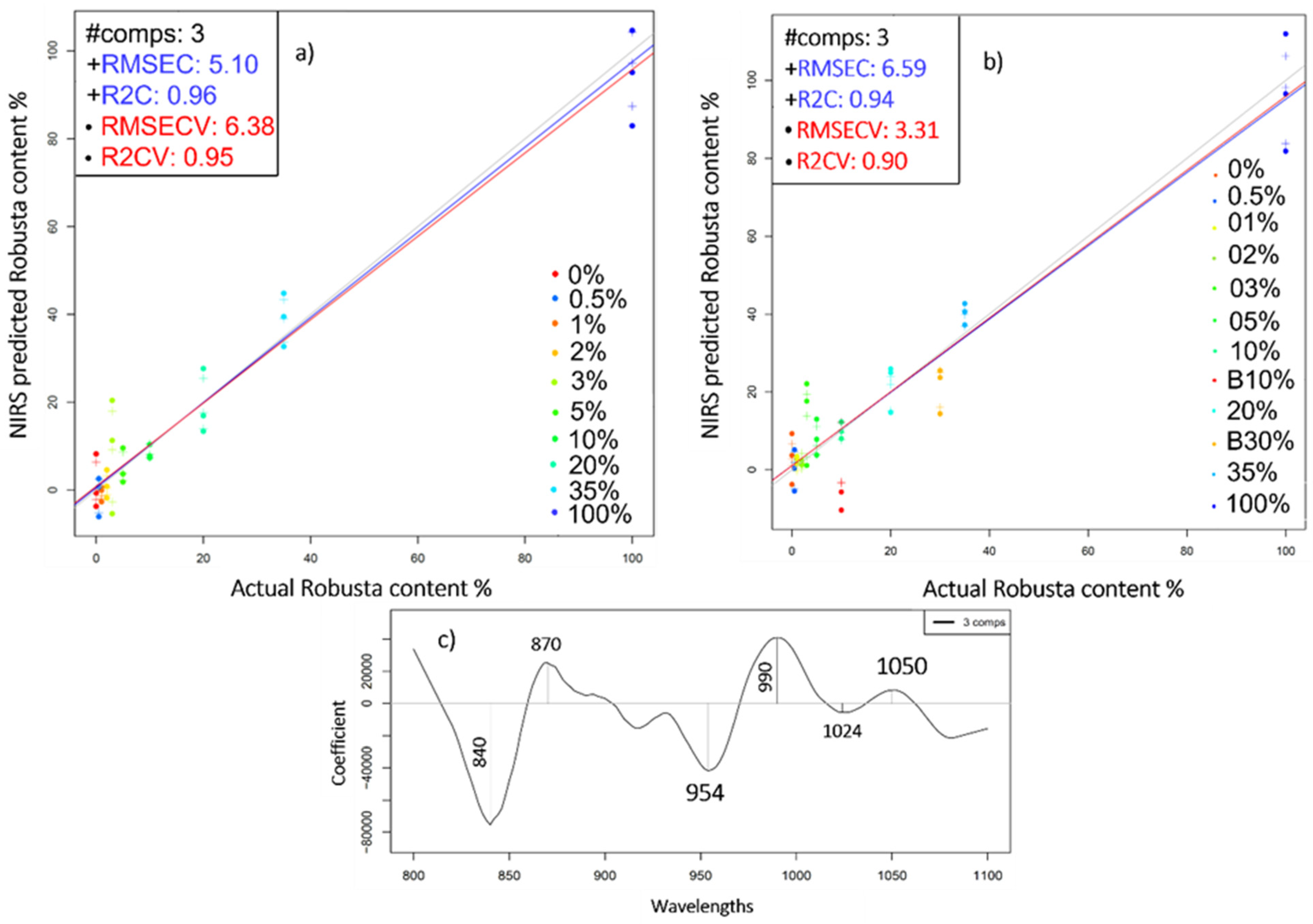
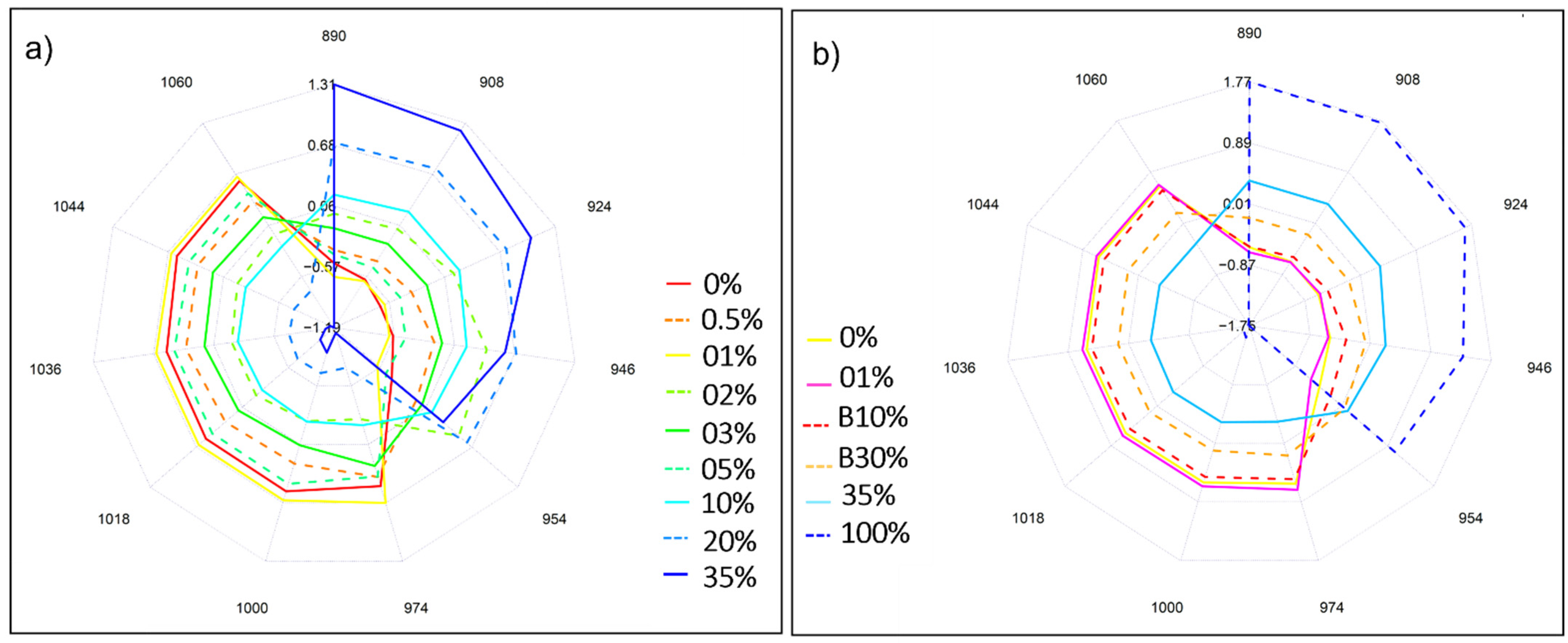
2.4. Near Infrared Analysis of Liquid Coffee Mixtures
3. Materials and Methods
3.1. Samples Preparation
3.2. Instrumental Analysis
3.3. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toci, A.T.; Farah, A.; Pezza, H.R. Critical Reviews in Analytical Chemistry Coffee Adulteration : More than Two Decades of Coffee Adulteration : More than Two Decades of Research. Crit. Rev. Anal. Chem. 2016, 46, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sezer, B.; Apaydin, H.; Bilge, G.; Boyaci, I.H. Coffee Arabica Adulteration: Detection of Wheat, Corn and Chickpea. Food Chem. 2018, 264, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.I.; Rossini, E.L.; Catelani, T.A.; Pezza, L.; Toci, A.T.; Pezza, H.R. Authentication of Roasted and Ground Coffee Samples Containing Multiple Adulterants Using NMR and a Chemometric Approach. Food Control 2020, 112, 107104. [Google Scholar] [CrossRef]
- Paola de Pádua Gandra, F.; Ribeiro Lima, A.; Batista Ferreira, E.; Cardoso De Angelis Pereira, M.; Gualberto Fonseca Alvarenga Pereira, R. Adding Adulterants to Coffee Reduces Bioactive Compound Levels and Antioxidant Activity. J. Food Nutr. Res. 2017, 5, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Wermelinger, T.; D’Ambrosio, L.; Klopprogge, B.; Yeretzian, C. Quantification of the Robusta Fraction in a Coffee Blend via Raman Spectroscopy: Proof of Principle. J. Agric. Food Chem. 2011, 59, 9074–9079. [Google Scholar] [CrossRef] [PubMed]
- Schievano, E.; Finotello, C.; De Angelis, E.; Mammi, S.; Navarini, L. Rapid Authentication of Coffee Blends and Quantification of 16-O-Methylcafestol in Roasted Coffee Beans by Nuclear Magnetic Resonance. J. Agric. Food Chem. 2014, 62, 12309–12314. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Member, S.; Dwulit, A. Recognition of Coffee Using Differential Electronic Nose. IEEE Trans. Instrum. Meas. 2012, 61, 1803–1810. [Google Scholar] [CrossRef]
- Pizarro, C.; Esteban-Díez, I.; González-Sáiz, J.M. Mixture Resolution According to the Percentage of Robusta Variety in Order to Detect Adulteration in Roasted Coffee by near Infrared Spectroscopy. Anal. Chim. Acta 2007, 585, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Spaniolas, S.; May, S.T.; Bennett, M.J.; Tucker, G.A. Authentication of Coffee by Means of PCR-RFLP Analysis and Lab-on-a-Chip Capillary Electrophoresis. J. Agric. Food Chem. 2006, 54, 7466–7470. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Arrieta, P.L.; Mendoza, J.M. Analysis of Coffee Adulterated with Roasted Corn and Roasted Soybean Using Voltammetric Electronic Tongue. Acta Sci. Pol. Technol. Aliment. 2019, 18, 35–41. [Google Scholar] [PubMed]
- Daniel, D.; Lopes, F.S.; dos Santos, V.B.; do Lago, C.L. Detection of Coffee Adulteration with Soybean and Corn by Capillary Electrophoresis-Tandem Mass Spectrometry. Food Chem. 2018, 243, 305–310. [Google Scholar] [CrossRef]
- Forchetti, D.A.P.; Poppi, R.J. Detection and Quantification of Adulterants in Roasted and Ground Coffee by NIR Hyperspectral Imaging and Multivariate Curve Resolution. Food Anal. Methods 2020, 13, 44–49. [Google Scholar] [CrossRef]
- Song, H.Y.; Jang, H.W.; Debnath, T.; Lee, K.G. Analytical Method to Detect Adulteration of Ground Roasted Coffee. Int. J. Food Sci. Technol. 2019, 54, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Suhandy, D.; Yulia, M. The Quantification of Adulteration in Arabica Coffee Using UV-Visible Spectroscopy in Combination with Two Different PLS Regressions. Aceh Int. J. Sci. Technol. 2017, 6, 59–67. [Google Scholar] [CrossRef]
- Muncan, J.; Tsenkova, R. Aquaphotomics-From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef] [Green Version]
- Atanassova, S.; Naydenova, N.; Kolev, T.; Iliev, T.; Mihaylova, G. Near Infrared Spectroscopy and Aquaphotomics for Monitoring Changes during Yellow Cheese Ripening. Agric. Sci. Technol. 2016, 3, 390. [Google Scholar]
- Kovacs, Z.; Bázár, G.; Oshima, M.; Shigeoka, S.; Tanaka, M.; Furukawa, A.; Nagai, A.; Osawa, M.; Itakura, Y.; Tsenkova, R. Water Spectral Pattern as Holistic Marker for Water Quality Monitoring. Talanta 2015, 147, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Muncan, J.; Tei, K.; Tsenkova, R. Real-Time Monitoring of Yogurt Fermentation Process by Aquaphotomics near-Infrared Spectroscopy. Sensors 2021, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Bázár, G.; Romvári, R.; Szabó, A.; Somogyi, T.; Éles, V.; Tsenkova, R. NIR Detection of Honey Adulteration Reveals Differences in Water Spectral Pattern. Food Chem. 2016, 194, 873–880. [Google Scholar] [CrossRef]
- Cui, X.; Liu, X.; Yu, X.; Cai, W.; Shao, X. Water Can Be a Probe for Sensing Glucose in Aqueous Solutions by Temperature Dependent near Infrared Spectra. Anal. Chim. Acta 2017, 957, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Cappai, C.; Franco, G.; Barzaghi, S.; Stellari, A.; Maria, T.; Cattaneo, P. Vibrational Spectroscopy and Aquaphotomics Holistic Approach to Determine Chemical Compounds Related to Sustainability in Soil pro Fi Les. Comput. Electron. Agric. 2019, 159, 92–96. [Google Scholar] [CrossRef]
- Mcglone, A.; Kaur, H.; Ku, R. Investigating Aquaphotomics for Temperature-Independent Prediction of Soluble Solids Content of Pure Apple Juice. J. Near Infrared Spectrosc. 2020, 28, 103–112. [Google Scholar]
- Matija, L.R.; Tsenkova, R.N.; Miyazaki, M.; Banba, K.; Muncan, J.S. Aquagrams: Water Spectral Pattern as Characterization of Hydrogenated Nanomaterial. FME Trans. 2012, 40, 51–56. [Google Scholar]
- Giraudo, A.; Grassi, S.; Savorani, F.; Gavoci, G.; Casiraghi, E.; Geobaldo, F. Determination of the Geographical Origin of Green Coffee Beans Using NIR Spectroscopy and Multivariate Data Analysis. Food Control 2019, 99, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Esteban-Díez, I.; González-Sáiz, J.M.; Pizarro, C. Prediction of Sensory Properties of Espresso from Roasted Coffee Samples by Near-Infrared Spectroscopy. Anal. Chim. Acta 2004, 525, 171–182. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and Traceability in Beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Ferreira, M.M.C.; Salva, T.J.G. Chemometric Models for the Quantitative Descriptive Sensory Analysis of Arabica Coffee Beverages Using near Infrared Spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, A.; Naumann, M.; Morlein, D.; Pawelzik, E. Reliable Discrimination of Green Coffee Beans Species: A Comparison of UV-Vis-Based Determination of Caffeine and Chlorogenic Acid with Non-Targeted near-Infrared Spectroscopy. Foods 2020, 9, 788. [Google Scholar] [CrossRef]
- Wu, D.; He, Y.; Feng, S. Short-Wave near-Infrared Spectroscopy Analysis of Major Compounds in Milk Powder and Wavelength Assignment. Anal. Chim. Acta 2008, 610, 232–242. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. The Lipid Fraction of the Coffee Bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Assis, C.; Oliveira, L.S.; Sena, M.M. Variable Selection Applied to the Development of a Robust Method for the Quantification of Coffee Blends Using Mid Infrared Spectroscopy. Food Anal. Methods 2018, 11, 578–588. [Google Scholar] [CrossRef]
- Tsenkova, R.; Muncan, J.; Pollner, B.; Kovacs, Z. Essentials of Aquaphotomics and Its Chemometrics Approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2002; Volume 6. [Google Scholar]
- Kovacs, Z.; Pollner, B.; Bazar, G.; Muncan, J.; Tsenkova, R. A Novel Tool for Visualization of Water Molecular Structure and Its Changes, Expressed on the Scale of Temperature Influence. Molecules 2020, 25, 2234. [Google Scholar] [CrossRef] [PubMed]
| Validation Accuracy % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robusta-to-Arabica Ratio | |||||||||||
| 0% | 0.5% | 1% | 2% | 3% | 5% | 10% | 20% | 35% | 100% | ||
| Robusta-to-Arabica ratio | 0% | 66.74 | 8.03 | 0 | 7.44 | 0 | 11.11 | 11.11 | 0 | 0 | 0 |
| 0.5% | 14.79 | 51.92 | 7.45 | 7.44 | 14.78 | 7.44 | 7.44 | 0 | 3.67 | 0 | |
| 1% | 0 | 3.96 | 70.41 | 0 | 7.44 | 0 | 0 | 11.11 | 0 | 0 | |
| 2% | 0 | 8.03 | 0 | 81.44 | 7.44 | 0 | 0 | 0 | 0 | 0 | |
| 3% | 3.67 | 20.02 | 3.67 | 0 | 55.56 | 7.44 | 3.67 | 0 | 0 | 0 | |
| 5% | 3.67 | 0 | 3.67 | 3.67 | 11.11 | 66.67 | 3.67 | 7.44 | 3.67 | 0 | |
| 10 % | 7.45 | 8.03 | 0 | 0 | 3.67 | 3.67 | 66.67 | 7.44 | 3.67 | 0 | |
| 20% | 3.67 | 0 | 14.79 | 0 | 0 | 3.67 | 7.44 | 70.33 | 3.67 | 0 | |
| 35% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.67 | 85.32 | 0 | |
| 100% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouadi, B.; Vitalis, F.; Bodor, Z.; Zinia Zaukuu, J.-L.; Kertesz, I.; Kovacs, Z. NIRS and Aquaphotomics Trace Robusta-to-Arabica Ratio in Liquid Coffee Blends. Molecules 2022, 27, 388. https://doi.org/10.3390/molecules27020388
Aouadi B, Vitalis F, Bodor Z, Zinia Zaukuu J-L, Kertesz I, Kovacs Z. NIRS and Aquaphotomics Trace Robusta-to-Arabica Ratio in Liquid Coffee Blends. Molecules. 2022; 27(2):388. https://doi.org/10.3390/molecules27020388
Chicago/Turabian StyleAouadi, Balkis, Flora Vitalis, Zsanett Bodor, John-Lewis Zinia Zaukuu, Istvan Kertesz, and Zoltan Kovacs. 2022. "NIRS and Aquaphotomics Trace Robusta-to-Arabica Ratio in Liquid Coffee Blends" Molecules 27, no. 2: 388. https://doi.org/10.3390/molecules27020388
APA StyleAouadi, B., Vitalis, F., Bodor, Z., Zinia Zaukuu, J.-L., Kertesz, I., & Kovacs, Z. (2022). NIRS and Aquaphotomics Trace Robusta-to-Arabica Ratio in Liquid Coffee Blends. Molecules, 27(2), 388. https://doi.org/10.3390/molecules27020388










