The Enteric Glia and Its Modulation by the Endocannabinoid System, a New Target for Cannabinoid-Based Nutraceuticals?
Abstract
1. Introduction
2. The Enteric Nervous System
2.1. Enteric Neurons
2.2. Enteric Glial Cells
2.2.1. EGCs and Intercellular Communication
2.2.2. EGCs and the Intestinal Barrier
2.2.3. EGCs and GI Motility
2.2.4. EGCs and Immune System Cross-Talk
2.2.5. EGCs and Visceral Sensitivity
2.2.6. EGCs and Altered GI Functions
3. The Endocannabinoid System
3.1. The Endocannabinoid System in the Gastrointestinal Tract
3.2. EGCs and the ECS
3.3. Nutraceuticals Acting on the ECS with Potential Effects on EGCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 15d-PGJ2 | 15-deoxy-Δ12,14-prostaglandin J2 |
| 2-AG | 2-arachidonoyl glycerol |
| 2-AGE | 2-arachidonoyl glyceryl ether |
| 5-FU | 5-fluorouracil |
| ABHD | α,β-hydrolase |
| ACE2 | angiotensin converting enzyme 2 |
| ACh | acetylcholine |
| AD | Alzheimer’s disease |
| AEA | anandamide, N-arachidonoyl ethanolamine |
| ANS | autonomic nervous system |
| ATP | adenosine triphosphate |
| BDNF | brain derived neurotrophic factor |
| BR1 | bradykinin receptor 1 |
| Ca2+ | calcium |
| cAMP | cyclic adenosine monophosphate |
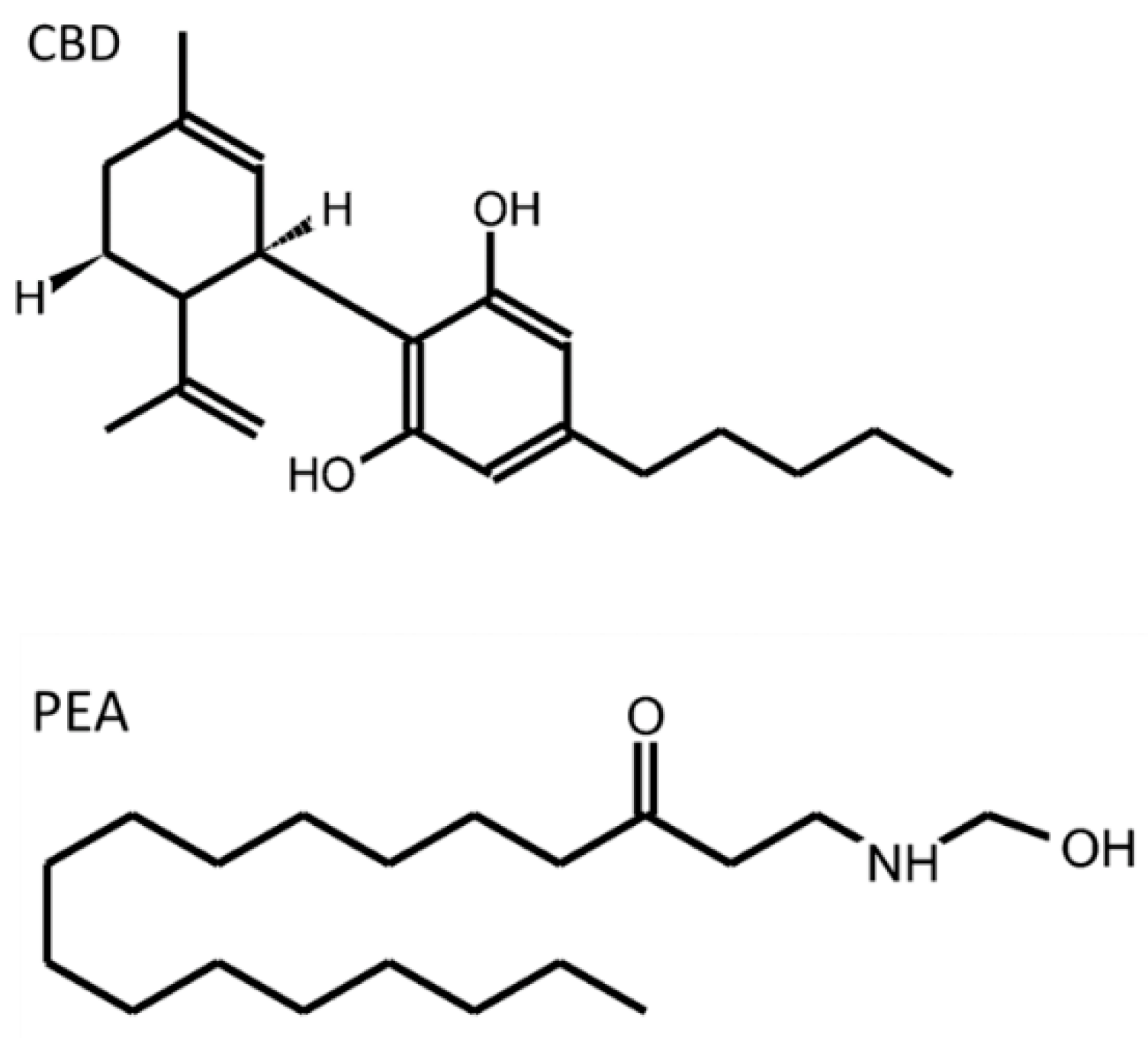
| CBD | cannabidiol |
| CD | Crohn’s disease |
| ChAT | choline acetyltransferase |
| CNS | central nervous system |
| COX | cyclooxygenase |
| CRD | colorectal distension |
| Cx43 | connexin 43 |
| DNBS | 2,4-dinitrobenzene sulfonic acid |
| DRG | dorsal root ganglion, dorsal root ganglia |
| DSS | dextran sulfate sodium |
| ECB | endocannabinoid |
| ECC | enterochromaffin cell |
| ECS | endogenous cannabinoid system, endocannabinoid system |
| EGC | enteric glial cell |
| ENS | enteric nervous system |
| ET-B | endothelin-1 receptor B |
| FAAH | fatty acid amide hydrolase |
| GABA | gamma amino butyric acid |
| GAT2 | GABA transporter |
| GDNF | glial cell-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| GI | gastrointestinal |
| GLP-2 | glucagon-like peptide 2 |
| GPCR | G-protein coupled receptor |
| GPR55 | G protein-coupled receptor 55 |
| GSH | glutathione |
| HA | haemagglutinin |
| HFD | high-fat diet |
| HIV | human immunodeficiency virus |
| IBD | inflammatory bowel disease |
| IBS-C | irritable bowel syndrome with constipation |
| IBS-D | irritable bowel syndrome with diarrhea |
| IBS-M | mixed or alternating irritable bowel syndrome |
| IBS | irritable bowel syndrome |
| ICC | interstitial cell of Cajal |
| IFN | interferon |
| IL-1R | interleukin 1 receptor |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| IPAN | intrinsic primary afferent neuron |
| KO | knock-out |
| LPI | lysophosphatidylinositol |
| LPS | lipopolysaccharide |
| MAGL | monoacylglycerol lipase |
| M-CSF | macrophage colony-stimulating factor |
| MCP1 | monocyte chemotactic protein 1 |
| MHC | major histocompatibility complex |
| MPO | myeloperoxidase |
| mRNA | messenger ribonucleic acid |
| NADA | N-arachidonoyl dopamine |
| NF-κB | nuclear factor kappa B |
| NGF | nerve growth factor |
| nNOS | neuronal nitric oxide synthase |
| NO | nitric oxide |
| O-AEA | O-arachidonoyl ethanolamine |
| OEA | oleoylethanolamide |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| Pdk1 | pyruvate dehydrogenase lipoamide kinase isozyme 1 |
| PEA | palmitoylethanolamide |
| PGE2 | prostaglandin E2 |
| PI-IBS | post-infectious irritable bowel syndrome |
| PI3K/Akt | phosphatidylinositol 3-kinase/protein kinase B signaling pathway |
| PKC | protein kinase C |
| PPAR | peroxisome proliferator-activated receptor |
| proEGF | proepidermal growth factor |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SP | substance P |
| TACE | tumor necrosis factor (TNF)-a converting enzyme |
| TGF | transforming growth factor |
| TLR | toll-like receptor |
| TMRPSS2 | transmembrane protease serine 2 |
| TNBS | 2,4,6-trinitrobenzene sulfonic acid |
| TNF | tumor necrosis factor |
| TrkA | tropomyosin receptor kinase A |
| TrkB | tropomyosin receptor kinase B |
| TRPV1 | transient receptor potential channel of subfamily V member 1 |
| UC | ulcerative colitis |
| VH | visceral hypersensitivity |
| VIP | vasoactive intestinal peptide |
| WIN | WIN 55, 212-2 |
References
- Soty, M.; Gautier-Stein, A.; Rajas, F.; Mithieux, G. Gut-Brain Glucose Signaling in Energy Homeostasis. Cell Metab. 2017, 25, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The Enteric Nervous System; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Labanski, A.; Langhorst, J.; Engler, H.; Elsenbruch, S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointes-tinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology 2020, 111, 104501. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Vanden Berghe, P. Functional circuits and signal processing in the enteric nervous system. Cell Mol. Life Sci. 2020, 77, 4505–4522. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef] [PubMed]
- Spear, E.T.; Mawe, G.M. Enteric neuroplasticity and dysmotility in inflammatory disease: Key players and possible therapeutic targets. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G853–G861. [Google Scholar] [CrossRef] [PubMed]
- Morales-Soto, W.; Gulbransen, B.D. Enteric Glia: A New Player in Abdominal Pain. CMGH 2019, 7, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, D.; Loris, E.; Maas-Omlor, S.; Huang, W.; Scheller, A.; Kirchhoff, F.; Schäfer, K.H. Enteric Glia: S100, GFAP, and Beyond. Anat. Rec. 2019, 302, 1333–1344. [Google Scholar] [CrossRef]
- DeFelice, S.L. The Nutraceutical Revolution: Fueling a Powerful, New International Market; The Foundation for Innovation in Medicine: Mountside, NJ, USA, 1989. [Google Scholar]
- Sharkey, K.A.; Wiley, J.W. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology 2016, 151, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Hasenoehrl, C.; Taschler, U.; Storr, M.; Schicho, R. The gastrointestinal tract-a central organ of cannabinoid signaling in health and disease. Neurogastroenterol. Motil. 2016, 28, 1765–1780. [Google Scholar] [CrossRef]
- Sałaga, M.; Abalo, R.; Fichna, J. Cannabis and Cannabinoids and the Effects on Gastrointestinal Function: An Overview. In Handbook of Cannabis and Related Pathologies; Treat; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 471–480. [Google Scholar]
- Uranga, J.A.; Vera, G.; Abalo, R. Cannabinoid pharmacology and therapy in gut disorders. Biochem. Pharmacol. 2018, 157, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Meletis, C. The important role of the endocannabinoid system and the endocannabinoidome in gut health. Altern. Ther. Health Med. 2019, 25, 24–27. [Google Scholar] [PubMed]
- Szczepaniak, A.; Fichna, J. What role do cannabinoids have in modern medicine as gastrointestinal anti-inflammatory drugs? Expert. Opin. Pharmacother. 2020, 21, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.B.; Neuman, M.G. Cannabis and the gastrointestinal tract. J. Pharm. Pharm. Sci. 2020, 23, 304–313. [Google Scholar] [CrossRef]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Pharmacology, Clinical Effects, and Therapeutic Potential of Cannabinoids for Gastrointestinal and Liver Diseases. Clin. Gastroenterol. Hepatol. 2020, 19, 1748–1758. [Google Scholar] [CrossRef]
- DeVuono, M.V.; Parker, L.A. Cannabinoid Hyperemesis Syndrome: A Review of Potential Mechanisms. Cannabis Cannabinoid Res. 2020, 5, 132–144. [Google Scholar] [CrossRef]
- Russo, E.B.; Spooner, C.; May, L.; Leslie, R.; Whiteley, V.L. Cannabinoid Hyperemesis Syndrome Survey and Genomic Investigation. Cannabis Cannabinoid Res. 2022, 7, 336–344. [Google Scholar] [CrossRef]
- Kakish, D.; Alaoudi, M.; Welch, B.; Fan, D.; Meghpara, M.; Mandava, N.; Kumthekar, N. Small bowel intussusception in marijuana users. J. Surg. Case Rep. 2020, 2020, rjaa335. [Google Scholar] [CrossRef]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Kunze, W.A.; Bornstein, J.C.; Furness, J.B. Electrical mapping of the projections of intrinsic primary afferent neurones to the mucosa of the guinea-pig small intestine. Neurogastroenterol. Motil. 1998, 10, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Kunze, W.A.; Bertrand, P.P.; Clerc, N.; Bornstein, J.C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 1998, 54, 1–18. [Google Scholar] [CrossRef]
- Lomax, A.E.; Furness, J.B. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000, 302, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Neal, K.B.; Bornstein, J.C. Targets of myenteric interneurons in the guinea-pig small intestine. Neurogastroenterol. Motil. 2008, 20, 566–575. [Google Scholar] [CrossRef]
- Grubišić, V.; Gulbransen, B.D. Enteric glia: The most alimentary of all glia. J. Physiol. 2017, 595, 557–570. [Google Scholar] [CrossRef]
- Rosenberg, H.J.; Rao, M. Enteric glia in homeostasis and disease: From fundamental biology to human pathology. iScience 2021, 24, 102863. [Google Scholar] [CrossRef]
- Hanani, M.; Reichenbach, A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 1994, 278, 153–160. [Google Scholar] [CrossRef]
- Ferri, G.L.; Probert, L.; Cocchia, D.; Michetti, F.; Marangos, P.J.; Polak, J.M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 1982, 297, 409–410. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980, 286, 736–737. [Google Scholar] [CrossRef]
- Hoff, S.; Zeller, F.; von Weyhern, C.W.; Wegner, M.; Schemann, M.; Michel, K.; Rühl, A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 2008, 509, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Zamir, O.; Baluk, P. Glial cells in the guinea pig myenteric plexus are dye coupled. Brain Res. 1989, 497, 245–249. [Google Scholar] [CrossRef]
- Christofi, F.L.; Wood, J.D. Effects of PACAP on morphologically identified myenteric neurons in guinea pig small bowel. Am. J. Physiol.-Gastrointest. Liver Physiol. 1993, 264, G414–G421. [Google Scholar] [CrossRef] [PubMed]
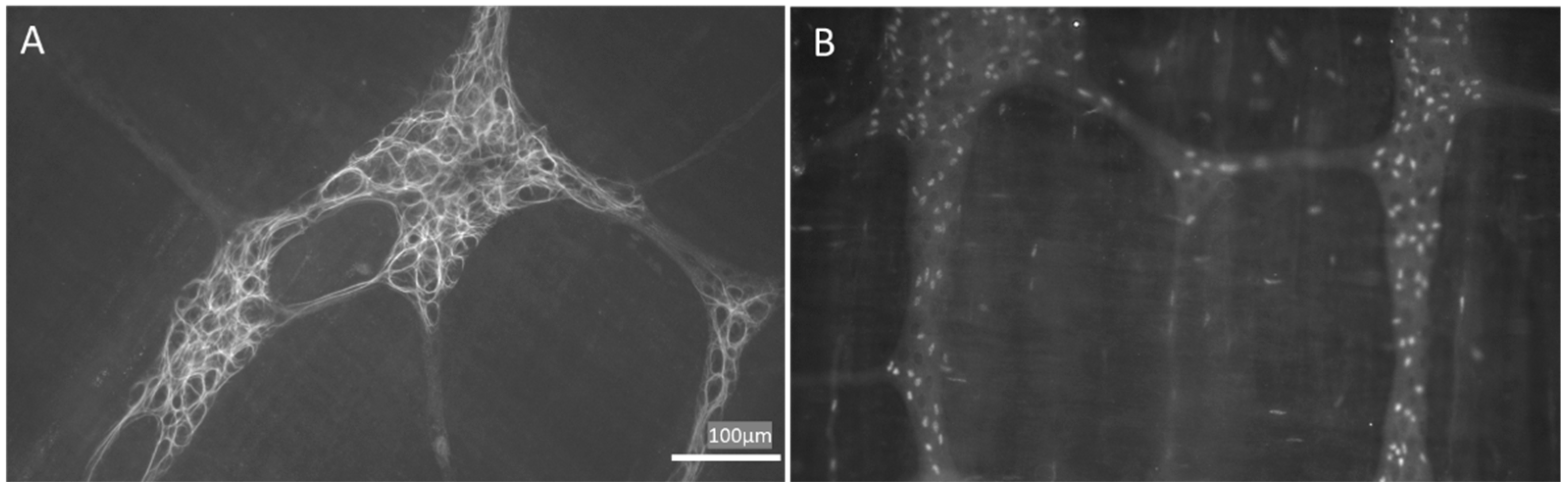
- López-Gómez, L.; Szymaszkiewicz, A.; Zielińska, M.; Abalo, R. Nutraceuticals and enteric glial cells. Molecules 2021, 26, 3762. [Google Scholar] [CrossRef]
- Liu, Y.A.; Chung, Y.C.; Pan, S.T.; Shen, M.Y.; Hou, Y.C.; Peng, S.J.; Pasricha, P.J.; Tang, S.C. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol. Motil. 2013, 25, e324–e338. [Google Scholar] [CrossRef]
- Bohórquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. Enteroendocrine cell-Enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881. [Google Scholar] [CrossRef]
- De Heuvel, E.; Wallace, L.; Sharkey, K.A.; Sigalet, D.L. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-7 signaling. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E994–E1005. [Google Scholar] [CrossRef] [PubMed]
- Maudlej, N.; Hanani, M. Modulation of dye coupling among glial cells in the myenteric and submucosal plexuses of the guinea pig. Brain Res. 1992, 578, 94–98. [Google Scholar] [CrossRef]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric Glia Regulate Intestinal Barrier Function and Inflammation Via Release of S-Nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef]
- Bach-Ngohou, K.; Mahé, M.M.; Aubert, P.; Abdo, H.; Boni, S.; Bourreille, A.; Denis, M.G.; Lardeux, B.; Neunlist, M.; Masson, D. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-? 12,14 -prostaglandin J2. J. Physiol. 2010, 588, 2533–2544. [Google Scholar] [CrossRef]
- Neunlist, M.; Aubert, P.; Bonnaud, S.; Van Landeghem, L.; Coron, E.; Wedel, T.; Naveilhan, P.; Ruhl, A.; Lardeux, B.; Savidge, T.; et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, 231–241. [Google Scholar] [CrossRef]
- Van Landeghem, L.; Chevalier, J.; Mahé, M.M.; Wedel, T.; Urvil, P.; Derkinderen, P.; Savidge, T.; Neunlist, M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G976–G987. [Google Scholar] [CrossRef] [PubMed]
- Aubé, A.C.; Cabarrocas, J.; Bauer, J.; Philippe, D.; Aubert, P.; Doulay, F.; Liblau, R.; Galmiche, J.P.; Neunlist, M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 2006, 55, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Y.; Fernandez, E.; Keenan, C.M.; Ho, W.; Oland, L.D.; Tibbles, L.A.; Schemann, M.; MacNaughton, W.K.; Rühl, A.; Sharkey, K.A. Role of enteric glia in intestinal physiology: Effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 291, G912–G927. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.L.; Grants, I.; Needleman, B.J.; Williams, K.C.; Soghomonyan, S.; Turco, F.; Christofi, F.L. Gliomodulation of Neuronal and Motor Behavior in the Human GI Tract. Gastroenterology 2015, 148, S-18. [Google Scholar] [CrossRef]
- Fields, R.D.; Ni, Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci. Signal. 2010, 3, ra-73. [Google Scholar] [CrossRef]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.G.; Savidge, T.C.; Freeman, T.C.; Cox, H.J.; Campbell, E.A.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Fulminant jejuno-ileitis following ablation of enteric gila in adult transgenic mice. Cell 1998, 93, 189–201. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Astrocyte-like glia in the peripheral nervous system: An immunohistochemical study of enteric glia. J. Neurosci. 1983, 3, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef]
- Bradley, J.S.; Parr, E.J.; Sharkey, K.A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 1997, 289, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is crohn’s disease a gliopathy? Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G1–G11. [Google Scholar] [CrossRef]
- Von Boyen, G.B.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Nerve Growth Factor Secretion in Cultured Enteric Glia Cells is Modulated by Proinflammatory Cytokines. J. Neuroendocrinol. 2006, 18, 820–825. [Google Scholar] [CrossRef]
- Von Boyen, G.B.; Degenkolb, N.; Hartmann, C.; Adler, G.; Steinkamp, M. The endothelin axis influences enteric glia cell functions. Med. Sci. Monit. 2010, 16, 161–167. [Google Scholar]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ohta, T.; Ito, S. Interleukin-1beta enhances the action of bradykinin in rat myenteric neurons through up-regulation of glial B1 receptor expression. Neuroscience 2008, 151, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Turco, F.; Sarnelli, G.; Cirillo, C.; Palumbo, I.; De Giorgi, F.; D’Alessandro, A.; Cammarota, M.; Giuliano, M.; Cuomo, R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2014, 63, 105–115. [Google Scholar] [CrossRef]
- Geboes, K.; Rutgeerts, P.; Ectors, N.; Mebis, J.; Penninckx, F.; Vantrappen, G.; Desmet, V.J. Major histocompatibility class II expression on the small intestinal nervous system in Crohn’s disease. Gastroenterology 1992, 103, 439–447. [Google Scholar] [CrossRef]
- Koretz, K.; Momburg, F.; Otto, H.F.; Möller, P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn’s disease. Am. J. Pathol. 1987, 129, 493–502. [Google Scholar] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Calì, G.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112. [Google Scholar] [CrossRef] [PubMed]
- Von Boyen, G.B.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 2004, 53, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, C.; Schick, M.A.; Wollborn, J.; Heider, A.; Scholz, C.J.; Cecil, A.; Niesler, B.; Hirrlinger, J.; Walles, H.; Metzger, M. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS ONE 2016, 11, e0151335. [Google Scholar]
- Cirillo, C.; Sarnelli, G.; Turco, F.; Mango, A.; Grosso, M.; Aprea, G.; Masone, S.; Cuomo, R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol. Motil. 2011, 23, e372–e382. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Cirillo, C.; Sarnelli, G.; De Filippis, D.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; Petruzzelli, R.; Grosso, M.; Izzo, P.; et al. Enteric Glial-Derived S100B Protein Stimulates Nitric Oxide Production in Celiac Disease. Gastroenterology 2007, 133, 918–925. [Google Scholar] [CrossRef]
- Burns, A.; Pachnis, V. Development of the enteric nervous system: Bringing together cells, signals and genes. Neurogastroenterol. Motil. 2009, 21, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Von Boyen, G.B.; Steinkamp, M.; Geerling, I.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: A key to the regulation of epithelial apoptosis in crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.A.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020, 32, 108100. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Seguella, L.; Vincenzi, M.; Parisio, C.; Micheli, L.; Toti, A.; Corpetti, C.; Del Re, A.; Squillace, S.; Maftei, D.; et al. Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines 2021, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Sarosi, G.A.; Barnhart, D.C.; Turner, D.J.; Mulholland, M.W. Capacitative Ca2+ entry in enteric glia induced by thapsigargin and extracellular ATP. Am. J. Physiol.-Gastrointest. Liver Physiol. 1998, 275, G550–G555. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, V.; Parpura, V. Two modes of enteric gliotransmission differentially affect gut physiology. Glia 2017, 65, 699–711. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.; Grubišić, V.; Fried, D.; Gomez-Suarez, R.A.; Leinninger, G.M.; Sévigny, J.; Parpura, V.; Gulbransen, B.D. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 2014, 146, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.C.; Mulholland, M.W. Enteric Glia Exhibit P2U Receptors that Increase Cytosolic Calcium by a Phospholipase C-Dependent Mechanism. J. Neurochem. 2002, 66, 604–612. [Google Scholar] [CrossRef]
- Van Nassauw, L.; Costagliola, A.; Van Op den Bosch, J.; Cecio, A.; Vanderwinden, J.M.; Burnstock, G.; Timmermans, J.P. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton. Neurosci. Basic Clin. 2006, 126, 299–306. [Google Scholar] [CrossRef]
- Vanderwinden, J.M.; Timmermans, J.P.; Schiffmann, S.N. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 2003, 312, 149–154. [Google Scholar] [CrossRef]
- Nasser, Y.; Keenan, C.M.; Ma, A.C.; McCafferty, D.M.; Sharkey, K.A. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia 2007, 55, 859–872. [Google Scholar] [CrossRef]
- Bornstein, J.C. Purinergic mechanisms in the control of gastrointestinal motility. Purinergic. Signal. 2008, 4, 197–212. [Google Scholar] [CrossRef]
- Gulbransen, B.D.; Bains, J.S.; Sharkey, K.A. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J. Neurosci. 2010, 30, 6801–6809. [Google Scholar] [CrossRef]
- Boesmans, W.; Hao, M.M.; Fung, C.; Li, Z.; Van den Haute, C.; Tack, J.; Pachnis, V.; Vanden Berghe, P. Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia 2019, 67, 1167–1178. [Google Scholar] [CrossRef]
- Giaroni, C.; Zanetti, E.; Chiaravalli, A.M.; Albarello, L.; Dominioni, L.; Capella, C.; Lecchini, S.; Frigo, G. Evidence for a glutamatergic modulation of the cholinergic function in the human enteric nervous system via NMDA receptors. Eur. J. Pharmacol. 2003, 476, 63–69. [Google Scholar] [CrossRef]
- Fletcher, E.L.; Clark, M.J.; Furness, J.B. Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res. 2002, 308, 339–346. [Google Scholar] [CrossRef]
- Galligan, J.J.; LePard, K.J.; Schneider, D.A.; Zhou, X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 97–103. [Google Scholar] [CrossRef]
- Aoki, E.; Semba, R.; Kashiwamata, S. Evidence for the presence of l-arginine in the glial components of the peripheral nervous system. Brain Res. 1991, 559, 159–162. [Google Scholar] [CrossRef]
- Nagahama, M.; Semba, R.; Tsuzuki, M.; Aoki, E. L-arginine immunoreactive enteric glial cells in the enteric nervous system of rat ileum. NeuroSignals 2001, 10, 336–340. [Google Scholar] [CrossRef]
- Gulbransen, B.D.; Sharkey, K.A. Purinergic Neuron-to-Glia Signaling in the Enteric Nervous System. Gastroenterology 2009, 136, 1349–1358. [Google Scholar] [CrossRef]
- Gulbransen, B.D.; Christofi, F.L. Are We Close to Targeting Enteric Glia in Gastrointestinal Diseases and Motility Disorders? Gastroenterology 2018, 155, 245–251. [Google Scholar] [CrossRef]
- Phillips, R.J.; Kieffer, E.J.; Powley, T.L. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat. Embryol. 2004, 209, 19–30. [Google Scholar] [CrossRef]
- Baudry, C.; Reichardt, F.; Marchix, J.; Bado, A.; Schemann, M.; des Varannes, S.B.; Neunlist, M.; Moriez, R. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: Involvement of leptin and glial cell line-derived neurotrophic factor. J. Physiol. 2012, 590, 533–544. [Google Scholar] [CrossRef]
- Schoffen, J.P.; Santi Rampazzo, A.P.; Cirilo, C.P.; Zapater, M.C.; Vicentini, F.A.; Comar, J.F.; Bracht, A.; Natali, M.R. Food restriction enhances oxidative status in aging rats with neuro-protective effects on myenteric neuron populations in the proximal colon. Exp. Gerontol. 2014, 51, 54–64. [Google Scholar] [CrossRef]
- Cornet, A.; Savidge, T.C.; Cabarrocas, J.; Deng, W.L.; Colombel, J.F.; Lassmann, H.; Desreumaux, P.; Liblau, R.S. Enterocolitis induced by autoimmune targeting of enteric glial cells: A possible mechanism in Crohn’s disease? Proc. Natl. Acad. Sci. USA 2001, 98, 13306–13311. [Google Scholar] [CrossRef] [PubMed]
- Von Boyen, G.B.; Schulte, N.; Pflüger, C.; Spaniol, U.; Hartmann, C.; Steinkamp, M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Bassotti, G.; Nascimbeni, R.; Antonelli, E.; Cadei, M.; Fisogni, S.; Salerni, B.; Geboes, K. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol. Motil. 2008, 20, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Villanacci, V.; Cathomas, G.; Maurer, C.A.; Fisogni, S.; Cadei, M.; Baron, L.; Morelli, A.; Valloncini, E.; Salerni, B. Enteric neuropathology of the terminal ileum in patients with intractable slow-transit constipation. Hum. Pathol. 2006, 37, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Iantorno, G.; Bassotti, G.; Kogan, Z.; Lumi, C.M.; Cabanne, A.M.; Fisogni, S.; Varrica, L.M.; Bilder, C.R.; Munoz, J.P.; Liserre, B.; et al. The Enteric Nervous System in Chagasic and Idiopathic Megacolon. Am. J. Surg. Pathol. 2007, 31, 460–468. [Google Scholar] [CrossRef]
- Bassotti, G.; Villanacci, V.; Maurer, C.A.; Fisogni, S.; Di Fabio, F.; Cadei, M.; Morelli, A.; Panagiotis, T.; Cathomas, G.; Salerni, B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut 2006, 55, 41–46. [Google Scholar] [CrossRef]
- Stoffels, B.; Hupa, K.J.; Snoek, S.A.; van Bree, S.; Stein, K.; Schwandt, T.; Vilz, T.O.; Lysson, M.; Veer, C.V.; Kummer, M.P.; et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 2014, 146, 176–187. [Google Scholar] [CrossRef]
- Lilli, N.L.; Quénéhervé, L.; Haddara, S.; Brochard, C.; Aubert, P.; Rolli-Derkinderen, M.; Durand, T.; Naveilhan, P.; Hardouin, J.B.; De Giorgio, R.; et al. Glioplasticity in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13232. [Google Scholar] [CrossRef]
- Fettucciari, K.; Ponsini, P.; Gioè, D.; Macchioni, L.; Palumbo, C.; Antonelli, E.; Coaccioli, S.; Villanacci, V.; Corazzi, L.; Marconi, P.; et al. Enteric glial cells are susceptible to Clostridium difficile toxin B. Cell Mol. Life Sci. 2017, 74, 1527–1551. [Google Scholar] [CrossRef]
- Hoehner, J.C.; Wester, T.; Påhlman, S.; Olsen, L. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology 1996, 110, 756–767. [Google Scholar] [CrossRef]
- Wang, P.; Du, C.; Chen, F.X.; Li, C.Q.; Yu, Y.B.; Han, T.; Akhtar, S.; Zuo, X.L.; Tan, X.D.; Li, Y.Q. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci. Rep. 2016, 6, 20320. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Li, M.; Li, L.X.; Sun, Y.Y.; Zhang, W.X.; Zhao, D.Y.; Li, Y.Q. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol. Motil. 2018, 30, e13227. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, S.; Hagbom, M.; Rajan, A.; Loitto, V.; Persson, B.D.; Allard, A.; Nordgren, J.; Sharma, S.; Magnusson, K.E.; Arnberg, N.; et al. Interaction of Human Enterochromaffin Cells with Human Enteric Adenovirus 41 Leads to Serotonin Release and Subsequent Activation of Enteric Glia Cells. J. Virol. 2018, 92, 26–44. [Google Scholar] [CrossRef]
- Da Cunha Franceschi, R.; Nardin, P.; Machado, C.V.; Tortorelli, L.S.; Martinez-Pereira, M.A.; Zanotto, C.; Gonçalves, C.A.; Zancan, D.M. Enteric glial reactivity to systemic LPS administration: Changes in GFAP and S100B protein. Neurosci. Res. 2017, 119, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; D’Antongiovanni, V.; Pellegrini, C.; Fornai, M.; Benvenuti, L.; di Carlo, A.; van den Wijngaard, R.; Caputi, V.; Cerantola, S.; Giron, M.C. Colonic dysmotility associated with high-fat diet induced obesity: Role of enteric glia. FASEB J. 2020, 34, 5512–5524. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, G.; He, F.; Zhang, L.; Yang, K.; Yu, H.; Zhou, J.; Gan, H. MicroRNA 375 modulates hyperglycemia-induced enteric glial cell apoptosis and Diabetes-induced gastrointestinal dysfunction by targeting Pdk1 and repressing PI3K/Akt pathway. Sci. Rep. 2018, 8, 12681. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, D.; Li, C.; He, W.X.; Zhang, C.L.; Chang, M.J. Enteric glial cell activation protects enteric neurons from damage due to diabetes in part via the promotion of neurotrophic factor release. Neurogastroenterol. Motil. 2018, 30, e13368. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Gondha, C.; Sutliff, R.; Parsadanian, A.; Mwangi, S.; Sitaraman, S.V.; Srinivasan, S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Investig. 2006, 116, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Bruley des Varannes, S.; Naveilhan, P. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef]
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.R.; Arantes, C.P.; Muras, A.G.; Nomizo, R.; Brentani, R.R.; Martins, V.R. Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. J. Neurochem. 2007, 103, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Ferrucci, M.; Lazzeri, G.; Paparelli, A.; Fornai, F. Transmission of prions within the gut and toward the central nervous system. Prion 2011, 5, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kujala, P.; Raymond, C.R.; Romeijn, M.; Godsave, S.F.; van Kasteren, S.I.; Wille, H.; Prusiner, S.B.; Mabbott, N.A.; Peters, P.J. Prion uptake in the gut: Identification of the first uptake and replication sites. PLoS Pathog. 2011, 7, e1002449. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Gigli, S.; Pesce, M.; Bruzzese, E.; D’Alessandro, A.; Cirillo, C.; di Cerbo, A.; Cuomo, R.; Seguella, L.; et al. HIV-1 Tat-induced diarrhea evokes an enteric glia-dependent neuroinflammatory response in the central nervous system. Sci. Rep. 2017, 7, 7735. [Google Scholar] [CrossRef] [PubMed]
- Guedia, J.; Brun, P.; Bhave, S.; Fitting, S.; Kang, M.; Dewey, W.L.; Hauser, K.F.; Akbarali, H. HIV-1 Tat exacerbates lipopolysaccharide-induced cytokine release via TLR4 signaling in the enteric nervous system. Sci. Rep. 2016, 6, 31203. [Google Scholar] [CrossRef]
- Deffner, F.; Scharr, M.; Klingenstein, S.; Klingenstein, M.; Milazzo, A.; Scherer, S.; Wagner, A.; Hirt, B.; Mack, A.F.; Neckel, P. Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV2. Front. Neuroanat. 2020, 14, 596439. [Google Scholar] [CrossRef]
- Bhave, S.; Gade, A.; Kang, M.; Hauser, K.F.; Dewey, W.L.; Akbarali, H.I. Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB J. 2017, 31, 2649–2660. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.M.; Stojanovska, V.; Rahman, A.A.; McQuade, R.M.; Senior, P.V.; Nurgali, K. Effects of Oxaliplatin Treatment on the Enteric Glial Cells and Neurons in the Mouse Ileum. J. Histochem. Cytochem. 2016, 64, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, V.; McQuade, R.M.; Miller, S.; Nurgali, K. Effects of Oxaliplatin Treatment on the Myenteric Plexus Innervation and Glia in the Murine Distal Colon. J. Histochem. Cytochem. 2018, 66, 723–736. [Google Scholar] [CrossRef]
- Costa, D.; Bon-Frauches, A.C.; Silva, A.; Lima-Júnior, R.; Martins, C.S.; Leitão, R.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway. Sci. Rep. 2019, 9, 665. [Google Scholar] [CrossRef]
- Nogueira, L.T.; Costa, D.V.; Gomes, A.S.; Martins, C.S.; Silva, A.M.; Coelho-Aguiar, J.M.; Castelucci, P.; Lima-Júnior, R.C.; Leitão, R.F.; Moura-Neto, V.; et al. The involvement of mast cells in the irinotecan-induced enteric neurons loss and reactive gliosis. J. Neuroinflammation 2017, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Nardini, P.; Pini, A.; Bessard, A.; Duchalais, E.; Niccolai, E.; Neunlist, M.; Vannucchi, M.G. GLP-2 Prevents Neuronal and Glial Changes in the Distal Colon of Mice Chronically Treated with Cisplatin. Int. J. Mol. Sci. 2020, 21, 8875. [Google Scholar] [CrossRef]
- Lin, T.; Zhang, W.; Garrido, R.; Segura, B.; Hu, Y.; Guzman, E.; Mulholland, M.Y. The role of the cytoskeleton in capacitative calcium entry in myenteric glia. Neurogastroenterol. Motil. 2003, 15, 277–287. [Google Scholar] [CrossRef][Green Version]
- Sheikh, N.K.; Dua, A. Cannabinoids. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Duggan, P.J. The chemistry of cannabis and cannabinoids. Aust. J. Chem. 2021, 74, 369–387. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Melck, D.; Bobrov, M.Y.; Gretskaya, N.M.; Bezuglov, V.V.; De Petrocellis, L.; Di Marzo, V. N-acyl-dopamines: Novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000, 351, 817–824. [Google Scholar] [CrossRef]
- Porter, A.C.; Sauer, J.M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R Soc. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef]
- Ahluwalia, J.; Urban, L.; Capogna, M.; Bevan, S.; Nagy, I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 2000, 100, 685–688. [Google Scholar] [CrossRef]
- Ahluwalia, J.; Urban, L.; Bevan, S.; Capogna, M.; Nagy, I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience 2002, 110, 747–753. [Google Scholar] [CrossRef]
- Binzen, U.; Greffrath, W.; Hennessy, S.; Bausen, M.; Saaler-Reinhardt, S.; Treede, R. Co-expression of the voltage-gated potassium channel Kv1.4 with transient receptor potential channels (TRPV1 and TRPV2) and the cannabinoid receptor CB1 in rat dorsal root ganglion neurons. Neuroscience 2006, 14, 527–539. [Google Scholar] [CrossRef]
- Ralevic, V.; Kendall, D. Cannabinoid Modulation of Perivascular Sympathetic and Sensory Neurotransmission. Curr. Vasc. Pharmacol. 2009, 7, 15–25. [Google Scholar] [CrossRef]
- Weller, K.; Reeh, P.W.; Sauer, S.K. TRPV1, TRPA1, and CB1 in the isolated vagus nerve-Axonal chemosensitivity and control of neuropeptide release. Neuropeptides 2011, 45, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; de Petrocellis, L.; Pryce, G.; Baker, D.; Guglielmotti, V.; Di Marzo, V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 2006, 139, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; De Petrocellis, L.; de Novellis, V.; Moriello, A.S.; Petrosino, S.; Palazzo, E.; Rossi, F.S.; Woodward, D.F.; Di Marzo, V. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br. J. Pharmacol. 2007, 150, 766–781. [Google Scholar] [CrossRef]
- Micale, V.; Cristino, L.; Tamburella, A.; Petrosino, S.; Leggio, G.M.; Drago, F.; Di Marzo, V. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology 2009, 34, 593–606. [Google Scholar] [CrossRef]
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endo-cannabinoid receptors. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef]
- Domenicali, M.; Ros, J.; Fernández-Varo, G.; Cejudo-Martín, P.; Crespo, M.; Morales-Ruiz, M.; Briones, A.M.; Campistol, J.M.; Arroyo, V.; Vila, E.; et al. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: Role of cannabinoid and vanilloid receptors. Gut 2005, 54, 522–527. [Google Scholar] [CrossRef]
- Lu, T.; Newton, C.; Perkins, I.; Friedman, H.; Klein, T.W. Role of cannabinoid receptors in Delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow-derived dendritic cells infected with Legionella pneumophila. Eur. J. Pharmacol. 2006, 532, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Cavuoto, P.; McAinch, A.J.; Hatzinikolas, G.; Janovská, A.; Game, P.; Wittert, G.A. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res. Commun. 2007, 364, 105–110. [Google Scholar] [CrossRef]
- Rossi, F.; Siniscalco, D.; Luongo, L.; De Petrocellis, L.; Bellini, G.; Petrosino, S.; Torella, M.; Santoro, C.; Nobili, B.; Perrotta, S.; et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone 2009, 44, 476–484. [Google Scholar] [CrossRef]
- Tóth, B.I.; Dobrosi, N.; Dajnoki, A.; Czifra, G.; Oláh, A.; Szöllosi, A.G.; Juhász, I.; Sugawara, K.; Paus, R.; Bíró, T. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J. Investig. Dermatol. 2011, 131, 1095–1104. [Google Scholar] [CrossRef]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agrò, A.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef]
- Oka, S.; Nakajima, K.; Yamashita, A.; Kishimoto, S.; Sugiura, T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007, 362, 928–934. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Feledziak, M.; Lambert, D.M.; Marchand-Brynaert, J.; Muccioli, G.G. Inhibitors of the endocannabinoid-degrading enzymes, or how to increase endocannabinoid’s activity by preventing their hydrolysis. Recent Pat. CNS Drug Discov. 2012, 7, 49–70. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011, 111, 5899–5921. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.; Davison, J.S.; Sharkey, K.A. Review article: Endocannabinoids and their receptors in the enteric nervous system. Aliment. Pharmacol. Ther. 2005, 22, 667–683. [Google Scholar] [CrossRef]
- Donnerer, J.; Liebmann, I. Effect of CB1 Ligands on Neurogenic and Myogenic Contractile Responses in the Guinea-Pig Ileum. Pharmacology 2018, 101, 330–336. [Google Scholar] [CrossRef]
- Karwad, M.A.; Couch, D.G.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. The role of CB1 in intestinal permeability and inflammation. FASEB J. 2017, 31, 3267–3277. [Google Scholar] [CrossRef]
- Wright, K.; Rooney, N.; Feeney, M.; Tate, J.; Robertson, D.; Welham, M.; Ward, S. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005, 129, 437–453. [Google Scholar] [CrossRef]
- Grill, M.; Hasenoehrl, C.; Kienzl, M.; Kargl, J.; Schicho, R. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem. Cell Biol. 2019, 151, 5–20. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10 -/- mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 256–267. [Google Scholar] [CrossRef]
- Ke, P.; Shao, B.Z.; Xu, Z.Q.; Wei, W.; Han, B.Z.; Chen, X.W.; Su, D.F.; Liu, C. Activation of cannabinoid receptor 2 ameliorates dss-induced colitis through inhibiting nlrp3 inflammasome in macrophages. PLoS ONE 2016, 11, e0155076. [Google Scholar] [CrossRef]
- Storr, M.A.; Keenan, C.M.; Zhang, H.; Patel, K.D.; Makriyannis, A.; Sharkey, K.A. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm. Bowel Dis. 2009, 15, 1678–1685. [Google Scholar] [CrossRef]
- D’Argenio, G.; Valenti, M.; Scaglione, G.; Cosenza, V.; Sorrentini, I.; Di Marzo, V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006, 20, 568–570. [Google Scholar] [CrossRef]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011, 25, 2711–2721. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, A.; Misicka, A.; Sacharczuk, M.; Fichna, J. Modulation of the endocannabinoid system by the fatty acid amide hydrolase, monoacylglycerol and diacylglycerol lipase inhibitors as an attractive target for secretory diarrhoea therapy. J. Physiol. Pharmacol. 2017, 68, 591–596. [Google Scholar]
- Abalo, R.; Chen, C.; Vera, G.; Fichna, J.; Thakur, G.A.; López-Pérez, A.E.; Makriyannis, A.; Martín-Fontelles, M.I.; Storr, M. In vitro and non-invasive in vivo effects of the cannabinoid-1 receptor agonist AM841 on gastrointestinal motor function in the rat. Neurogastroenterol. Motil. 2015, 27, 1721–1735. [Google Scholar] [CrossRef]
- Abalo, R.; Cabezos, P.A.; Vera, G.; López-Miranda, V.; Herradón, E.; Martín-Fontelles, M.I. Cannabinoid-induced delayed gastric emptying is selectively increased upon intermittent administration in the rat: Role of CB1 receptors. Neurogastroenterol. Motil. 2011, 23, 457-e177. [Google Scholar] [CrossRef]
- Abalo, R.; Cabezos, P.A.; Vera, G.; Fernández-Pujol, R.; Martín, M.I. The cannabinoid antagonist SR144528 enhances the acute effect of WIN 55,212-2 on gastrointestinal motility in the rat. Neurogastroenterol. Motil. 2010, 22, 694-e206. [Google Scholar] [CrossRef] [PubMed]
- Abalo, R.; Cabezos, P.A.; López-Miranda, V.; Vera, G.; González, C.; Castillo, M.; Fernández-Pujol, R.; Martín, M.I. Selective lack of tolerance to delayed gastric emptying after daily administration of WIN 55,212-2 in the rat. Neurogastroenterol. Motil. 2009, 21, 1002-e80. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ruano, S.; López-Pérez, A.E.; Girón, R.; Pérez-García, I.; Martín-Fontelles, M.I.; Abalo, R. Fluoroscopic characterization of colonic dysmotility associated to opioid and cannabinoid agonists in conscious rats. J. Neurogastroenterol. Motil. 2019, 25, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Fezza, F.; Capasso, R.; Bisogno, T.; Pinto, L.; Iuvone, T.; Esposito, G.; Mascolo, N.; Di Marzo, V.; Capasso, F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharmacol. 2001, 134, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Fichna, J.; Wood, J.T.; Papanastasiou, M.; Vadivel, S.K.; Oprocha, P.; Sałaga, M.; Sobczak, M.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K. Endocannabinoid and cannabinoid-like fatty acid amide levels correlate with pain-related symptoms in patients with IBS-D and IBS-C: A pilot study. PLoS ONE 2013, 8, e85073. [Google Scholar] [CrossRef]
- Park, J.M.; Choi, M.G.; Cho, Y.K.; Lee, I.S.; Kim, S.W.; Choi, K.Y.; Chung, I.S. Cannabinoid receptor 1 gene polymorphism and irritable bowel syndrome in the korean population: A hypothesis-generating study. J. Clin. Gastroenterol. 2011, 45, 45–49. [Google Scholar] [CrossRef]
- Camilleri, M.; Kolar, G.J.; Vazquez-Roque, M.I.; Carlson, P.; Burton, D.D.; Zinsmeister, A.R. Cannabinoid receptor 1 gene and irritable bowel syndrome: Phenotype and quantitative traits. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Carlson, P.; McKinzie, S.; Grudell, A.; Busciglio, I.; Burton, D.; Baxter, K.; Ryks, M.; Zinsmeister, A.R. Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am. J. Physiol. Liver Physiol. 2008, 294, G13–G19. [Google Scholar] [CrossRef] [PubMed]
- Abalo, R.; Uranga, J.A.; Pérez-García, I.; de Andrés, R.; Girón, R.; Vera, G.; López-Pérez, A.E.; Martín-Fontelles, M.I. May cannabinoids prevent the development of chemotherapy-induced diarrhea and intestinal mucositis? Experimental study in the rat. Neurogastroenterol. Motil. 2017, 29, e12952. [Google Scholar] [CrossRef]
- Vera, G.; López-Pérez, A.E.; Uranga, J.A.; Girón, R.; Martín-Fontelles, M.I.; Abalo, R. Involvement of Cannabinoid Signaling in Vincristine-Induced Gastrointestinal Dysmotility in the Rat. Front. Pharmacol. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, N.; Izzo, A.A.; Ligresti, A.; Costagliola, A.; Pinto, L.; Cascio, M.G.; Maffia, P.; Cecio, A.; Capasso, F.; Di Marzo, V. The endocannabinoid system and the molecular basis of paralytic ileus in mice. FASEB J. 2002, 16, 1973–1975. [Google Scholar] [CrossRef]
- Liu, Y.L.; Malik, N.; Sanger, G.J.; Friedman, M.I.; Andrews, P.L. Pica-A model of nausea? Species differences in response to cisplatin. Physiol. Behav. 2005, 85, 271–277. [Google Scholar] [CrossRef]
- Vera, G.; López-Pérez, A.E.; Martínez-Villaluenga, M.; Cabezos, P.A.; Abalo, R. X-ray analysis of the effect of the 5-HT3 receptor antagonist granisetron on gastrointestinal motility in rats repeatedly treated with the antitumoral drug cisplatin. Exp. Brain Res. 2014, 232, 2601–2612. [Google Scholar] [CrossRef]
- Vera, G.; Chiarlone, A.; Cabezos, P.A.; Pascual, D.; Martín, M.I.; Abalo, R. WIN 55,212-2 prevents mechanical allodynia but not alterations in feeding behaviour induced by chronic cisplatin in the rat. Life Sci. 2007, 81, 468–479. [Google Scholar] [CrossRef]
- Abalo, R.; Cabezos, P.A.; Vera, G.; López-Pérez, A.E.; Martín, M.I. Cannabinoids may worsen gastric dysmotility induced by chronic cisplatin in the rat. Neurogastroenterol. Motil. 2013, 25, 373-e292. [Google Scholar] [CrossRef]
- Perisetti, A. Cannabis hyperemesis syndrome: An update on the pathophysiology and management. Ann. Gastroenterol. 2020, 33, 571–578. [Google Scholar] [CrossRef]
- Abalo, R.; Martín-Fontelles, M.I. Cannabis, Cannabinoids, and Visceral Pain. In Handbook of Cannabis and Related Pathologies; Treat; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 439–449. [Google Scholar]
- Sanson, M.; Bueno, L.; Fioramonti, J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol. Motil. 2006, 18, 949–956. [Google Scholar] [CrossRef]
- Kikuchi, A.; Ohashi, K.; Sugie, Y.; Sugimoto, H.; Omura, H. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J. Pharmacol. Sci. 2008, 106, 219–224. [Google Scholar] [CrossRef]
- Brusberg, M.; Arvidsson, S.; Kang, D.; Larsson, H.; Lindström, E.; Martinez, V. CB1 receptors mediate the analgesic effects of cannabinoids on colorectal disten-sion-induced visceral pain in rodents. J. Neurosci. 2009, 29, 1554–1564. [Google Scholar] [CrossRef]
- Jones, R.C.W.; Xu, L.; Gebhart, G.F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J. Neurosci. 2005, 25, 10981–10989. [Google Scholar] [CrossRef]
- Ravnefjord, A.; Brusberg, M.; Kang, D.; Bauer, U.; Larsson, H.; Lindström, E.; Martinez, V. Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur. J. Pharmacol. 2009, 611, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Fan, J.; Kemmerer, E.S.; Evans, S.; Li, Y.; Wiley, J.W. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 2009, 58, 202–210. [Google Scholar] [CrossRef]
- Sakin, Y.S.; Dogrul, A.; Ilkaya, F.; Seyrek, M.; Ulas, U.H.; Gulsen, M.; Bagci, S. effect of FAAH, MAGL, and Dual FAAH/MAGL inhibition on inflammatory and colorectal distension-induced visceral pain models in Rodents. Neurogastroenterol. Motil. 2015, 27, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Lyubashina, O.A.; Sivachenko, I.B.; Panteleev, S.S. Supraspinal Mechanisms of Intestinal Hypersensitivity. Cell Mol. Neurobiol. 2020, 42, 389–441. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Alonso-Esteban, J.A.; Gallego-Barceló, P.; Garcia, P.; Abalo, R.; Castillo, M.D. Nutrition Security of Hemp for Human Consumption. In Sustainable Food Science: A Comprehensive Approach; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Sibaev, A.; Yüce, B.; Kemmer, M.; Van Nassauw, L.; Broedl, U.; Allescher, H.D.; Göke, B.; Timmermans, J.P.; Storr, M. Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G119–G128. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, A.; Galiazzo, G.; Giancola, F.; Tagliavia, C.; De Silva, M.; Pietra, M.; Fracassi, F.; Chiocchetti, R. Localization of cannabinoid and cannabinoid related receptors in the cat gastro-intestinal tract. Histochem. Cell Biol. 2020, 153, 339–356. [Google Scholar] [CrossRef]
- Galiazzo, G.; Giancola, F.; Stanzani, A.; Fracassi, F.; Bernardini, C.; Forni, M.; Pietra, M.; Chiocchetti, R. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPAR αin the canine gastrointestinal tract. Histochem. Cell Biol. 2018, 150, 187–205. [Google Scholar] [CrossRef]
- Galiazzo, G.; Tagliavia, C.; Giancola, F.; Rinnovati, R.; Sadeghinezhad, J.; Bombardi, C.; Grandis, A.; Pietra, M.; Chiocchetti, R. Localisation of Cannabinoid and Cannabinoid-Related Receptors in the Horse Ileum. J. Equine Vet. Sci. 2021, 104, 103688. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nishiyama, M.; Iizuka, S.; Suzuki, S.; Suzuki, N.; Aiso, S.; Nakahara, J. Transient receptor potential vanilloid 1-immunoreactive signals in murine enteric glial cells. World J. Gastroenterol. 2016, 22, 9752–9764. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Sarnelli, G.; Seguella, L.; Pesce, M.; Lu, J.; Gigli, S.; Bruzzese, E.; Lattanzi, R.; D’Alessandro, A.; Cuomo, R.; Steardo, L.; et al. HIV-1 Tat-induced diarrhea is improved by the PPARalpha agonist, palmitoylethanolamide, by suppressing the activation of enteric glia. J. Neuroinflammation 2018, 15, 94. [Google Scholar] [CrossRef]
- D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Benvenuti, L.; Di Salvo, C.; Flori, L.; Piccarducci, R.; Daniele, S.; Martelli, A.; Calderone, V.; et al. Palmitoylethanolamide Counteracts Enteric Inflammation and Bowel Motor Dysfunctions in a Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 748021. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.; Bigioni, I.; Mattioli, R.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Mariani, P.F.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Harpagophytum procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation. Pharmaceuticals 2022, 15, 457. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Fiorenzani, P.; Pessina, F.; Pinassi, J.; Aglianò, M.; Miragliotta, V.; Aloisi, A.M. The CB2 Agonist β-Caryophyllene in Male and Female Rats Exposed to a Model of Persistent Inflammatory Pain. Front. Neurosci. 2020, 14, 850. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Tutino, V.; Tafaro, A.; Bianco, G.; Guglielmi, E.; Caruso, M.G. Dietary olive oil induces cannabinoid CB2 receptor expression in adipose tissue of ApcMin/+ transgenic mice. Nutr. Healthy Aging 2016, 4, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Palani Kumar, M.K.; Halami, P.M.; Serva Peddha, M. Effect of Lactobacillus fermentum MCC2760-Based Probiotic Curd on Hypercholesterolemic C57BL6 Mice. ACS Omega 2021, 6, 7701–7710. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Goldsmith, J.R.; Carroll, I.M.; Barros, S.P.; Palsson, O.; Jobin, C.; Ringel, Y. Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain-a randomised clinical study. Aliment. Pharmacol. Ther. 2014, 40, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Son, D.B.; Choi, W.; Kim, M.; Go, E.J.; Jeong, D.; Park, C.K.; Kim, Y.H.; Lee, H.; Suh, J.W. Decursin alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells 2021, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Veigas, J.M.; Williams, P.J.; Halade, G.; Rahman, M.M.; Yoneda, T.; Fernandes, G. Fish oil concentrate delays sensitivity to thermal nociception in mice. Pharmacol. Res. 2011, 63, 377–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matta, J.A.; Miyares, R.L.; Ahern, G.P. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 2007, 578 Pt 2, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Dai, C.; Jiang, M. Mechanisms of Probiotic VSL#3 in a Rat Model of Visceral Hypersensitivity Involves the Mast Cell-PAR2-TRPV1 Pathway. Dig. Dis. Sci. 2019, 64, 1182–1192. [Google Scholar] [PubMed]
- Zhang, J.; Chen, B.; Liu, B.; Zhou, X.; Mu, J.; Wang, Q.; Zhao, X.; Yang, Z. Preventive Effect of Lactobacillus fermentum CQPC03 on Activated Carbon-Induced Consti-pation in ICR Mice. Medicina 2018, 54, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Suo, H.Y.; Qian, Y.; Li, G.J.; Liu, Z.H.; Li, J. Therapeutic effects of Lactobacillus casei Qian treatment in activated carbon-induced con-stipated mice. Mol. Med. Rep. 2015, 12, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burgos, A.; Wang, L.; McVey Neufeld, K.A.; Mao, Y.K.; Ahmadzai, M.; Janssen, L.J.; Stanisz, A.M.; Bienenstock, J.; Kunze, W.A. The TRPV1 channel in rodents is a major target for antinociceptive effect of the probiotic Lactobacillus reuteri DSM 17938. J. Physiol. 2015, 593, 3943–3957. [Google Scholar] [CrossRef] [PubMed]
- Bowen, K.J.; Kris-Etherton, P.M.; Shearer, G.C.; West, S.G.; Reddivari, L.; Jones, P. Oleic acid-derived oleoylethanolamide: A nutritional science perspective. Prog. Lipid Res. 2017, 67, 1–15. [Google Scholar] [CrossRef]
- Sihag, J.; Jones, P.J.H. Oleoylethanolamide: The role of a bioactive lipid amide in modulating eating behaviour. Obes. Rev. 2018, 19, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, Y.; Gong, X.; Cheng, G.; Pu, S.; Cai, S. The preventive effect of phenolic-rich extracts from Chinese sumac fruits against nonalcoholic fatty liver disease in rats induced by a high-fat diet. Food Funct. 2020, 11, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wei, Y.; Xu, W.; Lu, F.; Ma, H. Corn peptides improved obesity-induced non-alcoholic fatty liver disease through relieving lipid metabolism, insulin resistance and oxidative stress. Food Funct. 2022, 13, 5782–5793. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kang, I.B.; Kim, H.; Song, K.Y.; Seo, K.H. Dual function of Lactobacillus kefiri DH5 in preventing high-fat-diet-induced obesity: Direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue. Mol. Nutr. Food Res. 2017, 61, 1700252. [Google Scholar] [CrossRef]
- Zhu, K.; Tan, F.; Mu, J.; Yi, R.; Zhou, X.; Zhao, X. Anti-Obesity Effects of Lactobacillus fermentum CQPC05 Isolated from Sichuan Pickle in High-Fat Diet-Induced Obese Mice through PPAR-α Signaling Pathway. Microorganisms 2019, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Beekmann, K.; Rubió, L.; de Haan, L.H.; Actis-Goretta, L.; van der Burg, B.; van Bladeren, P.J.; Rietjens, I.M. The effect of quercetin and kaempferol aglycones and glucuronides on peroxisome proliferator-activated receptor-gamma (PPAR-γ). Food Funct. 2015, 6, 1098–1107. [Google Scholar] [CrossRef]
- Kooshki, R.; Anaeigoudari, A.; Abbasnejad, M.; Askari-Zahabi, K.; Esmaeili-Mahani, S. Abscisic acid interplays with PPARγ receptors and ameliorates diabe-tes-induced cognitive deficits in rats. Avicenna J. Phytomed. 2021, 11, 247–257. [Google Scholar]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Lee, G.H.; Kim, Y.A.; Chung, Y.C.; Jeong, H.G. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 2017, 65, 317–326. [Google Scholar] [CrossRef]
- Lu, R.; Zheng, Z.; Yin, Y.; Jiang, Z. Genistein prevents bone loss in type 2 diabetic rats induced by streptozotocin. Food Nutr. Res. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Saini, M.K.; Sanyal, S.N. Piroxicam and c-phycocyanin prevent colon carcinogenesis by inhibition of membrane fluidity and canonical Wnt/β-catenin signaling while up-regulating ligand dependent transcription factor PPARγ. Biomed. Pharmacother. 2014, 68, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, F.; Huang, Z.; Wang, Y.; Wong, C. Kaempferol is an estrogen-related receptor alpha and gamma inverse agonist. FEBS Lett. 2009, 583, 643–647. [Google Scholar] [CrossRef]
- De Souza Basso, B.; Haute, G.V.; Ortega-Ribera, M.; Luft, C.; Antunes, G.L.; Bastos, M.S.; Carlessi, L.P.; Levorse, V.G.; Cassel, E.; Fagundes Donadio, M.V.; et al. Methoxyeugenol deactivates hepatic stellate cells and attenuates liver fibrosis and inflammation through a PPAR-γ and NF-kB mechanism. J. Ethnopharmacol. 2021, 280, 114433. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M. Crocin prevents metabolic syndrome in rats via enhancing PPAR-gamma and AMPK. Saudi J. Biol. Sci. 2020, 27, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Reynolds, K.; Martino-Catt, S.; Cui, Y.; Hennighausen, L.; Gonzalez, F.; Rohrer, J.; Benninghoff, A.U.; Hontecillas, R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 2004, 127, 777–791. [Google Scholar] [CrossRef]
- Kumar, S.; Sinha, K.; Sharma, R.; Purohit, R.; Padwad, Y. Phloretin and phloridzin improve insulin sensitivity and enhance glucose uptake by subverting PPARγ/Cdk5 interaction in differentiated adipocytes. Exp. Cell Res. 2019, 383, 111480. [Google Scholar] [CrossRef]
- Cho, K.W.; Lee, O.H.; Banz, W.J.; Moustaid-Moussa, N.; Shay, N.F.; Kim, Y.C. Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARgamma transcriptional activity. J. Nutr. Biochem. 2010, 21, 841–847. [Google Scholar] [CrossRef]
- Sheng, X.; Zhang, Y.; Gong, Z.; Huang, C.; Zang, Y.Q. Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors. PPAR Res. 2008, 2008, 581348. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.; Zong, X.; Yang, X.; Zhang, Y.; Man, C.; Jiang, Y. Preventive Effect and Molecular Mechanism of Lactobacillus rhamnosus JL1 on Food-Borne Obesity in Mice. Nutrients 2021, 13, 3989. [Google Scholar] [CrossRef]
- Zhou, X.; Shang, G.S.; Tan, Q.; He, Q.; Tan, X.; Park, K.Y.; Zhao, X. Effect of Lactobacillus fermentum TKSN041 on improving streptozotocin-induced type 2 diabetes in rats. Food Funct. 2021, 12, 7938–7953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Zhang, J.; Li, Y.; He, Q.; Li, H.; Guo, X.; Guo, J.; Zhang, H. Probiotic Lactobacillus casei Zhang ameliorates high-fructose-induced impaired glucose tolerance in hyperinsulinemia rats. Eur. J. Nutr. 2014, 53, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Jan, R.L.; Wu, L.S.; Chen, P.C.; Kao, H.F.; Kuo, W.S.; Wang, J.Y. Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells. J. Mol. Med. 2018, 96, 39–51. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Helal, S.A. Upregulation of PPAR-γ mediates the renoprotective effect of omega-3 PUFA and ferulic acid in gentamicin-intoxicated rats. Biomed. Pharmacother. 2018, 99, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Cugno, C.; Kizhakayil, D.; Calzone, R.; Rahman, S.M.; Halade, G.V.; Rahman, M. Omega-3 fatty acid-rich fish oil supplementation prevents rosiglitazone-induced osteopenia in aging C57BL/6 mice and in vitro studies. Sci. Rep. 2021, 11, 10364. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Protein Digests and Pure Peptides from Chia Seed Prevented Adipogenesis and Inflammation by Inhibiting PPARγ and NF-κB Pathways in 3T3L-1 Adipocytes. Nutrients 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg white hydrolysate shows insulin mimetic and sensitizing effects in 3T3-F442A pre-adipocytes. PLoS ONE 2017, 12, e0185653. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Mercer, A.; Mawhinney, H.; Pulinilkunnil, T.; Udenigwe, C.C.; Kienesberger, P.C. Whey Peptides Stimulate Differentiation and Lipid Metabolism in Adipocytes and Ameliorate Lipotoxicity-Induced Insulin Resistance in Muscle Cells. Nutrients 2020, 12, 425. [Google Scholar] [CrossRef]
- Marcone, S.; Haughton, K.; Simpson, P.J.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides inhibit human endotheli-al-monocyte interactions via PPAR-γ dependent regulation of NF-κB. J. Inflamm. 2015, 12, 1. [Google Scholar] [CrossRef]
- Li, Q.; Liao, S.; Pang, D.; Li, E.; Liu, T.; Liu, F.; Zou, Y. The transported active mulberry leaf phenolics inhibited adipogenesis through PPAR-γ and Leptin signaling pathway. J. Food Biochem. 2022, 15, e14270. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhiza glabra roots and their PPAR-gamma ligand-binding activity. Bioorg. Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, R.H.; Kamel, E.M.; Mahmoud, A.M.; El-Bassuony, A.A.; Bin-Jumah, M.; Lamsabhi, A.M.; Ahmed, S.A. Rumex dentatus L. phenolics ameliorate hyperglycemia by modulating hepatic key enzymes of carbohydrate metabolism, oxidative stress and PPARγ in diabetic rats. Food Chem. Toxicol. 2020, 138, 111202. [Google Scholar] [CrossRef]
- Shiner, M.; Fuhrman, B.; Aviram, M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis 2007, 195, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Rehman, A.U.; Luckett, D.J.; Blanchard, C.L.; Obied, H.K.; Strappe, P. Phenolic Compounds with Antioxidant Properties from Canola Meal Extracts Inhibit Adipogenesis. Int. J. Mol. Sci. 2019, 21, 1. [Google Scholar] [CrossRef]
- Ramírez, N.M.; Toledo, R.; Moreira, M.; Martino, H.; Benjamin, L.; de Queiroz, J.H.; Ribeiro, A.Q.; Ribeiro, S. Anti-obesity effects of tea from Mangifera indica L. leaves of the Ubá variety in high-fat diet-induced obese rats. Biomed. Pharmacother. 2017, 91, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Abalo, R.; Nurgali, K. Targeting Enteric Neurons and Plexitis for the Management of Inflammatory Bowel Disease. Curr. Drug Targets 2020, 21, 1428–1439. [Google Scholar] [CrossRef]

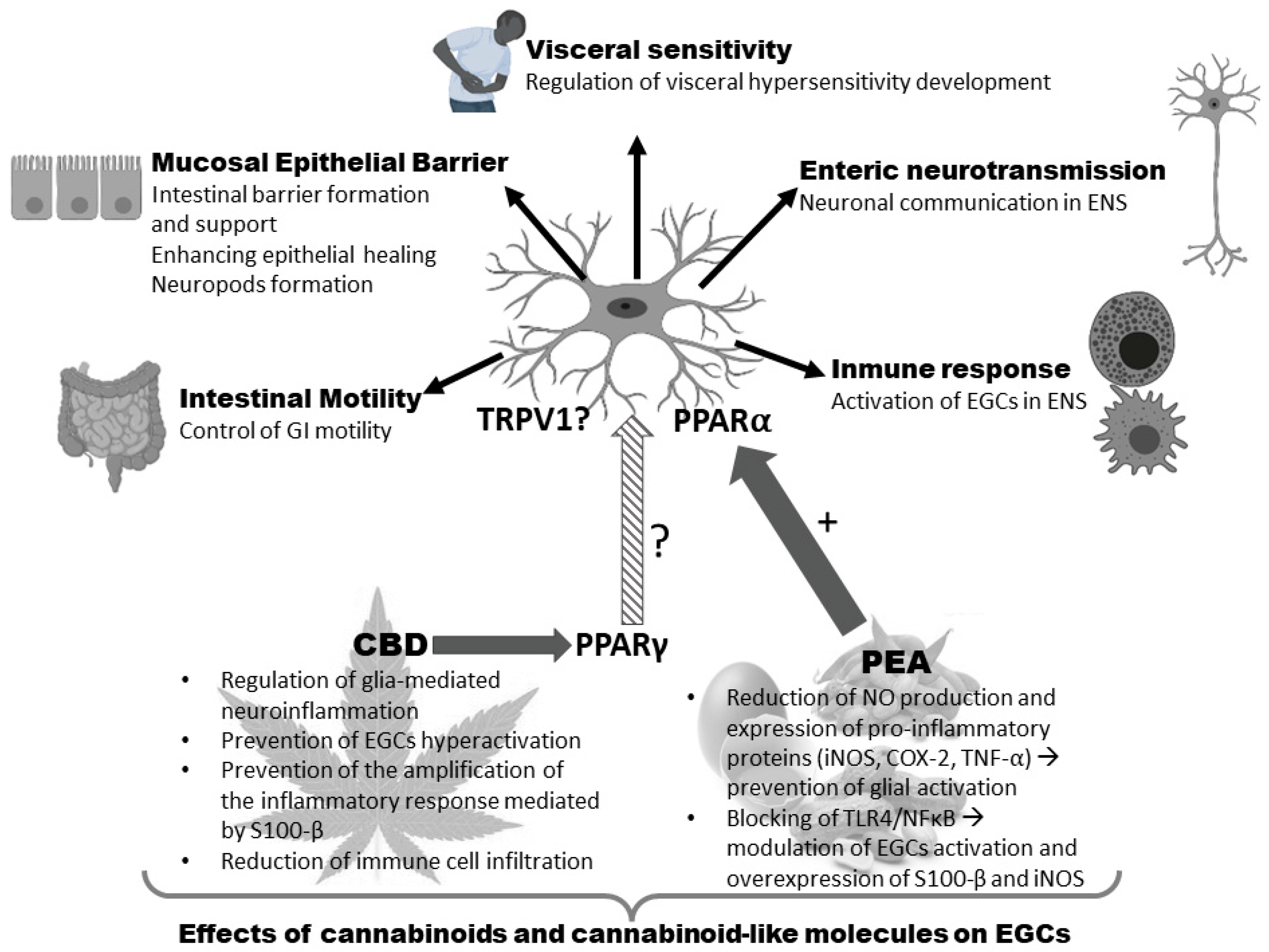
| Aspect | Function | Localization | Mediators | References |
|---|---|---|---|---|
| Epithelial barrier | Intestinal barrier formation and support Enhancing epithelial healing Neuropods formation | Mucosa | proEGF TGF-β S-nitrosoglutathione 15d-PGJ2 NGF-β * Artemin * | [38,39,40,41,42,43,44,45] |
| Intestinal motility | Control of GI motility # | Myenteric plexus | ATP | [46,47,48] |
| Enteric neurotransmission | Neuronal communication | ENS | ATP NFG GSH | [49] |
| Immune response | Activation of EGCs | ENS | MHC II class IL-1β IL-6 TGF-β proEGF GSH PGE2 | [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Visceral sensitivity | Sensitizing/activating nociceptors | ENS | ATP GABA IL-1β neurotrophins | [8,71,72] |
| Condition | Species | Changes on EGCs | References |
|---|---|---|---|
| PHYSIOLOGICAL | |||
| Aging | Rat | Loss of myenteric EGCs | [91] |
| Diet modification | Mice Rat | HFD reduces EGC density in duodenal mucosa and submucosal plexus Food restriction is detrimental to EGCs (but not neurons) | [92,93] |
| GI DISEASES | |||
| IBD | Human | Glial markers (GFAP and S100-β) and GDNF are increased in inflamed areas of biopsies. In co-cultures, EGCs from CD patients increased intestinal permeability and epithelial proliferation | [55,94,95,96] |
| Celiac disease | Human | In EGCs from duodenal biopsies, increased S100-β and NO production | [67] |
| Chronic constipation | Human | Loss of ileal and colonic EGCs, in constipated patients, particularly in infectious megacolon (Chagas disease) | [97,98,99] |
| Postoperative ileus | Mice | In cultured EGCs, activation of IL-1R promotes an inflammatory response with increased IL-6 and MCP1 levels | [100] |
| IBS | Human Rat | Reduced immunoreactivity of S100-β in colonic biopsies (Independently from the IBS subtype) Exposure of EGCs to supernatants from mucosal human biopsies: IBS-C → reduced EGC→ proliferation; IBS-D and IBS-M→ impaired ATP-induced Ca2+ response of EGCs | [101,102] |
| PI-IBS associated with C. difficile | Rat | Exposure to toxin B produced cytotoxic and pro-apoptotic effects on cultured EGC | |
| Visceral hypersensitivity in IBS | Human Mice Rat | Increased expression of S-100, SP and TrkB (receptor for BDNF) in the colonic mucosa of IBS patients Increased expression of GFAP, SP and TrkB and induced VH in wild type but not BDNF+/− mice after administration of fecal supernatants from IBS-D patients. Butyrate enemas increased colocalization of GFAP and NGF in colonic EGCs, as well as NGF secretion. | [103,104,105] |
| Viral gastroenteritis | Human | EGCs stimulated with supernatants from ECCs infected with the human adenovirus 41 showed altered GFAP expression. | [106] |
| SYSTEMIC DISEASES AFFECTING GI TRACT | |||
| Endotoxemia (systemic inflammation) | Rat | LPS systemically administered produced a dose-, time- and region-specific activation of EGCs (increased expression of S100-β and GFAP) | [107] |
| Obesity | Mice | In colonic whole-mount preparations, overexpression of S100-β (but not GFAP) and gliosis, with release of pro-inflammatory mediators. In cultured EGCs mimicking HFD-associated low-grade inflammation, increased SP and IL-1β production that may be related to dysmotility associated with obesity. | [108] |
| Diabetes | Mice Rat | Hyperglycemia promotes EGCs apoptosis involving Pdk1 and PI3K/Akt pathways. Lack of GDNF due to EGC loss, affects neuronal surviving, and GDNF supplementation limits neuronal loss. | [109,110,111] |
| Parkinson’s disease | Human | In colonic biopsies, increased expression of glial markers GFAP, S100-β, Sox10, accompanied by elevation of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL-6) at mRNA level. In colonic biopsies, GFAP over-expression. | [112,113] |
| Prion’s disease | Human | The spreading of pathological isoforms of cellular prion protein affects EGC in the GI tract. | [114,115,116] |
| HIV infection | Rat Mice | Intracolonic application of HIV1-tat protein produced lidocaine-sensitive S100-β and GFAP overexpression in submucosal plexus. Calcium signals from EGCs passed through Cx43 to glial cells of the spinal cord and the cerebral cortex, causing an inflammatory reaction, and cognitive loss. GI dysmotility and enhanced immune activation after treatment with HIV-1Tat + LPS, related to EGC release of IL-6, IL-1β and TNF-α and NF-κB activation; but not in glia from TLR4 KO mice | [117,118] |
| SARS-CoV-2 infection | Human | Enteric neurons and EGCs express ACE2 and TMRPSS2 and may be susceptible to invasion by the virus, this may lead to compromised immune response, cytokine storm facilitation, as well as alterations in intestinal motility. | [119] |
| DRUG-INDUCED GI DISORDERS | |||
| Opioid-induced hyperalgesia and “narcotic bowel syndrome” | Mice | Upregulation of purinergic signaling in EGCs induced by prolonged opioid use and proinflammatory cytokine release, leading to gut barrier dysfunction and constipation | [8,120] |
| Cancer chemotherapy: oxaliplatin | Mice | In ileal whole-mount preparations, GFAP decreased in submucosal and myenteric plexus and S100-β increased in the myenteric plexus and mucosa. In distal colon, GFAP immunolabelling decreased whereas S100-β increased. | [121,122] |
| Cancer chemotherapy: 5-FU | Mice | Increased expression of S100-β protein in GFAP-positive cells during mucositis Pentamidine inhibits S100-β induced by 5-FU and this inhibits gliosis. | [123] |
| Cancer chemotherapy: irinotecan | Mice | Increased co-expression of GFAP and S100-β in irinotecan-treated tissues (duodenum, jejunum, ileum). Indirect relationship of mast cells with EGCs: forced mast cell degranulation, decreased the expression of GFAP and S100-β | [124] |
| Cancer chemotherapy: cisplatin | Mice | Chronic treatment with cisplatin reduces expression of S100-β, GFAP and SOX-10 in EGCs as well as that of ChAT and nNOS in myenteric neurons. | [125] |
| Cancer chemotherapy: others | Guinea pig | In cultured ECGs exposed to cytochalasin D (alters microfilaments), and nocodazole (alters microtubules), entry of calcium is reduced → other antineoplastic drug directed against elements of the cytoskeleton (taxanes, vinca alkaloids) might impair entry of calcium, and therefore alter EGC activity | [126] |
| ECS Component | Nutraceutical (And its Natural Source) | Effect/Reference |
|---|---|---|
| CB2 | Harpagophytum procumbens root extract | Activation [203] |
| β-caryophyllene (oregano, cinnamon, and black pepper) | Agonist [204] | |
| Olive oil | Increase CB2 expression [205] | |
| Lactobacillus fermentum MCC2760 * | Increase CB2 expression [206] | |
| Lactobacillus acidophilus NCFM * | Decrease CB2 expression [207] | |
| TRPV1 | Capsaicin (chili peppers) | Agonist [208] |
| Decursin (eggs) | Antagonist [209] | |
| Fish oil | Decrease TRPV1 expression [210] | |
| Omega 3 fatty acids | Activation [211] | |
| Probiotics: VSL#3 | ||
| Lactobacillus fermentum CQPC03 * | Decrease TRPV1 expression [212] | |
| Lactobacillus casei Qian * | Decrease TRPV1 expression [213] | |
| Lactobacillus reuteri DSM 17938 * | Decrease TRPV1 expression [214] Antagonist [215] | |
| PPAR α | Oleic acid | Agonist [216] |
| Oleoylethanolamide (oleic acid derivative) | Agonist [217] | |
| Extracts from Chinese sumac (Rhus chinensis Mill.) | Increase PPAR α expression [218] | |
| Bioactive peptides from corn | Increased expression [219] | |
| Lactobacillus kefiri DH5 * | Upregulation [220] | |
| Lactobacillus fermentum CQPC06 * | Increase PPAR α expression [221] | |
| PPAR γ | Quercetin (red wine, tea, cherries, grapes) | Activation [222] |
| Abscisic acid (fruits and vegetables) | Activation [223] | |
| Gallic acid (tea and fruits) | Partial agonist [224] | |
| Capsaicin (chili peppers) | Agonist [225] | |
| Genistein (soybeans and legumes) | Decrease PPARγ levels [226] | |
| Phycocyanin (blue-green algae) | Downregulation [227] | |
| Kaempferol | Inverse agonist [228] | |
| Methoxyeugenol (nutmeg and Brazilian red propolis) | Agonist [229] | |
| Crocin (saffron) | Activation [230] | |
| Punicic acid (pomegranate) | Activation [231] | |
| Linoleic acid (sunflower, soybean, corn, and canola oils, nuts and seeds) | Activation [232] | |
| Phloretin (apples) | Inhibition [233] | |
| Phloridzin (apples) | Inhibition [233] | |
| Equol (eggs and dairy) | Activation [234] | |
| Daidzein (soybean and legumes) | Activation [234] | |
| Cinnamon | Activation [235] | |
| Lactobacillus rhamnosus JL1 * | Increased expression [236] | |
| Lactobacillus fermentum TKSN04 * | Upregulation [237] | |
| Lactobacillus casei Zhang * | Increased expression [238] | |
| Lactobacillus gasseri * | Activation [239] | |
| Omega 3 fatty acids | Upregulation [240] | |
| Fish oil | Decreased expression [241] | |
| Bioactive peptides: Chia seed peptides Egg white peptides Whey peptides Milk peptides | Inhibition [242] Activation [243] Activation [244] Inhibition [245] | |
| Phenolic compunds: Mulberry Leaf | Inhibition [246] | |
| Glycyrrhiza glabra | Activation [247] | |
| Rumex dentatus | Upregulation [248] | |
| Pomegranate juice | Activation [249] | |
| Canola Meal | Downregulation [250] | |
| Mango Leaf | Upregulation [251] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, L.; Szymaszkiewicz, A.; Zielińska, M.; Abalo, R. The Enteric Glia and Its Modulation by the Endocannabinoid System, a New Target for Cannabinoid-Based Nutraceuticals? Molecules 2022, 27, 6773. https://doi.org/10.3390/molecules27196773
López-Gómez L, Szymaszkiewicz A, Zielińska M, Abalo R. The Enteric Glia and Its Modulation by the Endocannabinoid System, a New Target for Cannabinoid-Based Nutraceuticals? Molecules. 2022; 27(19):6773. https://doi.org/10.3390/molecules27196773
Chicago/Turabian StyleLópez-Gómez, Laura, Agata Szymaszkiewicz, Marta Zielińska, and Raquel Abalo. 2022. "The Enteric Glia and Its Modulation by the Endocannabinoid System, a New Target for Cannabinoid-Based Nutraceuticals?" Molecules 27, no. 19: 6773. https://doi.org/10.3390/molecules27196773
APA StyleLópez-Gómez, L., Szymaszkiewicz, A., Zielińska, M., & Abalo, R. (2022). The Enteric Glia and Its Modulation by the Endocannabinoid System, a New Target for Cannabinoid-Based Nutraceuticals? Molecules, 27(19), 6773. https://doi.org/10.3390/molecules27196773








