Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review
Abstract
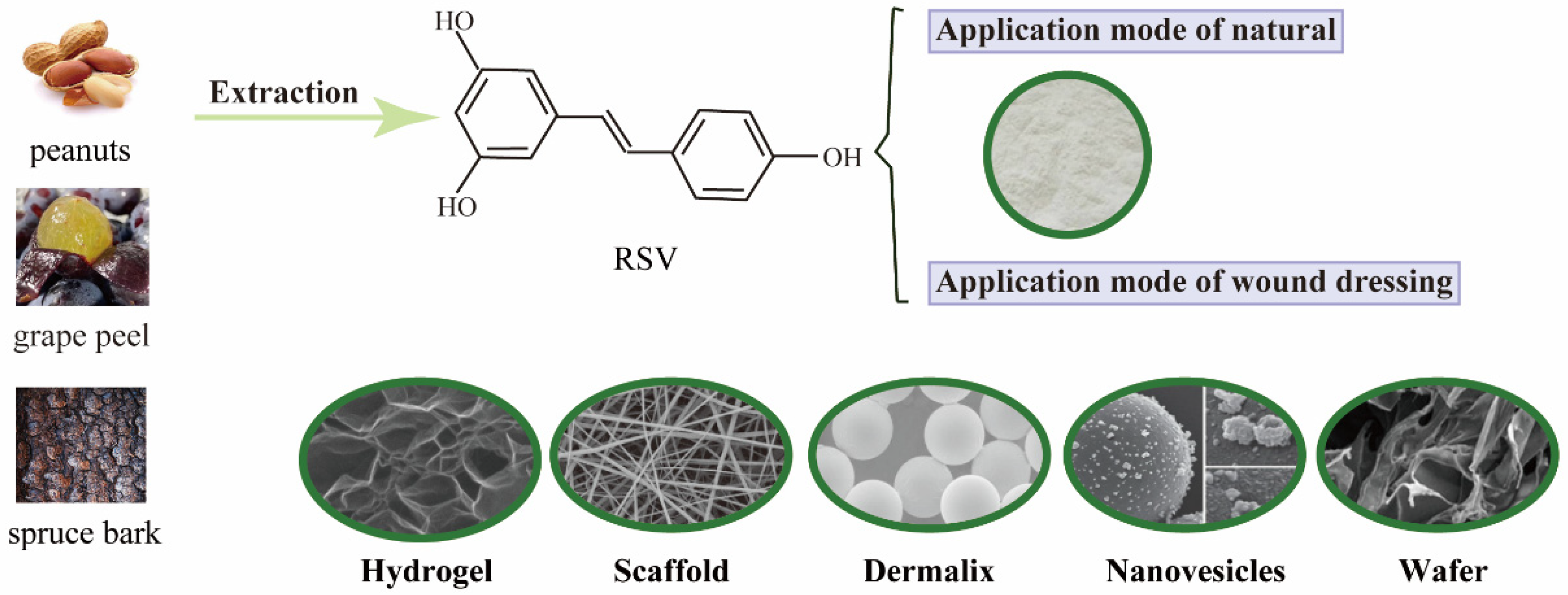
1. Introduction
2. The Performance Forms of RSV to Wound Healing
2.1. Hydrogel Chitosan-Based Wound Dressing
2.2. Electrospun Scaffolds
2.3. Others
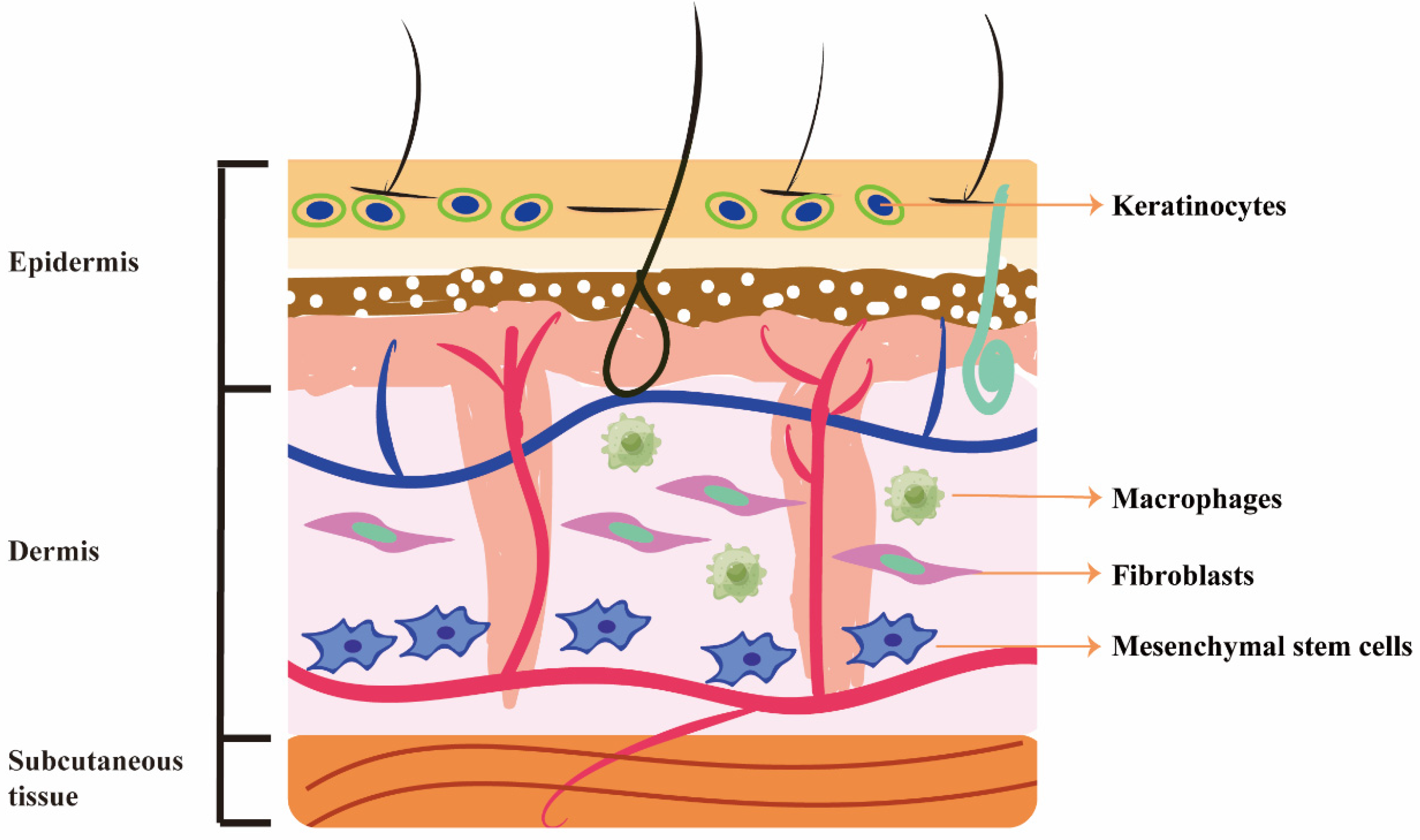
3. Beneficial Effects of RSV on Different Cells
3.1. Fibroblasts
3.2. Keratinocytes
3.3. Macrophages
3.4. Mesenchymal Stem Cells
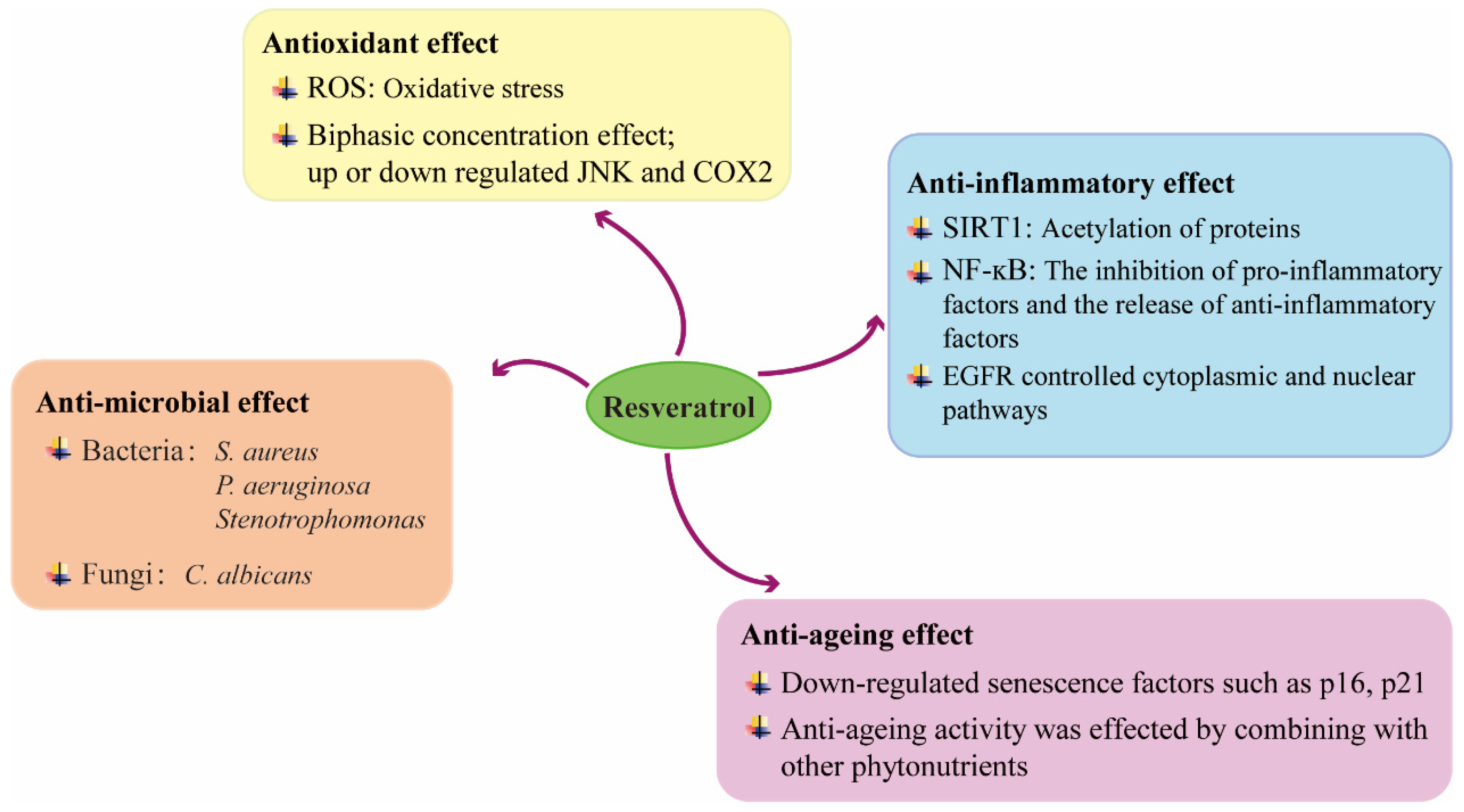
4. Mechanisms Underlying the Beneficial Effects of RSV on Wound Healing
4.1. Anti-Inflammatory Effect
4.2. Anti-Microbial Effect
4.3. Antioxidant Effect
4.4. Anti-Ageing Effect
5. Cytotoxicity and Adverse Effects of RSV on Wound Healing
6. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butkeviciute, A.; Ramanauskiene, K.; Kurapkiene, V.; Janulis, V. Dermal Penetration Studies of Potential Phenolic Compounds Ex Vivo and Their Antioxidant Activity In Vitro. Plants 2022, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Bryan, D.; Walker, K.B.; Ferguson, M.; Thorpe, R. Cytokine gene expression in a murine wound healing model. Cytokine 2005, 31, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, İ.; Gökçe, E.H.; Tsapis, N.; Tanrıverdi, S.T.; Gökçe, G.; Fattal, E.; Özer, Ö. Evaluation of characteristics and in vitro antioxidant properties of RSV loaded hyaluronic acid-DPPC microparticles as a wound healing system. Colloids Surf. B Biointerfaces 2015, 126, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; O’Meara, M.; Zhang, X.; Zhang, K.; Seyoum, B.; Yi, Z.; Kaufman, R.J.; Monks, T.J.; Wang, J.M. Ameliorating Methylglyoxal-Induced Progenitor Cell Dysfunction for Tissue Repair in Diabetes. Diabetes 2019, 68, 1287–1302. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Chen, G.; Niu, C.; Wang, Y.; Zhao, C.; Sun, J.; Huang, H.; Huang, S.; Liang, Y.; et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharmacol. 2019, 10, 421. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The Role of Resveratrol in Eye Diseases-A Review of the Literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Vaňková, E.; Paldrychová, M.; Kašparová, P.; Lokočová, K.; Kodeš, Z.; Maťátková, O.; Kolouchová, I.; Masák, J. Natural antioxidant pterostilbene as an effective antibiofilm agent, particularly for gram-positive cocci. World J. Microbiol. Biotechnol. 2020, 36, 101. [Google Scholar] [CrossRef]
- Tripathi, V.; Chhabria, S.; Jadhav, V.; Bhartiya, D.; Tripathi, A. Stem Cells and Progenitors in Human Peripheral Blood Get Activated by Extremely Active Resveratrol (XAR™). Stem Cell Rev. Rep. 2018, 14, 213–222. [Google Scholar] [CrossRef]
- Ávila-Salas, F.; Marican, A.; Pinochet, S.; Carreño, G.; Valdés, O.; Venegas, B.; Donoso, W.; Cabrera-Barjas, G.; Vijayakumar, S.; Durán-Lara, E.F. Film Dressings Based on Hydrogels: Simultaneous and Sustained-Release of Bioactive Compounds with Wound Healing Properties. Pharmaceutics 2019, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Šoltés, L. Self-Associating Polymers Chitosan and Hyaluronan for Constructing Composite Membranes as Skin-Wound Dressings Carrying Therapeutics. Molecules 2021, 26, 2535. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sivaraj, D.; Davitt, M.F.; Leeolou, M.C.; Henn, D.; Steele, S.R.; Huskins, S.L.; Trotsyuk, A.A.; Kussie, H.C.; Greco, A.H.; et al. Pullulan-Collagen hydrogel wound dressing promotes dermal remodelling and wound healing compared to commercially available collagen dressings. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2022, 30, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A cellulose nanofibril-reinforced hydrogel with robust mechanical, self-healing, pH-responsive and antibacterial characteristics for wound dressing applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef]
- Dickmeis, C.; Kauth, L.; Commandeur, U. From infection to healing: The use of plant viruses in bioactive hydrogels. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1662. [Google Scholar] [CrossRef]
- Boominathan, T.; Sivaramakrishna, A. Recent Advances in the Synthesis, Properties, and Applications of Modified Chitosan Derivatives: Challenges and Opportunities. Top. Curr. Chem. 2021, 379, 19. [Google Scholar] [CrossRef]
- Lan, W.; Zhao, J.; Sun, Y.; Liu, J.; Xie, J. Chitosan-grafted-phenolic acid copolymers against Shewanella putrefaciens by disrupting the permeability of cell membrane. World J. Microbiol. Biotechnol. 2022, 38, 73. [Google Scholar] [CrossRef]
- Fuster, M.G.; Montalbán, M.G.; Carissimi, G.; Lima, B.; Feresin, G.E.; Cano, M.; Giner-Casares, J.J.; López-Cascales, J.J.; Enriz, R.D.; Víllora, G. Antibacterial Effect of Chitosan-Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model. Nanomaterials 2020, 10, 2340. [Google Scholar] [CrossRef]
- Berce, C.; Muresan, M.S.; Soritau, O.; Petrushev, B.; Tefas, L.; Rigo, I.; Ungureanu, G.; Catoi, C.; Irimie, A.; Tomuleasa, C. Cutaneous wound healing using polymeric surgical dressings based on chitosan, sodium hyaluronate and resveratrol. A preclinical experimental study. Colloids Surf. B Biointerfaces 2018, 163, 155–166. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, W.; Liu, H.; Huang, S.; Bian, L.; Guo, R. Injectable supramolecular gelatin hydrogel loading of resveratrol and histatin-1 for burn wound therapy. Biomater. Sci. 2020, 8, 4810–4820. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Krupa, A.; Majda, D.; Kulinowski, P.; Kurek, M.; Węglarz, W.P.; Jachowicz, R. Poly(Vinyl Alcohol) Cryogel Membranes Loaded with Resveratrol as Potential Active Wound Dressings. AAPS PharmSciTech 2021, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Campbell, J.; Ukani, G.; O’Reilly Beringhs, A.; Selvaraju, V.; Thirunavukkarasu, M.; Lu, X.; Palesty, J.A.; Maulik, N. Evaluation of dermal tissue regeneration using resveratrol loaded fibrous matrix in a preclinical mouse model of full-thickness ischemic wound. Int. J. Pharm. 2019, 558, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhou, M.; Dong, W.; Zhao, S.; Wang, Y.; Yao, J.; Liu, Z.; Han, H.; Sun, D.; Zhang, M. A bi-layered scaffold of a poly(lactic-co-glycolic acid) nanofiber mat and an alginate-gelatin hydrogel for wound healing. J. Mater. Chem. B 2021, 9, 7492–7505. [Google Scholar] [CrossRef]
- Çetinkalp, Ş.; Gökçe, E.H.; Şimşir, I.; Tuncay Tanrıverdi, S.; Doğan, F.; Biray Avcı, Ç.; Eroğlu, İ.; Utku, T.; Gündüz, C.; Özer, Ö. Comparative Evaluation of Clinical Efficacy and Safety of Collagen Laminin-Based Dermal Matrix Combined With Resveratrol Microparticles (Dermalix) and Standard Wound Care for Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2021, 20, 217–226. [Google Scholar] [CrossRef]
- Amanat, S.; Taymouri, S.; Varshosaz, J.; Minaiyan, M.; Talebi, A. Carboxymethyl cellulose-based wafer enriched with resveratrol-loaded nanoparticles for enhanced wound healing. Drug Deliv. Transl. Res. 2020, 10, 1241–1254. [Google Scholar] [CrossRef]
- Vitonyte, J.; Manca, M.L.; Caddeo, C.; Valenti, D.; Peris, J.E.; Usach, I.; Nacher, A.; Matos, M.; Gutiérrez, G.; Orrù, G.; et al. Bifunctional viscous nanovesicles co-loaded with resveratrol and gallic acid for skin protection against microbial and oxidative injuries. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2017, 114, 278–287. [Google Scholar] [CrossRef]
- Gokce, E.H.; Tuncay Tanrıverdi, S.; Eroglu, I.; Tsapis, N.; Gokce, G.; Tekmen, I.; Fattal, E.; Ozer, O. Wound healing effects of collagen-laminin dermal matrix impregnated with resveratrol loaded hyaluronic acid-DPPC microparticles in diabetic rats. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2017, 119, 17–27. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Trojanowska, M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology 2008, 47 (Suppl. 5), v2–v4. [Google Scholar] [CrossRef]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast pathology in inflammatory diseases. J. Clin. Investig. 2021, 131, e149538. [Google Scholar] [CrossRef] [PubMed]
- Kaleci, B.; Koyuturk, M. Efficacy of resveratrol in the wound healing process by reducing oxidative stress and promoting fibroblast cell proliferation and migration. Dermatol. Ther. 2020, 33, e14357. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Li, B.; Tang, Z.; Yang, W.; Hao, L.; Shi, Z.; Zhang, J.; Cai, L.; Li, R.; Liu, Y.; et al. Resveratrol inhibits hypertrophic scars formation by activating autophagy via the miR-4654/Rheb axis. Mol. Med. Rep. 2020, 22, 3440–3452. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Zhang, M.; Guan, E.; Han, Q.; Liu, Y.; Long, X.; Long, F.; Zhao, R.C.; Huang, J.; Liu, Z.; et al. Resveratrol inhibits proliferation and promotes apoptosis of keloid fibroblasts by targeting HIF-1α. J. Plast. Surg. Hand Surg. 2020, 54, 290–296. [Google Scholar] [CrossRef]
- Zhai, X.X.; Ding, J.C.; Tang, Z.M. Resveratrol Inhibits Proliferation and Induces Apoptosis of Pathological Scar Fibroblasts Through the Mechanism Involving TGF-β1/Smads Signaling Pathway. Cell Biochem. Biophys. 2015, 71, 1267–1272. [Google Scholar] [CrossRef]
- Bai, X.Z.; Liu, J.Q.; Yang, L.L.; Fan, L.; He, T.; Su, L.L.; Shi, J.H.; Tang, C.W.; Zheng, Z.; Hu, D.H. Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars. Br. J. Pharmacol. 2016, 173, 1589–1601. [Google Scholar] [CrossRef]
- Tang, Z.M.; Zhai, X.X.; Ding, J.C. Expression of mTOR/70S6K signaling pathway in pathological scar fibroblasts and the effects of resveratrol intervention. Mol. Med. Rep. 2017, 15, 2546–2550. [Google Scholar] [CrossRef]
- Bártolo, I.; Reis, R.L.; Marques, A.P.; Cerqueira, M.T. Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization. Tissue Eng. Part B Rev. 2022, 28, 665–676. [Google Scholar] [CrossRef]
- Werner, S.; Smola, H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 2001, 11, 143–146. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, W.; Wang, C.W.; Shi, J.P.; Shi, Z.Q.; Zhou, J.D. Resveratrol promotes skin wound healing by regulating the miR-212/CASP8 axis. Lab. Investig. A J. Tech. Methods Pathol. 2021, 101, 1363–1370. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, H.S.; Na, J.I.; Huh, C.H.; Park, K.C.; Choi, H.R. Resveratrol Inhibits Particulate Matter-Induced Inflammatory Responses in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 3446. [Google Scholar] [CrossRef]
- Khanna, S.; Venojarvi, M.; Roy, S.; Sharma, N.; Trikha, P.; Bagchi, D.; Bagchi, M.; Sen, C.K. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free. Radic. Biol. Med. 2002, 33, 1089–1096. [Google Scholar] [CrossRef]
- Spallotta, F.; Cencioni, C.; Straino, S.; Nanni, S.; Rosati, J.; Artuso, S.; Manni, I.; Colussi, C.; Piaggio, G.; Martelli, F.; et al. A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. J. Biol. Chem. 2013, 288, 11004–11012. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Maurelli, R.; Dellambra, E.; De Luca, C.; Korkina, L.G. Resveratrol induces long-lasting IL-8 expression and peculiar EGFR activation/distribution in human keratinocytes: Mechanisms and implications for skin administration. PLoS ONE 2013, 8, e59632. [Google Scholar] [CrossRef]
- Bird, M.D.; Kovacs, E.J. Organ-specific inflammation following acute ethanol and burn injury. J. Leukoc. Biol. 2008, 84, 607–613. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.C.; Zhu, L.; Wu, Z.; Yang, R.; Xu, N.; Liang, L. Resveratrol-loaded peptide-hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen. Biomater. 2020, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Bai, X.; Jia, W.; Liu, Y.; Zhu, X.; Han, J.; Dong, M.; Li, J.; Chen, D.; Hu, D. Effects of resveratrol on the treatment of inflammatory response induced by severe burn. Inflammation 2015, 38, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Tseng, C.H.; Wang, P.W.; Lu, P.L.; Weng, Y.H.; Yen, F.L.; Fang, J.Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, X.; Hu, W.; Chu, Z.; Ruan, Q.; Zhang, H.; Li, M.; Zhang, H.; Huang, X.; Yao, P. Pterostilbene accelerates wound healing by modulating diabetes-induced estrogen receptor β suppression in hematopoietic stem cells. Burn. Trauma 2021, 9, tkaa045. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Sterodimas, A.; Calabrese, C.; Garcovich, S. Systematic review: Advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose-derived mesenchymal stem cells in wound and scar treatment. Stem Cell Res. Ther. 2021, 12, 318. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Rindiastuti, Y.; Wirohadidjojo, Y.W.; Komaratih, E.; Nurwasis; Dinaryati, A.; Lestari, N.M.I.; Rantam, F.A. Resveratrol promotes secretion of wound healing related growth factors of mesenchymal stem cells originated from adult and fetal tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1160–1167. [Google Scholar] [CrossRef]
- Hu, J.; Liu, X.; Chi, J.; Che, K.; Ma, X.; Qiu, M.; Fu, Z.; Wang, Y.; Wang, Y.; Wang, W. Resveratrol Enhances Wound Healing in Type 1 Diabetes Mellitus by Promoting the Expression of Extracellular Vesicle-Carried MicroRNA-129 Derived from Mesenchymal Stem Cells. J. Proteome Res. 2022, 21, 313–324. [Google Scholar] [CrossRef]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef]
- Latruffe, N.; Lançon, A.; Frazzi, R.; Aires, V.; Delmas, D.; Michaille, J.J.; Djouadi, F.; Bastin, J.; Cherkaoui-Malki, M. Exploring new ways of regulation by resveratrol involving miRNAs, with emphasis on inflammation. Ann. N. Y. Acad. Sci. 2015, 1348, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pignet, A.L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Lephart, E.D. Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin-Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. Int. J. Mol. Sci. 2021, 22, 11218. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Huang, Y.; Liu, W.; Cai, G.; Huang, S.; Zeng, Y.; Ren, S.; Zhan, H.; Wu, W. Resveratrol mediates mechanical allodynia through modulating inflammatory response via the TREM2-autophagy axis in SNI rat model. J. Neuroinflammation 2020, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.Z.; Jandova, J.; Janda, J.; Vleugels, F.R.; Elliott, D.A.; Sligh, J.E. Resveratrol-sensitized UVA induced apoptosis in human keratinocytes through mitochondrial oxidative stress and pore opening. J. Photochem. Photobiol. B Biol. 2012, 113, 42–50. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zou, Y.; Peng, Y.; McClements, D.J.; Hu, K. Resveratrol-loaded biopolymer core-shell nanoparticles: Bioavailability and anti-inflammatory effects. Food Funct. 2020, 11, 4014–4025. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Fidanza, P.; Potapovich, A.I.; Kostyuk, V.A.; De Luca, C.; Mikhal’chik, E.; Korkina, L.G. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxid. Redox Signal. 2012, 16, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L.; Clayton, A.; Stephens, P. The role of bacterial extracellular vesicles in chronic wound infections: Current knowledge and future challenges. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2021, 29, 864–880. [Google Scholar] [CrossRef]
- Wang, X.C.; Huang, H.B.; Gong, W.; He, W.Y.; Li, X.; Xu, Y.; Gong, X.J.; Hu, J.N. Resveratrol Triggered the Quick Self-Assembly of Gallic Acid into Therapeutic Hydrogels for Healing of Bacterially Infected Wounds. Biomacromolecules 2022, 23, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, A.B.; La Porta, N.; Isakova, E.P.; Martens, S.; Biryukova, Y.K.; Belous, A.S.; Sivokhin, D.A.; Trubnikova, E.V.; Zylkova, M.V.; Belyakova, A.V.; et al. In Vivo Antimicrobial and Wound-Healing Activity of Resveratrol, Dihydroquercetin, and Dihydromyricetin against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans. Pathogens 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.S.; Vaidyanathan, L. Effects of resveratrol on the growth and enzyme production of Stenotrophomonas maltophilia: A burn wound pathogen. J. Wound Care 2020, 29, S38–S43. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Chu, G.C.; Chao, T.; Heard, T.C.; Gómez, B.I.; Sousse, L.E.; Natesan, S.; Christy, R.J. ASCs derived from burn patients are more prone to increased oxidative metabolism and reactive oxygen species upon passaging. Stem Cell Res. Ther. 2021, 12, 270. [Google Scholar] [CrossRef]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burn. J. Int. Soc. Burn. Inj. 2021, 47, 133–139. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Luo, J.; Jiang, Y.; Zhao, Z.; Chen, Y.; Huang, Q.; Zhang, L.; Wu, T.; Pang, J. Resveratrol, a novel inhibitor of GLUT9, ameliorates liver and kidney injuries in a D-galactose-induced ageing mouse model via the regulation of uric acid metabolism. Food Funct. 2021, 12, 8274–8287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Sui, B.D.; Liu, N.; Lv, Y.J.; Zheng, C.X.; Lu, Y.B.; Huang, W.T.; Zhou, C.H.; Chen, J.; Pang, D.L.; et al. Anti-aging pharmacology in cutaneous wound healing: Effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017, 16, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, D.; Lazzeretti, A.; Tocabens, P.; Nobile, V.; Cestone, E.; Santin, G.; Bottone, M.G.; Marzatico, F. Resveratrol-procyanidin blend: Nutraceutical and antiaging efficacy evaluated in a placebocontrolled, double-blind study. Clin. Cosmet. Investig. Dermatol. 2012, 5, 159–165. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Jorfi, M.; Buchanan, K.L.; Park, D.J.; Foster, E.J.; Tyler, D.J.; Rowan, S.J.; Weder, C.; Capadona, J.R. Influence of resveratrol release on the tissue response to mechanically adaptive cortical implants. Acta Biomater. 2016, 29, 81–93. [Google Scholar] [CrossRef]
- Lin, L.X.; Wang, P.; Wang, Y.T.; Huang, Y.; Jiang, L.; Wang, X.M. Aloe vera and Vitis vinifera improve wound healing in an in vivo rat burn wound model. Mol. Med. Rep. 2016, 13, 1070–1076. [Google Scholar] [CrossRef]
- Saleh, H.A.; Ramdan, E.; Elmazar, M.M.; Azzazy, H.M.E.; Abdelnaser, A. Comparing the protective effects of resveratrol, curcumin and sulforaphane against LPS/IFN-γ-mediated inflammation in doxorubicin-treated macrophages. Sci. Rep. 2021, 11, 545. [Google Scholar] [CrossRef]
- Radkar, V.; Hardej, D.; Lau-Cam, C.; Billack, B. Evaluation of resveratrol and piceatannol cytotoxicity in macrophages, T cells, and skin cells. Arh. Za Hig. Rada I Toksikol. 2007, 58, 293–304. [Google Scholar] [CrossRef][Green Version]
- Ikeda, K.; Torigoe, T.; Matsumoto, Y.; Fujita, T.; Sato, N.; Yotsuyanagi, T. Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2013, 21, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.L.; Teng, Y.Y.; Chen, Z.H.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Wu, J.J.; Yuan, Z.D.; Tang, X.Y.; Yu, S.; et al. The uPA System Differentially Alters Fibroblast Fate and Profibrotic Ability in Skin Fibrosis. Front. Immunol. 2022, 13, 845956. [Google Scholar] [CrossRef]
- Zou, M.L.; Teng, Y.Y.; Wu, J.J.; Liu, S.Y.; Tang, X.Y.; Jia, Y.; Chen, Z.H.; Zhang, K.W.; Sun, Z.L.; Li, X.; et al. Fibroblasts: Heterogeneous Cells With Potential in Regenerative Therapy for Scarless Wound Healing. Front. Cell Dev. Biol. 2021, 9, 713605. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Shao, J.-H.; Zhang, K.-W.; Zou, M.-L.; Teng, Y.-Y.; Tian, F.; Chen, M.-N.; Chen, W.-W.; Yuan, Z.-D.; Wu, J.-J.; et al. Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review. Molecules 2022, 27, 6736. https://doi.org/10.3390/molecules27196736
Jia Y, Shao J-H, Zhang K-W, Zou M-L, Teng Y-Y, Tian F, Chen M-N, Chen W-W, Yuan Z-D, Wu J-J, et al. Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review. Molecules. 2022; 27(19):6736. https://doi.org/10.3390/molecules27196736
Chicago/Turabian StyleJia, Yuan, Jia-Hao Shao, Kai-Wen Zhang, Ming-Li Zou, Ying-Ying Teng, Fan Tian, Meng-Nan Chen, Wei-Wei Chen, Zheng-Dong Yuan, Jun-Jie Wu, and et al. 2022. "Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review" Molecules 27, no. 19: 6736. https://doi.org/10.3390/molecules27196736
APA StyleJia, Y., Shao, J.-H., Zhang, K.-W., Zou, M.-L., Teng, Y.-Y., Tian, F., Chen, M.-N., Chen, W.-W., Yuan, Z.-D., Wu, J.-J., & Yuan, F.-L. (2022). Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review. Molecules, 27(19), 6736. https://doi.org/10.3390/molecules27196736





