Synthesis, Electrochemical and Fluorescence Properties of the First Fluorescent Member of the Ferrocifen Family and of Its Oxidized Derivatives
Abstract
1. Introduction
2. Results and Discussion
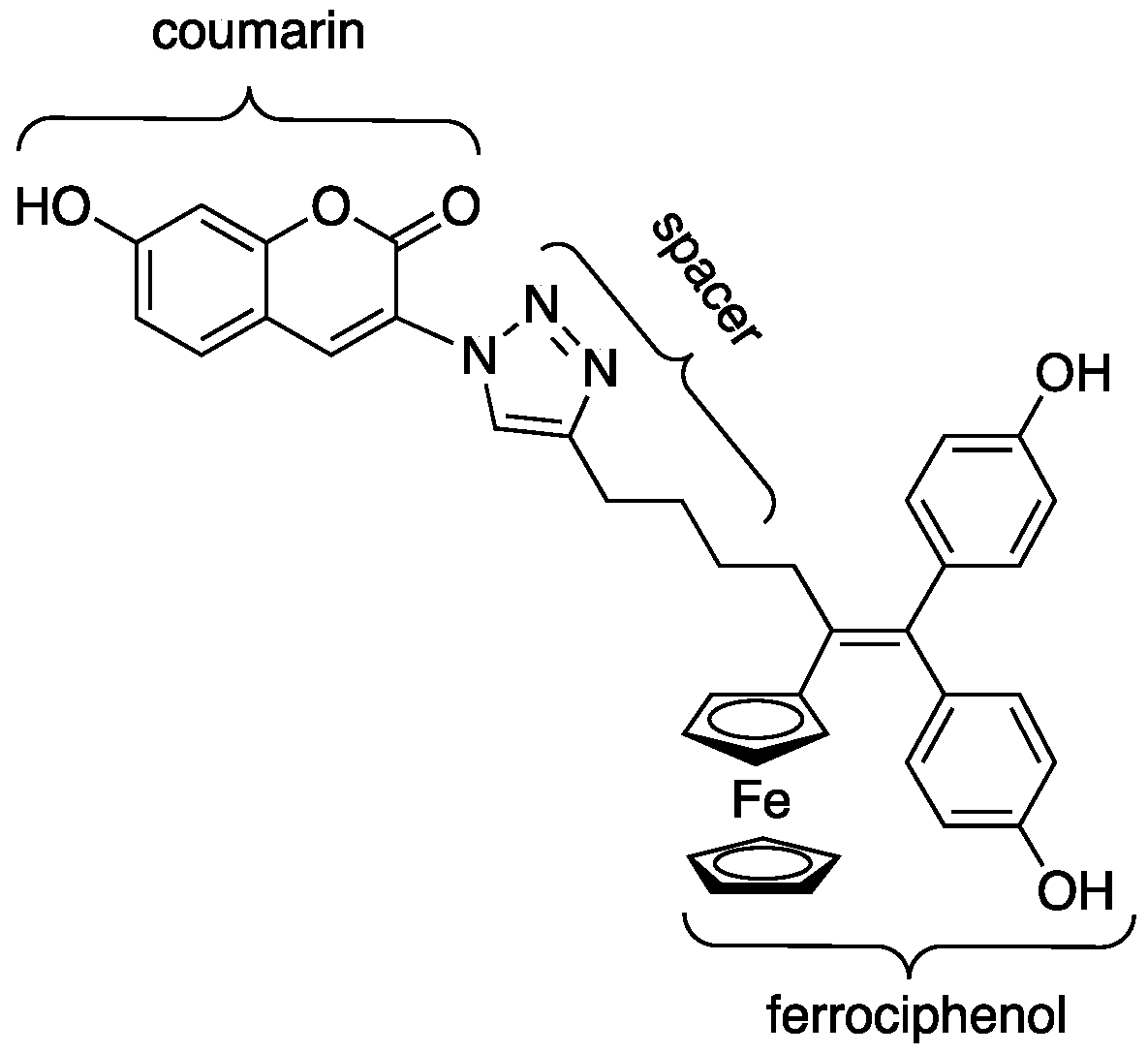
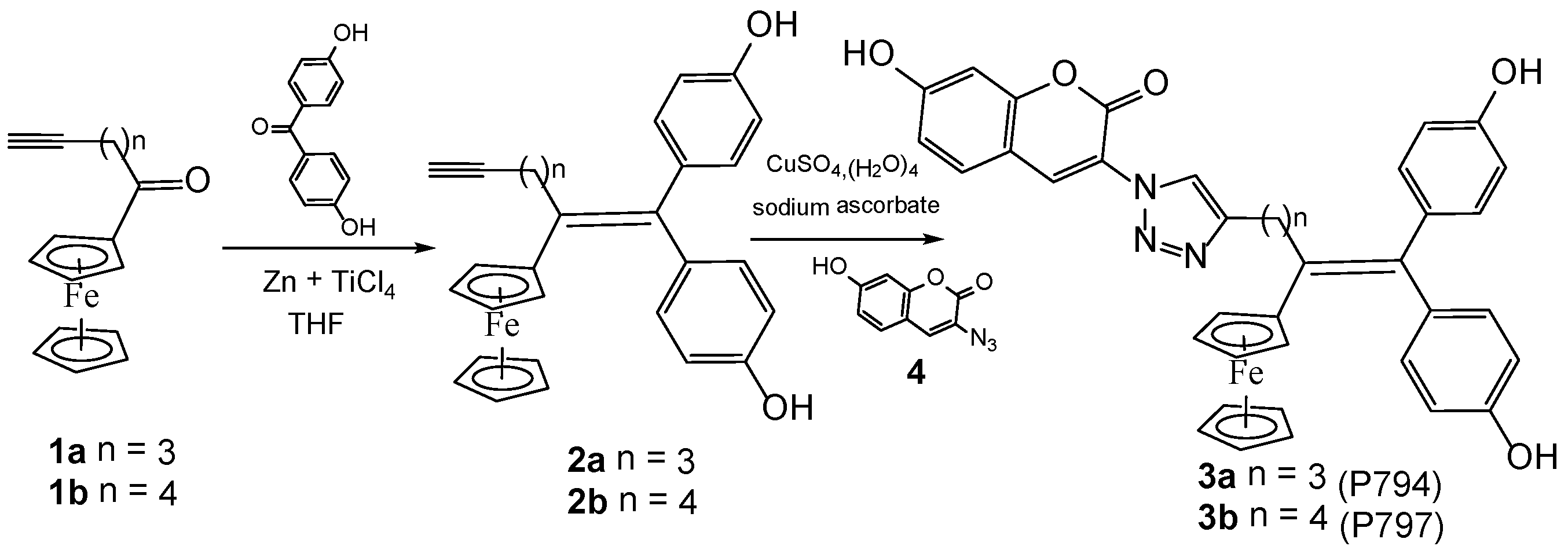
2.1. Synthesis of Ferrociphenol Coumarin Dyads
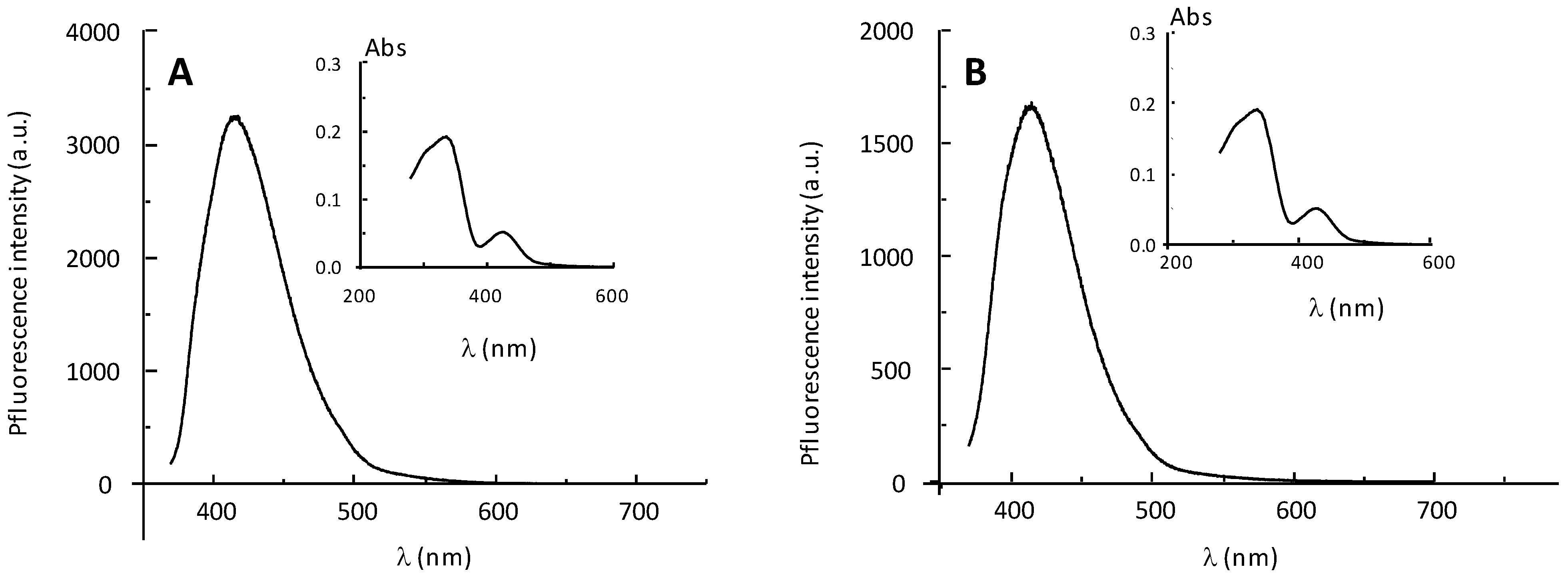
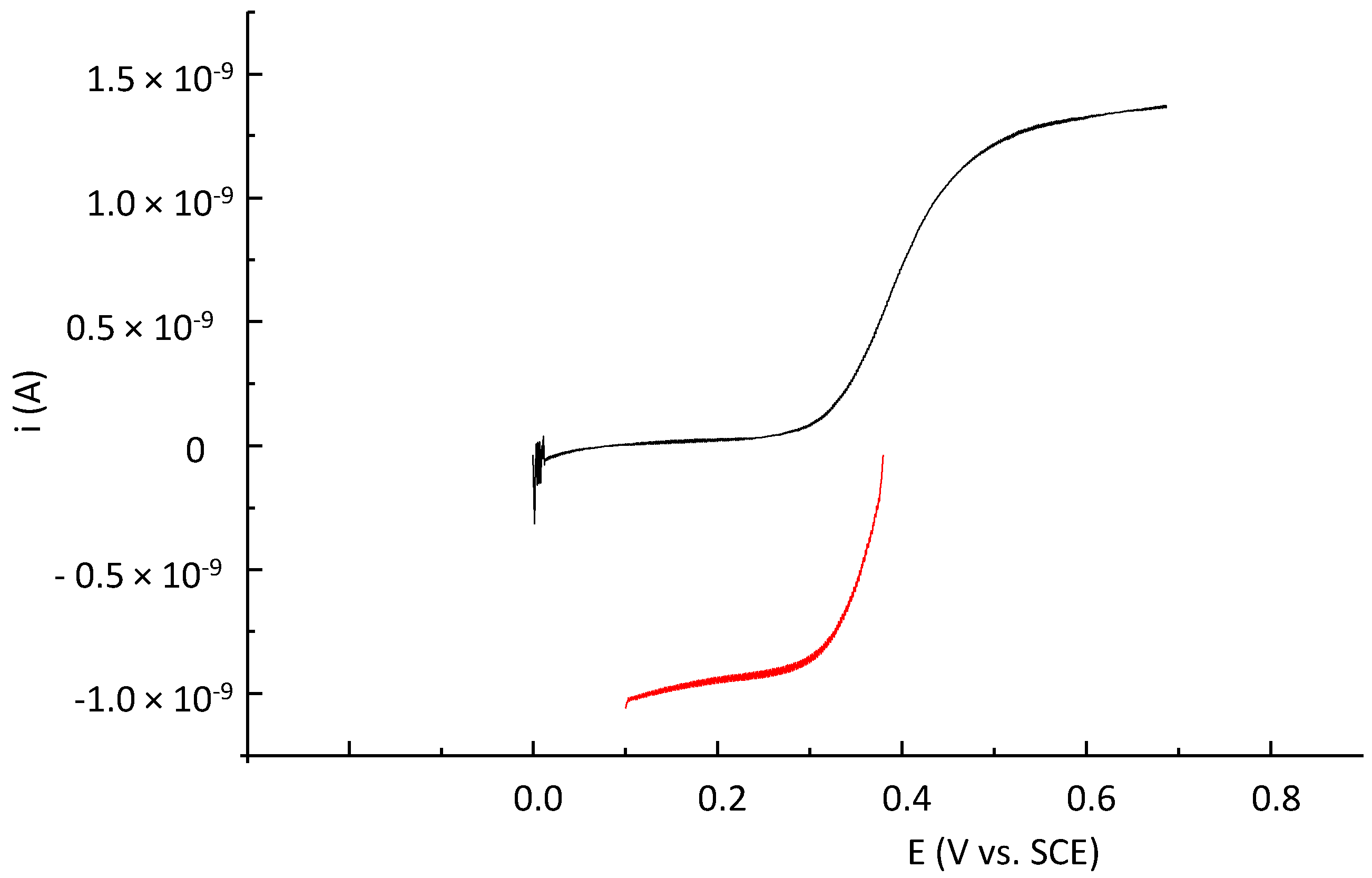
2.2. Fluorescence Properties of P794 and P797
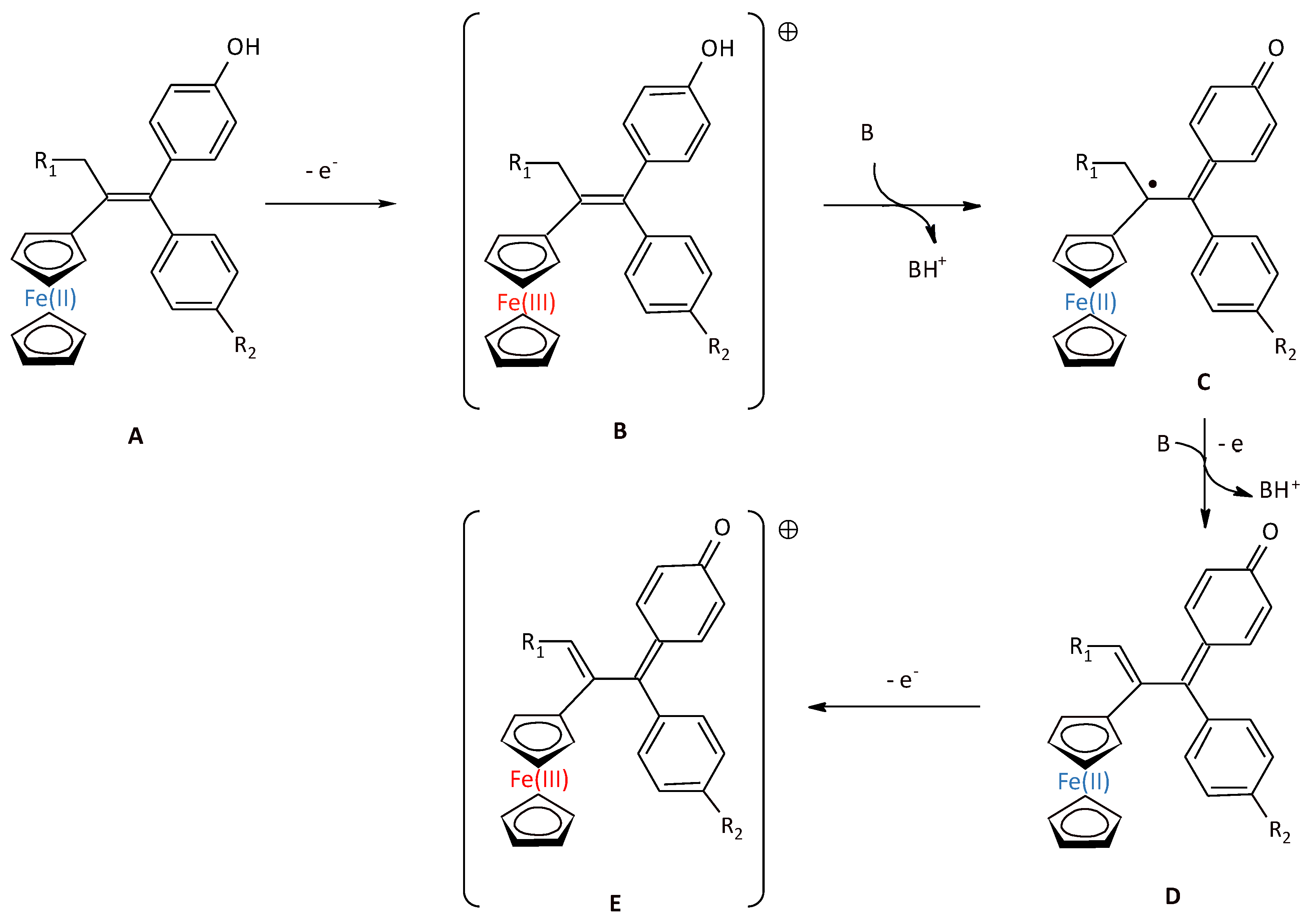
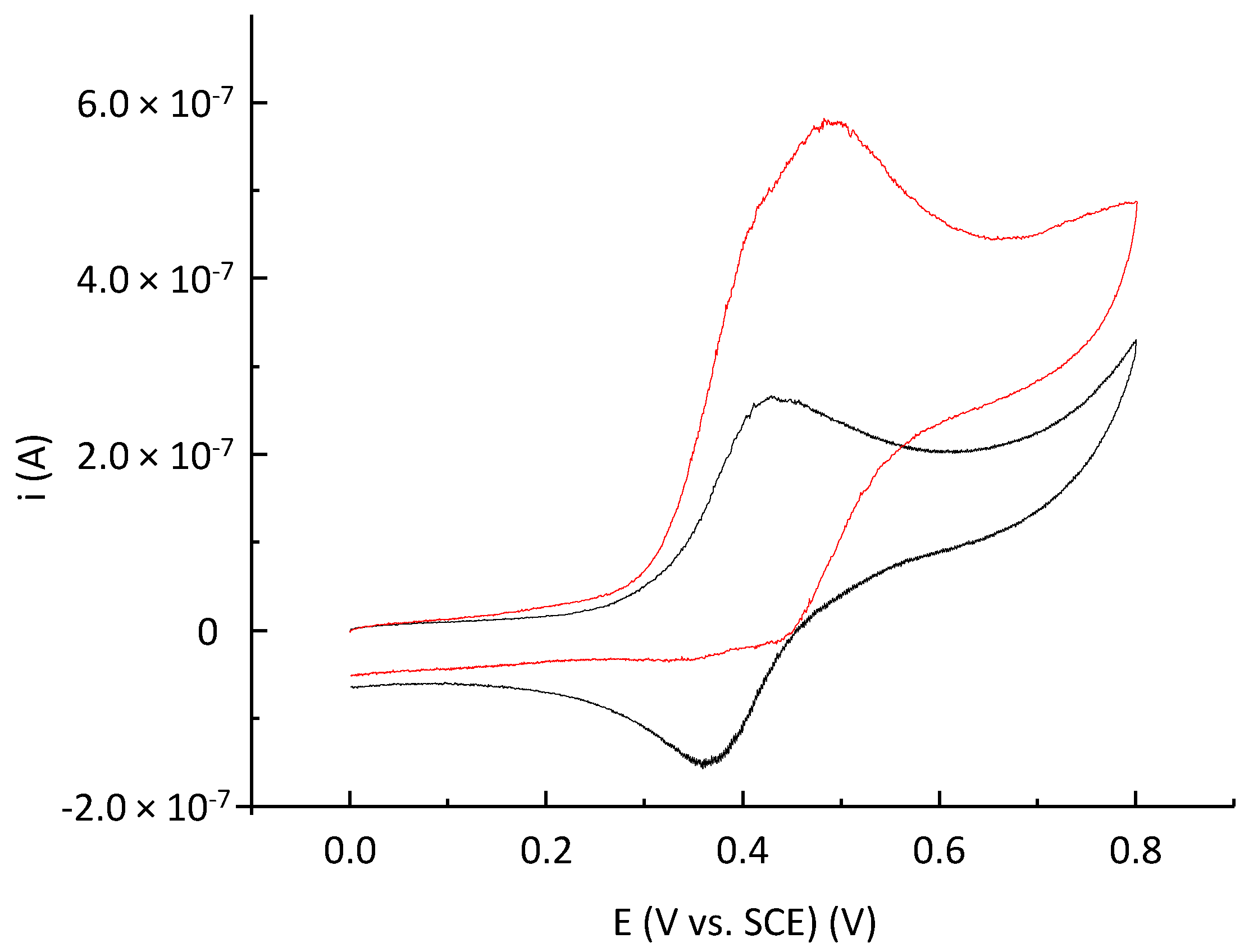
2.3. Electrochemical Behavior of P797 in Acetonitrile in the Absence and Presence of Imidazole Used as a Base
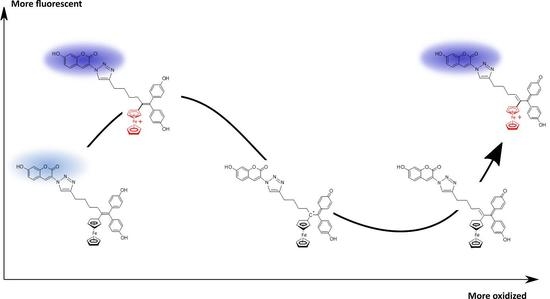
2.4. Electrochemical Preparation of P797 Oxidized States
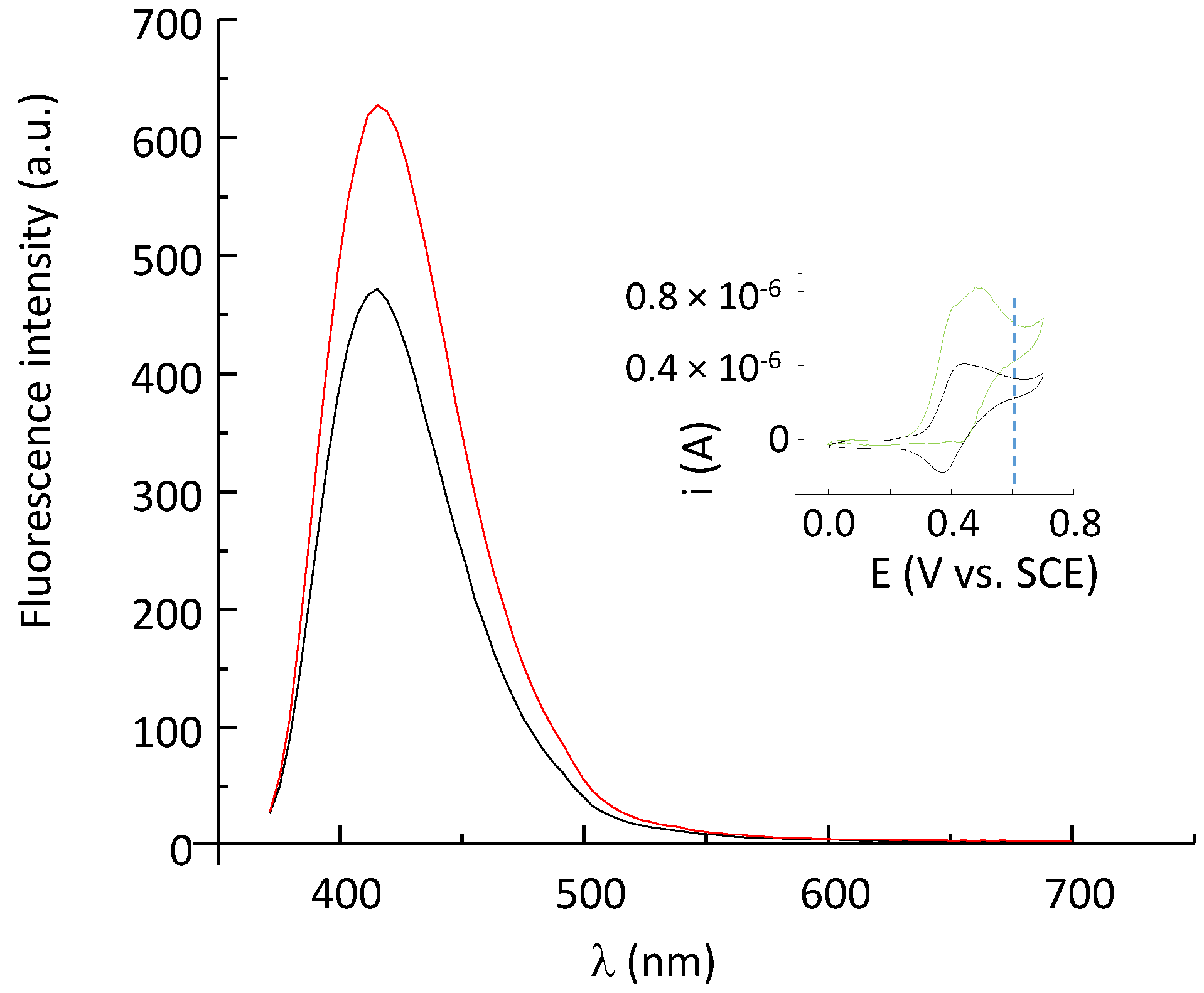
2.5. Fluorescence Properties of P797 in Its Reduced and Oxidized Forms
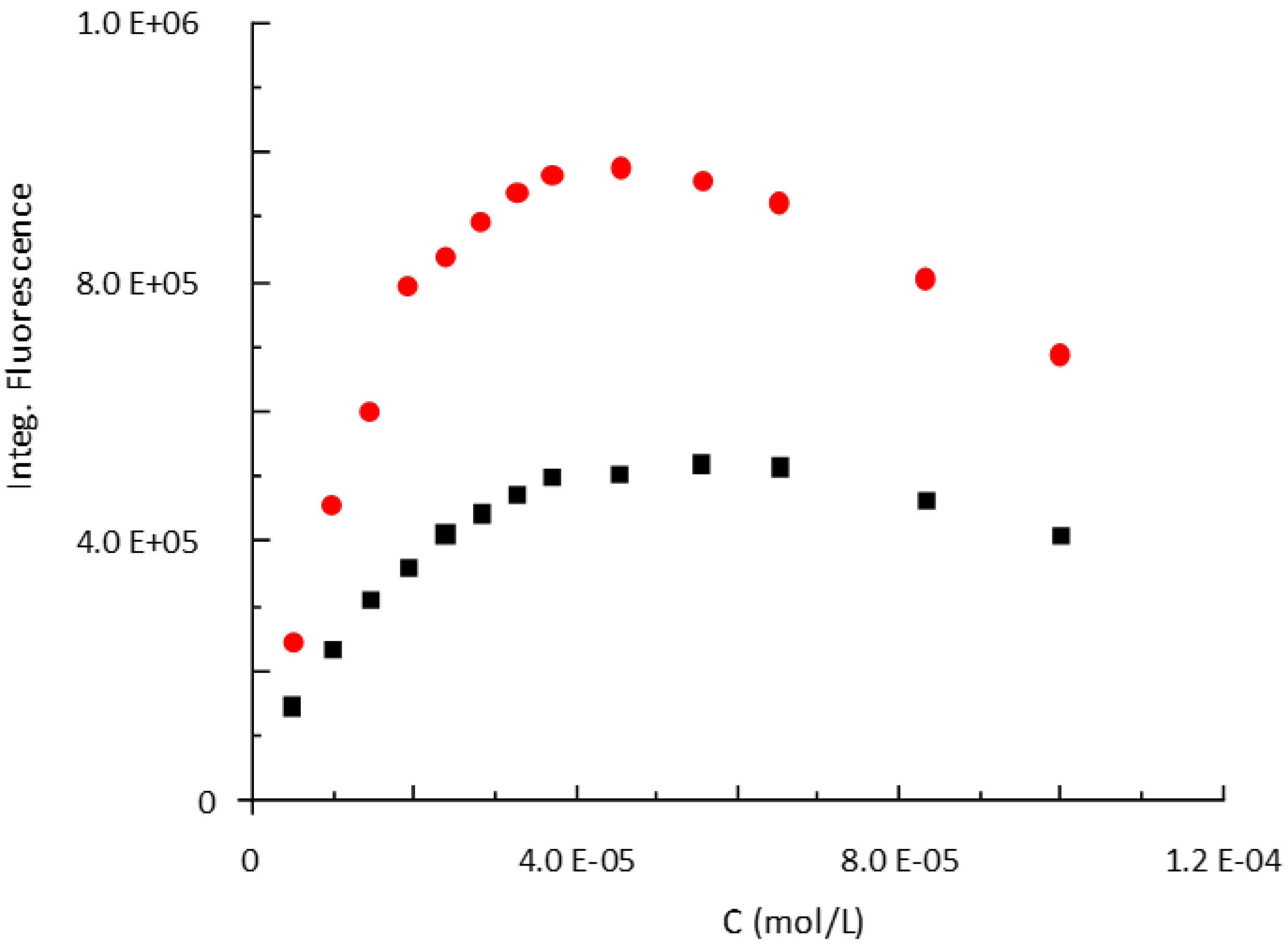
2.6. Determination of the Quantum Yields of P797 in Its Reduced and Oxidized Form
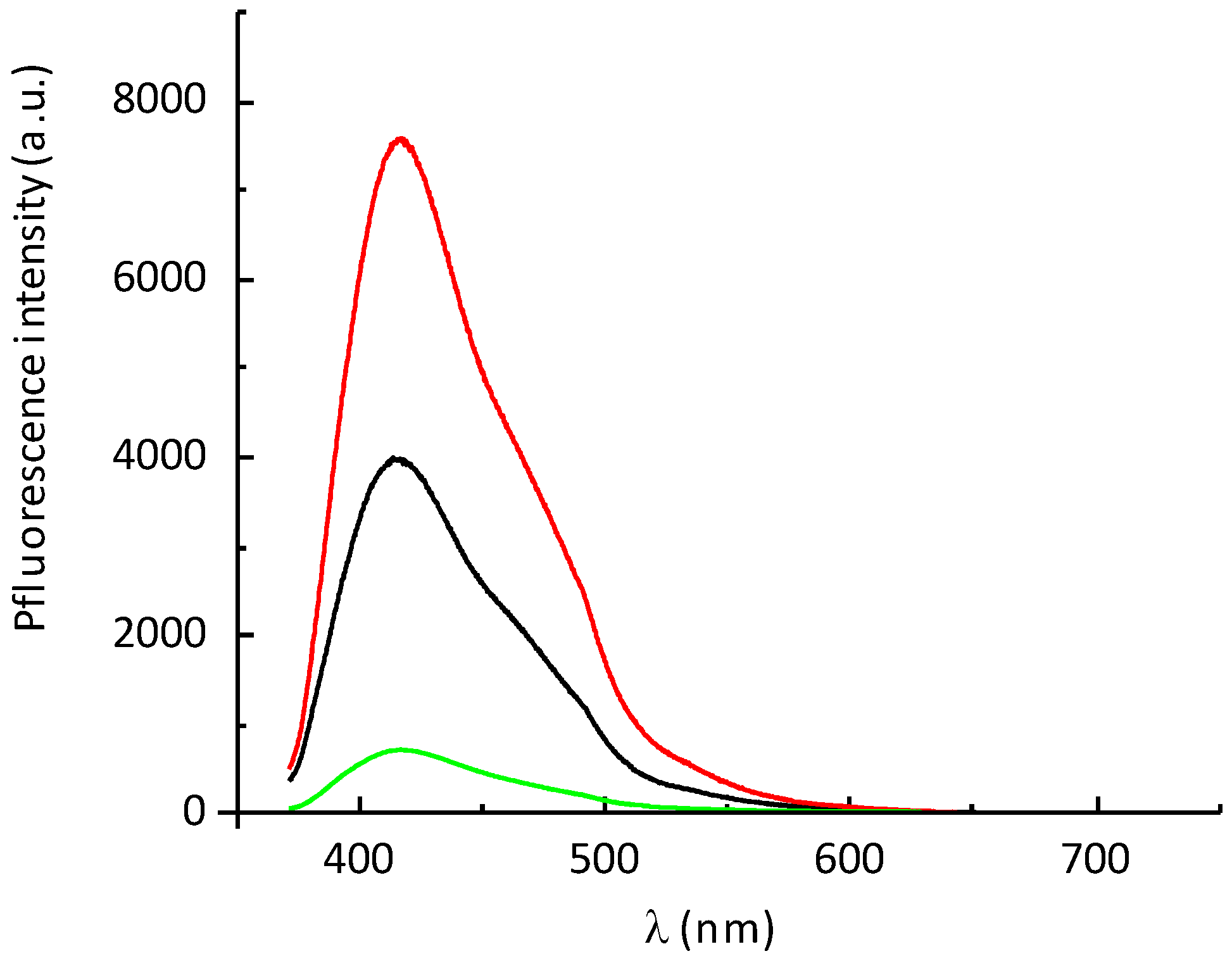
2.7. Fluorescence Properties of P797 in the Presence of Imidazole
3. Materials and Methods
3.1. Synthesis of Coumarin-Functionalized Ferrociphenols P794 and P797
3.1.1. Synthesis of 2a and 2b via a McMurry Coupling
2-Ferrocenyl-1,1-bis-(4-hydroxyphenyl)-hept-1-en-6-yne (2a)
2-Ferrocenyl-1,1-bis-(4-hydroxyphenyl)-oct-1-en-7-yne (2b)
3.1.2. Synthesis of Ferrocifen Coumarin Dyads 3a and 3b via a Click Coupling Procedure
7-Hydroxy-3-(4-[35]-[1,2,3]triazol-1-yl)-chromen-2-one (3a, P794) (0.24 g, 81% Yield, Orange Solid)
7-Hydroxy-3-(4-[15,33,36]-[1,2,3]triazol-1-yl)-chromen-2-one (3b, P797). (0.25 g, 68% Yield, Orange Solid)
3.2. Electrochemical and Fluorescence Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Etrych, T.; Lucas, H.; Janoušková, O.; Chytil, P.; Mueller, T.; Mäder, K. Fluorescence optical imaging in anticancer drug delivery. J. Control. Release 2016, 226, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Ballou, B.; Ernst, L.A.; Waggoner, A.S. Fluorescence imaging of tumors in vivo. Curr. Med. Chem. 2005, 12, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Passador, K.; Goze, C.; Denat, F.; Bodio, E.; Salmain, M. Metal-based BODIPY derivatives as multimodal tools for life sciences. Coord. Chem. Rev. 2018, 358, 108–124. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Ahumada, G.; Borkowska, M. Fluorescent Polymers Conspectus. Polymers 2022, 14, 1118. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef]
- Licandro, E.; Panigati, M.; Salmain, M.; Vessieres, A. Organometallic Bioprobes for Cellular Imaging. In Bioorganometallic Chemistry; Jauen, G., Salmain, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 341–391. [Google Scholar]
- Qian, X.; Xu, Z. Fluorescence imaging of metal ions implicated in diseases. Chem. Soc. Rev. 2015, 44, 4487–4493. [Google Scholar] [CrossRef]
- Bergonzi, R.; Fabbrizzi, L.; Licchelli, M.; Mangano, C. Molecular switches of fluorescence operating through metal centred redox couples. Coord. Chem. Rev. 1998, 170, 31–46. [Google Scholar] [CrossRef]
- Kaur, A.; Kolanowski, J.L.; New, E.J. Reversible Fluorescent Probes for Biological Redox States. Angew. Chem. Int. Ed. 2016, 55, 1602–1613. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Delavaux-Nicot, B. Ferrocene and ferrocenyl derivatives in luminescent systems. J. Photochem. Photobiol. A-Chem. 2000, 132, 137–159. [Google Scholar] [CrossRef]
- Escudero, D. Revising intramolecular photoinduced electron transfer (PET) from first-principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef]
- Sabuzi, F.; Coletti, A.; Pomarico, G.; Floris, B.; Galloni, P.; Conte, V. Modulating electron transfer in ferrocene-naphthoquinone dyads: New insights in parameters influencing ET efficiency. J. Organomet. Chem. 2019, 885, 49–58. [Google Scholar] [CrossRef]
- Spiteri, J.C.; Johnson, A.D.; Denisov, S.A.; Jonusauskas, G.; McClenaghan, N.D.; Magri, D.C. A fluorescent AND logic gate based on a ferrocene-naphthalimide-piperazine format responsive to acidity and oxidizability. Dye. Pigment. 2018, 157, 278–283. [Google Scholar] [CrossRef]
- Karmakar, M.; Bhatta, S.R.; Giri, S.; Thakur, A. Oxidation-Induced Differentially Selective Turn-On Fluorescence via Photoinduced Electron Transfer Based on a Ferrocene-Appended Coumarin-Quinoline Platform: Application in Cascaded Molecular Logic. Inorg. Chem. 2020, 59, 4493–4507. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Bheemireddy, V.; Thalcur, A. A Redox-Driven Fluorescence “Off-On” Molecular Switch Based on a 1,1’-Unsymmetrically Substituted Ferrocenyl Coumarin System: Mimicking Combinational Logic Operation. Organometallics 2017, 36, 829–838. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Ozkan, H.G.; Daum, S.; Janko, C.; Alexiou, C.; Sauer, C.; Heinrich, M.R.; Mokhir, A. N-Alkylaminoferrocene-Based Prodrugs Targeting Mitochondria of Cancer Cells. Molecules 2020, 25, 2545. [Google Scholar] [CrossRef]
- Xu, H.G.; Schikora, M.; Sisa, M.; Daum, S.; Klemt, I.; Janko, C.; Alexiou, C.; Bila, G.; Bilyy, R.; Gong, W.J.; et al. An Endoplasmic Reticulum Specific Pro-amplifier of Reactive Oxygen Species in Cancer Cells. Angew. Chem.-Int. Ed. 2021, 60, 11158–11162. [Google Scholar] [CrossRef]
- Gizem Özkan, H.; Toms, J.; Maschauer, S.; Prante, O.; Mokhir, A. Aminoferrocene-Based Anticancer Prodrugs Labelled with Cyanine Dyes for in vivo Imaging. Eur. J. Inorg. Chem. 2021, 2021, 5096–5102. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessieres, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef]
- Hillard, E.; Vessieres, A.; Thouin, L.; Jaouen, G.; Amatore, C. Ferrocene-mediated proton-coupled electron transfer in a series of ferrocifen-type breast-cancer drug candidates. Angew. Chem. -Int. Ed. 2006, 45, 285–290. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Jaouen, G. Organometallic Antitumor Compounds: Ferrocifens as Precursors to Quinone Methides. Angew. Chem.-Int. Ed. 2015, 54, 10230–10233. [Google Scholar] [CrossRef]
- Pigeon, P.; Top, S.; Zekri, O.; Hillard, E.A.; Vessieres, A.; Plamont, M.-A.; Buriez, O.; Labbé, E.; Huche, M.; Boutamine, S.; et al. The replacement of a phenol group by an aniline or acetanilide group enhances the cytotoxicity of 2-ferrocenyl-1,1-diphenyl-but-1-ene compounds against breast cancer cells. J. Organomet. Chem. 2009, 694, 895–901. [Google Scholar] [CrossRef]
- Cazares-Marinero, J.D.; Labbé, E.; Top, S.; Buriez, O.; Amatore, C.; Jaouen, G. The effect of protic electron donor aromatic substituents on ferrocenic and 3 ferrocenophanic anilines and anilides: Some aspects of structure-activity relationship studies on organometallic compounds with strong antiproliferative effects. J. Organomet. Chem. 2013, 744, 92–100. [Google Scholar] [CrossRef]
- Cazares-Marinero, J.d.J.; Buriez, O.; Labbé, E.; Top, S.; Amatore, C.; Jaouen, G. Synthesis, Characterization, and Antiproliferative Activities of Novel Ferrocenophanic Suberamides against Human Triple-Negative MDA-MB-231 and Hormone-Dependent MCF-7 Breast Cancer Cells. Organometallics 2013, 32, 5926–5934. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999, 576, 254–278. [Google Scholar] [CrossRef]
- Jutand, A. Contribution of electrochemistry to organometallic catalysis. Chem. Rev. 2008, 108, 2300–2347. [Google Scholar] [CrossRef]
- Čížková, M.; Cattiaux, L.; Pandard, J.; Guille-Collignon, M.; Lemaître, F.; Delacotte, J.; Mallet, J.M.; Labbé, E.; Buriez, O. Redox switchable rhodamine-ferrocene dyad: Exploring imaging possibilities in cells. Electrochem. Commun. 2018, 97, 46–50. [Google Scholar] [CrossRef]
- de Oliveira, R.; Durand, M.; Challier, L.; Messina, P.; Swiecicki, J.M.; Di Pisa, M.; Chassaing, G.; Lavielle, S.; Buriez, O.; Labbe, E. Electrochemical quenching of the fluorescence produced by NBD-labelled cell penetrating peptides: A contribution to the study of their internalization in large unilamellar vesicles. J. Electroanal. Chem. 2017, 788, 225–231. [Google Scholar] [CrossRef]
- Buriez, O.; Perez Jimenez, A.I.; Challier, L.; Aït-Yahiatène, E.; Delacotte, J.; Labbé, E. Selective Electrochemical Bleaching of the Outer Leaflet of Fluorescently Labelled Giant Liposomes. Chem. A Eur. J. 2017, 23, 6781–6787. [Google Scholar] [CrossRef]
- Fox, M.A. Photoinduced Electron Transfer. Photochem. Photobiol. 1990, 52, 617–627. [Google Scholar] [CrossRef]
- Kondo, T.; Kanai, T.; Iso-o, K.; Uosaki, K. Effects of Alkylchain Length on the Efficiency of Photoinduced Electron Transfer at Gold Electrodes Modified with Self-Assembled Monolayers of Molecules Containing Porphyrin, Ferrocene and Thiol Separated each other by Alkylchains. J. Z. Für Phys. Chem. 1999, 212, 23–30. [Google Scholar] [CrossRef]
- Plazuk, D.; Vessieres, A.; Hillard, E.A.; Buriez, O.; Labbé, E.; Pigeon, P.; Plamont, M.-A.; Amatore, C.; Zakrzewski, J.; Jaouen, G. A 3 Ferrocenophane Polyphenol Showing a Remarkable Antiproliferative Activity on Breast and Prostate Cancer Cell Lines. J. Med. Chem. 2009, 52, 4964–4967. [Google Scholar] [CrossRef]
- Tan, Y.L.K.; Pigeon, P.; Top, S.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessieres, A.; Amatore, C.; Leong, W.K.; Jaouen, G. Ferrocenyl catechols: Synthesis, oxidation chemistry and anti-proliferative effects on MDA-MB-231 breast cancer cells. Dalton Trans. 2012, 41, 7537–7549. [Google Scholar] [CrossRef]
- Messina, P.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessieres, A.; Hamels, D.; Top, S.; Jaouen, G.; Frapart, Y.M.; Mansuy, D.; et al. Deciphering the Activation Sequence of Ferrociphenol Anticancer Drug Candidates. Chem.-A Eur. J. 2012, 18, 6581–6587. [Google Scholar] [CrossRef]
- Buriez, O.; Labbé, E.; Amatore, C. Electrochemistry and Supramolecular Interactions of “Ferrocifen” Anticancer Drugs with Cyclodextrins and Lipid Bilayers: An Electrochemical Overview. In Advances in Organometallic Chemistry and Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 631–651. [Google Scholar] [CrossRef]
- Buriez, O.; Labbé, E. Disclosing the redox metabolism of drugs: The essential role of electrochemistry. Curr. Opin. Electrochem. 2020, 24, 63–68. [Google Scholar] [CrossRef]
- Amatore, C.; Labbé, E.; Buriez, O. Molecular electrochemistry: A central method to understand the metabolic activation of therapeutic agents. The example of metallocifen anti-cancer drug candidates. Curr. Opin. Electrochem. 2017, 2, 7–12. [Google Scholar] [CrossRef]
- Liu, X.; Savy, A.; Maurin, S.; Grimaud, L.; Darchen, F.; Quinton, D.; Labbé, E.; Buriez, O.; Delacotte, J.; Lemaitre, F.; et al. A Dual Functional Electroactive and Fluorescent Probe for Coupled Measurements of Vesicular Exocytosis with High Spatial and Temporal Resolution. Angew. Chem. Int. Ed. Engl. 2017, 56, 2366–2370. [Google Scholar] [CrossRef]
- Liu, X.; Hu, L.; Pan, N.; Grimaud, L.; Labbé, E.; Buriez, O.; Delacotte, J.; Lemaître, F.; Guille-Collignon, M. Coupling electrochemistry and TIRF-microscopy with the fluorescent false neurotransmitter FFN102 supports the fluorescence signals during single vesicle exocytosis detection. Biophys. Chem. 2018, 235, 48–55. [Google Scholar] [CrossRef]
- Pandard, J.; Pan, N.; Ebene, D.H.; Le Saux, T.; Ait-Yahiatène, E.; Liu, X.; Grimaud, L.; Buriez, O.; Labbé, E.; Lemaître, F.; et al. A Fluorescent False Neurotransmitter as a Dual Electrofluorescent Probe for Secretory Cell Models. ChemPlusChem 2019, 84, 1578–1586. [Google Scholar] [CrossRef]
- Girault, H. Analytical and Physical Electrochemistry; EFPL Press: Lausanne, Switzerland, 2004. [Google Scholar] [CrossRef]
- Vessières, A.; Wang, Y.; McGlinchey, M.J.; Jaouen, G. Multifaceted chemical behaviour of metallocene (M = Fe, Os) quinone methides. Their contribution to biology. Coord. Chem. Rev. 2021, 430, 213658. [Google Scholar] [CrossRef]
- Lu, C.; Heldt, J.M.; Guille-Collignon, M.; Lemaitre, F.; Jaouen, G.; Vessieres, A.; Amatore, C. Quantitative Analyses of ROS and RNS Production in Breast Cancer Cell Lines Incubated with Ferrocifens. Chemmedchem 2014, 9, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, G.; Schoof, S.; Gusten, H. 9, 10-diphenylanthracene as a fluorescence quantum yield standard. J. Photochem. 1974, 3, 315–320. [Google Scholar] [CrossRef]
- Morris, J.V.; Mahaney, M.A.; Huber, J.R. Fluorescence quantum yield determinations—9,10-diphenylanthracene as a reference-standard in different solvents. J. Phys. Chem. 1976, 80, 969–974. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Green, J.A.; Singer, L.A.; Parks, J.H. Fluorescence quenching by stable free-radical di-tert-butylnitroxide. J. Chem. Phys. 1973, 58, 2690–2695. [Google Scholar] [CrossRef]
- Plażuk, D.; Zakrzewski, J. Friedel–Crafts acylation of ferrocene with alkynoic acids. J. Organomet. Chem. 2009, 694, 1802–1806. [Google Scholar] [CrossRef]
- Sivakumar, K.; Xie, F.; Cash, B.M.; Long, S.; Barnhill, H.N.; Wang, Q. A Fluorogenic 1,3-Dipolar Cycloaddition Reaction of 3-Azidocoumarins and Acetylenes. Org. Lett. 2004, 6, 4603–4606. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayolle, C.; Pigeon, P.; Fischer-Durand, N.; Salmain, M.; Buriez, O.; Vessières, A.; Labbé, E. Synthesis, Electrochemical and Fluorescence Properties of the First Fluorescent Member of the Ferrocifen Family and of Its Oxidized Derivatives. Molecules 2022, 27, 6690. https://doi.org/10.3390/molecules27196690
Fayolle C, Pigeon P, Fischer-Durand N, Salmain M, Buriez O, Vessières A, Labbé E. Synthesis, Electrochemical and Fluorescence Properties of the First Fluorescent Member of the Ferrocifen Family and of Its Oxidized Derivatives. Molecules. 2022; 27(19):6690. https://doi.org/10.3390/molecules27196690
Chicago/Turabian StyleFayolle, Charles, Pascal Pigeon, Nathalie Fischer-Durand, Michèle Salmain, Olivier Buriez, Anne Vessières, and Eric Labbé. 2022. "Synthesis, Electrochemical and Fluorescence Properties of the First Fluorescent Member of the Ferrocifen Family and of Its Oxidized Derivatives" Molecules 27, no. 19: 6690. https://doi.org/10.3390/molecules27196690
APA StyleFayolle, C., Pigeon, P., Fischer-Durand, N., Salmain, M., Buriez, O., Vessières, A., & Labbé, E. (2022). Synthesis, Electrochemical and Fluorescence Properties of the First Fluorescent Member of the Ferrocifen Family and of Its Oxidized Derivatives. Molecules, 27(19), 6690. https://doi.org/10.3390/molecules27196690