Abstract
(1) Background: Encapsulation of surfactants is an innovative approach that allows not only protection of the active substance, but also its controlled and gradual release. This is primarily used to protect metallic surfaces against corrosion or to create biologically active surfaces. Gemini surfactants are known for their excellent anticorrosion, antimicrobial and surface properties; (2) Methods: In this study, we present an efficient methods of preparation of encapsulated gemini surfactants in form of alginate and gelatin capsules; (3) Results: The analysis of infrared spectra and images of the scanning electron microscope confirm the effectiveness of encapsulation; (4) Conclusions: Gemini surfactants in encapsulated form are promising candidates for corrosion inhibitors and antimicrobials with the possibility of protecting the active substance against environmental factors and the possibility of controlled outflow.
1. Introduction
Surfactants are chemical compounds that upon adsorption at the air–water interface reduce the surface tension of water. Their molecules contain at least two moieties, hydrophobic and hydrophilic. The hydrophobic part is usually a straight or branched hydrocarbon or fluorocarbon chain with 8–18 carbon atoms, whereas a hydrophilic moiety is a polar or ionic group. The head group can be cationic, anionic, amphoteric or nonionic [1]. The balance between hydrophobic and hydrophilic parts, and the hydrophilic-lipophilic balance, is responsible for the self-assembly process of these amphiphilic compounds in solutions [2]. It affects the properties of the compounds, which generates their use in many areas of life and industry, starting from cleaning agents and detergents [3,4,5,6], through to protective applications such as antimicrobials [7,8,9,10] or anticorrosion products [11,12,13,14,15], to modern biomedical applications [16,17,18,19,20,21] or enhanced oil recovery [22,23,24,25,26]. Surfactants may also have two hydrocarbon chains attached to a polar head and are named double chain surfactants. They can be also anionic, cationic, amphoteric or nonionic, but of greatest practical importance are their double quaternary ammonium salts, named as gemini surfactants by Menger and Littau [27]. Cationic gemini surfactants are made of two single alkylammonium monomeric salts connected by a spacer. The nature of the spacer can be very different; it can be a flexible polymethylene chain or a rigid aromatic ring, or a short or long hydrocarbon chain. Spacers can be hydrophobic or hydrophilic in nature with additional polar organic groups [2,28,29,30,31]. The type of spacer determines the properties of the gemini surfactants, significantly influencing their hydrophilic-lipophilic balance and potential application [32,33,34,35,36]. The substituents in gemini surfactants are also responsible to a great extent for the behavior of these compounds in solution and their possible applications [2,37]. From an ecological point of view, the functionalization of substituents with groups of natural origin is of great importance, because it can increase their biological profile, improving biodegradability or reducing ecotoxicity [8,38]. Gemini surfactants are referred to as multifunctional, because one compound may be an exceptionally effective surface active agent [39,40,41], corrosion and biocorrosion inhibitor [42,43,44,45,46,47], but also has remarkably high antibacterial [48,49,50,51] and antifungal [52,53,54,55,56,57] activity and is effective in preventing biofilm eradication [52,58]. It is worth emphasizing that gemini surfactants work effectively at a very low concentration, often several times smaller in comparison to single quaternary ammonium salts [59,60,61,62,63]. This is very important from an ecological point of view, in that to achieve the desired utility effect, the minimal concentration of compounds are used [64,65,66,67].
Encapsulation is a process of enclosing or entrapping a core material (active agent) inside a solid shell or within a solid or liquid matrix. The different encapsulation techniques can provide various microcapsule morphologies (Figure 1) [68,69].
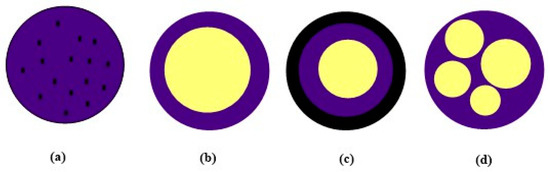
Figure 1.
Morphologies of microcapsules: (a) microsphere (matrix type), (b) core/shell, (c) core/multi-shell and (d) multicore/shell.
Recent decades have witnessed the rapid development of encapsulation techniques leading to the industrial use of capsules, especially in food and pharmaceutical/medicinal industries. Encapsulation in the food industry ensures several functions, including masking of undesirable color/flavor/taste, preservation of unstable components, incorporation of additional functional and nutritional constituents and site-specific release of encapsulated ingredients at a controlled time and rate [70,71,72]. In the pharmaceutical industry, encapsulation is commonly adopted in drug delivery systems to form capsules that improve functionality and solubility in order to protect the active agent of the medicine and prevent it from leaching out before reaching the targeted site [73,74,75,76]. Generally, the main purpose of the encapsulation is to achieve a surface with a permanent acting active agent or a surface with a slowed and, in many cases, controlled release of the substance. The release can be spontaneous or triggered by some kind of stimuli, such as humidity, pH, temperature, light, magnetic field, CO2 (acidification), redox and enzymes or mechanical impact [69]. The immobilization of biocidal agents by the encapsulation technique is another way to overcome some problems associated with the toxicity and the lifetime of conventional strategies for the incorporation of agents. For many substances, e.g., antimicrobials are chemically fixed in a microcapsule shell where the uncontrolled release is strongly restricted. Therefore, encapsulated microbiocides are potentially non-toxic and environmentally friendly materials [77,78,79]. Recently, encapsulated corrosion inhibitors have started to be put into use to ensure constant access of the active substance to protect the material according to slow and controlled release or to fill the surface with self-healing properties [80,81,82,83,84,85]. Very popular carriers in encapsulation processes are natural polymers, especially proteins and polysaccharides including agarose, alginate, carrageenan, chitosan, hyaluronic acid, collagen, elastin, gelatin, fibrin, and silk fibroin [86]. Thus, it is essential to select the appropriate biopolymer material to formulate nanoparticles for practice applications. Polysaccharides have attracted considerable interest in this area due to their good biocompatibility and biodegradability and low costs [87].
The purpose of this paper is to report the fabrication of biodegradable encapsulated gemini surfactants. In reference to the need to discover materials with good biological profiles, we used gelatin and sodium alginate as a shell materials and gemini surfactants functionalized by ester groups in substituents parts. These kinds of gemini surfactants exhibit great antimicrobial [88,89,90] or anticorrosion [91] properties and better biodegradability than conventional analogues [36,92,93,94]. To our knowledge, the encapsulation technique using alginate [95,96,97] and gelatin [98,99] is well-known and described, but information about encapsulated gemini surfactants is lacking.
2. Results and Discussion
2.1. Synthesis
Gemini surfactants (GS) were obtained by innovative synthesis without a solvent (Scheme 1), according to the preparation developed in our laboratory and described previously [100]. This method ensures the best results in the shortest period of time. The reactions proceed according to the mechanism of nucleophilic substitution SN2, which go most quickly when the concentrations of the reagents are the highest. This is what makes synthesis possible without a solvent. Moreover, such reactions are in line with the assumptions of green chemistry, as the use of reagents and energy is limited. The synthetic details are given in the Supplementary Materials.
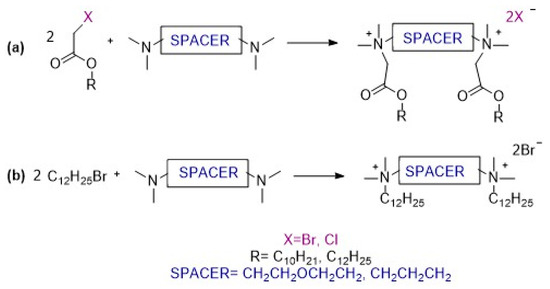
Scheme 1.
Synthesis of gemini surfactants: (a) functionalized by ester groups in substituent part; (b) with dodecyl substituents.
In the presented syntheses, we obtained six products, four gemini surfactants with ester bonds in substituents. For comparison purposes, classic gemini surfactants with a hydrocarbon chain in the substituent were also prepared. Particularly noteworthy is the compound 3-oxa-1,5-pentane-bis(N-dodecyl-N,N-dimethylammonium bromide) (12-O-12), which exhibits the unique antimicrobial and surface activity described by us earlier [29,67]. The structural formulas and designations of the obtained products are shown in Figure 2.
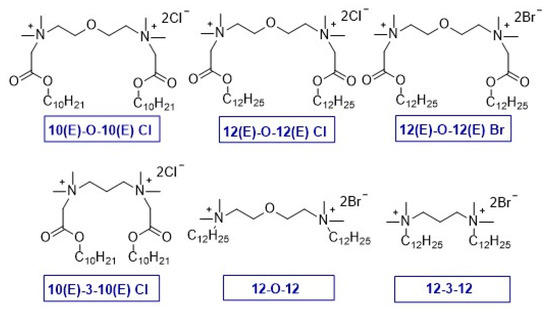
Figure 2.
Chemical formulas of obtained gemini surfactants.
2.2. Analysis of Gemini Surfactants
The purities of synthesized gemini surfactants were also confirmed by spectroscopy methods (1H NMR, 13C NMR, FTIR).
10(E)-3-10(E) Cl: mp 155–156 °C; 1H NMR (403 MHz, CDCl3) δ 4.85 (4H, N+CH2COO-), 4.17 (4H, O-CH2-), 4.00 (4H, N+CH2- spacer), 3.65 (12H, N+CH3), 2.72 (2H, -CH2- spacer), 1.66 (4H, O-CH2-CH2-), 1.30-1.26 (28H, O-CH2-CH2-(CH2)7-), 0.88 (6H, -CH2CH3); 13C NMR (101MHz, CDCl3) δ 164.90 (C=O), 66.87 (N+CH2COO-), 61.76 (O-CH2-), 61.41(N+CH2- spacer), 52.05 (N+CH3), 29.53, 29.47, 29.28, 29.19, 28.24, 22.65 (O-CH2-(CH2)8-), 25.61 (-CH2- spacer), 14.09 (-CH2CH3).
10(E)-O-10(E) Cl: mp 149–151 °C; 1H NMR (403 MHz, CDCl3) δ 4.97 (4H, N+CH2COO-), 4.33 (8H -CH2- spacer), 4.16 (4H, O-CH2-), 3.71 (12H, N+CH3), 1.65 (4H, O-CH2-CH2-), 1.31–1.25 (28H, O-CH2-CH2-(CH2)7-), 0.88 (6H, -CH2CH3); 13C NMR (101MHz, CDCl3) δ 165.06 (C=O), 66.71 (N+CH2COO-), 64.54 (-CH2O spacer), 62.03 (N+CH2 spacer), 61.36 (O-CH2-), 52.41 (N+CH3), 29.52, 29.45, 29.26, 29.17,28.27, 22.62 (O-CH2-(CH2)8-), 14.08 (-CH2CH3).
12(E)-O-12(E) Cl: mp 162–163 °C; 1H NMR (403 MHz, CDCl3) δ 4.92 (4H, N+CH2COO-), 4.16 (8H -CH2- spacer), 3.75 (4H, O-CH2-), 3.69 (12H, N+CH3), 1.70 (4H, O-CH2-CH2-), 1.39–1.25 (36H, O-CH2-CH2-(CH2)9-), 0.87 (6H, -CH2CH3); 13C NMR (101MHz, CDCl3) δ 165.05 (C=O), 66.75 (N+CH2COO-), 64.43 (-CH2O spacer), 62.15 (N+CH2 spacer), 59.00 (O-CH2-), 52.59 (N+CH3), 31.82, 29.47, 29.42, 29.16, 28.22, 25.61, 22.64 (O-CH2-(CH2)10-), 14.10 (-CH2CH3).
12(E)-O-12(E) Br: mp 171–172 °C; 1H NMR (403 MHz, CDCl3) δ 4.95 (4H, N+CH2COO-), 4.37 (8H -CH2- spacer), 4.17 (4H, O-CH2-), 3.74 (12H, N+CH3), 1.66 (4H, O-CH2-CH2-), 1.30–1.26 (36H, O-CH2-CH2-(CH2)9-), 0.88 (6H, -CH2CH3); 13C NMR (101MHz, CDCl3) δ 164.84 (C=O), 66.84 (N+CH2COO-), 64.43 (-CH2O spacer), 62.57 (N+CH2 spacer), 62.15 (O-CH2-), 52.68 (N+CH3), 31.88, 29.61, 29.32, 29.17, 28.27, 25.62, 22.66 (O-CH2-(CH2)10-), 14.08 (-CH2CH3).
12-3-12: mp 213–215 °C; 1H NMR (403 MHz, CDCl3) δ 3.88 (4H, N+CH2-), 3.54 (4H, N+CH2CH2-), 3.42 (12H, N+CH3), 2.74 (2H -CH2- spacer), 1.80 (4H, N+-CH2-CH2-), 1.36–1.26 (36H, N+-CH2-CH2-(CH2)9-), 0.88 (6H, -CH2CH3); 13C NMR (101MHz, CDCl3) δ 66.51 (N+CH2-), 60.92 (N+-CH2- spacer), 51.16 (N+CH3), 31.81, 29.53, 29.42, 29.37, 29.25, 29.18, 26.88 (N+-CH2-(CH2)10-), 22.60 (-CH2- spacer), 14.05 (-CH2CH3).
12-O-12: mp 248–249 °C; 1H NMR (403 MHz, CDCl3) δ 4.35 (4H,-OCH2 spacer), 4.04 (4H, N+CH2CH2O- spacer), 3.63 (4H, N+CH2-) 3.46 (12H, N+CH3), 1.73 (4H, N+CH2CH2-), 1.45–1.17 (36H, N+-CH2-CH2-(CH2)9-) -), 0.88 (6H, -CH2CH3); 13C NMR (101MHz, CDCl3) δ 65.81 (N+CH2CH2O spacer), 64.57 (-OCH2 spacer), 63.94 (N+CH2-), 51.60 (N+CH3), 31.82, 29.53, 29.46, 29.38, 29.28, 29.26, 26.27,22.60 (N+-CH2-(CH2)10-), 14.05 (-CH2CH3).
The Fourier transform infrared spectroscopy (FTIR) spectra for all obtained gemini surfactants have typical bands for these kind of chemical compounds (Table 1).

Table 1.
Characteristic FTIR absorption bands for synthesized gemini surfactants.
2.3. Fabrication and Analysis of Alginate Capsules (Al@GS)
In encapsulation of gemini surfactants by sodium alginate, we used complex formation, which can occur in an aqueous solution. We used calcium chloride as a crosslinker. Firstly, in a clear aqueous solution of gemini surfactant, sodium alginate was dissolved. The mixture was heated and stirred until homogeneous. It was then injected into a cross-linking solution containing calcium chloride. Sodium alginate is a polyelectrolyte biopolymer extracted from brown algae. It can be characterized as an anionic copolymer, which consists of 1–4 linked α-L-guluronic and β-D-mannuronic residues with a very diverse composition and structure sequence. Alginates are considered block copolymers. Alginates form a gel in the presence of divalent cations such as Ca2+. After interaction with the ions of divalent metals, they form an ordered structure. These cations act as cross linkers between the functional groups of the alginate chains. Calcium alginate gelation is an irreversible and almost immediate process. This process is governed by the relative rate of diffusion of calcium ions and polymer molecules into the gelling zone and can therefore be expressed by the relationships used for other diffusion limited reaction systems [101]. After filtration from the aqueous solution, the encapsulated gemini surfactants were dried in an incubator, thus reducing their diameter several times, and reducing the weight by over 95% (Figure 3). The encapsulation efficiency was calculated as the ratio of the amount of encapsulated to the amount of gemini surfactant used, and in each case it exceeded 90%.

Figure 3.
Photograph with a digital microscope: (a) wet and dry Al@12-O-12 +BaSO4; (b) wet and dry Al@12-O-12.
Initial analysis of the capsule surface morphology was performed using a digital microscope. In Figure 3, we presented capsules of gemini surfactant 12-O-12. Subpoint (a) and (b) present ones with the addition of barium sulphate, the addition of which strengthens the coating and maintains its shape after drying. Subpoint (c) and (d) show capsules obtained without the addition of barium sulphate, which are transparent and visibly more deformed when dried. In order to compare the sizes, wet (a) and (c) and dry (b) and (d) capsules were put together. The addition of barium sulphate improves the strength of the capsules and also gives them a more uniform shape. However, it does not affect the size of the capsules; the average diameter of wet microcapsules is 10 mm a dry is 1.5 mm. The structure of the gemini surfactant used does not affect the size or appearance of the alginate capsules. Introduction of hydrophilic ester groups into gemini surfactants substituents has no effect on preparation and morphology of both wet and dry capsules (Figure 4).

Figure 4.
Photograph with a digital microscope: (a) wet Al@10(E)-O-10(E) Cl; (b) dry Al@10(E)-O-10(E) Cl.
Detailed studies of the surface morphology of alginate capsules were performed using a scanning electron microscope. By comparing the appearance and morphology of the surface of the empty capsules, it can be confirmed that the addition of barium sulphate results in the strengthening of the shell. It is visible in the macroscopic image as a white color. Moreover, alginate capsules prepared with BaSO4 are characterized by a more symmetrical, spherical structure (Figure 5).
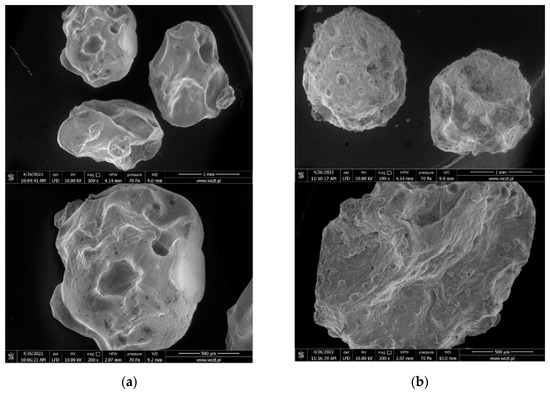
Figure 5.
Scanning electron micrographs of alginate capsules without gemini surfactant: (a) Al@empty; (b) Al@empty+BaSO4.
The alginate capsules filled with gemini surfactants apparently have a different, more varied morphology than the empty capsules (Figure 6). This proves that they contain an active substance. It can be stated that in the SEM images, the empty capsules are smoother, whereas those containing gemini surfactants are more pleated. The addition of barium sulphate in the case of capsules filled with gemini surfactant 12-O-12 (Al@12-O-12) strengthens the shell. The surface morphology of the capsules does not depend on the structure of the gemini surfactant (Figure 7).
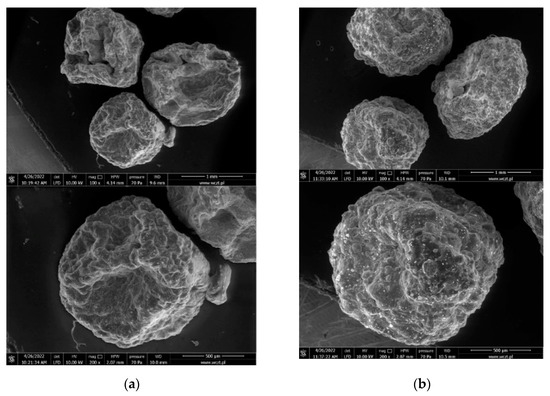
Figure 6.
Scanning electron micrographs of alginate capsules with gemini surfactants. (a) Al@12-O-12; (b) Al@12-O-12+BaSO4.
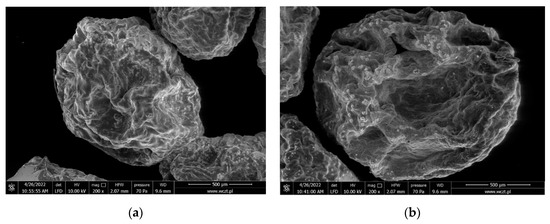
Figure 7.
Comparison of scanning electron micrographs of alginate capsules with different gemini surfactants: (a) Al@12-O-12; (b) Al@10(E)-3-10(E) Cl.
In order to confirm the encapsulation, FTIR spectra (ATR technic) were made after the capsules were thoroughly washed with water and dried. In Figure 8a, there is a spectrum of empty alginate capsules (Al@empty). The most intense band in the spectrum at 3347 cm−1 comes from the O-H stretching vibrations of the O-H hydroxyl group. The broadening of the band indicates the presence of hydrogen bonds. Another characteristic band at 1598 cm−1 is derived from the carbonyl group of the carboxylate. Remaining spectra derived from capsules filled with gemini surfactants prove the correct encapsulation path. In Figure 8b, there is a spectrum of alginate capsules with gemini surfactant 12-O-12 (Al@12-O-12) and in addition to the characteristic bands of alginate, bands derived from gemini surfactant can be seen, especially the bands coming from the C-H stretching vibrations of the methylene and methyl groups at 2854 cm−1 and 2922 cm−1. The last spectrum from capsules filled with gemini surfactant with ester groups (Al@10(E)-O-10(E) Cl) looks analogous, which has an additional band derived from ester carbonyl at 1748 cm−1.
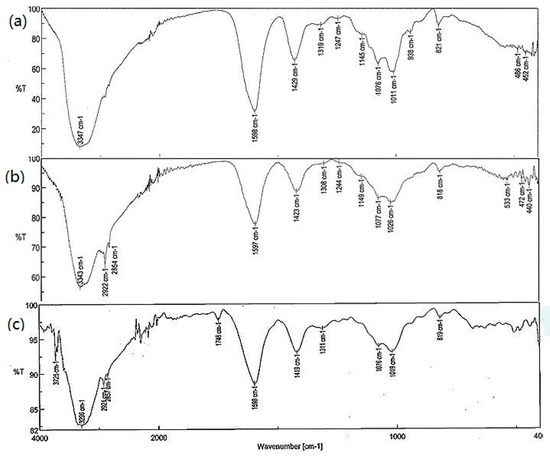
Figure 8.
FTIR spectra of alginate capsules without and with the gemini surfactants: (a) Al@empty; (b) Al@12-O-12; (c) Al@10(E)-O-10(E) Cl.
2.4. Fabrication and Analysis of Gelatine Capsules (Ge@GS)
Gelatin is a protein derived by hydrolysis from collagen, a naturally occurring protein. In the formation of gels, particles and microspheres gelatin is used particularly frequently in the pharmaceutical industry due to biocompatibility and non-toxicity [99]. Cross-linking of gelatin fibers can be carried out by various chemical methods (using sugars, dialdehydes, phenolic compounds), enzymatic and physical means or a combination of these [102]. In our work, we carried out thermal cross-linking with glucose. In this reaction mechanism, the aldehyde group of reducing sugars can react with the free amino groups of gelatin molecule resulting in the formation of an aminoglycoside. The aminoglycoside can further react with another amine group creating a cross-linked structure [99]. A water-in-oil emulsion was used. The dispersion medium was sunflower oil, and the surfactant used was Span 80 (sorbitan oleate), which was used to minimize aggregation of gelatin microcapsules in the oil solution. After filtration, the gelatin capsules with gemini surfactants were dried in an er, thus the desiccator reduced their diameter several times, and reduced the weight by over 95% (Figure 9).

Figure 9.
Photograph with a digital microscope: (a) wet Ge@12(E)-O-12(E) Cl; (b) dry Ge@12(E)-O-12(E).
Each capsule was characterized by a compact and wrinkled consistency, and on its surface one could see gelatin microcapsules stuck together. The most durable and, at the same time, the hardest form were empty gelatin capsules, while those filled with gemini surfactants were softer. Additionally, capsules with gemini surfactants showed less aggregation. All of the obtained capsules turned white. The encapsulation efficiency was calculated as the ratio of the amount of encapsulated to the amount of gemini surfactant used and, in each case, it exceeded 60%. The surface morphology of the gelatin capsules was analyzed on the basis of SEM images. Figure 10 presents a comparison of gelatin microspheres with and without gemini surfactant. The structure of gemini surfactant has an influence on morphology of gelatin microspheres (Figure 11).
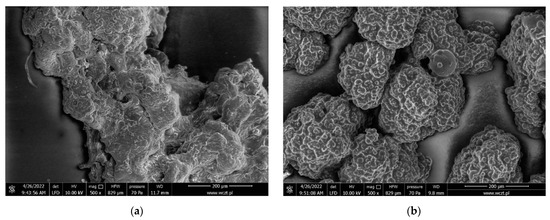
Figure 10.
Scanning electron micrographs of gelatin capsules without and with gemini surfactants. (a) Ge@empty; (b) Ge@12(E)-O-12(E) Cl.
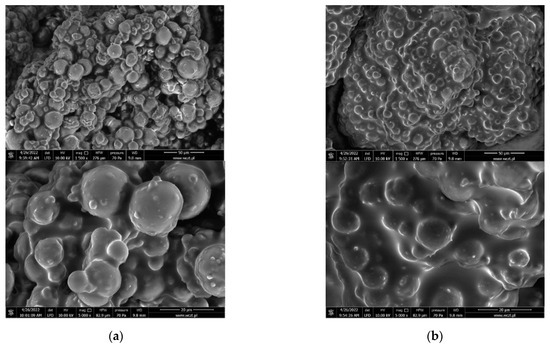
Figure 11.
Scanning electron micrographs of gelatin capsules with different gemini surfactants. (a) Ge@12(E)-O-12(E) Br; (b) Ge@12(E)-O-12(E) Cl.
SEM analysis of gelatin capsules filled with gemini surfactants have a very symmetrical and spherical structure in comparison to empty capsules. This suggests that gemini surfactants are effective in stabilizing gelatin capsules, which is particularly evident in the case of compound 12(E)-O-12(E) Br. The type of active substance used also has a great influence on the size of the capsules. They have an average diameter 12.5 µm and 20 µm for Ge@12(E)-O-12(E) Cl and Ge@12(E)-O-12(E) Br, respectively.
The FTIR spectra of empty capsules and capsules filled with gemini surfactant confirm the effectiveness of the encapsulation preparation (Figure 12).
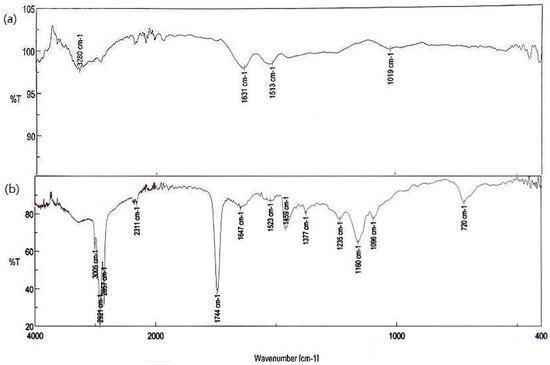
Figure 12.
FTIR spectra of gelatin capsules without and with the gemini surfactants. (a) Ge@empty; (b) Ge@12(E)-O-12(E) Br.
In Figure 12a, it can be seen that the band in the area of 3700–3300 cm−1 is due to the N-H stretching of amine and the amine group, which is further widened by the presence of hydrogen bonds. Bands at 1631 and 1515 cm−1 come from stretching vibrations of carbonyl and C-N bonds. In the second spectrum, separate from the bands characteristic of the cross-linked gelatin, bands originating from gemini surfactant can be found.
3. Materials and Methods
3.1. Materials
1-Decanol (98%) and 1-dodecanol (98%) were obtained from Merck KGaA (Darmstadt, Germany). Chloroacetic acid (99%), bromoacetic acid (97%), 1-bromododecane (97%), N,N,N′,N′-tetramethyl-1,3-propanediamine (TMPDA) (99%), bis[2 -(N,N-dimethylamino)ethyl] ether (BDMAEE) (97%), D-glucose (99.5%) and Span 80 were obtained from Sigma-Aldrich (Poznan, Poland). Sulphuric acid and isopropanol (99%) were purchased from Chempur (Piekary Slaskie, Poland). Acetonitrile (≥99%), methanol (≥99%), calcium chloride (99%) and phosphorus pentoxide (98%) were obtained from VWR Chemicals (Gdansk, Poland). Barium sulfate (95%) and gelatin (95%) were purchased from WarChem (Warsaw, Poland). Sodium alginate from brown algae was obtained from Biomus (Lublin, Poland). Sunflower oil (food grade) was bought from Bunge Polska (Kruszwica, Poland).
3.2. Synthesis
Halogenoesters (decyl chloroacetate 10Cl, dodecyl chloroacetate 12Cl, dodecyl bromoacetate 12Br) were obtained from alcohols: 1–decanol or 1-dodecanol with appropriate amount of halogenoacetic acid under acidic conditions (catalytic amount of sulphuric acid) according to of the described esterification reaction procedure [91].
In all gemini surfactants synthesis, 1 equivalent of the appropriate diamine (TMPDA for 10(E)-3-10(E) Cl and 12-3-12; BDMAEE for 10(E)-O-10(E) Cl, 12-O-12, 12(E)-O-12(E) Cl and 12(E)-O-12(E) Br) was mixed with 2 equivalents of halogenoester (10Cl for 10(E)-3-10(E) Cl and 10(E)-O-10(E) Cl; 12Cl for 12(E)-O-12(E) Cl; 12Br for 12(E)-O-12(E) Br), or bromododecane for 12-3-12 and 12-O-12. The reactions were carried out without a solvent, at room temperature, by stirring using a magnetic stirrer until the reaction mixture solidified (max 6 h). The crude products were crystallized from a mixture of acetonitrile:methanol in the volume ratio of 10:1 and dried in an incubator (60 °C) and over P4O10 in a vacuum desiccator.
3.3. Analytical Methods
The melting point (mp) of the products was measured on Stuart SMP30 (Staffordshire, UK) by using a one-sealed side capillary. Measurement accuracy is up to 1 °C.
The NMR spectra for the synthesized compounds were obtained by using a Varian model VNMR-S 400 MHz (Oxford, UK), operating at 403 and 101 MHz for 1H and 13C, respectively, by using the software, VNMR VERSION 2.3 REVISION A (Varian, Oxford, UK). The 13C and 1H chemical shifts were measured in CDCl3 with TMS as an internal standard.
The FTIR spectra of all gemini surfactants were recorded with a Thermo Scientific Nicolet iS5 FT-IR spectrometer (Waltham, MA, USA). The samples were in a solid phase and prepared as tablets with potassium bromide, whereas the FTIR analysis of alginate and gelatin capsules was performed using a JASCO FT/IR-4600 spectrometer (JASCO, Tokyo, Japan). The samples were tested in the form of solids with a suitable powder attachment (Attenuated Total Reflectance-ATR).
The elemental analysis (EA) measurements were carried out on a FLASH 2000 elemental analyzer (Thermo Fisher Scientific, Warsaw, Poland) with a thermal conductivity detector.
Digital photos were taken with Levenhuk DTX 50 Digital Microscope (Tampa, FL, USA).
Scanning electron microscopy (SEM) studies were performed using a high-resolution environmental electron scanning microscope Quanta FEG 250 FEI (Quanta, Eindhoven, The Netherlands) in low vacuum conditions at a pressure of 70 Pa and a beam accelerating voltage of 10 kV.
3.4. Preparation of Alginate Capsules
To fabricate gel capsules of gemini surfactants, an excess amount of suitable gemini surfactant (10(E)-3-10(E) Cl, 10(E)-O-10(E) Cl, 12-3-12, 12-O-12) higher than its critical micelle concentration was added to a mixture 50 mL of sodium alginate (0.6 g) with vigorous stirring at 70 °C for 20 min until reaching homogeneity. After that, the synthesized homogeneous viscous mixture was injected into a calcium chloride solution (2% wt) to form the capsules. Subsequently, the capsules were filtered from the solution and kept in incubator (60 °C) to dry. Empty capsules without gemini surfactant were prepared in an analogous manner.
To prepare more durable capsules of gemini surfactant 12-O-12, an excess amount of was added to a mixture 50 mL of sodium alginate (0.6 g) and barium sulfate (1.2 g) with vigorous stirring at 70 °C for 20 min until reaching homogeneity. After that, the homogeneous viscous mixture was injected into calcium chloride solution (2% wt) to form the capsules. Subsequently, the capsules were collected from the solution and kept in incubator (60 °C) to dry. Empty capsules without gemini surfactant were prepared in an analogous manner.
3.5. Preparation of Gelatin Capsules
In total, 1.5 g of α-D-glucose was added to the aqueous gelatin solution (15%, 15 mL, 80 °C) and allowed to react for 5 min. Then, the individual gemini surfactants (about 0.1 g) were added to the aqueous solution containing gelatin and sugar. The resulting mixture was carefully added to sunflower oil (150 mL, 80 °C) containing Span 80 (1.5 mL). The dispersion was mixed with a magnetic stirrer to obtain an emulsion. Quick cooling to 5 °C with an ice bath hardens the gelatin droplets. After 15 min, isopropanol (15 × 4 mL, 5 °C) was added at regular intervals to dehydrate the droplets. Stirring was continued for 2 h, followed by filtration. The microspheres were washed with isopropanol to remove any adhered oil. The microspheres prepared in this way were allowed to dry in a desiccator over P2O5. Empty capsules without gemini surfactant were prepared in an analogous manner.
4. Conclusions
Ensuring environmental safety and the user’s safety requires the use of necessary chemical compounds with the highest activity at the lowest concentrations. Moreover, their operations should be limited to a specific place and activated by a specific stimulus, such as pH, pressure, water etc. Gemini surfactants are modern compounds with a very high biological activity and high anticorrosion efficacy. Immobilization of these compounds in alginate and gelatin microcapsules allow you to introduce them to a specific carrier and obtain smart materials. In this work, we presented effective encapsulated methods of the synthesized gemini surfactants in alginate and gelatin microcapsules. The encapsulation efficiency and the capsule morphology were confirmed by physicochemical tests. The resulting microcapsules meet the smart materials criteria and are promising candidates as corrosion inhibitors and antimicrobials.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27196664/s1, Supplementary Materials contain synthetic details and elemental analysis results.
Author Contributions
Conceptualization, B.B. and A.S.; methodology, A.S.; software, A.S.; validation, J.B.; formal analysis, B.B. and A.S.; investigation, B.B.; resources, I.K.; data curation, A.S.; writing—original draft preparation, A.S.; writing—review and editing, B.B. and J.B.; visualization, A.S.; supervision, B.B.; project administration, I.K.; funding acquisition, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by a subsidy from the Faculty of Chemistry, Adam Mickiewicz University for maintaining research potential.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all the compounds are available from the authors.
References
- Polarz, S.; Kunkel, M.; Donner, A.; Schlötter, M. Added-Value Surfactants. Chem. Eur. J. 2018, 24, 18842–18856. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: London, UK, 2017. [Google Scholar]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. 2 Surfactants and Their Applications. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Babajanzadeh, B.; Sherizadeh, S.; Hasan, R. Detergents and Surfactants: A Brief Review. Open Access J. Sci. 2019, 3, 94–99. [Google Scholar] [CrossRef]
- Nazdrajic, S.; Bratovcic, A. The Role of Surfactants in Liquid Soaps and Its Antimicrobial Properties. Int. J. Adv. Res. 2019, 7, 501–507. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Dimeric Surfactants: Promising Ingredients of Cosmetics and Toiletries. Cosmetics 2014, 1, 3–13. [Google Scholar] [CrossRef]
- Asadov, Z.H.; Ahmadova, G.A.; Rahimov, R.A.; Hashimzade, S.-Z.F.; Abdullayev, Y.; Ismailov, E.H.; Suleymanova, S.A.; Asadova, N.Z.; Zubkov, F.I.; Autschbach, J. Aggregation and Antimicrobial Properties of Gemini Surfactants with Mono- and Di-(2-Hydroxypropyl)Ammonium Head-Groups: Effect of the Spacer Length and Computational Studies. J. Mol. Liq. 2020, 302, 112579. [Google Scholar] [CrossRef]
- Kowalczyk, I.; Pakiet, M.; Szulc, A.; Koziróg, A. Antimicrobial Activity of Gemini Surfactants with Azapolymethylene Spacer. Molecules 2020, 25, 4054. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic Lipids and Surfactants as Antifungal Agents: Mode of Action. J. Antimicrob. Chemother. 2006, 58, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable Cationic Antibacterial Amphiphiles: Synthesis, Mechanism of Action, and Cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Szulc, A. Gemini Surfactants as Corrosion Inhibitors. A Review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Mao, T.; Huang, H.; Liu, D.; Shang, X.; Wang, W.; Wang, L. Novel Cationic Gemini Ester Surfactant as an Efficient and Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl Solution. J. Mol. Liq. 2021, 339, 117174. [Google Scholar] [CrossRef]
- Malik, M.A.; Hashim, M.A.; Nabi, F.; AL-Thabaiti, S.A.; Khan, Z. Anti-Corrosion Ability of Surfactants: A Review. Int. J. Electrochem. Sci. 2011, 6, 1927–1948. [Google Scholar]
- Heakal, F.E.-T.; Elkholy, A.E. Gemini Surfactants as Corrosion Inhibitors for Carbon Steel. J. Mol. Liq. 2017, 230, 395–407. [Google Scholar] [CrossRef]
- Shaban, S.M.; Elsamad, S.A.; Tawfik, S.M.; Abdel-Rahman, A.A.-H.; Aiad, I. Studying Surface and Thermodynamic Behavior of a New Multi-Hydroxyl Gemini Cationic Surfactant and Investigating Their Performance as Corrosion Inhibitor and Biocide. J. Mol. Liq. 2020, 316, 113881. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic Gemini Surfactant Properties, Its Potential as a Promising Bioapplication Candidate, and Strategies for Improving Its Biocompatibility: A Review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef]
- Pavlov, R.V.; Gaynanova, G.A.; Kuznetsova, D.A.; Vasileva, L.A.; Zueva, I.V.; Sapunova, A.S.; Buzyurova, D.N.; Babaev, V.M.; Voloshina, A.D.; Lukashenko, S.S.; et al. Biomedical Potentialities of Cationic Geminis as Modulating Agents of Liposome in Drug Delivery across Biological Barriers and Cellular Uptake. Int. J. Pharm. 2020, 587, 119640. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A.; Babkova, M. Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents. Appl. Sci. 2020, 11, 154. [Google Scholar] [CrossRef]
- Kirby, A.J.; Camilleri, P.; Engberts, J.B.F.N.; Feiters, M.C.; Nolte, R.J.M.; Söderman, O.; Bergsma, M.; Bell, P.C.; Fielden, M.L.; García Rodríguez, C.L.; et al. Gemini Surfactants: New Synthetic Vectors for Gene Transfection. Angew. Chem. Int. Ed. 2003, 42, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Kamel, A.O.; Wettig, S.D. Interactions between DNA and Gemini Surfactant: Impact on Gene Therapy: Part I. Nanomedicine 2016, 11, 289–306. [Google Scholar] [CrossRef]
- Wettig, S.; Verrall, R.; Foldvari, M. Gemini Surfactants: A New Family of Building Blocks for Non-Viral Gene Delivery Systems. Curr. Gene Ther. 2008, 8, 9–23. [Google Scholar] [CrossRef]
- Kamal, M.S. A Review of Gemini Surfactants: Potential Application in Enhanced Oil Recovery. J. Surfact. Deterg. 2016, 19, 223–236. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Industrial Applications of Dimeric Surfactants: A Review. J. Dispers. Sci. Technol. 2014, 35, 205–214. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Studies on the Physicochemical Properties of Synthesized Tailor-Made Gemini Surfactants for Application in Enhanced Oil Recovery. J. Mol. Liq. 2018, 258, 211–224. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The Use of Surfactants in Enhanced Oil Recovery: A Review of Recent Advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Han, X.; Lu, M.; Fan, Y.; Li, Y.; Holmberg, K. Recent Developments on Surfactants for Enhanced Oil Recovery. Tenside Surfactants Deterg. 2021, 58, 164–176. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini-Surfactants: Synthesis and Properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A.; Koenig, H.; Kowalczyk, I.; Pospieszny, T.; Górka, S. Effect of the Alkyl Chain Length on Micelle Formation for Bis(N-Alkyl-N,N-Dimethylethylammonium)Ether Dibromides. Comptes Rendus Chim. 2019, 22, 386–392. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Kozirog, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-Bis(N,N- Dimethyl-N-Dodecyloammonium Bromides). Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the Synthesis, Molecular Architectures and Potential Applications of Gemini Surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Mivehi, L.; Bordes, R.; Holmberg, K. Adsorption of Cationic Gemini Surfactants at Solid Surfaces Studied by QCM-D and SPR: Effect of the Rigidity of the Spacer. Langmuir 2011, 27, 7549–7557. [Google Scholar] [CrossRef]
- Łudzik, K.; Kustrzepa, K.; Kowalewicz-Kulbat, M.; Kontek, R.; Kontek, B.; Wróblewska, A.; Jóźwiak, M.; Lulo, D. Antimicrobial and Cytotoxic Properties of Bisquaternary Ammonium Bromides of Different Spacer Length. J. Surfactants Deterg. 2018, 21, 91–99. [Google Scholar] [CrossRef]
- Sarrión, B.; Bernal, E.; Martín, V.I.; López-López, M.; López-Cornejo, P.; García-Calderón, M.; Moyá, M.L. Binding of 12-s-12 Dimeric Surfactants to Calf Thymus DNA: Evaluation of the Spacer Length Influence. Colloids Surf. B 2016, 144, 311–318. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Brycki, B.; Ribosa, I.; Comelles, F.; Garcia, M.T. Cationic Gemini Surfactants Containing an O-Substituted Spacer and Hydroxyethyl Moiety in the Polar Heads: Self-Assembly, Biodegradability and Aquatic Toxicity. J. Ind. Eng. Chem. 2018, 59, 141–148. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K.; van Ginkel, C.G.; Kean, M. Cationic Gemini Surfactants with Cleavable Spacer: Chemical Hydrolysis, Biodegradation, and Toxicity. J. Colloid Interface Sci. 2015, 449, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hajy Alimohammadi, M.; Javadian, S.; Gharibi, H.; Tehrani-Bagha, A.R.; Alavijeh, M.R.; Kakaei, K. Aggregation Behavior and Intermicellar Interactions of Cationic Gemini Surfactants: Effects of Alkyl Chain, Spacer Lengths and Temperature. J. Chem. Thermodyn. 2012, 44, 107–115. [Google Scholar] [CrossRef]
- Brycki, B.; Waligórska, M.; Szulc, A. The Biodegradation of Monomeric and Dimeric Alkylammonium Surfactants. J. Hazard. Mater. 2014, 280, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Zhuang, W.; Yao, M.; Pan, Y.; Chen, X. Spacer Length Effect on the Aggregation Behaviours of Gemini Surfactants in EAN. Colloid. Polym. Sci. 2021, 299, 685–692. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.S.; Rizvanova, L.Z.; Konovalov, A.I. Geminal Alkylammonium Surfactants: Aggregation Properties and Catalytic Activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y. Aggregation Behavior of Gemini Surfactants and Their Interaction with Macromolecules in Aqueous Solution. Phys. Chem. Chem. Phys. 2011, 13, 1939–1956. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Deyab, M.A.; Osman, M.M.; Nessim, M.I.; Elkholy, A.E. Synthesis and Assessment of New Cationic Gemini Surfactants as Inhibitors for Carbon Steel Corrosion in Oilfield Water. RSC Adv. 2017, 7, 47335–47352. [Google Scholar] [CrossRef]
- Mahdavian, M.; Tehrani-Bagha, A.R.; Alibakhshi, E.; Ashhari, S.; Palimi, M.J.; Farashi, S.; Javadian, S.; Ektefa, F. Corrosion of Mild Steel in Hydrochloric Acid Solution in the Presence of Two Cationic Gemini Surfactants with and without Hydroxyl Substituted Spacers. Corros. Sci. 2018, 137, 62–75. [Google Scholar] [CrossRef]
- Labena, A.; Hegazy, M.A.; Horn, H.; Müller, E. Sulfidogenic-Corrosion Inhibitory Effect of Cationic Monomeric and Gemini Surfactants: Planktonic and Sessile Diversity. RSC Adv. 2016, 6, 42263–42278. [Google Scholar] [CrossRef]
- Labena, A.; Hegazy, M.A.; Horn, H.; Müller, E. Cationic Gemini Surfactant as a Corrosion Inhibitor and a Biocide for High Salinity Sulfidogenic Bacteria Originating from an Oil-Field Water Tank. J. Surfactants Deterg. 2014, 17, 419–431. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Lu, X.; Wang, J.; Hu, Z.; Ma, X. Efficiency of Gemini Surfactant Containing Semi-Rigid Spacer as Microbial Corrosion Inhibitor for Carbon Steel in Simulated Seawater. Bioelectrochemistry 2021, 140, 107809. [Google Scholar] [CrossRef]
- Aiad, I.; Emam, D.; El-Deeb, A.; Abd-Alrahman, E. Novel Imidazolium-Based Gemini Surfactants: Synthesis, Surface Properties, Corrosion Inhibition and Biocidal Activity Against Sulfate-Reducing Bacteria. J. Surfactants Deterg. 2013, 16, 927–935. [Google Scholar] [CrossRef]
- Laatiris, A.; El Achouri, M.; Rosa Infante, M.; Bensouda, Y. Antibacterial Activity, Structure and CMC Relationships of Alkanediyl α,ω-Bis(Dimethylammonium Bromide) Surfactants. Microbiol. Res. 2008, 163, 645–650. [Google Scholar] [CrossRef]
- Sarıkaya, İ.; Bilgen, S.; Ünver, Y.; İnan Bektaş, K.; Akbaş, H. Synthesis, Characterization, Antibacterial Activity, and Interfacial and Micellar Features of Novel Cationic Gemini Surfactants with Different Spacers. J. Surfactants Deterg. 2021, 24, jsde.12532. [Google Scholar] [CrossRef]
- Rahimov, R.A.; Ahmadova, G.A.; Hashimzade, S.F.; Imanov, E.; Khasiyev, H.G.; Karimova, N.K.; Zubkov, F.I. Surface and Biocidal Properties of Gemini Cationic Surfactants Based on Propoxylated 1,6-Diaminohexane and Alkyl Bromides. J. Surfactants Deterg. 2021, 24, 433–444. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Yuan, H.; Yin, J.; Hu, M. Antibacterial Mechanism of Octamethylene-1,8-Bis(Dodecyldimethylammonium Bromide) Against E. Coli. J. Surfactants Deterg. 2017, 20, 717–723. [Google Scholar] [CrossRef]
- Hsu, L.-H.; Kwaśniewska, D.; Wang, S.-C.; Shen, T.-L.; Wieczorek, D.; Chen, Y.-L. Gemini Quaternary Ammonium Compound PMT12-BF4 Inhibits Candida Albicans via Regulating Iron Homeostasis. Sci. Rep. 2020, 10, 2911. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, F.; Lacko, I.; Mlynarčík, D.; Racansky, V.; Krasnec, L. Relationship between Critical Micelle Concentration and Minimum Inhibitory Concentration for Some Non-Aromatic Quaternary Ammonium Salts and Amine Oxides. Tenside Surfactants Deterg. 1985, 22, 10–15. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal Activity of Gemini Quaternary Ammonium Salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Taleb, K.; Mohamed-Benkada, M.; Benhamed, N.; Saidi-Besbes, S.; Grohens, Y.; Derdour, A. Benzene Ring Containing Cationic Gemini Surfactants: Synthesis, Surface Properties and Antibacterial Activity. J. Mol. Liq. 2017, 241, 81–90. [Google Scholar] [CrossRef]
- Machuca, L.M.; Reno, U.; Plem, S.C.; Gagneten, A.M.; Murguía, M.C. N-Acetylated Gemini Surfactants: Synthesis, Surface-Active Properties, Antifungal Activity, and Ecotoxicity Bioassays. Adv. Chem. Engineer. Sci. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Dani, U.; Bahadur, A.; Kuperkar, K. Micellization, Antimicrobial Activity and Curcumin Solubilization in Gemini Surfactants: Influence of Spacer and Non-Polar Tail. Colloids Interface Sci. Commun. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Koziróg, A.; Otlewska, A.; Brycki, B. Viability, Enzymatic and Protein Profiles of Pseudomonas Aeruginosa Biofilm and Planktonic Cells after Monomeric/Gemini Surfactant Treatment. Molecules 2018, 23, 1294. [Google Scholar] [CrossRef]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-Active Properties and Antimicrobial Study of Conventional Cationic and Synthesized Symmetrical Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 107–115. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Bhadani, A.; Singh, S. Novel Gemini Pyridinium Surfactants: Synthesis and Study of Their Surface Activity, DNA Binding, and Cytotoxicity. Langmuir 2009, 25, 11703–11712. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y. Structure–Activity Relationship of Cationic Surfactants as Antimicrobial Agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Egorova, E.M.; Kaba, S.I. The Effect of Surfactant Micellization on the Cytotoxicity of Silver Nanoparticles Stabilized with Aerosol-OT. Toxicol. Vitr. 2019, 57, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.H.; Roy, A.; Malik, S.; Ghosh, A.; Saha, B. Review on Chemically Bonded Geminis with Cationic Heads: Second-Generation Interfactants. Res. Chem. Intermed. 2016, 42, 1913–1928. [Google Scholar] [CrossRef]
- Mondal, M.H.; Malik, S.; Roy, A.; Saha, R.; Saha, B. Modernization of Surfactant Chemistry in the Age of Gemini and Bio-Surfactants: A Review. RSC Adv. 2015, 5, 92707–92718. [Google Scholar] [CrossRef]
- Brycki, B.E.; Szulc, A.; Kowalczyk, I.; Koziróg, A.; Sobolewska, E. Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part. Molecules 2021, 26, 5759. [Google Scholar] [CrossRef] [PubMed]
- Andersson Trojer, M.; Nordstierna, L.; Bergek, J.; Blanck, H.; Holmberg, K.; Nydén, M. Use of Microcapsules as Controlled Release Devices for Coatings. Adv. Colloid Interface Sci. 2015, 222, 18–43. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.; Szulc, A. Smart Antimicrobial Materials with the Immobilized Gemini Surfactants. In Antimicrobial Research: Novel Bioknowledge and Educational Programs; Mendez-VilasA, A., Ed.; Formatex Research Center: Badajoz, Spain, 2017; pp. 427–437. [Google Scholar]
- Timilsena, Y.P.; Adhikari, A.H.B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Sci. Nutr. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia. Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Augustin, M.A.; Oliver, C.M. Use of Milk Proteins for Encapsulation of Food Ingredients. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 211–226. [Google Scholar]
- Favaro-Trindade, C.S. Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling. Pharmaceutics 2021, 13, 1186. [Google Scholar] [CrossRef]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of Pharmaceutical and Nutraceutical Active Ingredients Using Electrospinning Processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides Using Colloidal Systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef]
- Li, W.; Lei, X.; Feng, H.; Li, B.; Kong, J.; Xing, M. Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics 2022, 14, 297. [Google Scholar] [CrossRef]
- Sørensen, G.; Nielsen, A.L.; Møller Pedersen, M.; Poulsen, S.; Nissen, H.; Poulsen, M.; Nygaard, S.D. Controlled Release of Biocide from Silica Microparticles in Wood Paint. Prog. Org. Coat. 2010, 68, 29–306. [Google Scholar] [CrossRef]
- Nardello-Rataj, V.; Leclercq, L. Encapsulation of Biocides by Cyclodextrins: Toward Synergistic Effects against Pathogens. Beilstein J. Org. Chem. 2014, 10, 2603–2622. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.; Silva, A.P.; Fernandes, S.; Cunha, A.; Almeida, A.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Incorporation of Biocides in Nanocapsules for Protective Coatings Used in Maritime Applications. Chem. Eng. J. 2015, 270, 150–157. [Google Scholar] [CrossRef]
- Alsmaeil, A.W.; Hammami, M.A.; Enotiadis, A.; Kanj, M.Y.; Giannelis, E.P. Encapsulation of an Anionic Surfactant into Hollow Spherical Nanosized Capsules: Size Control, Slow Release, and Potential Use for Enhanced Oil Recovery Applications and Environmental Remediation. ACS Omega 2021, 6, 5689–5697. [Google Scholar] [CrossRef] [PubMed]
- Raja, P.B.; Assad, M.A.; Ismail, M. Inhibitor-Encapsulated Smart Nanocontainers for the Controlled Release of Corrosion Inhibitors. In Corrosion Protection at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–105. [Google Scholar]
- Falcón, J.M.; Batista, F.F.; Aoki, I.V. Encapsulation of Dodecylamine Corrosion Inhibitor on Silica Nanoparticles. Electrochim. Acta 2014, 124, 109–118. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Ramazani, S.A.A.; Mahdavian, M.; Taheri, P.; Mol, J.M.C. Fabrication and Characterization of Graphene-Based Carbon Hollow Spheres for Encapsulation of Organic Corrosion Inhibitors. Chem. Eng. J. 2018, 352, 909–922. [Google Scholar] [CrossRef]
- Kim, C.; Karayan, A.I.; Milla, J.; Hassan, M.; Castaneda, H. Smart Coating Embedded with PH-Responsive Nanocapsules Containing a Corrosion Inhibiting Agent. ACS Appl. Mater. Interfaces 2020, 12, 6451–6459. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Patidar, B.; Bhawsar, J. Potential of Nanoparticles as a Corrosion Inhibitor: A Review. J. Bio. Tribo. Corros. 2020, 6, 43. [Google Scholar] [CrossRef]
- Mania, S.; Tylingo, R.; Michałowska, A. The Drop-in-Drop Encapsulation in Chitosan and Sodium Alginate as a Method of Prolonging the Quality of Linseed Oil. Polymers 2018, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Banno, T.; Kawada, K.; Matsumura, S. Creation of Novel Green and Sustainable Gemini-Type Cationics Containing Carbonate Linkages. J. Surfactants Deterg. 2010, 13, 387–398. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Guz-Regner, K.; Dworniczek, E. Antibacterial Activity of Gemini Quaternary Ammonium Salts. FEMS Microbiol. Lett. 2014, 350, 190–198. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Dworniczek, E.; Olejniczak, T. The Influence of Biodegradable Gemini Surfactants, N,N’-Bis(1-Decyloxy-1-Oxopronan-2-Yl)-N,N,N’,N’ Tetramethylpropane-1,3-Diammonium Dibromide and N,N’-Bis(1-Dodecyloxy-1-Oxopronan-2-Yl) N,N,N’,N’-Tetramethylethane-1,2-Diammonium Dibromide, on Fungal Biofilm and Adhesion. J. Oleo Sci. 2015, 64, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Pakiet, M.; Kowalczyk, I.; Leiva Garcia, R.; Akid, R.; Brycki, B. Cationic Clevelable Surfactants as Highly Efficient Corrosion Inhibitors of Stainless Steel AISI 304: Electrochemical Study. J. Mol. Liq. 2020, 315, 113675. [Google Scholar] [CrossRef]
- Xu, D.; Ni, X.; Zhang, C.; Mao, J.; Song, C. Synthesis and Properties of Biodegradable Cationic Gemini Surfactants with Diester and Flexible Spacers. J. Mol. Liq. 2017, 240, 542–548. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Oskarsson, H.; van Ginkel, C.G.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Chemical Hydrolysis and Biodegradation. J. Colloid Interface Sci. 2007, 312, 444–452. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Kabir-ud-Din; Beg, M. Ester-Bonded Cationic Gemini Surfactants: Assessment of Their Cytotoxicity and Antimicrobial Activity. J. Mol. Liq. 2016, 222, 390–394. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil Encapsulation Techniques Using Alginate as Encapsulating Agent: Applications and Drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.M.; Sagis, L.M.C. Preparation Methods of Alginate Nanoparticles. Adv. Coll. Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Chat, O.A.; Maswal, M.; Ashraf, U.; Rather, G.M.; Dar, A.A. Hydrogels of Sodium Alginate in Cationic Surfactants: Surfactant Dependent Modulation of Encapsulation/Release toward Ibuprofen. Carbohydr. Polym. 2015, 133, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Producing Gelatin Nanoparticles as Delivery System for Bovine Serum Albumin. Iran. Biomed. J. 1996, 18, 34–40. [Google Scholar] [CrossRef]
- Cortesi, R.; Nastruzzi, C.; Davis, S.S. Sugar Cross-Linked Gelatin for Controlled Release: Microspheres and Disks. Biomaterials 1998, 19, 1641–1649. [Google Scholar] [CrossRef]
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, Spectroscopic Studies, Aggregation and Surface Behavior of Hexamethylene-1,6-Bis(N,N-Dimethyl-N-Dodecylammonium Bromide). J. Mol. Liq. 2016, 221, 1086–1096. [Google Scholar] [CrossRef]
- Blandino, A.; Macias, M.; Cantero, D. Formation of Calcium Alginate Gel Capsules: Influence of Sodium Alginate and CaCl2 Concentration on Gelation Kinetics. J. Biosci. Bioenerg. 1999, 88, 686–689. [Google Scholar] [CrossRef]
- Siimon, K.; Reemann, P.; Põder, A.; Pook, M.; Kangur, T.; Kingo, K.; Jaks, V.; Mäeorg, U.; Järvekülg, M. Effect of Glucose Content on Thermally Cross-Linked Fibrous Gelatin Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2014, 42, 538–545. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).