Capric Acid Behaves Agonistic Effect on Calcitriol to Control Inflammatory Mediators in Colon Cancer Cells
Abstract
1. Introduction
2. Results
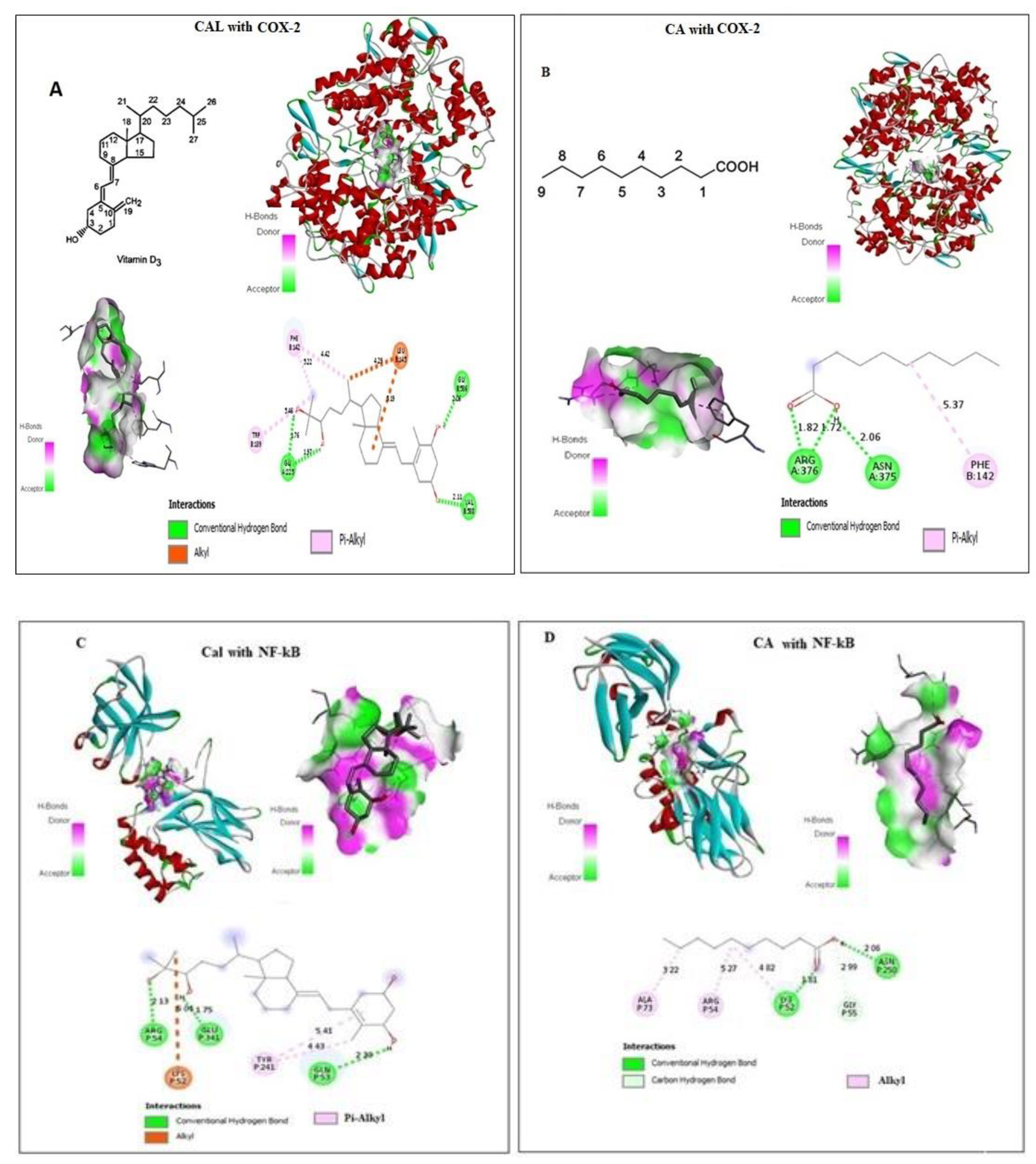
2.1. Effect of CA and Cal on Virtual Binding of Apoptotic and Inflammatory Markers COX-2 and NF-κB
2.2. Antiproliferative Effect of CA and Cal on HCT116 Cancer Cell Lines
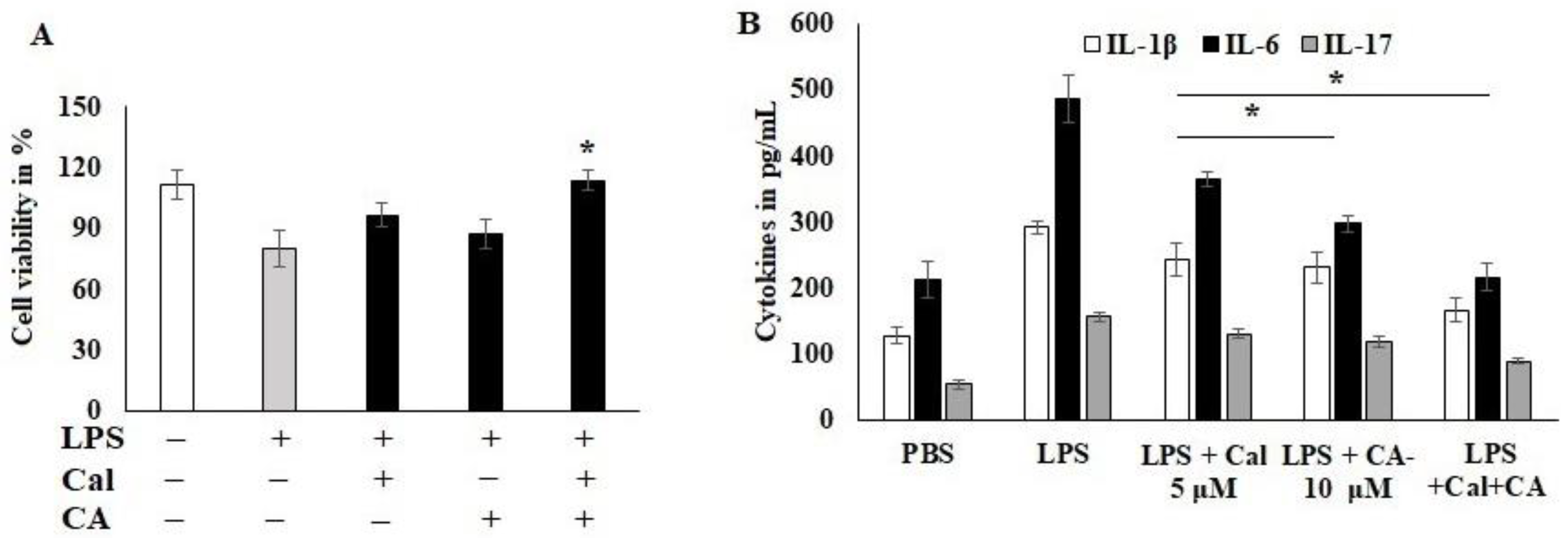
2.3. Effect of CA and Cal on LPS-Induced HCT116 Cancer Cell Lines
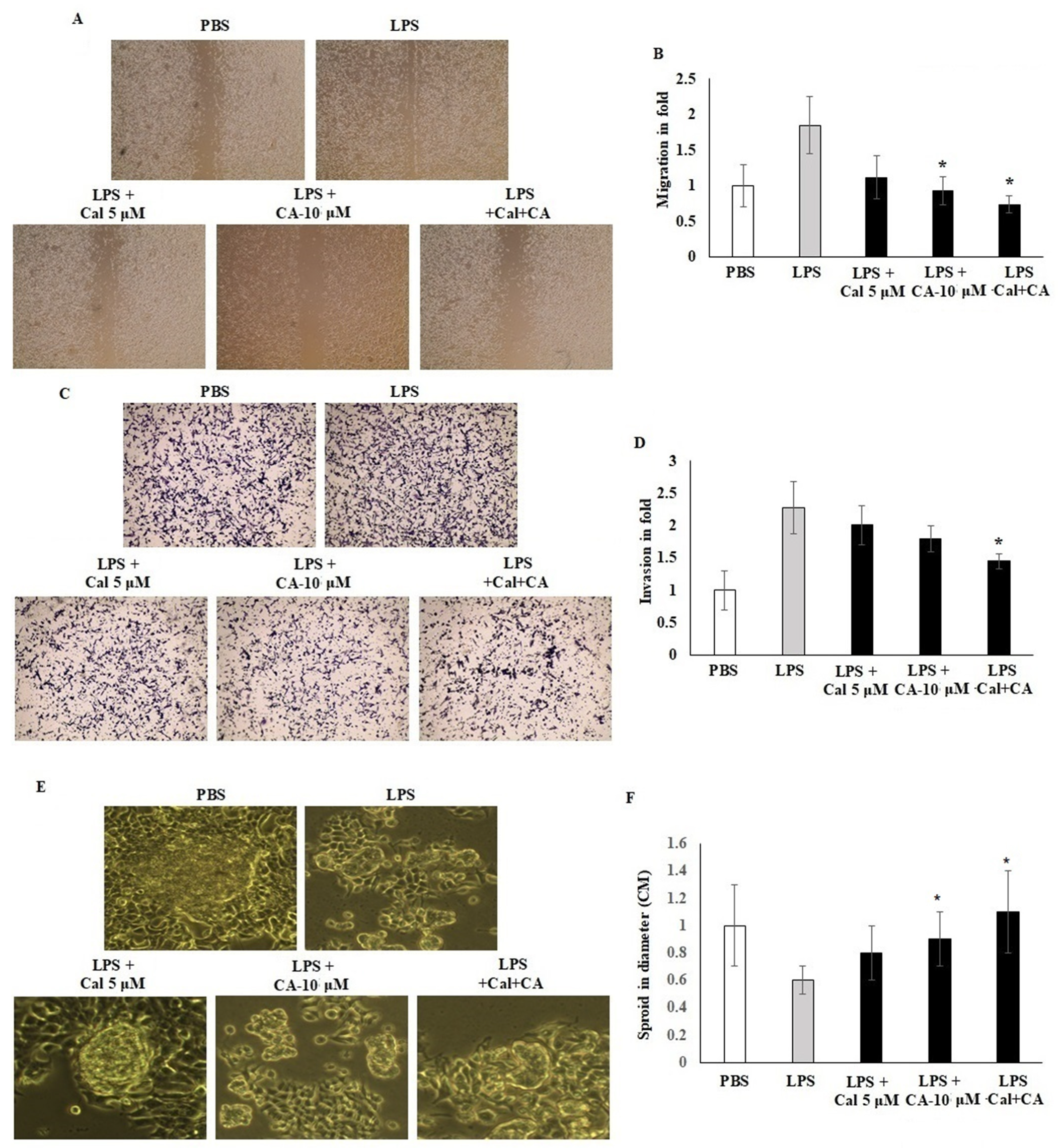
2.4. Synergistic Effect of CA and Cal on Cell Migration, Invasion and Sphere Formation Properties of HCT116
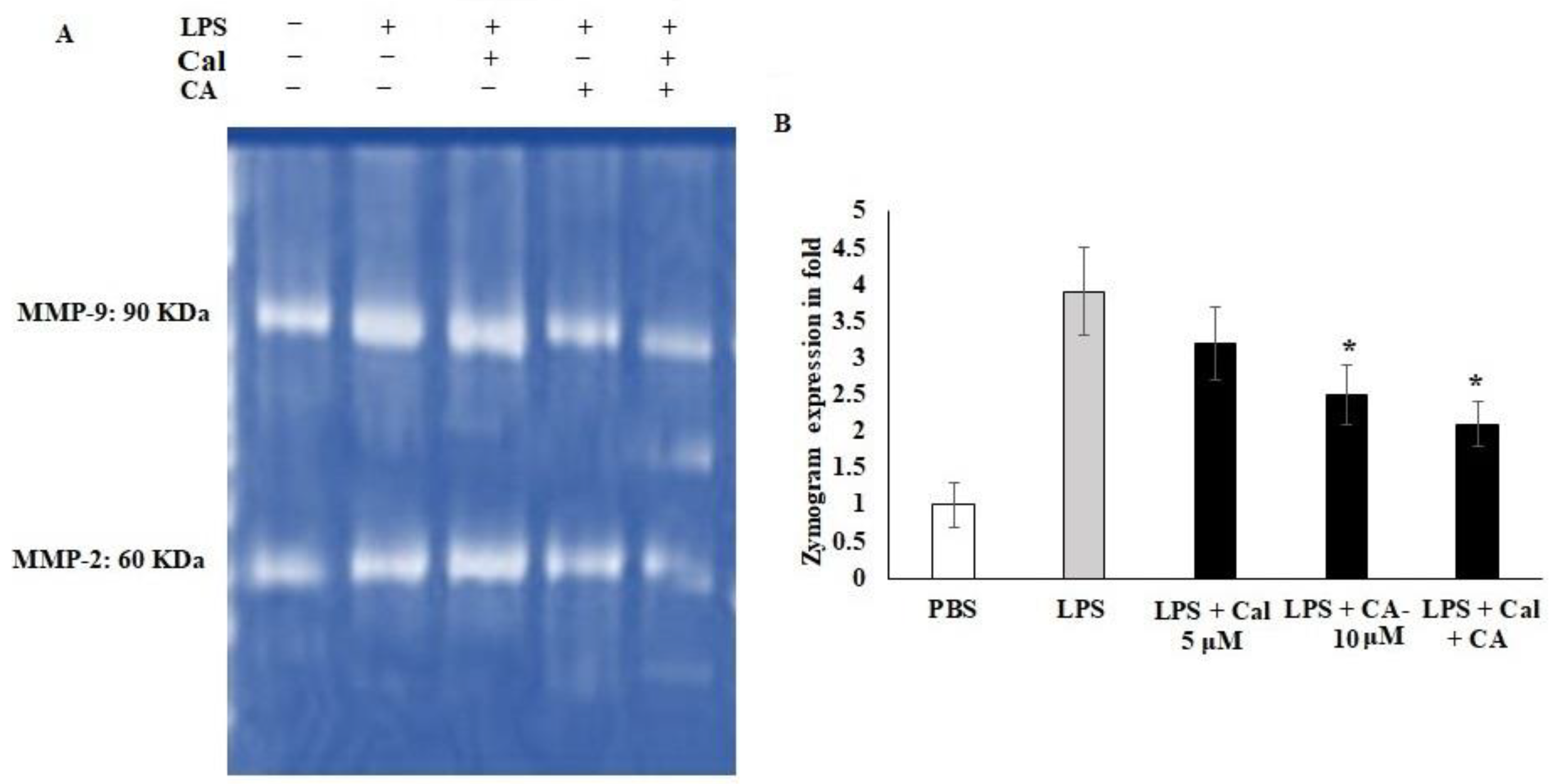
2.5. Synergistic Effect of CA and Cal on MMP-2 and MMP-9 Inhibition in LPS-Stimulated HCT116 Tumorigenic Cells
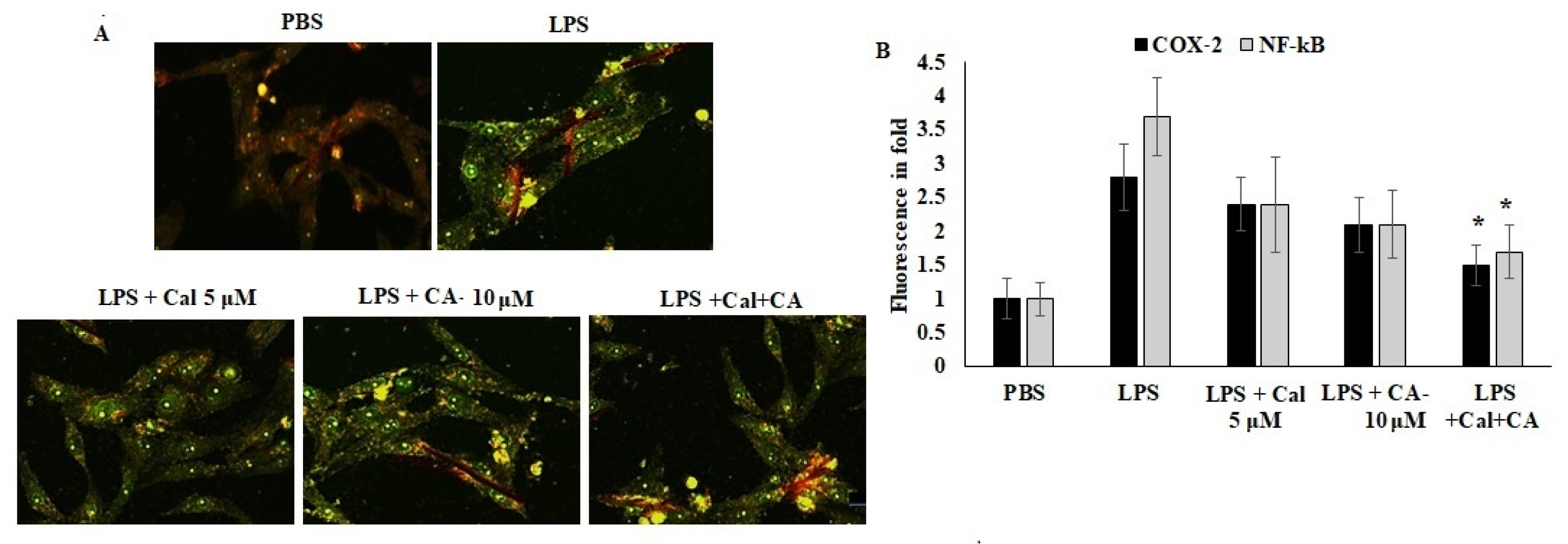
2.6. Synergistic Effect of CA and Cal Inhibit Inflammation in HCT116 via COX-2/ NF-κB Pathway
3. Discussion
4. Materials and Methods
4.1. Ligand Preparation and In Silico Docking Analysis
4.2. Cell Culture and MTT Assay
4.3. In Vitro Wound-Healing Assay (Scratch Wound Method)
4.4. Matrigel Invasion Assay
4.5. Spheroid Formation Assay
4.6. Zymographic Assays for MMP-2 and MMP-9
4.7. Effect of CA and Cal on Inflammatory mRNA Mediators of HCT116
4.8. Effect of CA and Cal on Inflammatory Protein Mediators of HCT116
4.9. COX-2 and NF-κB Immunofluorescence
4.10. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Rayburn, E.R.; Ezell, S.J.; Zhang, R. Anti-inflammatory agents for cancer therapy. Mol. Cell. Pharmacol. 2009, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Hoepelman, A. Inflammation: Basic Principles and Clinical Correlates’ 3rd Edition, 1999, Ed. by J.I. Gallin and R. Snyderman, Lippincott Williams & Wilkins Publishers, ISBN: 0 39 751 759 9, pp. 1335. Neth. J. Med. 2001, 1, 41. [Google Scholar]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2004; pp. 433–439. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Guo, Y.; Nie, Q.; MacLean, A.L.; Li, Y.; Lei, J.; Li, S. Multiscale modeling of inflammation-induced tumorigenesis reveals competing oncogenic and oncoprotective roles for inflammation. Cancer Res. 2017, 77, 6429–6441. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Ohnishi, S.; Ma, N.; Hiraku, Y.; Murata, M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, J.; Song, L.; Liu, Z.; Han, G.; Yuan, D.; Wang, T.; Dun, Y.; Zhou, Z.; Liu, Z. Oleanolic acid rejuvenates testicular function through attenuating germ cell DNA damage and apoptosis via deactivation of NF-κB, p53 and p38 signalling pathways. J. Pharm. Pharmacol. 2017, 69, 295–304. [Google Scholar] [CrossRef]
- Petrenko, O.; Moll, U.M. Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol. Cell 2005, 17, 225–236. [Google Scholar] [CrossRef]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, M.; Wang, F.; Song, M.; Huang, Q.; Ge, B. miR-224 Controls Human Colorectal Cancer Cell Line HCT116 Proliferation by Targeting Smad4. Int. J. Med. Sci. 2017, 14, 937–942. [Google Scholar] [CrossRef]
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy-Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, A.; Mahdi, M.R.; Zahran, M.H.; Abdelbaset-Ismail, A.; El-Dosoky, M.; Negm, A. Baicalein and Alphalpha-Tocopherol Inhibit Toll-like Receptor Pathways in Cisplatin-Induced Nephrotoxicity. Molecules 2022, 27, 2179. [Google Scholar] [CrossRef] [PubMed]
- Hosny, S.; Sahyon, H.; Youssef, M.; Negm, A. Oleanolic Acid Suppressed DMBA-Induced Liver Carcinogenesis through Induction of Mitochondrial-Mediated Apoptosis and Autophagy. Nutr. Cancer 2021, 73, 968–982. [Google Scholar] [CrossRef] [PubMed]
- Hosny, S.; Sahyon, H.; Youssef, M.; Negm, A. Prunus Armeniaca L. Seed Extract and Its Amygdalin Containing Fraction Induced Mitochondrial-Mediated Apoptosis and Autophagy in Liver Carcinogenesis. Anticancer Agents Med. Chem. 2021, 21, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Shpitz, B.; Giladi, N.; Sagiv, E.; Lev-Ari, S.; Liberman, E.; Kazanov, D.; Arber, N. Celecoxib and curcumin additively inhibit the growth of colorectal cancer in a rat model. Digestion 2006, 74, 140–144. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, S.A.; Choi, Y.M.; Kwon, H.K.; Shim, W.; Lee, G.; Choi, S. Capric acid inhibits NO production and STAT3 activation during LPS-induced osteoclastogenesis. PLoS ONE 2011, 6, e27739. [Google Scholar] [CrossRef]
- Yamada, K.; Hata, S.; Kato, I.; Hisata, T.; Shimisu, S. Capric acid inhalation therapy in pulmonary tuberculosis. Chiryo 1961, 43, 1984–1990. [Google Scholar]
- White, R.P.; el-Bauomy, A.M.; Wood, W.B. Capric acid as a potent dilator of canine vessels in vitro and in vivo. Gen Pharm. 1991, 22, 741–748. [Google Scholar] [CrossRef]
- White, R.P.; Ricca, G.F.; el-Bauomy, A.M.; Robertson, J.T. Identification of capric acid as a potent vasorelaxant of human basilar arteries. Stroke 1991, 22, 469–476. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Kumar, V.; Jain, S.; Sharma, P.C. Thiazolidin-4-one and hydrazone derivatives of capric acid as possible anti-inflammatory, analgesic and hydrogen peroxide-scavenging agents. J Enzym. Inhib Med Chem 2011, 26, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Ghosh, M. Comparison of native and capric acid-enriched mustard oil effects on oxidative stress and antioxidant protection in rats. Br. J. Nutr. 2011, 107, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.T. Ajoene (natural garlic compound): A new anti-leukaemia agent for AML therapy. Leuk. Res. 2004, 28, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.W.; Lee, D.T.; Chiu, Y.T.; Chua, C.W.; Wang, X.; Wong, Y.C. Evidence of a novel docetaxel sensitizer, garlic-derived S-allylmercaptocysteine, as a treatment option for hormone refractory prostate cancer. Int. J. Cancer 2008, 122, 1941–1948. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Wargovich, M.J. Inflammation, cancer, and targets of ginseng. J. Nutr. 2007, 137, 183S–185S. [Google Scholar] [CrossRef]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Anticarcinogenic Properties of Medium Chain Fatty Acids on Human Colorectal, Skin and Breast Cancer Cells in Vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Feldman, D. Mechanisms of the Anti-Cancer and Anti-Inflammatory Actions of Vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef]
- Castellone, R.D.; Leffler, N.R.; Dong, L.; Yang, L.V. Inhibition of tumor cell migration and metastasis by the proton-sensing GPR4 receptor. Cancer Lett. 2011, 312, 197–208. [Google Scholar] [CrossRef]
- Hanieh, H.; Mohafez, O.; Hairul-Islam, V.I.; Alzahrani, A.; Bani Ismail, M.; Thirugnanasambantham, K. Novel aryl hydrocarbon receptor agonist suppresses migration and invasion of breast cancer cells. PLoS ONE 2016, 11, e0167650. [Google Scholar] [CrossRef]
- Rodriguez-Escaja, C.; Navascués, C.A.; Gonzalez-Dieguez, L.; Cadahia, V.; Varela, M.; de Jorge, M.A.; Castano-Garcia, A.; Rodriguez, M. Diabetes is not associated with an increased risk of hepatocellular carcinoma in patients with alcoholic or hepatitis C virus cirrhosis. Rev. Esp. Enferm. Dig. 2021, 113, 505–511. [Google Scholar] [CrossRef]
- Nelson, A.R.; Fingleton, B.; Rothenberg, M.L.; Matrisian, L.M. Matrix metalloproteinases: Biologic activity and clinical implications. J. Clin. Oncol. 2000, 18, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Lee, S.-G.; Oh, J.H.; Kim, J.; Kong, C.-S. Inhibition of MMP-2 and MMP-9 activities by solvent-partitioned Sargassum horneri extracts. Fish. Aquat. Sci. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar] [PubMed]
- Kumar, V.S.; Kumaresan, S.; Tamizh, M.M.; Islam, M.I.H.; Thirugnanasambantham, K. Anticancer potential of NF-κB targeting apoptotic molecule “flavipin” isolated from endophytic Chaetomium globosum. Phytomedicine 2019, 61, 152830. [Google Scholar] [CrossRef]
- Hoque, A.; Lippman, S.M.; Wu, T.-T.; Xu, Y.; Liang, Z.D.; Swisher, S.; Zhang, H.; Cao, L.; Ajani, J.A.; Xu, X.-C. Increased 5-lipoxygenase expression and induction of apoptosis by its inhibitors in esophageal cancer: A potential target for prevention. Carcinogenesis 2005, 26, 785–791. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.-I.M.; Ahmed, E.A.; Emeka, P.M.; Alhaider, I.A. Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals 2022, 15, 154. [Google Scholar] [CrossRef]
- Kummer, N.T.; Nowicki, T.S.; Azzi, J.P.; Reyes, I.; Iacob, C.; Xie, S.; Swati, I.; Darzynkiewicz, Z.; Gotlinger, K.H.; Suslina, N. Arachidonate 5 lipoxygenase expression in papillary thyroid carcinoma promotes invasion via MMP-9 induction. J. Cell. Biochem. 2012, 113, 1998–2008. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, T.H.; Chuang, L.T.; Li, Y.Y.; Zouboulis, C.C.; Tsai, P.J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J Derm. Sci 2013, 73, 232–240. [Google Scholar] [CrossRef]
- Negm, A.; Gouda, M.; Ibrahim, H.M. Carboxymethyl Cellulose/Zn-Organic Framework Down-Regulates Proliferation and Up-Regulates Apoptosis and DNA Damage in Colon and Lung Cancer Cell Lines. Polymers 2022, 14, 2015. [Google Scholar] [CrossRef]
- Fenu, M.; Bettermann, T.; Vogl, C.; Darwish-Miranda, N.; Schramel, J.; Jenner, F.; Ribitsch, I. A novel magnet-based scratch method for standardisation of wound-healing assays. Sci. Rep. 2019, 9, 12625. [Google Scholar] [CrossRef] [PubMed]
- Debbie, M.; Brooks, S.A. In vitro invasion assay using matrigel(R). In Metastasis Research Protocols; Humana Press: Totowa, NJ, USA, 2001; Volume 58, pp. 61–70. [Google Scholar] [CrossRef]
- Aslan, M.; Hsu, E.C.; Liu, S.; Stoyanova, T. Quantifying the invasion and migration ability of cancer cells with a 3D Matrigel drop invasion assay. Biol. Methods Protoc. 2021, 6, bpab014. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Chakrabarti, R. Assessment of Breast Cancer Stem Cell Activity Using a Spheroid Formation Assay. Methods Mol. Biol. 2022, 2429, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Ahmed, M.; Lorenzi, F.; Nateri, A.S. Spheroid-Formation (Colonosphere) Assay for in Vitro Assessment and Expansion of Stem Cells in Colon Cancer. Stem Cell Rev. Rep. 2016, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Fridman, R. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography. In Metastasis Research Protocols. Methods in Molecular Medicine; Brooks, S.A., Schumacher, U., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 57, pp. 163–174. [Google Scholar] [CrossRef]
- Toth, M.; Sohail, A.; Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. In Metastasis Research Protocols. Methods in Molecular Biology; Dwek, M., Brooks, S., Schumacher, U., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 878, pp. 121–135. [Google Scholar] [CrossRef]
- Li, N.; Xu, H.; Ou, Y.; Feng, Z.; Zhang, Q.; Zhu, Q.; Cai, Z. LPS-induced CXCR7 expression promotes gastric Cancer proliferation and migration via the TLR4/MD-2 pathway. Diagn. Pathol. 2019, 14, 3. [Google Scholar] [CrossRef]
- Lee, A.S.; Jung, Y.J.; Thanh, T.N.; Lee, S.; Kim, W.; Kang, K.P.; Park, S.K. Paricalcitol attenuates lipopolysaccharide-induced myocardial inflammation by regulating the NF-κB signaling pathway. Int. J. Mol. Med. 2016, 37, 1023–1029. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negm, A.; Sedky, A.; Elsawy, H. Capric Acid Behaves Agonistic Effect on Calcitriol to Control Inflammatory Mediators in Colon Cancer Cells. Molecules 2022, 27, 6624. https://doi.org/10.3390/molecules27196624
Negm A, Sedky A, Elsawy H. Capric Acid Behaves Agonistic Effect on Calcitriol to Control Inflammatory Mediators in Colon Cancer Cells. Molecules. 2022; 27(19):6624. https://doi.org/10.3390/molecules27196624
Chicago/Turabian StyleNegm, Amr, Azza Sedky, and Hany Elsawy. 2022. "Capric Acid Behaves Agonistic Effect on Calcitriol to Control Inflammatory Mediators in Colon Cancer Cells" Molecules 27, no. 19: 6624. https://doi.org/10.3390/molecules27196624
APA StyleNegm, A., Sedky, A., & Elsawy, H. (2022). Capric Acid Behaves Agonistic Effect on Calcitriol to Control Inflammatory Mediators in Colon Cancer Cells. Molecules, 27(19), 6624. https://doi.org/10.3390/molecules27196624





