Effects of Extracts and Flavonoids from Drosera rotundifolia L. on Ciliary Beat Frequency and Murine Airway Smooth Muscle
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characterization of Extracts and Quantification of Flavonoids
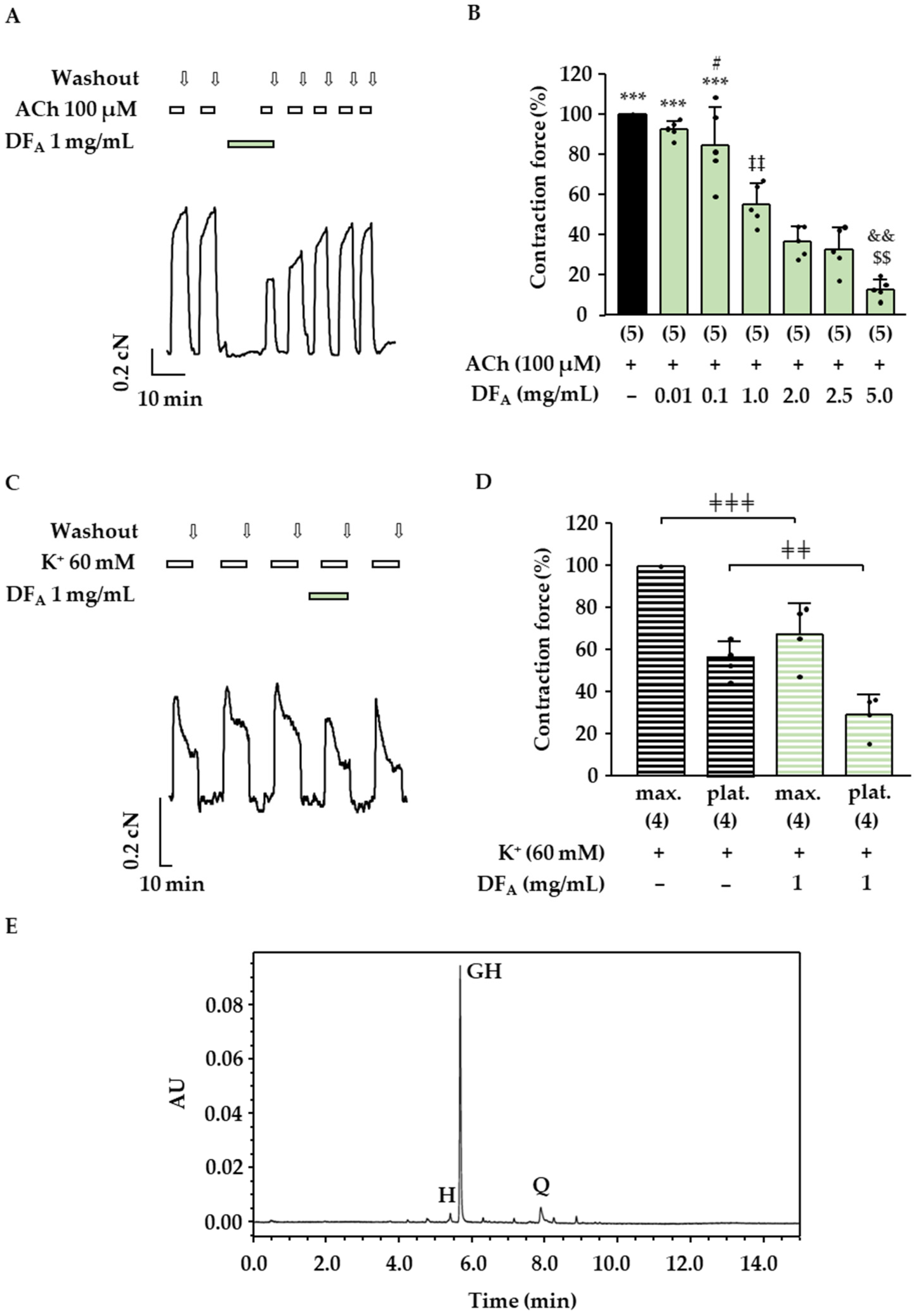
2.2. Effects of the Drosera Extract, the Aqueous Drosera Fraction and Isolated Flavonoids on ASM Contractions
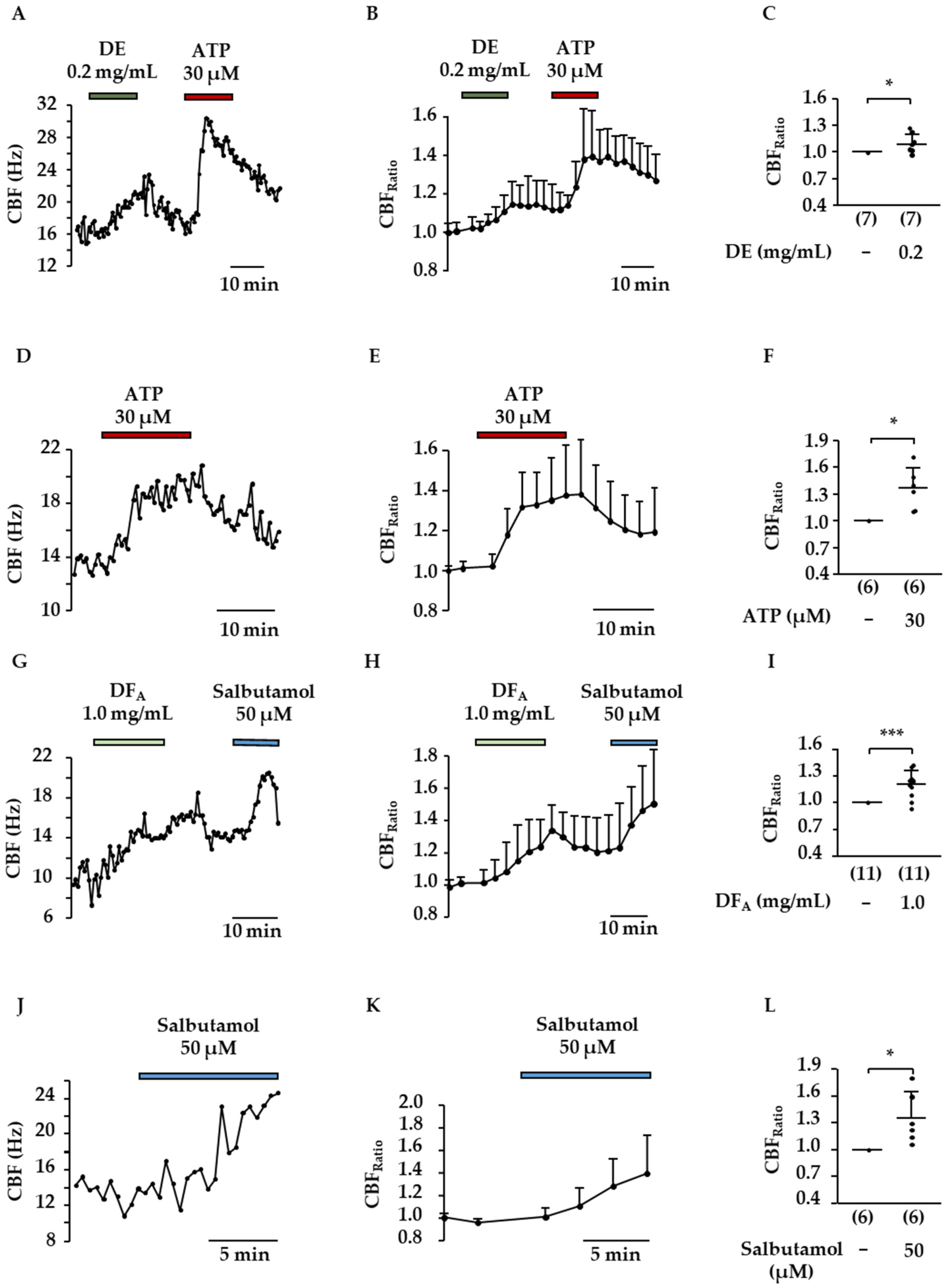
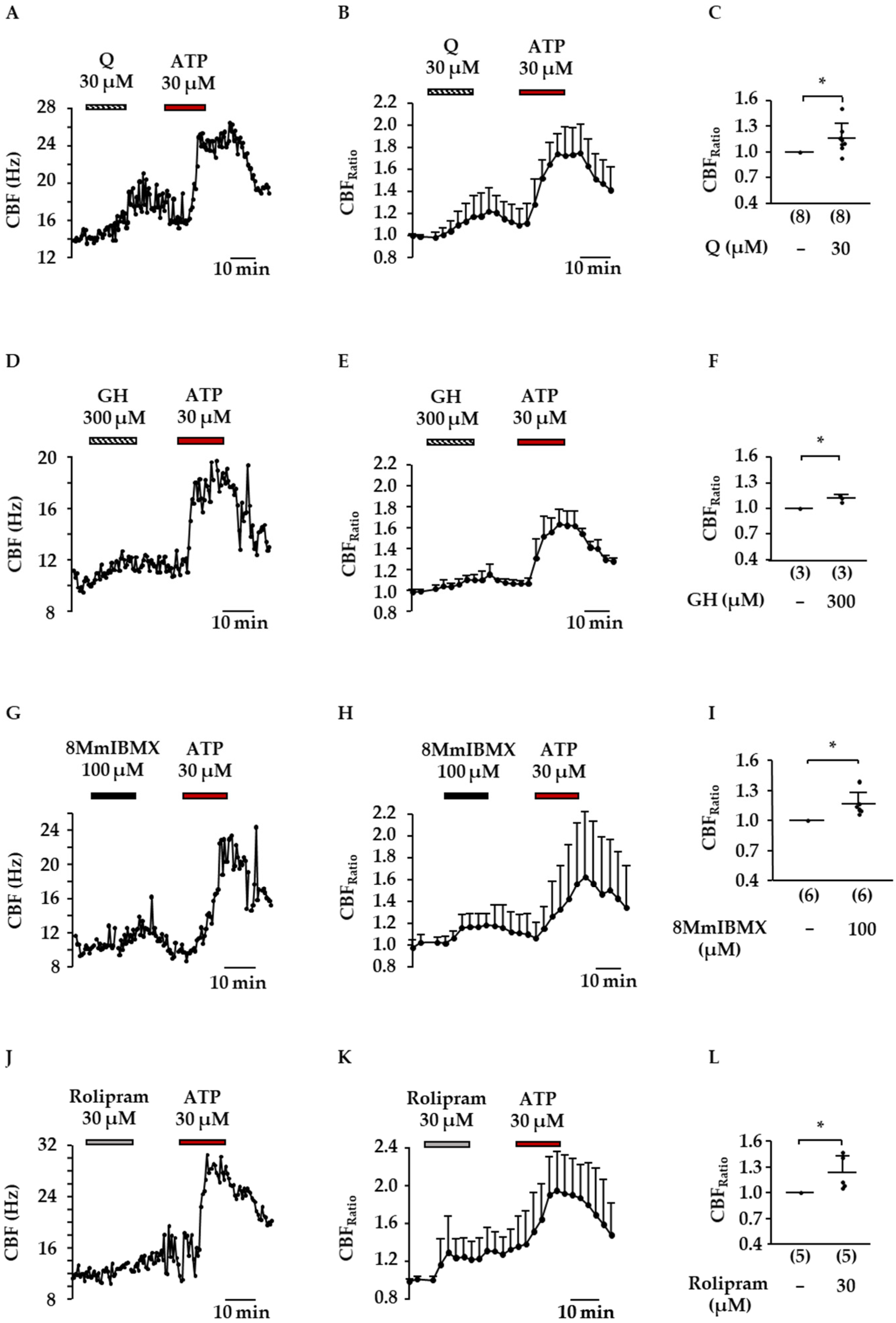
2.3. Influence of DE and Flavonoids on CBF
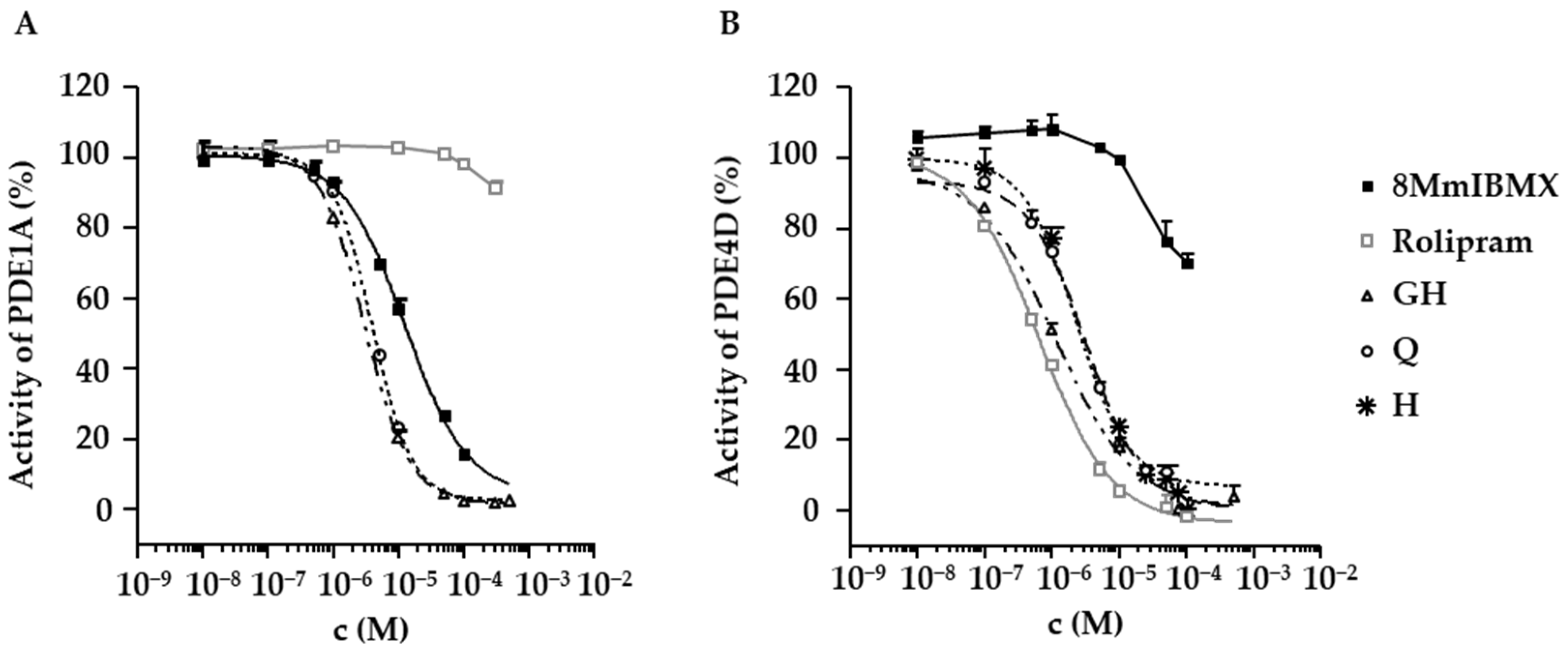
2.4. Inhibition of PDE1A and PDE4D by Flavonoids Present in D. rotundifolia
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extraction
4.3. Phytochemical Characterization of the Drosera Dry Extract
4.4. Isolation and Identification of 2″-O-galloylhyperoside
4.5. UHPLC-PDA Analysis: Quantification of 2″-O-galloylhyperoside, Quercetin and Hyperoside
4.6. Animals and Dissection of Mouse Tracheal Slices
4.7. Tissue Bath Experiments
4.8. Measurements of the Ciliary Beat Frequency (CBF)
4.9. Phosphodiesterase Inhibitor Assays
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baranyai, B.; Joosten, H. Biology, ecology, use, conservation and cultivation of round-leaved sundew (Drosera rotundifolia L.): A review. Mires Peat 2016, 18, 1–28. [Google Scholar]
- Council of Europe. Methods of Preparation of Homeopathic Stocks and Potentisation; European Pharmacopoiea 10th Ed.; Council of Europe: Strasbourg, France, January 2020. [Google Scholar]
- Homöopathisches Arzneibuch: Monographie Drosera; Deutscher Apotheker Verlag: Stuttgart, Germany, 2011.
- Zehl, M.; Braunberger, C.; Conrad, J.; Crnogorac, M.; Krasteva, S.; Vogler, B.; Beifuss, U.; Krenn, L. Identification and quantification of flavonoids and ellagic acid derivatives in therapeutically important Drosera species by LC-DAD, LC-NMR, NMR, and LC-MS. Anal. Bioanal. Chem. 2011, 400, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Braunberger, C.; Zehl, M.; Conrad, J.; Wawrosch, C.; Strohbach, J.; Beifuss, U.; Krenn, L. Flavonoids as chemotaxonomic markers in the genus Drosera. Phytochemistry 2015, 118, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.A.; van der Koo, F. Phytochemistry of the carnivorous sundew genus Drosera (Droseraceae)—Future perspectives and ethnopharmacological relevance. Chem. Biodivers. 2013, 10, 1774–1790. [Google Scholar] [CrossRef] [PubMed]
- Tienaho, J.; Reshamwala, D.; Karonen, M.; Silvan, N.; Korpela, L.; Marjomäki, V.; Sarjala, T. Field-Grown in vitro propagated round-leaved Sundew (Drosera rotundifolia L.) show differences in metabolic profiles and biological activities. Molecules 2021, 26, 3581. [Google Scholar] [CrossRef] [PubMed]
- Melzig, M.F.; Pertz, H.H.; Krenn, L. Anti-inflammatory and spasmolytic activity of extracts from Droserae herba. Phytomedicine 2001, 8, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Beyer, G.; Pertz, H.H.; Karall, E.; Kremser, M.; Galambosi, B.; Melzig, M.F. In vitro antispasmodic and anti-inflammatory effects of Drosera rotundifolia. Arzneimittelforschung 2004, 54, 402–405. [Google Scholar]
- van den Broucke, C.O.; Lemli, J.A. Pharmacological and chemical investigation of thyme liquid extracts. Planta Med. 1981, 41, 129–135. [Google Scholar] [CrossRef]
- Paper, D.H.; Karall, E.; Kremser, M.; Krenn, L. Comparison of the antiinflammatory effects of Drosera rotundifolia and Drosera madagascariensis in the HET-CAM assay. Phytother. Res. 2005, 19, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Nagai, K.; Hoshi, Y.; Masumoto, S.; Mikami, I.; Takahashi, Y.; Oike, H.; Kobori, M. Drosera rotundifolia and Drosera tokaiensis suppress the activation of HMC-1 human mast cells. J. Ethnopharmacol. 2009, 125, 90–96. [Google Scholar] [CrossRef]
- Didry, N.; Dubreuil, L.; Trotin, F.; Pinkas, M. Antimicrobial activity of aerial parts of Drosera peltata Smith on oral bacteria. J. Ethnopharmacol. 1998, 60, 91–96. [Google Scholar] [CrossRef]
- Arruda-Silva, F.; Bellavite, P.; Marzotto, M. Low-dose Drosera rotundifolia induces gene expression changes in 16HBE human bronchial epithelial cells. Sci. Rep. 2021, 11, 2356. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef] [PubMed]
- Komatani-Tamiya, N.; Daikokua, E.; Yoshizumi, T.; Shimamoto, C.; Nakano, T.; Iwasakia, Y.; Kohda, Y.; Matsumura, H.; Marunaka, Y.; Nakahari, T. Procaterol-stimulated increases in ciliary bend amplitude and ciliary beat frequency in mouse bronchioles. J. Cell. Physiol. Biochem. 2012, 29, 511–522. [Google Scholar] [CrossRef]
- Joskova, M.; Mokry, J.; Franova, S. Respiratory cilia as a therapeutic target of phosphodiesterase inhibitors. Front. Pharmacol. 2020, 11, 609. [Google Scholar] [CrossRef]
- Seybold, Z.V.; Mariassy, A.T.; Stroh, D.; Kim, C.S.; Gazeroglu, H.; Wanner, A. Mucociliary interaction in vitro: Effects of physiological and inflammatory stimuli. J. Appl. Physiol. 1990, 68, 1421–1426. [Google Scholar] [CrossRef]
- Begrow, F.; Böckenholt, C.; Ehmen, M.; Wittig, T.; Verspohl, E.J. Effect of myrtol standardized and other substances on the respiratory tract: Ciliary beat frequency and mucociliary clearance as parameters. Adv. Ther. 2012, 29, 350–358. [Google Scholar] [CrossRef]
- Milara, J.; Armengot, M.; Bañuls, P.; Tenor, H.; Beume, R.; Artigues, E.; Cortijo, J. Roflumilast N-oxide, a PDE4 inhibitor, improves cilia motility and ciliated human bronchial epithelial cells compromised by cigarette smoke in vitro. Br. J. Pharmacol. 2012, 166, 2243–2262. [Google Scholar] [CrossRef]
- Bienenfeld, W.; Katzlmeier, H. Flavonoide aus Drosera rotundifolia L. Arch. Pharm. 1966, 299, 598–602. [Google Scholar] [CrossRef]
- de Boer, J.; Philpott, A.J.; van Amsterdam, R.G.M.; Shahid, M.; Zaagsma, J.; Nicholson, C.D. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: Biochemical and pharmacological analysis using selective inhibitors. Br. J. Pharmacol. 1992, 106, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Bernareggi, M.M.; Belvisi, M.G.; Patel, H.; Barnes, P.J.; Giembycz, M.A. Anti-spasmogenic activity of isoenzyme-selective phosphodiesterase inhibitors in guinea-pig trachealis. Br. J. Pharmacol. 1999, 128, 327–336. [Google Scholar] [CrossRef][Green Version]
- Méhats, C.; Jin, S.-L.C.; Wahlstrom, J.; Law, E.; Umetsu, D.T.; Conti, M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003, 17, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, A.; Raeburn, D. The effect of isoenzyme-selective PDE inhibitors on methacholine-induced contraction of guineapig and rat ileum. Br. J. Pharmacol. 1996, 118, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Koloziej, H.; Pertz, H.H.; Humke, A. Main constituents of a commercial Drosera fluid extract and their antagonist activity at muscarinic M3 receptors in guinea-pig ileum. Pharmazie 2002, 57, 201–203. [Google Scholar]
- Schlemper, V.; Ribas, A.; Nicolau, M.; Cechinel Filho, V. Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine 1996, 3, 211–216. [Google Scholar] [CrossRef]
- Krenn, L.; Blaeser, U.; Hausknost-Chenicek, N. Determination of Naphthoquinones in Droserae herba by Reversed-Phase High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 3149–3160. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Havel, L.; Kizek, R. Noteworthy Secondary Metabolites Naphthoquinones—Their Occurrence, Pharmacological Properties and Analysis. CPA 2009, 5, 47–68. [Google Scholar] [CrossRef]
- Tikkanen, L.; Matsushima, T.; Natori, S.; Yoshihira, K. Mutagenicity of natural naphthoquinones and benzoquinones in the Salmonella/microsome test. Mutat. Res. Genet. Toxicol. 1983, 124, 25–34. [Google Scholar] [CrossRef]
- Townsend, E.A.; Emala, C.W. Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, 396–403. [Google Scholar] [CrossRef]
- Zhang, S.-D.; Wang, P.; Zhang, J.; Wang, W.; Yao, L.-P.; Gu, C.-B.; Efferth, T.; Fu, Y.-J. 2′O-galloylhyperin attenuates LPS-induced acute lung injury via up-regulation antioxidation and inhibition of inflammatory responses in vivo. Chem. Biol. Interact. 2019, 304, 20–27. [Google Scholar] [CrossRef]
- Khan, A.; Gilani, A.-H.; Najeeb-ur-Rehman. Pharmacological studies on Hypericum perforatum fractions and constituents. Pharm. Biol. 2011, 49, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-C.; Shih, C.-M.; Lai, Y.-H.; Chen, J.-H.; Huang, H.-L. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem. Pharmacol. 2004, 68, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Nmngoong, Y.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of naturally occuring flavonoids on mitogen-induced lymphocyte proliferation and mixed lymphocyte culture. Life Sci. 1993, 54, 313–320. [Google Scholar] [CrossRef]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Nakano, T.; Sano, K.-I.; Inui, T.; Marunaka, Y.; et al. A low Ca2+i -induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflügers Arch. 2017, 469, 1215–1227. [Google Scholar] [CrossRef]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Marunaka, Y.; Nakahari, T. Ca2+i modulation of cAMP-stimulated ciliary beat frequency via PDE1 in airway ciliary cells of mice. Exp. Physiol. 2018, 103, 381–390. [Google Scholar] [CrossRef]
- Zhang, S.; Smith, N.; Schuster, D.; Azbell, C.; Sorscher, E.J.; Rowe, S.M.; Woodworth, B.A. Quercetin increases cystic fibrosis transmembrane conductance regulator–mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am. J. Rhinol. Allergy 2011, 25, 307–311. [Google Scholar] [CrossRef]
- Reinboth, M.; Wolffram, S.; Abraham, G.; Ungemach, F.R.; Cermak, R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br. J. Nutr. 2010, 104, 198–203. [Google Scholar] [CrossRef]
- Boeckenholt, C.; Begrow, F.; Verspohl, E.J. Effect of silymarin and harpagoside on inflammation reaction of BEAS-2B cells, on ciliary beat frequency (CBF) of trachea explants and on mucociliary clearance (MCC). Planta Med. 2012, 78, 761–766. [Google Scholar] [CrossRef]
- Sisson, J.H.; Stoner, J.A.; Ammons, B.A. All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 2003, 211, 103–111. [Google Scholar] [CrossRef]
- Delmotte, P.; Sanderson, M.J. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef]
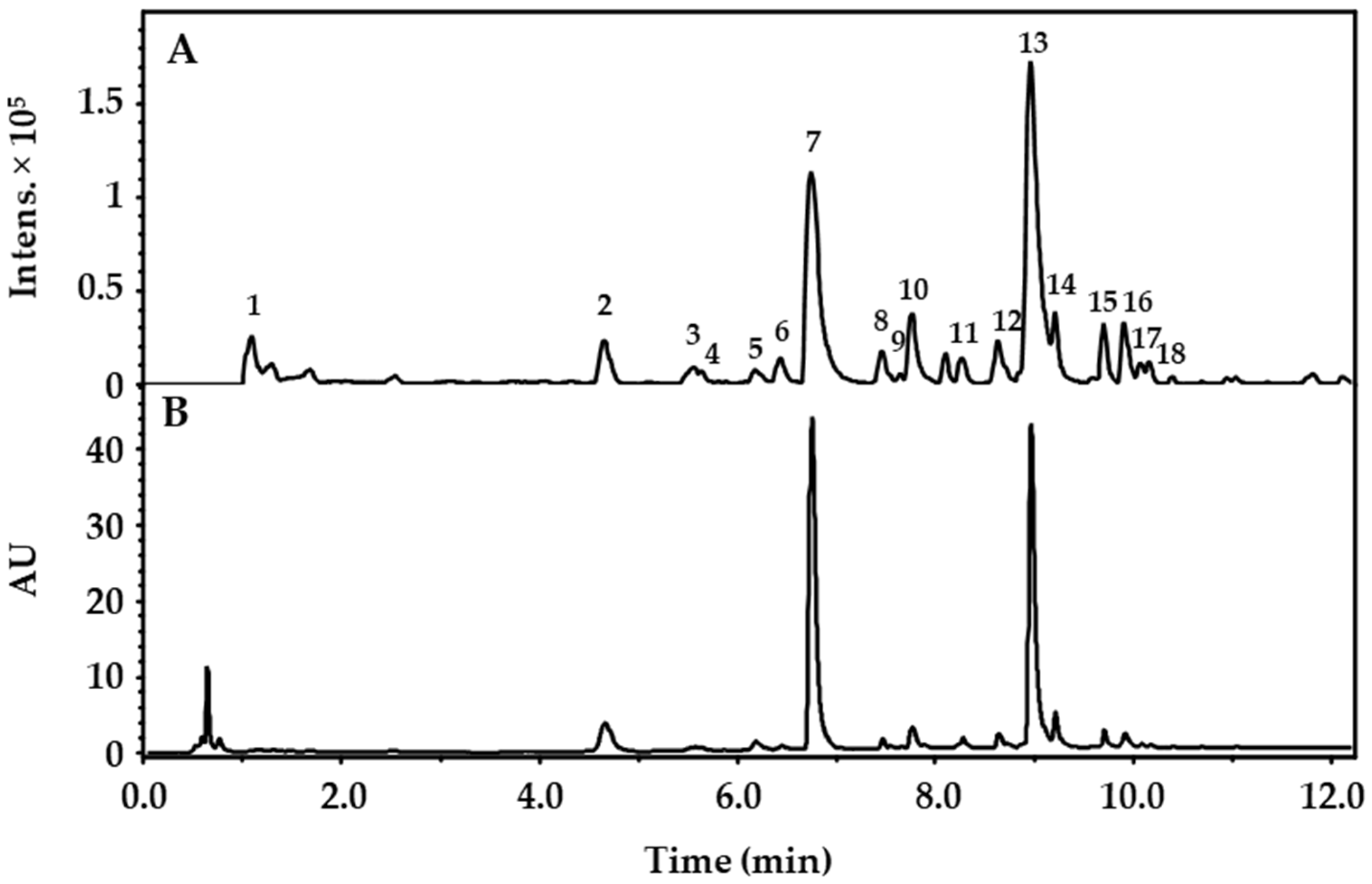


| Peak No. | tR (min) | λmax (nm) | [M+H]+ (m/z) | MS2 Fragments (m/z) | Ion Formula | Error (mDa) | mSigma | Tentative Compound Identity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.377 | - | 221.0676 | 203.0558, 175.0605, 157.0409, 129.0178, 115.0398 | C8H13O7 | −2.0 | 54.2 | 1,5-Dimethylcitrate | |
| 2 | 4.654 | 220, 272 | 199.0608 | 171.0270, 153.0166 | C9H11O5 | −0.6 | 1.6 | Ethyl gallate | |
| 3 | 5.557 | 216, 270, 357 | 633.1103 | 319.0437, 315.0719, 153.0167, 127.0427 | C28H25O17 | −1.6 | 4.3 | Myricetin-3-O-(6″-O-galloyl)-hexoside | [21] |
| 4 | 5.641 | 212, 269, 360 | 779.1669 | 617.1122, 477.1330, 315.0704, 303.0497, 153.0156 | C34H35O21 | −0.3 | 10.5 | tentatively identified Quercetin- (galloylhexosyl)-hexoside | |
| 5 | 6.186 | 214, 252, 363 | 303.0142 | 285.0069, 275.0145, 257.0081, 247.0264, 229.0135, 219.0262, 201.0202, 173.0215, | C14H7O8 | 0.6 | 18.8 | Ellagic acid | [21] |
| 6 | 6.435 | 212, 259, 356 | 465.1036 | 303.0496 | C21H21O12 | 0.9 | 7.5 | Hyperoside | [4] |
| 7 | 6.736 | 216, 264, 356 | 617.1173 | 303.0527, 233.0455, 153.0181 | C28H25O16 | 0.6 | 106.1 | 2″-O-galloylhyperoside | [4] |
| 8 | 7.455 | 216, 268, 354 | 601.1183 | 315.0710, 287.0545, 233.0410, 205.0485, 153.0160 | C28H25O15 | 0.5 | 16.6 | Kaempferol-3-O-(2-O-galloyl)-β- galactopyranoside | [4,5] |
| 9 | 7.6480 | 216, 262, 360 | 601.1191 | 465.1033, 303.0501, 299.0769, 137.0214 | C28H25O15 | 0.3 | 10.4 | tentatively identified Quercetin- (dihydroxybenzoyl-)hexoside | |
| 10 | 7.759 | 212, 256, 368 | 319.0441 | 273.0387, 245.0431, 217.0476, 179.0311, 165.0150, 153.0150 | C15H11O8 | −0.8 | 1.8 | Myricetin | |
| 11 | 5.741 | 206, 262, 364 | 585.1246 | 303.0509, 283.0835, 265.0676, 121.0268 | C28H25O14 | −0.7 | 8.8 | tentatively identified Quercetin- (hydroxybenzoyl-)hexoside | |
| 12 | 8.846 | 212, 256, 368 | 611.1389 | 309.0973, 303.0471, 147.0428 | C30H27O14 | 0.7 | 8.3 | tentatively identified Quercetin- (cumaroyl-)hexoside | |
| 13 | 8.956 | 220, 256, 368 | 303.0497 | 229.0487, 153.0161, 137.0223 | C15H11O7 | 0.7 | 37.6 | Quercetin | [4] |
| 14 | 9.222 | 220, 243, 371 | 331.0463 | 316.0227, 300.9989, 271.0255, 253.0097 | C16H11O8 | 0.8 | 3.8 | 3,3′-Di-O-methylellagic acid | [4] |
| 15 | 9.699 | 220, 269, 356 | 569.1301 | 303.0504, 267.0856, 123.0420, 105.0330 | C28H25O13 | −1.2 | 6.3 | Quercetin-(benzoyl-)hexoside | [21] |
| 16 | 9.910 | 220, 265, 362 | 287.0558 | 213.0574, 165.0160, 153.0168, 121.0290 | C15H11O6 | −0.1 | 35.0 | Kaempferol | [4] |
| 17 | 10.075 | 220, 268, 355 | 553.1359 | 287.0558, 267.0863, 123.0409, 105.0338 | C28H25O12 | −1.8 | 11.4 | tentatively identified Kaempferol-(benzoyl-)hexoside | |
| 18 | 10.157 | 220, 268, 355 | 553.1374 | 287.0546, 267.0870, 123.0462, 105.0334 | C28H25O12 | −3.3 | 55.3 | tentatively identified Kaempferol-(benzoyl-)hexoside |
| Sample | GH (µM) | Q (µM) | H (µM) |
|---|---|---|---|
| DE | 88 ± 3 | 71 ± 30 | 1.8 ± 0.2 |
| DFA | 67 ± 5 | 9 ± 3 | 2.3 ± 0.3 |
| GH IC50 (µM) | Q IC50 (µM) | H IC50 (µM) | 8MmIBMX IC50 (µM) | Rolipram IC50 (µM) | |
|---|---|---|---|---|---|
| PDE1A | 3.0 (CI: 2.8–3.3) | 4 (CI: 3–5) | not tested | 12 (CI: 9–18) | − |
| PDE4D | 1.1 (CI: 0.7–1.5) | 2 (CI: 2–3) | 3 (CI: 3–4) | − | 0.6 (CI: 0.5–0.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hake, A.; Begrow, F.; Spiegler, V.; Symma, N.; Hensel, A.; Düfer, M. Effects of Extracts and Flavonoids from Drosera rotundifolia L. on Ciliary Beat Frequency and Murine Airway Smooth Muscle. Molecules 2022, 27, 6622. https://doi.org/10.3390/molecules27196622
Hake A, Begrow F, Spiegler V, Symma N, Hensel A, Düfer M. Effects of Extracts and Flavonoids from Drosera rotundifolia L. on Ciliary Beat Frequency and Murine Airway Smooth Muscle. Molecules. 2022; 27(19):6622. https://doi.org/10.3390/molecules27196622
Chicago/Turabian StyleHake, Alexander, Frank Begrow, Verena Spiegler, Nico Symma, Andreas Hensel, and Martina Düfer. 2022. "Effects of Extracts and Flavonoids from Drosera rotundifolia L. on Ciliary Beat Frequency and Murine Airway Smooth Muscle" Molecules 27, no. 19: 6622. https://doi.org/10.3390/molecules27196622
APA StyleHake, A., Begrow, F., Spiegler, V., Symma, N., Hensel, A., & Düfer, M. (2022). Effects of Extracts and Flavonoids from Drosera rotundifolia L. on Ciliary Beat Frequency and Murine Airway Smooth Muscle. Molecules, 27(19), 6622. https://doi.org/10.3390/molecules27196622





