Introducing a Linear Empirical Correlation for Predicting the Mass Heat Capacity of Biomaterials
Abstract
1. Introduction
2. Results and Discussion
2.1. Developed Correlations in This Study
2.2. Comparison with the Literature Suggested Models
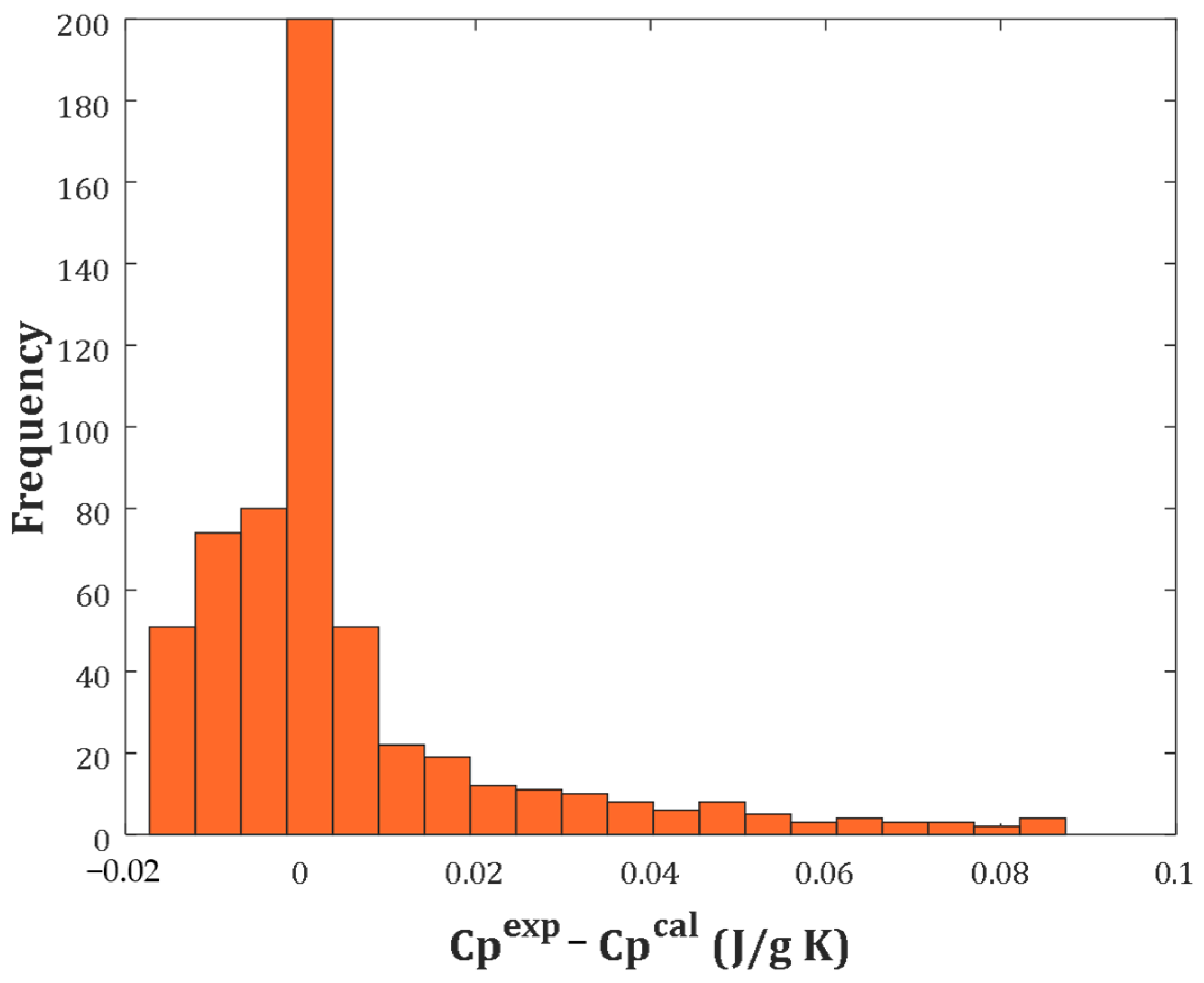
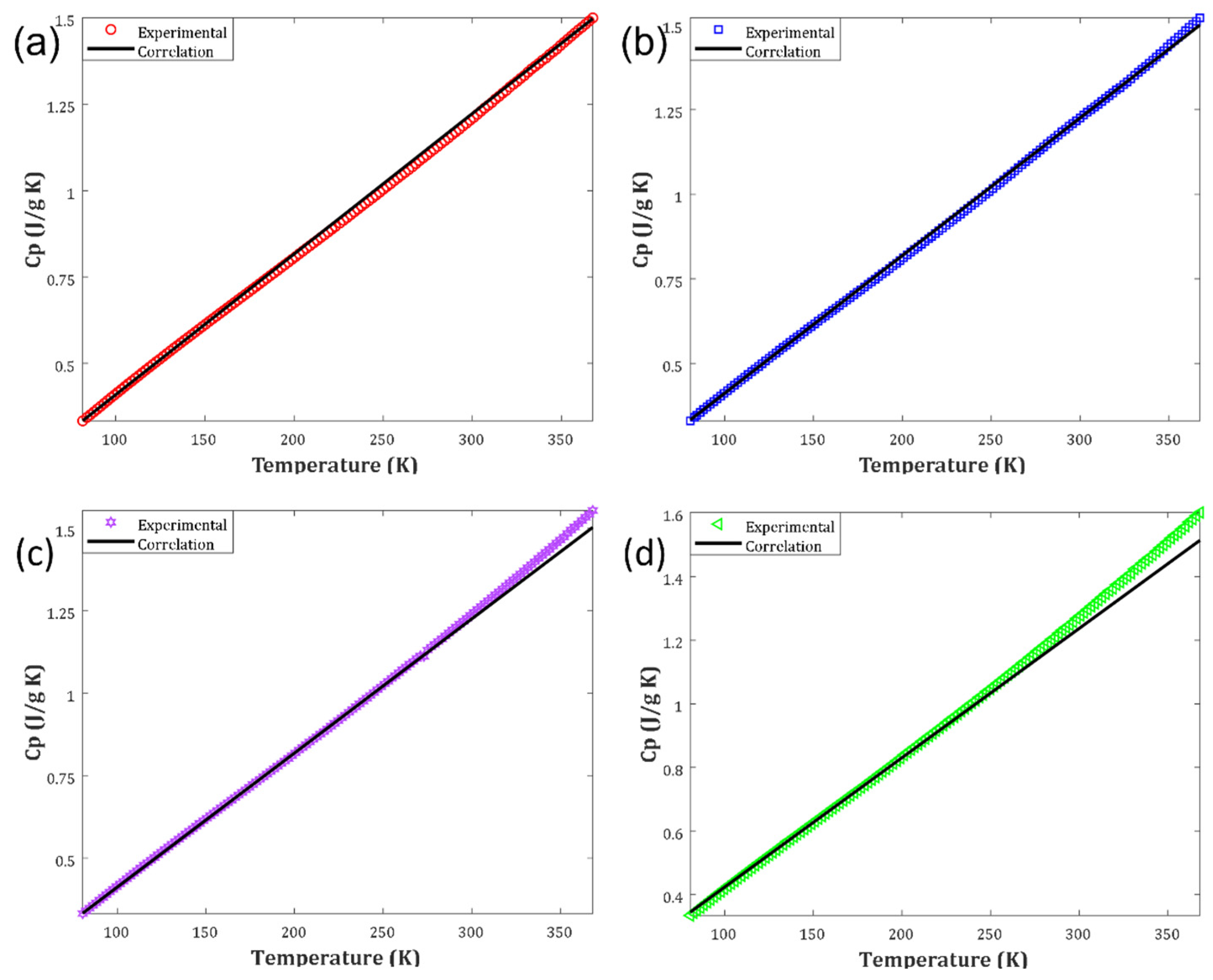
2.3. Visually Inspecting the Performance of the Three-Parameter Correlation
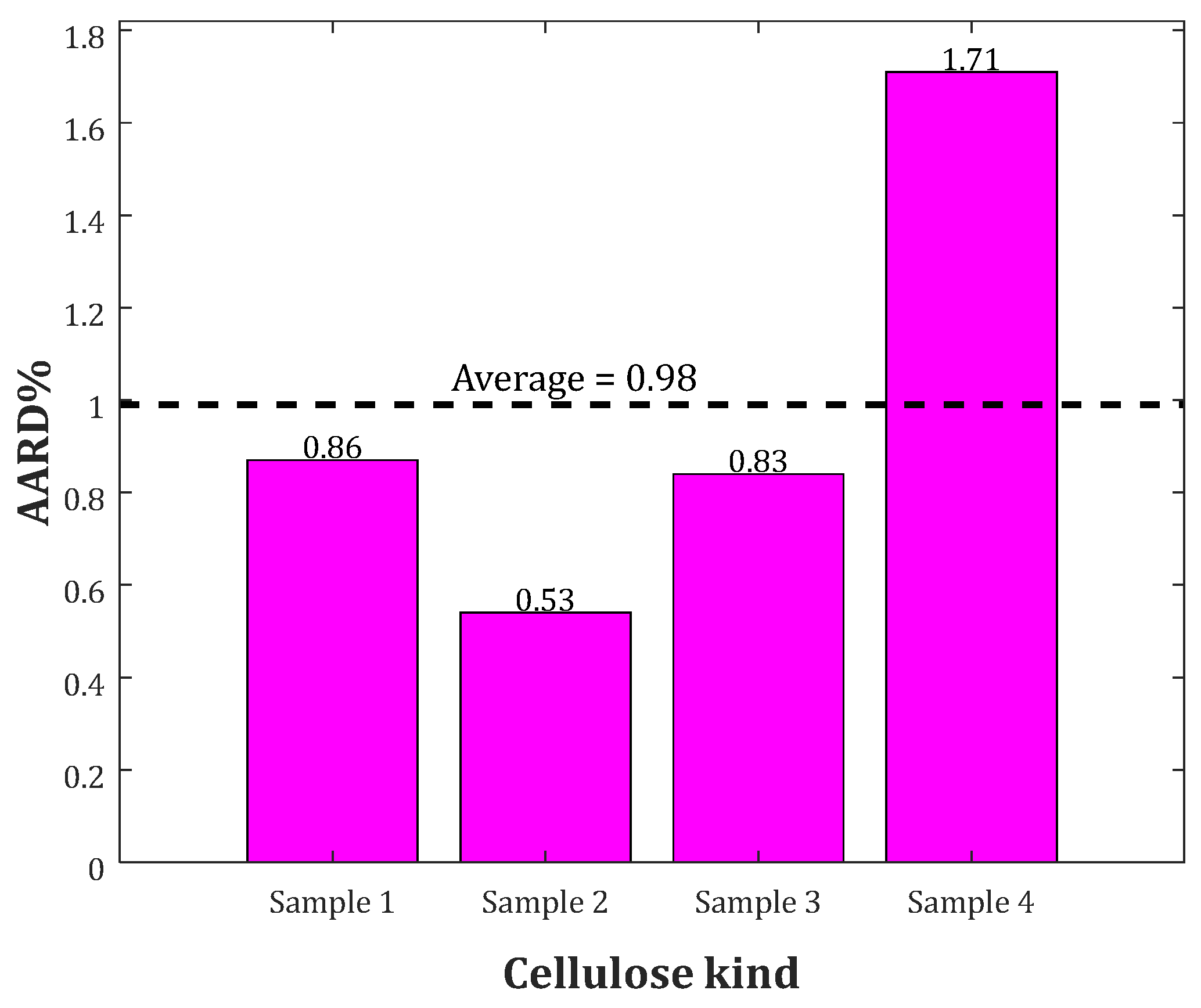
2.4. Analyzing the Cp of Bio-Samples with Different Origins
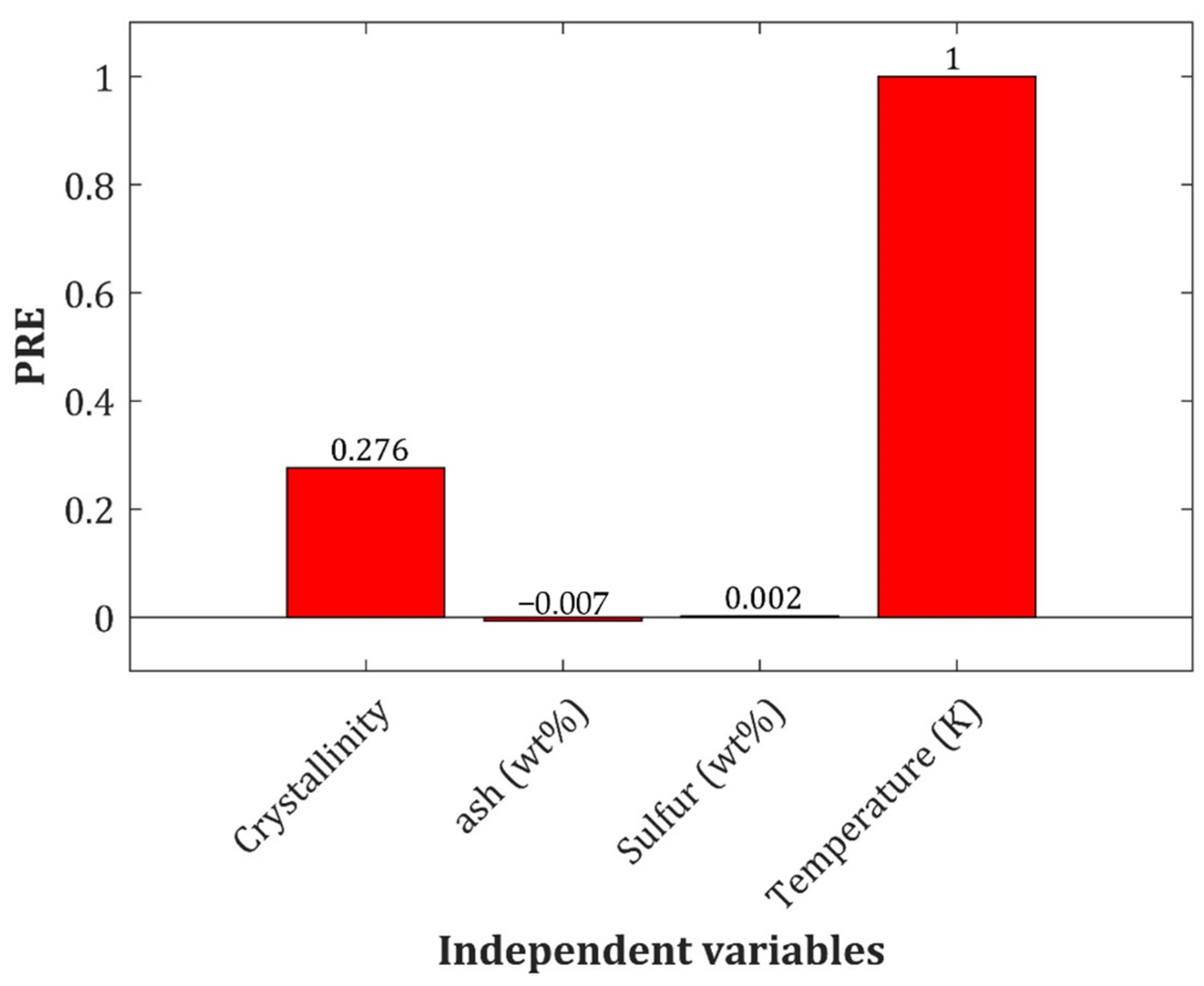
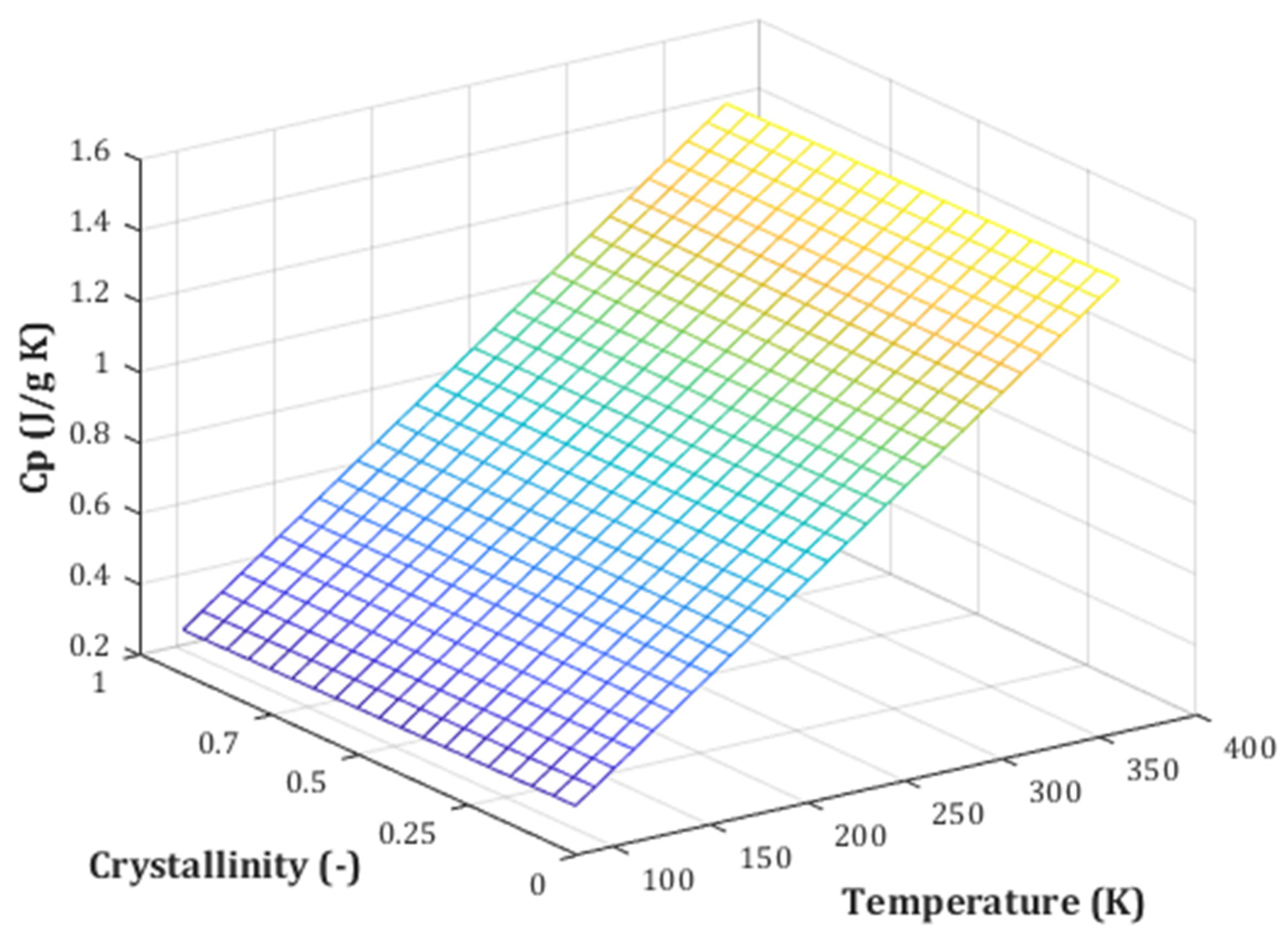
2.5. Pair Effect of Temperature and Crystallinity Index on the Biomass Cp
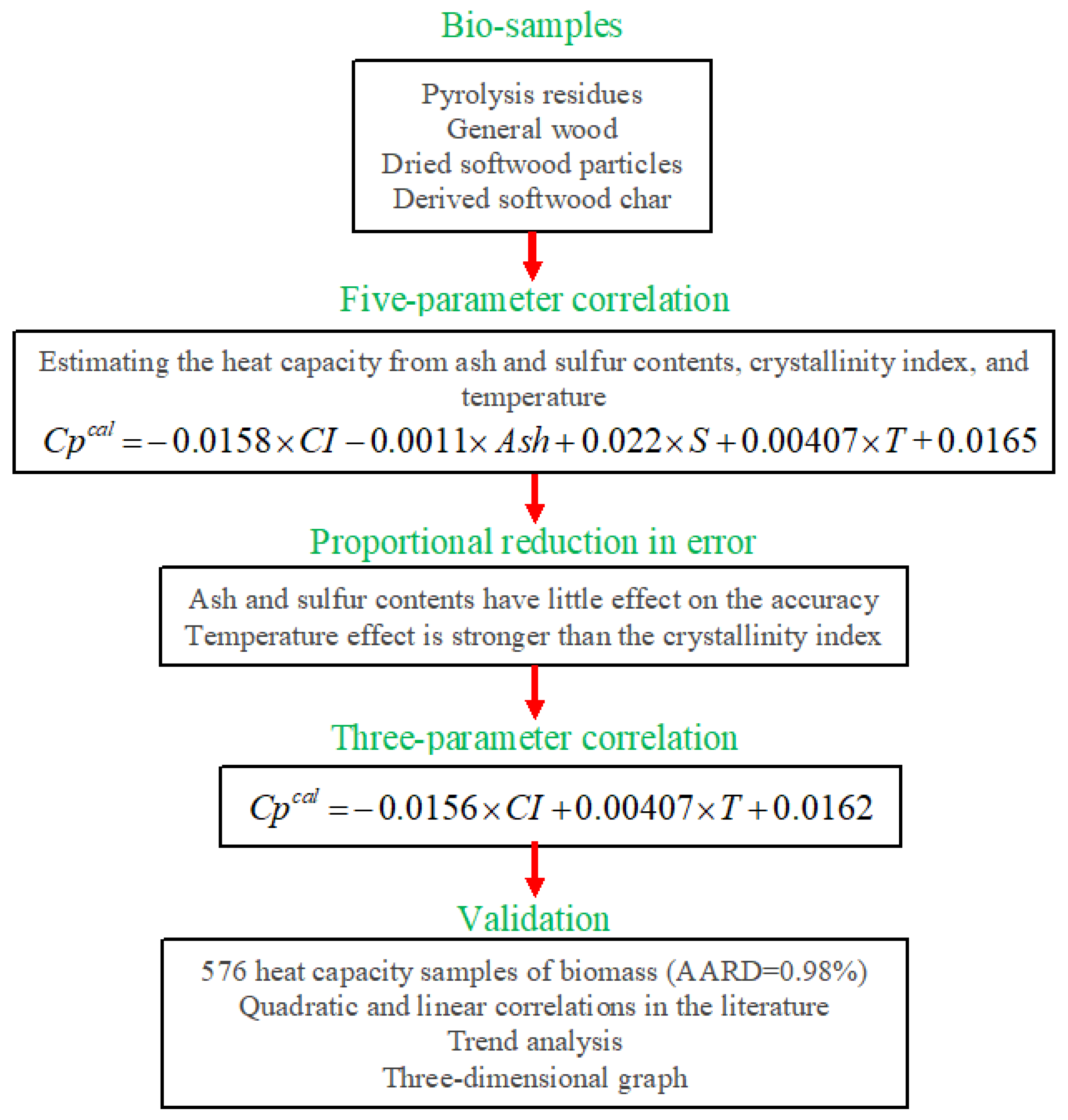
2.6. Summary of the Study in the Flowchart Form
3. Materials and Methods
3.1. Collected Database
3.2. Literature Suggested Correlations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Karimi, M.; Rahimpour, M.R.; Rafiei, R.; Jafari, M.; Iranshahi, D.; Shariati, A. Reducing environmental problems and increasing saving energy by proposing new configuration for moving bed thermally coupled reactors. J. Nat. Gas Sci. Eng. 2014, 17, 136–150. [Google Scholar] [CrossRef]
- Karimi, M.; Diaz de Tuesta, J.L.; Carmem, C.N.; Gomes, H.T.; Rodrigues, A.E.; Silva, J.A.C. Compost from Municipal Solid Wastes as a Source of Biochar for CO2 Capture. Chem. Eng. Technol. 2020, 43, 1336–1349. [Google Scholar] [CrossRef]
- Vaferi, B.; Eslamloueyan, R.; Ghaffarian, N. Hydrocarbon reservoir model detection from pressure transient data using coupled artificial neural network—Wavelet transform approach. Appl. Soft Comput. J. 2016, 47, 63–75. [Google Scholar] [CrossRef]
- Karimi, M.; Silva, J.A.C.; Gonçalves, C.N.D.P.; Diaz De Tuesta, J.L.; Rodrigues, A.E.; Gomes, H.T. CO2 Capture in Chemically and Thermally Modified Activated Carbons Using Breakthrough Measurements: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2018, 57, 11154–11166. [Google Scholar] [CrossRef]
- Esmaeili-Faraj, S.H.; Vaferi, B.; Bolhasani, A.; Karamian, S.; Hosseini, S.; Rashedi, R. Design of a Neuro-Based Computing Paradigm for Simulation of Industrial Olefin Plants. Chem. Eng. Technol. 2021, 44, 1382–1389. [Google Scholar] [CrossRef]
- Karimi, M.; Zafanelli, L.F.A.S.; Almeida, J.P.P.; Ströher, G.R.; Rodrigues, A.E.; Silva, J.A.C. Novel Insights into Activated Carbon Derived from Municipal Solid Waste for CO2 Uptake: Synthesis, Adsorption Isotherms and Scale-up. J. Environ. Chem. Eng. 2020, 8, 104069. [Google Scholar] [CrossRef]
- Henrique, A.; Karimi, M.; Silva, J.A.C.; Rodrigues, A.E. Analyses of Adsorption Behavior of CO2, CH4, and N2 on Different Types of BETA Zeolites. Chem. Eng. Technol. 2019, 42, 327–342. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Alobiad, F. CO2 capture from biogas by biomass-based adsorbents: A review. Fuel 2022, 328, 125276. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Karimi, M.; Rodrigues, A.E.; Silva, J.A.C. Designing a simple volumetric apparatus for measuring gas adsorption equilibria and kinetics of sorption. Application and validation for CO2, CH4 and N2 adsorption in binder-free beads of 4A zeolite. Chem. Eng. J. 2021, 425, 130538. [Google Scholar] [CrossRef]
- Esmaeili-Faraj, S.H.; Hassanzadeh, A.; Shakeriankhoo, F.; Hosseini, S.; Vaferi, B. Diesel fuel desulfurization by alumina/polymer nanocomposite membrane: Experimental analysis and modeling by the response surface methodology. Chem. Eng. Process.-Process Intensif. 2021, 164, 108396. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology-The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Karimi, M.; Hosin Alibak, A.; Seyed Alizadeh, S.M.; Sharif, M.; Vaferi, B. Intelligent modeling for considering the effect of bio-source type and appearance shape on the biomass heat capacity. Meas. J. Int. Meas. Confed. 2022, 189, 110529. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Prest, J.; Skryabin, I. Biogas as an energy vector. Biomass Bioenergy 2021, 144, 105935. [Google Scholar] [CrossRef]
- Cao, Y.; Kamrani, E.; Mirzaei, S.; Khandakar, A.; Vaferi, B. Electrical efficiency of the photovoltaic/thermal collectors cooled by nanofluids: Machine learning simulation and optimization by evolutionary algorithm. Energy Rep. 2022, 8, 24–36. [Google Scholar] [CrossRef]
- da Costa, T.P.; Quinteiro, P.; da Cruz Tarelho, L.A.; Arroja, L.; Dias, A.C. Environmental impacts of forest biomass-to-energy conversion technologies: Grate furnace vs. fluidised bed furnace. J. Clean. Prod. 2018, 171, 153–162. [Google Scholar] [CrossRef]
- UNEP. Converting Waste Agricultural Biomass into a Resource. In Compendium of Technologies. Osaka, United Nations Environment Programme; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2009; pp. 1–437. [Google Scholar]
- Dobele, G.; Dizhbite, T.; Gil, M.V.; Volperts, A.; Centeno, T.A. Production of nanoporous carbons from wood processing wastes and their use in supercapacitors and CO2 capture. Biomass Bioenergy 2012, 46, 145–154. [Google Scholar] [CrossRef]
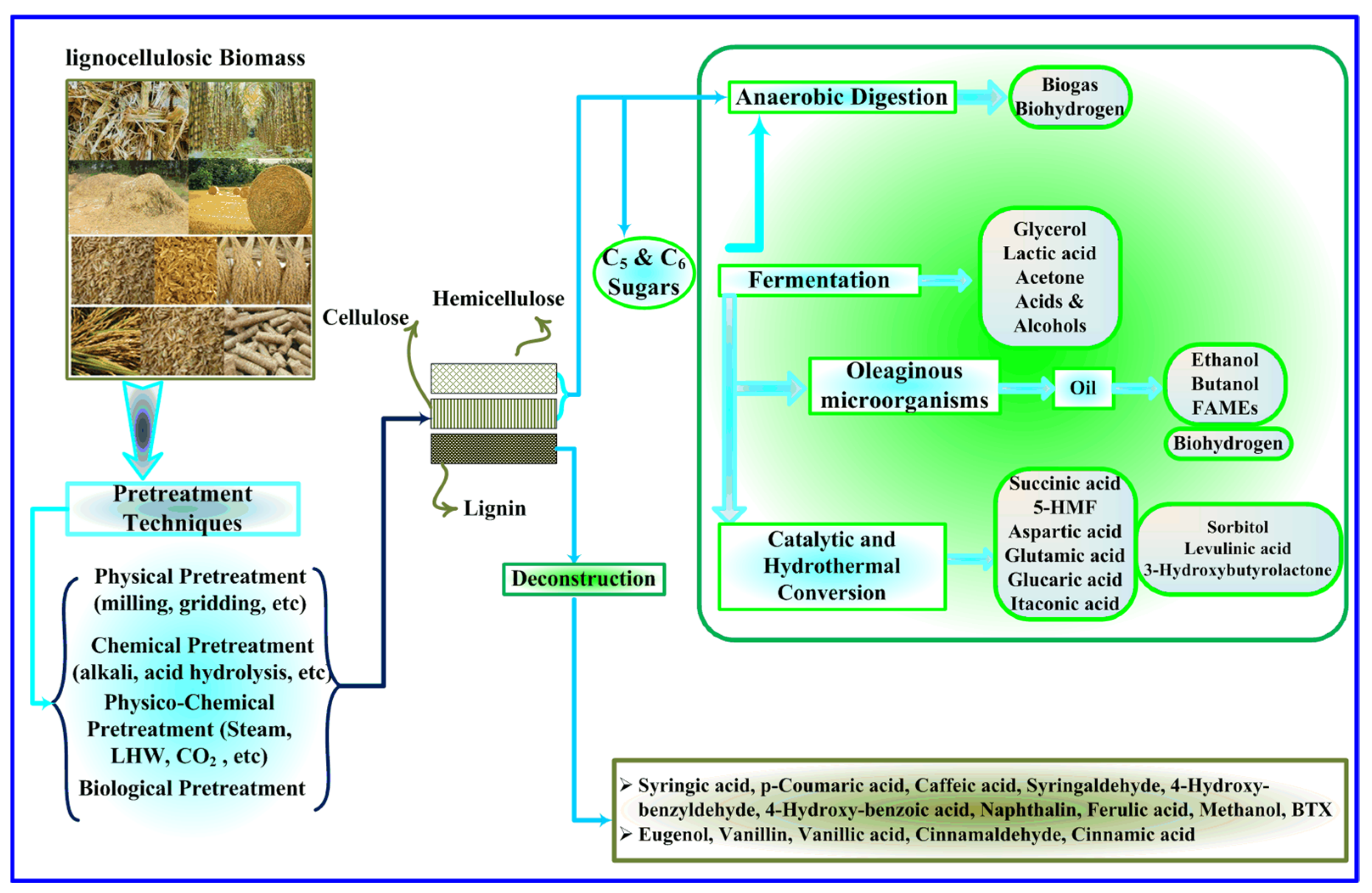
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Tan, X.; Wu, H.; Li, H.; Yang, S. Advances in Pretreatment of Straw Biomass for Sugar Production. Front. Chem. 2021, 9, 696030. [Google Scholar] [CrossRef]
- Singh, S.; Sinha, R.; Kundu, S. Role of organosolv pretreatment on enzymatic hydrolysis of mustard biomass for increased saccharification. Biomass Convers. Biorefinery 2022, 12, 1657–1668. [Google Scholar] [CrossRef]
- Dupont, C.; Chiriac, R.; Gauthier, G.; Toche, F. Heat capacity measurements of various biomass types and pyrolysis residues. Fuel 2014, 115, 644–651. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Ryms, M.; Kosakowski, W. Thermal biomass conversion: A review. Processes 2020, 8, 516. [Google Scholar] [CrossRef]
- Karimi, M.; Aminzadehsarikhanbeglou, E.; Vaferi, B. Robust intelligent topology for estimation of heat capacity of biochar pyrolysis residues. Meas. J. Int. Meas. Confed. 2021, 183, 109857. [Google Scholar] [CrossRef]
- Shirzad, M.; Karimi, M.; Silva, J.A.C.; Rodrigues, A.E. Moving Bed Reactors: Challenges and Progress of Experimental and Theoretical Studies in a Century of Research. Ind. Eng. Chem. Res. 2019, 58, 9179–9198. [Google Scholar] [CrossRef]
- Olatunji, O.O.; Akinlabi, S.; Madushele, N.; Adedeji, P.A. Estimation of the Elemental Composition of Biomass Using Hybrid Adaptive Neuro-Fuzzy Inference System. Bioenergy Res. 2019, 12, 642–652. [Google Scholar] [CrossRef]
- Suleymani, M.; Bemani, A. Application of ANFIS-PSO algorithm as a novel method for estimation of higher heating value of biomass. Energy Sources, Part A Recover. Util. Environ. Eff. 2018, 40, 288–293. [Google Scholar] [CrossRef]
- Zhu, L.T.; Gu, Y.F.; Wu, G.S. Biomass thermal conductivity measurement system design. J. For. Eng. 2020, 5, 97–102. [Google Scholar] [CrossRef]
- Pecchi, M.; Patuzzi, F.; Benedetti, V.; Di Maggio, R.; Baratieri, M. Kinetic analysis of hydrothermal carbonization using high-pressure differential scanning calorimetry applied to biomass. Appl. Energy 2020, 265, 114810. [Google Scholar] [CrossRef]
- Bitra, V.S.P.; Banu, S.; Ramakrishna, P.; Narender, G.; Womac, A.R. Moisture dependent thermal properties of peanut pods, kernels, and shells. Biosyst. Eng. 2010, 106, 503–512. [Google Scholar] [CrossRef]
- Mothé, C.G.; De Miranda, I.C. Characterization of sugarcane and coconut fibers by thermal analysis and FTIR. J. Therm. Anal. Calorim. 2009, 97, 661–665. [Google Scholar] [CrossRef]
- Voitkevich, O.V.; Kabo, G.J.; Blokhin, A.V.; Paulechka, Y.U.; Shishonok, M.V. Thermodynamic properties of plant biomass components. Heat capacity, combustion energy, and gasification equilibria of lignin. J. Chem. Eng. Data 2012, 57, 1903–1909. [Google Scholar] [CrossRef]
- Kiiskinen, H.T.; Lyytikäinen, K.; Hämäläinen, J.P. Specific heats of dry scandinavian wood pulps. J. Pulp Pap. Sci. 1998, 24, 219–223. [Google Scholar]
- Uryash, V.F.; Larina, V.N.; Kokurina, N.Y.; Novoselova, N.V. The thermochemical characteristics of cellulose and its mixtures with water. Russ. J. Phys. Chem. A 2010, 84, 915–921. [Google Scholar] [CrossRef]
- Wilfred Jr, A.; Kollmann, F.F.P. Principles of Wood Science and Technology; Springer: Berlin/Heidelberg, Germany, 1968. [Google Scholar]
- Gupta, M.; Yang, J.; Roy, C. Specific heat and thermal conductivity of softwood bark and softwood char particles. Fuel 2003, 82, 919–927. [Google Scholar] [CrossRef]
- Iranshahi, D.; Jafari, M.; Rafiei, R.; Karimi, M.; Amiri, S.; Rahimpour, M.R. Optimal design of a radial-flow membrane reactor as a novel configuration for continuous catalytic regenerative naphtha reforming process considering a detailed kinetic model. Int. J. Hydrog. Energy 2013, 38, 8384–8399. [Google Scholar] [CrossRef]
- Alibak, A.H.; Khodarahmi, M.; Fayyazsanavi, P.; Alizadeh, S.M.; Hadi, A.J.; Aminzadehsarikhanbeglou, E. Simulation the adsorption capacity of polyvinyl alcohol/carboxymethyl cellulose based hydrogels towards methylene blue in aqueous solutions using cascade correlation neural network (CCNN) technique. J. Clean. Prod. 2022, 337, 130509. [Google Scholar] [CrossRef]
- Madani, M.; Lin, K.; Tarakanova, A. DSResSol: A sequence-based solubility predictor created with dilated squeeze excitation residual networks. Int. J. Mol. Sci. 2021, 22, 13555. [Google Scholar] [CrossRef]
- Judd, C.M.; McClelland, G.H.; Ryan, C.S. Data Analysis: A Model Comparison Approach; Routledge: Oxfordshire, UK, 2011; ISBN 0203892054. [Google Scholar]
- Hosseini, S.; Vaferi, B. Determination of methanol loss due to vaporization in gas hydrate inhibition process using intelligent connectionist paradigms. Arab. J. Sci. Eng. 2022, 47, 5811–5819. [Google Scholar] [CrossRef]

| Correlation | A | B | C | AARD% |
|---|---|---|---|---|
| Linear | 0 | 0.00406 | 0.0061 | 1.29 |
| Quadratic | 6.11 × 10−5 | −0.0210 | 2.232 | 39.19 |
| Biomass Type | CI (-) | Temperature (K) | Ash (wt%) | S (wt%) | Cp (J/g∙K) | Number Data |
|---|---|---|---|---|---|---|
| Sample 1 | 0.90 | 81.50–367.50 | 0.10 | 0.02 | 0.3335–1.500 | 143 |
| Sample 2 | 0.80 | 80.73–367.40 | 0.10 | 0.43 | 0.3304–1.521 | 144 |
| Sample 3 | 0.74 | 80.53–368.25 | 0.49 | 0.11 | 0.3314–1.554 | 145 |
| Sample 4 | 0 | 80.61–368.09 | 0.07 | 0.02 | 0.3342–1.602 | 144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iranmanesh, R.; Pourahmad, A.; Faress, F.; Tutunchian, S.; Ariana, M.A.; Sadeqi, H.; Hosseini, S.; Alobaid, F.; Aghel, B. Introducing a Linear Empirical Correlation for Predicting the Mass Heat Capacity of Biomaterials. Molecules 2022, 27, 6540. https://doi.org/10.3390/molecules27196540
Iranmanesh R, Pourahmad A, Faress F, Tutunchian S, Ariana MA, Sadeqi H, Hosseini S, Alobaid F, Aghel B. Introducing a Linear Empirical Correlation for Predicting the Mass Heat Capacity of Biomaterials. Molecules. 2022; 27(19):6540. https://doi.org/10.3390/molecules27196540
Chicago/Turabian StyleIranmanesh, Reza, Afham Pourahmad, Fardad Faress, Sevil Tutunchian, Mohammad Amin Ariana, Hamed Sadeqi, Saleh Hosseini, Falah Alobaid, and Babak Aghel. 2022. "Introducing a Linear Empirical Correlation for Predicting the Mass Heat Capacity of Biomaterials" Molecules 27, no. 19: 6540. https://doi.org/10.3390/molecules27196540
APA StyleIranmanesh, R., Pourahmad, A., Faress, F., Tutunchian, S., Ariana, M. A., Sadeqi, H., Hosseini, S., Alobaid, F., & Aghel, B. (2022). Introducing a Linear Empirical Correlation for Predicting the Mass Heat Capacity of Biomaterials. Molecules, 27(19), 6540. https://doi.org/10.3390/molecules27196540











