Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Preparation and Characterization
2.2. Calibration Curves and Ozonation Experiments
2.3. Reaction Mixture Analysis
2.4. Intermediate Identification by LC-ToF-MS
3. Results and Discussion
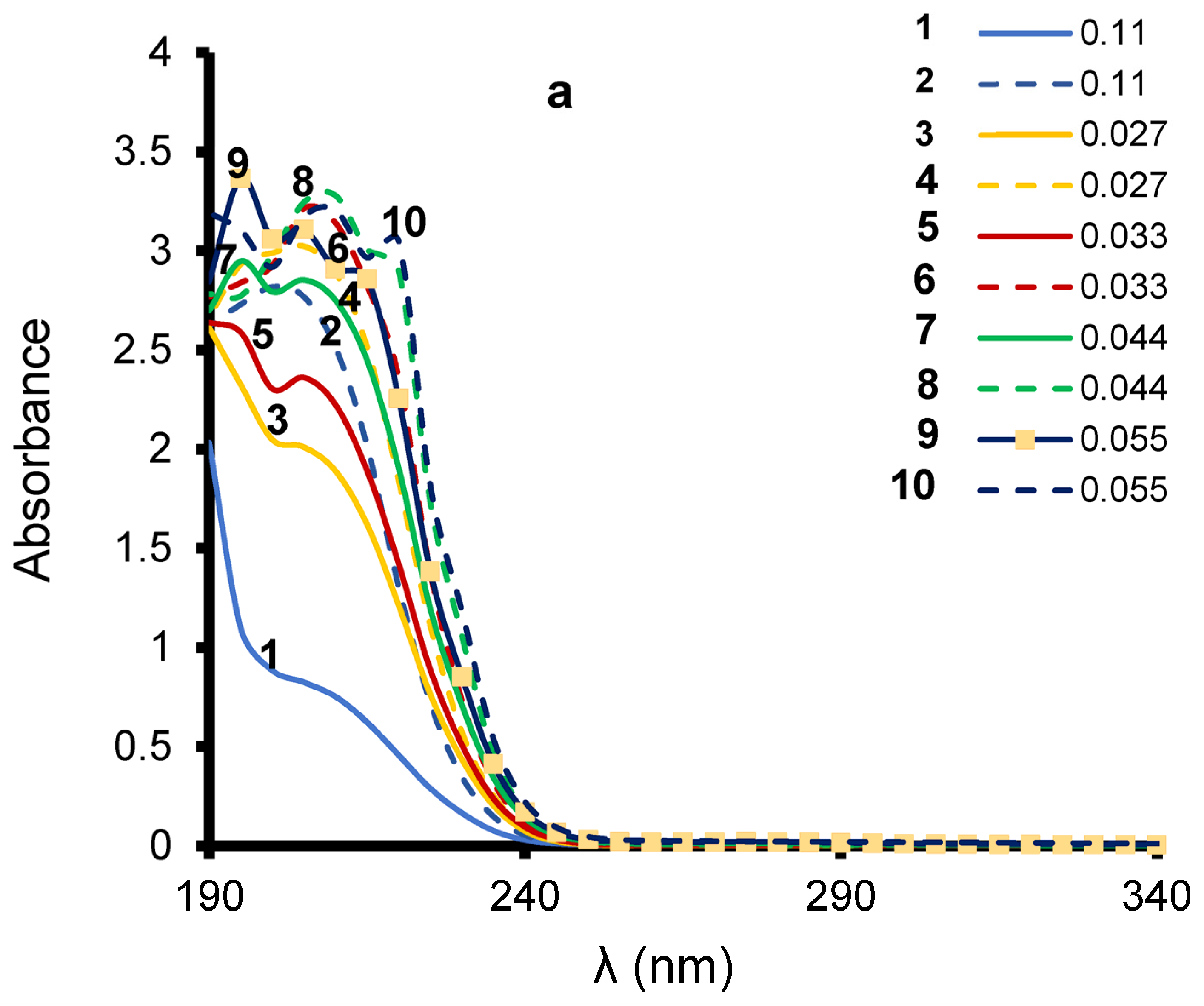
3.1. LA UV-Vis Spectrum Changes in Clay Suspension before Ozonation
3.2. Effect of Clay-Induced pH on LA Adsorption
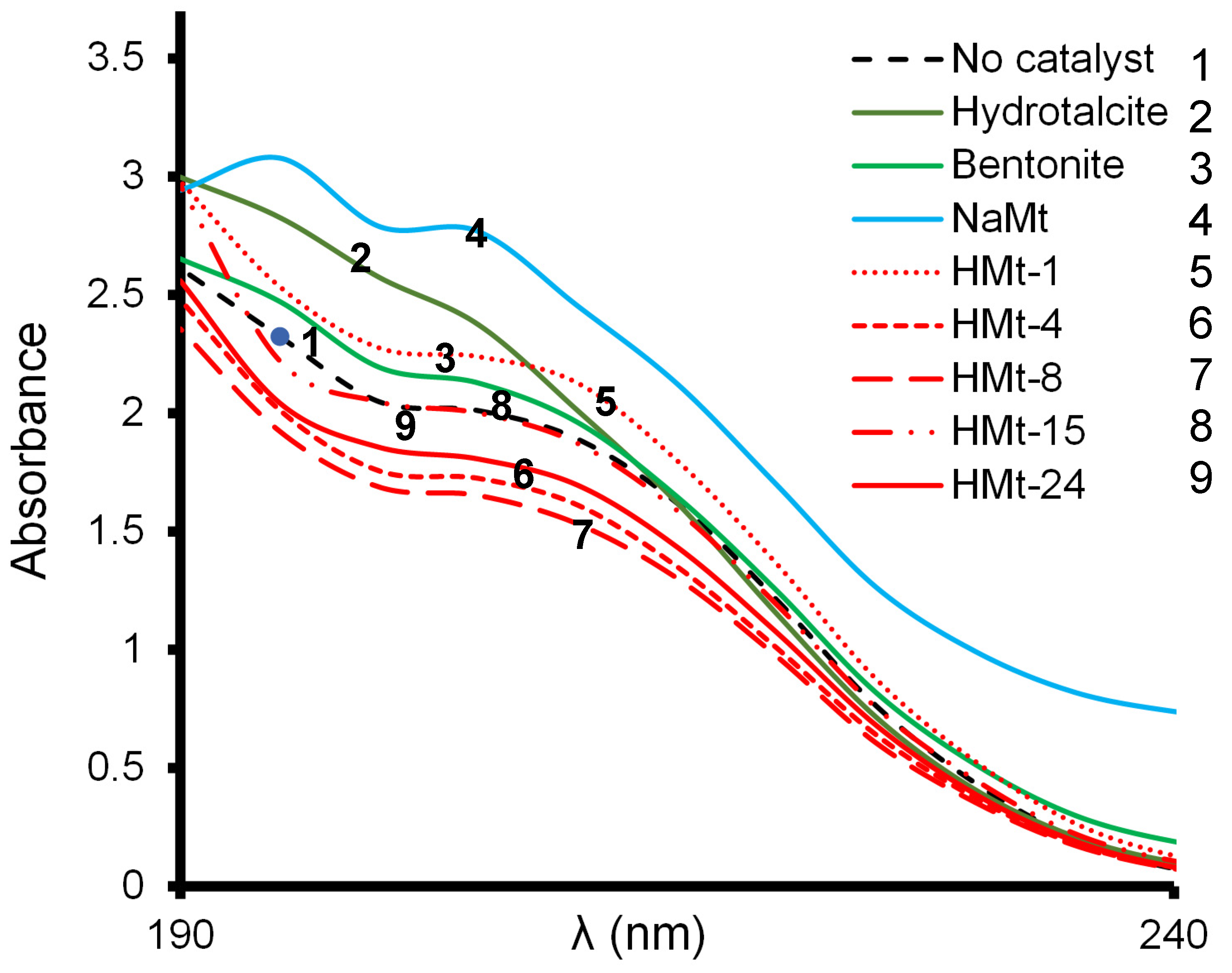
3.3. Effect of Catalyst Addition on LA UV-Vis Spectrum
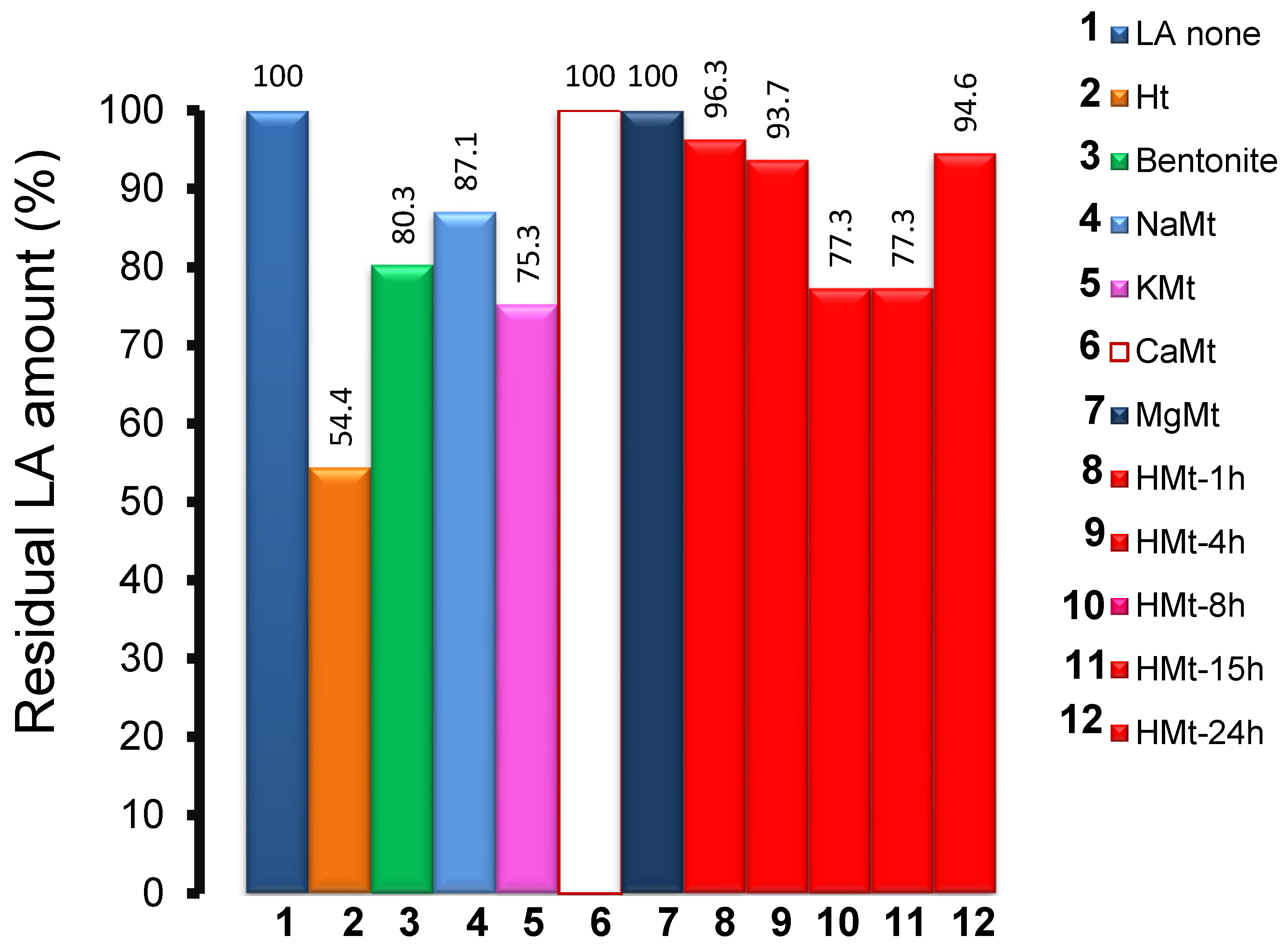
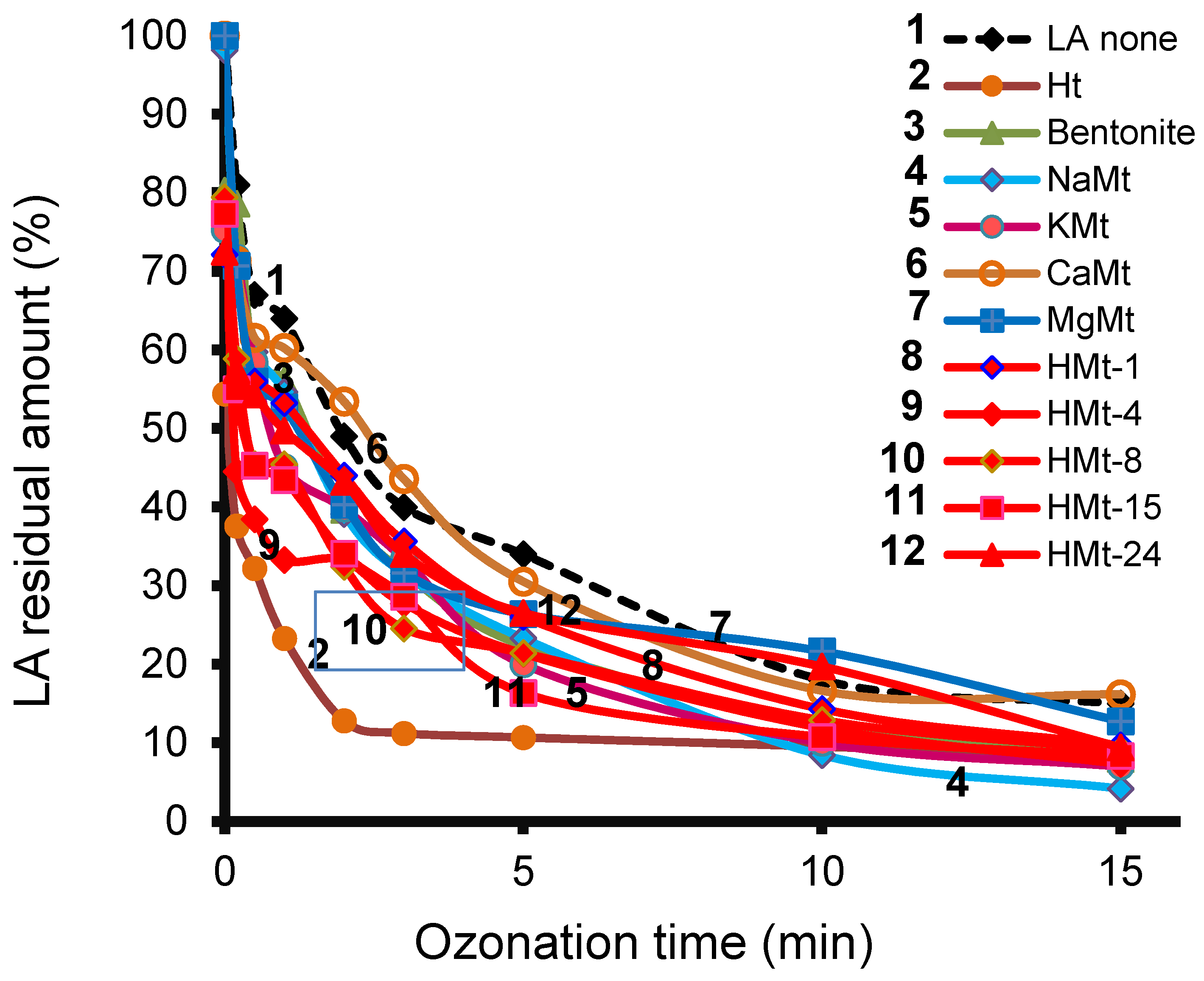
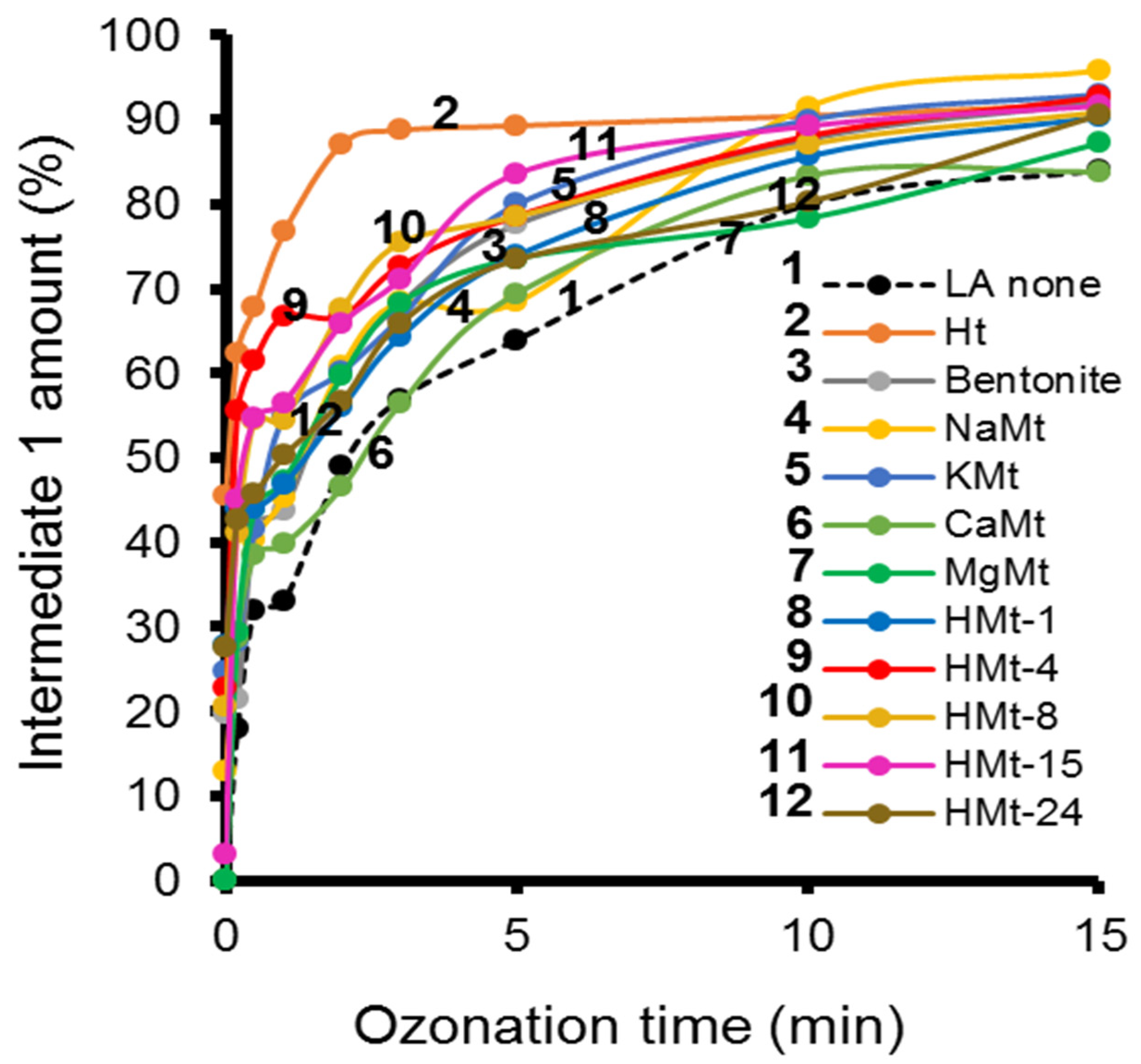
3.4. LA Depletion during Ozonation and Product Identification
3.5. Ht-Catalyzed Ozonation
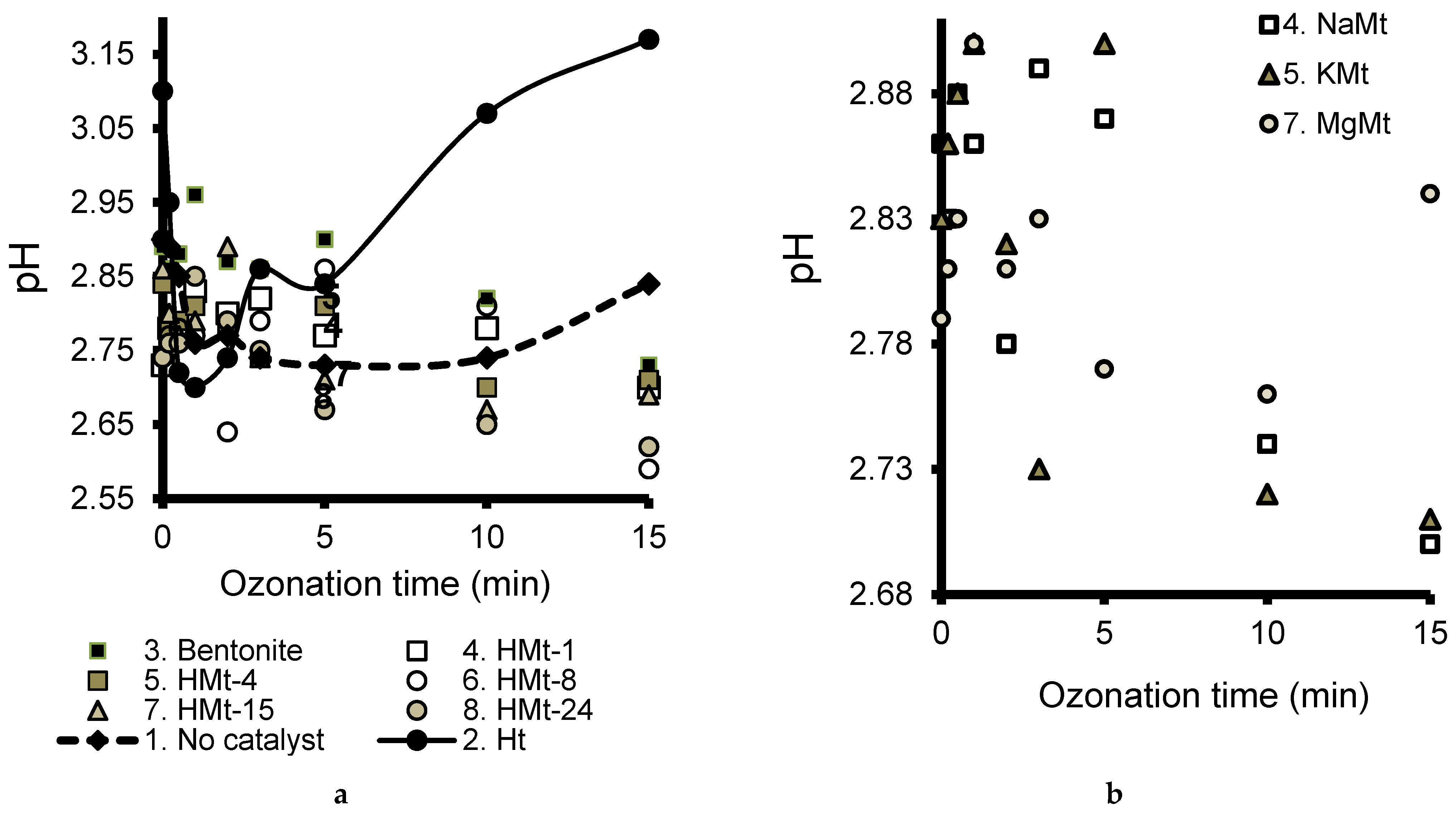
3.6. Effects of pH Evolution
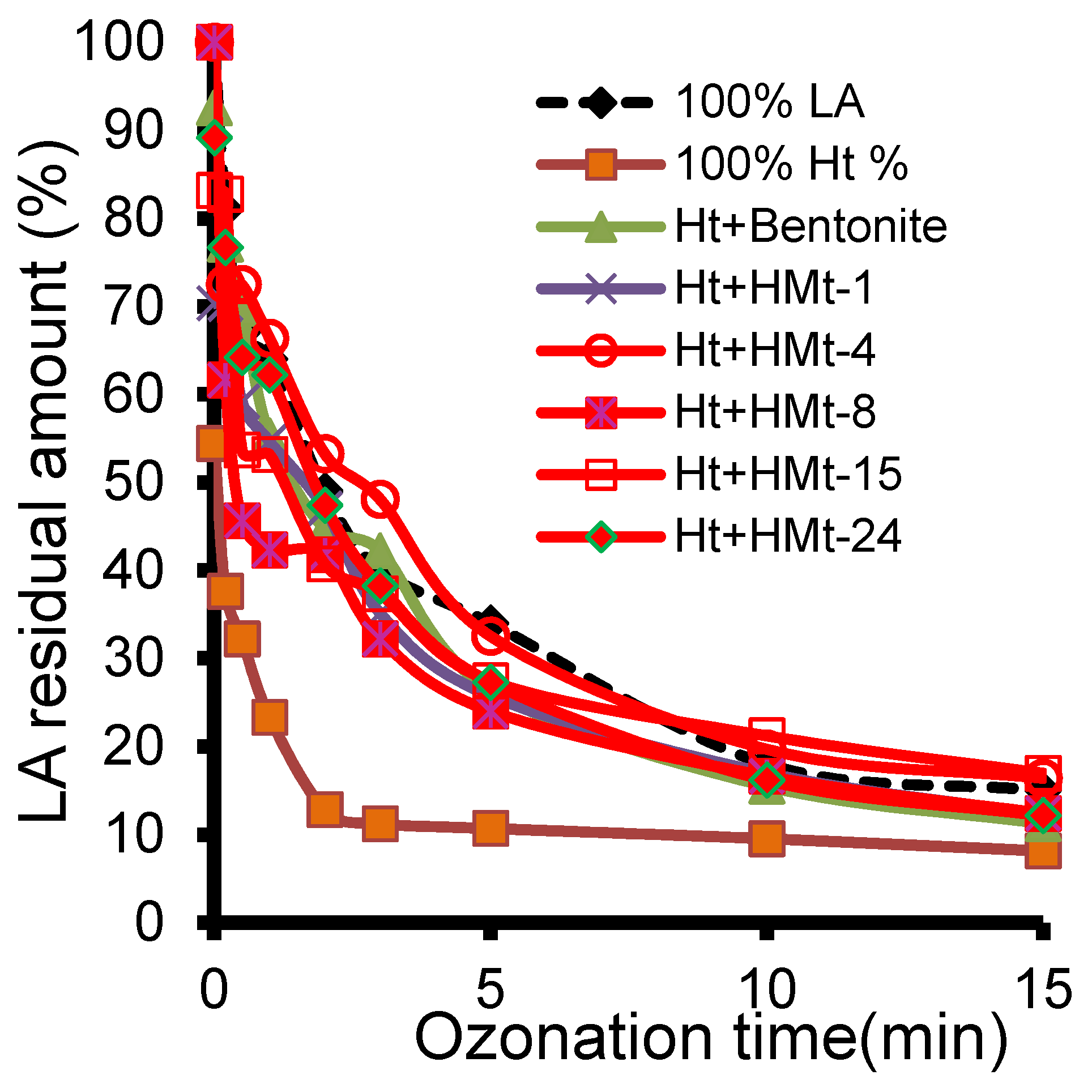
3.7. Ozonation with Ht-cationic Clay Mixtures
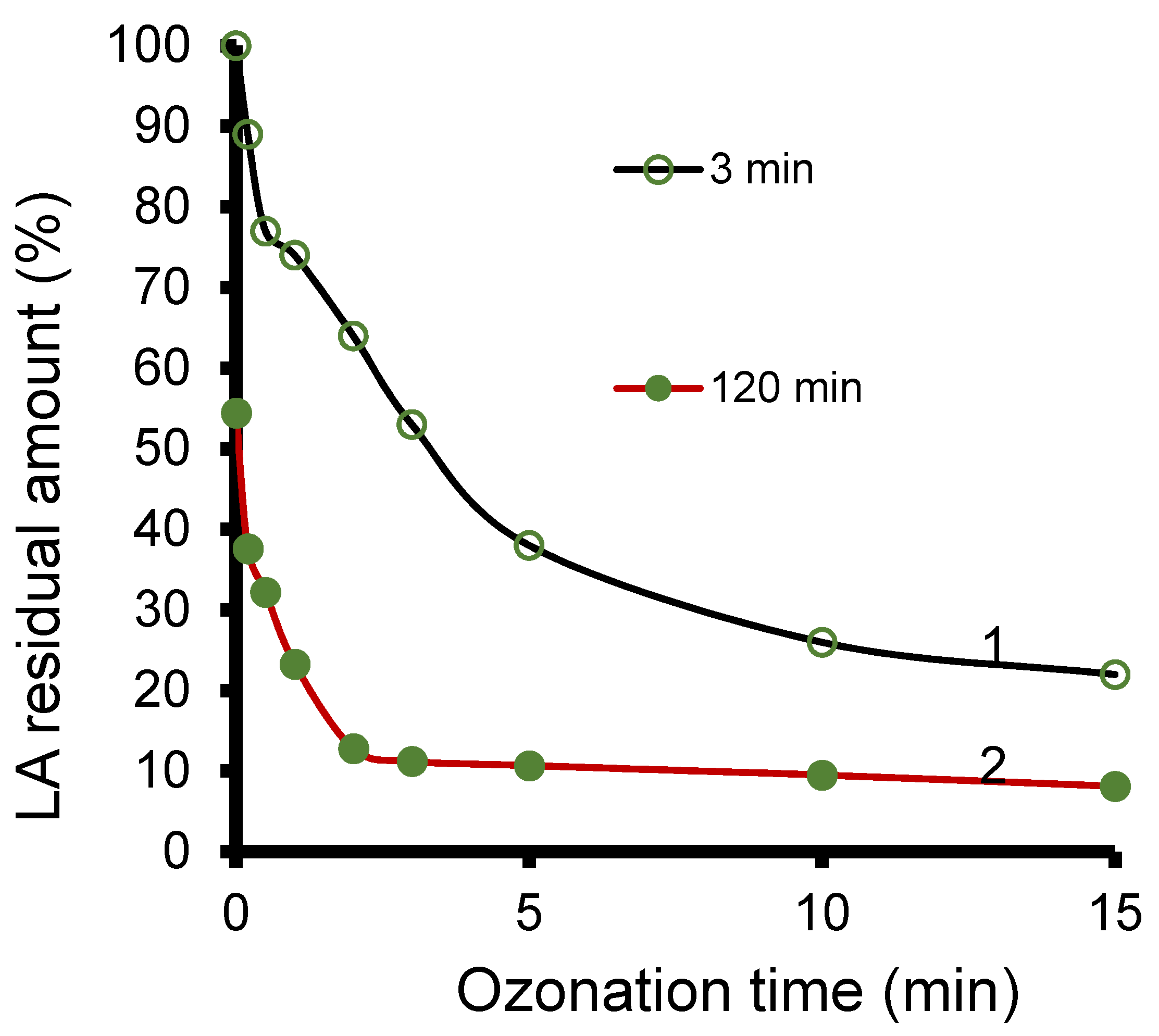
3.8. Ozonation Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Varadarajan, S.; Miller, D.J. Catalytic Upgrading of Fermentation-Derived Organic Acids. Biotechnol. Prog. 1999, 15, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Vidmantiene, D.; Basinskiene, L.; Cernauskas, D.; Bartkiene, E.; Cizeikiene, D. Green metrics for sustainability of biobased lactic acid from starchy biomass vs chemical synthesis. Catal. Today 2015, 239, 11–16. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Davies, A.; Carlomusto, M.; Pillai, S.; Zhang, K.; Kosturko, R.; Verdejo, P.; Feinberg, C.; Nguyen, L.; Chandar, P. Effect of lactic acid isomers on keratinocyte ceramide synthesis, stratum corneum lipid levels and stratum corneum barrier function. Arch. Dermatol. Res. 1996, 288, 383–390. [Google Scholar] [CrossRef]
- McHugh, J.; Dumont, S.N.; Paradis, J.; Loan Nguyen, A.; Levesque, S.; Carrier, A. New and Simple HPLC Method for the Determination of Lactic Acid Content in Ciprofloxacin Injection. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1905–1916. [Google Scholar] [CrossRef]
- Halim, M.; Mohd Mustafa, N.A.; Othman, M.; Wasoh, H.; Kapri, M.R.; Ariff, A.B. Effect of encapsulant and cryoprotectant on the viability of probiotic Pediococcus acidilactici ATCC 8042 during freeze-drying and exposure to high acidity, bile salts and heat. LWT Food Sci. Technol. 2017, 81, 210–216. [Google Scholar] [CrossRef]
- Luedeking, R.; Piret, E.L. A kinetic study of the lactic acid fermentation. Batch process at controlled pH. J. Biochem. Microbiol. Technol. Eng. 1959, 1, 393–412. [Google Scholar] [CrossRef]
- Aguirre-Ezkauriatza, E.J.; Aguilar-Yáñez, J.M.; Ramírez-Medrano, A.; Alvarez, M.M. Production of probiotic biomass (Lactobacillus casei) in goat milk whey: Comparison of batch, continuous and fed-batch cultures. Bioresour. Technol. 2010, 101, 2837–2844. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Broadbent, J.R.; Larsen, R.L.; Deibel, V.; Steele, J.L. Physiological and Transcriptional Response of Lactobacillus casei ATCC 334 to Acid Stress. J. Bacteriol. 2010, 192, 2445. [Google Scholar] [CrossRef]
- Wee, Y.; Kim, J.; Ryu, H. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Boyaval, P.; Corre, C.; Terre, S. Continuous lactic acid fermentation with concentrated product recovery by ultrafiltration and electrodialysis. Biotechnol. Lett. 1987, 9, 207–212. [Google Scholar] [CrossRef]
- Nomura, Y.; Iwahara, M.; Hongo, M. Lactic acid production by electrodialysis fermentation using immobilized growing cells. Biotechnol. Bioeng. 1987, 30, 788–793. [Google Scholar] [CrossRef]
- Yabannavar, V.M.; Wang, D.I.C. Extractive fermentation for lactic acid production. Biotechnol. Bioeng. 1991, 37, 1095–1100. [Google Scholar] [CrossRef]
- Srivastava, A.; Roychoudhury, P.K.; Sahai, V. Extractive lactic acid fermentation using ion-exchange resin. Biotechnol. Bioeng. 1992, 39, 607–613. [Google Scholar] [CrossRef]
- Kim, Y.H.; Moon, S.-H. Lactic acid recovery from fermentation broth using one-stage electrodialysis. J. Chem. Technol. Biotechnol. 2001, 76, 169–178. [Google Scholar] [CrossRef]
- Hábová, V.; Melzoch, K.; Rychtera, M.; Sekavová, B. Electrodialysis as a useful technique for lactic acid separation from a model solution and a fermentation broth. Desalination 2004, 162, 361–372. [Google Scholar] [CrossRef]
- Boonmee, M.; Cotano, O.; Amnuaypanich, S.; Grisadanurak, N. Improved Lactic Acid Production by In Situ Removal of Lactic Acid During Fermentation and a Proposed Scheme for Its Recovery. Arab. J. Sci. Eng. 2016, 41, 2067–2075. [Google Scholar] [CrossRef]
- Krzyżaniak, A.; Leeman, M.; Vossebeld, F.; Visser, T.J.; Schuur, B.; de Haan, A.B. Novel extractants for the recovery of fermentation derived lactic acid. Sep. Purif. Technol. 2013, 111, 82–89. [Google Scholar] [CrossRef]
- Boontawan, P.; Kanchanathawee, S.; Boontawan, A. Extractive fermentation of l-(+)-lactic acid by Pediococcus pentosaceus using electrodeionization (EDI) technique. Biochem. Eng. J. 2011, 54, 192–199. [Google Scholar] [CrossRef]
- Gao, M.-T.; Shimamura, T.; Ishida, N.; Takahashi, H. pH-Uncontrolled lactic acid fermentation with activated carbon as an adsorbent. Enzym. Microb. Technol. 2011, 48, 526–530. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, A.; Dong, H.; Li, Q.; Niu, C. A novel process for recovery and refining of l-lactic acid from fermentation broth. Bioresour. Technol. 2012, 112, 280–284. [Google Scholar] [CrossRef]
- Gao, M.-T.; Shimamura, T.; Ishida, N.; Nagamori, E.; Takahashi, H.; Umemoto, S.; Omasa, T.; Ohtake, H. Extractive lactic acid fermentation with tri-n-decylamine as the extractant. Enzym. Microb. Technol. 2009, 44, 350–354. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive Fermentation of Lactic Acid in Lactic Acid Bacteria Cultivation: A Review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttilä, M. Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef]
- Schiraldi, C.; Adduci, V.; Valli, V.; Maresca, C.; Giuliano, M.; Lamberti, M.; Cartenì, M.; De Rosa, M. High cell density cultivation of probiotics and lactic acid production. Biotechnol. Bioeng. 2003, 82, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Arus, V.A.; Platon, N.; Nistor, I.D. Lactic Acid Retention by CO3-Mg-Al Layered Double Hydroxides for Improved Milk Clotting An approach through acidity measurements. Rev. Chim. 2016, 67, 1348–1355. [Google Scholar]
- Azzouz, A.; Tudor, S.; Ileana-Denisa, N.; Alexandru, C.; Gheorghe, D. Methods for Cell Growth and Productivity Improvement of Lactic Seeds (Various Proposal). MD 2969 G2. 28 February 2006. [Google Scholar]
- Bergaya, F.; Lagaly, G. Chapter 1 General Introduction: Clays, Clay Minerals, and Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1–18. [Google Scholar]
- Bejoy, N. Hydrotalcite. Resonance 2001, 6, 57–61. [Google Scholar] [CrossRef]
- Rackley, S.A. 7—Adsorption capture systems. In Carbon Capture and Storage, 2nd ed.; Rackley, S.A., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2017; pp. 151–185. [Google Scholar] [CrossRef]
- Walter, R.H.; Sherman, R.M. Ozonation of Lactic Acid Fermentation Effluent. J. Water Pollut. Control Fed. 1974, 46, 1800–1803. [Google Scholar]
- Walter, R.H.; Sherman, R.M. Kinetics of the lactate-ozone reaction in an open-loop system. J. Water Pollut. Control Fed. 1976, 48, 748–752. [Google Scholar]
- Azzouz, A.; Kotbi, A.; Niquette, P.; Sajin, T.; Ursu, A.; Rami, A.; Monette, F.; Hausler, R. Ozonation of oxalic acid catalyzed by ion-exchanged montmorillonite in moderately acidic media. React. Kinet. Mech. Catal. 2010, 99, 289–302. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirilà, D.; Arus, V.-A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.-D.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation–Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Shahidi, D.; Moheb, A.; Abbas, R.; Larouk, S.; Roy, R.; Azzouz, A. Total mineralization of sulfamethoxazole and aromatic pollutants through Fe2+-montmorillonite catalyzed ozonation. J. Hazard. Mater. 2015, 298, 338–350. [Google Scholar] [CrossRef]
- Shahidi, D.; Roy, R.; Azzouz, A. Total removal of oxalic acid via synergistic parameter interaction in montmorillonite catalyzed ozonation. J. Environ. Chem. Eng. 2014, 2, 20–30. [Google Scholar] [CrossRef]
- Shahidi, D.; Roy, R.; Azzouz, A. Advances in catalytic oxidation of organic pollutants—Prospects for thorough mineralization by natural clay catalysts. Appl. Catal. B Environ. 2015, 174–175, 277–292. [Google Scholar] [CrossRef]
- Larouk, S.; Ouargli, R.; Shahidi, D.; Olhund, L.; Shiao, T.C.; Chergui, N.; Sehili, T.; Roy, R.; Azzouz, A. Catalytic ozonation of Orange-G through highly interactive contributions of hematite and SBA-16–To better understand azo-dye oxidation in nature. Chemosphere 2017, 168, 1648–1657. [Google Scholar] [CrossRef]
- Mirilă, D.-C.; Boudissa, F.; Beltrao-Nuñes, A.-P.; Platon, N.; Didi, M.-A.; Nistor, I.-D.; Roy, R.; Azzouz, A. Organic Dye Ozonation Catalyzed by Chemically Modified Montmorillonite K10– Role of Surface Basicity and Hydrophilic Character. Ozone Sci. Eng. 2020, 42, 517–530. [Google Scholar] [CrossRef]
- Benghaffour, A.; Foka-Wembe, E.N.; Dami, M.; Dewez, D.; Azzouz, A. Insight into natural medium remediation through ecotoxicity correlation with clay catalyst selectivity in organic molecule ozonation. Dalton Trans. 2022, 51, 4366–4376. [Google Scholar] [CrossRef]
- Zekkari, M.; Ouargli-Saker, R.; Boudissa, F.; Lachachi, A.K.; El Houda Sekkal, K.N.; Tayeb, R.; Boukoussa, B.; Azzouz, A. Silica-catalyzed ozonation of 17α -ethinyl-estradiol in aqueous media-to better understand the role of silica in soils. Chemosphere 2022, 298, 134312. [Google Scholar] [CrossRef] [PubMed]
- Pusch, R. Bentonite Clay: Environmental Properties and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bouberka, Z.; Khenifi, A.; Benderdouche, N.; Derriche, Z. Removal of Supranol Yellow 4GL by adsorption onto Cr-intercalated montmorillonite. J. Hazard. Mater. 2006, 133, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Pramada, P. Rice husk ash as an adsorbent for methylene blue—Effect of ashing temperature. Adsorption 2006, 12, 27. [Google Scholar] [CrossRef]
- Hamdaoui, O. Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J. Hazard. Mater. 2006, 135, 264–273. [Google Scholar] [CrossRef]
- Boudissa, F.; Zekkari, M.; Arus, V.-A.; Ouargli-Saker, R.; Nabil, B.; Roy, R.; Azzouz, A. Clay-catalyzed ozonation of endocrine-disrupting compounds in solvent-free media–to better understand soil catalytic capacity. Dalton Trans. 2020, 49, 16693–16706. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 273–278. [Google Scholar] [CrossRef]
- Komadel, P.; Janek, M.; Madejová, J.; Weekes, A.; Breen, C. Acidity and catalytic activity of mildly acid-treated Mg-rich montmorillonite and hectorite. J. Chem. Soc. Faraday Trans. 1997, 93, 4207–4210. [Google Scholar] [CrossRef]
- Arus, V.A.; Nousir, S.; Sennour, R.; Shiao, T.C.; Nistor, I.D.; Roy, R.; Azzouz, A. Intrinsic affinity of acid-activated bentonite towards hydrogen and carbon dioxide. Int. J. Hydrog. Energy 2018, 43, 7964–7972. [Google Scholar] [CrossRef]
- Ouargli, R.; Larouk, S.; Terrab, I.; Hamacha, R.; Benharrats, N.; Bengheddach, A.; Azzouz, A. Intrinsic activity of SBA-like silica in the catalytic ozonation of organic pollutants. Ozone: Sci. Eng. 2016, 38, 48–61. [Google Scholar] [CrossRef]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J. Lactic acid can be easily and precisely determined by reversed-phase high performance liquid chromatography with pre-column derivatization. J. Anim. Feed Sci. 2008, 17, 268–279. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 54178311, Lactoyllactic Acid. Retrieved 29 November 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lactoyllactic-acid (accessed on 8 September 2022).
- Pereira, C.S.; Silva, V.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Matsuura, Y.; Onda, A.; Yanagisawa, K. Selective conversion of lactic acid into acrylic acid over hydroxyapatite catalysts. Catal. Commun. 2014, 48, 5–10. [Google Scholar] [CrossRef]
- Chahal, S.P.; Starr, J.N. Lactic acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Splittstoesser, D.; Wilkison, M. Some factors affecting the activity of diethylpyrocarbonate as a sterilant. Appl. Microbiol. 1973, 25, 853–857. [Google Scholar] [CrossRef]
- Ehrenberg, L.; Fedorcsak, I.; Solymosy, F. Diethyl pyrocarbonate in nucleic acid research. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1976; Volume 16, pp. 189–262. [Google Scholar]
- Lowenstein, J. Ammonia production in muscle and other tissues: The purine nucleotide cycle. Physiol. Rev. 1972, 52, 382–414. [Google Scholar] [CrossRef]
- Psaltou, S.; Zouboulis, A. Catalytic ozonation and membrane contactors—A review concerning fouling occurrence and pollutant removal. Water 2020, 12, 2964. [Google Scholar] [CrossRef]
- Azzouz, A.; Belkhadem, F.; Benselka, N.; Drici, N.; Hadj-Abdelkader, N.E.H.; Ouargli-Saker, R. Structure-properties correlation with LDH applications. In Applications of Layered Double Hydroxides; Goswamee, R.L., Saikia, P., Eds.; Nova Science Publisher: New York, NY, USA, 2021; pp. 193–244. [Google Scholar] [CrossRef]
- Foster, C.; Shaw, S.; Neill, T.S.; Bryan, N.; Sherriff, N.; Natrajan, L.S.; Wilson, H.; Lopez-Odriozola, L.; Rigby, B.; Haigh, S.J.; et al. Hydrotalcite Colloidal Stability and Interactions with Uranium(VI) at Neutral to Alkaline pH. Langmuir 2022, 38, 2576–2589. [Google Scholar] [CrossRef]
- Sable, S.S.; Medina, F.; Contreras, S. Clofibric acid degradation by catalytic ozonation using hydrotalcite-derived catalysts. Appl. Catal. B Environ. 2014, 150–151, 30–36. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Wang, Y.; Liang, M.; Yang, Y.; Jin, Z.; Yang, J.; Hu, S.; Li, L. Ozonation Catalyzed by CoxFe1 Layered Double Hydroxide for the Degradation of P-toluenesulfonic Acid. Ozone: Sci. Eng. 2021, 43, 163–172. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, J.; Tang, M.; Wang, D.; Nie, Y.; Yang, L.; Dai, C.; Yang, C.; Lu, L. Surface acidity and basicity of Mg/Al hydrotalcite for 2, 4-dichlorophenoxyacetic acid degradation with ozone: Mineralization, mechanism, and implications to practical water treatment. J. Hazard. Mater. 2021, 402, 123475. [Google Scholar] [CrossRef] [PubMed]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
| Sample | Si/Al Mole Ratio | pH a | Average Particle Size (µm) e | Average Zeta Potential (mv) | ||||
|---|---|---|---|---|---|---|---|---|
| Water b | LA 0 min c | LA 1 min d | H2O | LA | H2O | LA | ||
| Bentonite | 2.50 | 6.23 | 2.80 | 2.84 | 1.78 | 1.01 | −30.87 | −25.73 |
| HMt-1 | 2.69 | 5.27 | 2.69 | 2.87 | 2.81 | 1.98 | −18.72 | −24.46 |
| HMt-4 | 3.00 | 4.13 | 2.67 | 2.74 | 2.69 | 0.78 | −24.93 | −25.53 |
| HMt-8 | 3.47 | 4.38 | 2.67 | 2.69 | 2.98 | 1.24 | −27.10 | −22.09 |
| HMt-15 | 4.03 | 3.82 | 2.42 | 2.73 | 3.32 | 2.28 | −35.93 | −31.88 |
| HMt-24 | 4.36 | 4.48 | 2.60 | 2.67 | 3.71 | 1.16 | −21.48 | −23.75 |
| NaMt | 2.45 | 6.88 | 2.76 | 2.82 | 0.63 | 0.63 | −25.07 | −37.07 |
| MgMt | 2.45 | 5.72 | 2.83 | 2.84 | 1.68 | 4.25 | −15.85 | −12.06 |
| KMt | 2.45 | 6.22 | 2.74 | 2.76 | 0.90 | 0.91 | −18.91 | −30.67 |
| CaMt | 2.45 | 6.07 | 2.74 | 2.74 | 1.27 | 1.12 | −23.14 | −19.62 |
| Ht | Mg/Al=2 | 6.27 | 4.64 | 4.19 | 1.40 | 9.98 | −09.33 | +16.23 |
| Ht Content (%) | LARA (%) after 60 min Ozonation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bent | HMt-1 | HMt-4 | HMt-8 | HMt-15 | HMt-24 | NaMt | KMt | CaMt | MgMt | |
| 100 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 |
| 80 | 3.93 | 3.40 | 5.30 | 3.20 | 4.06 | 3.19 | 5.65 | - | - | - |
| 60 | 3.85 | 6.35 | 4.25 | 2.93 | 2.67 | 2.66 | 3.92 | - | - | - |
| 40 | 2 | 4.14 | 3.95 | 3.68 | 2.67 | 3.80 | 3.5 | - | - | - |
| 20 | 4.98 | 3.21 | 3.57 | 3.28 | 2.63 | 2.99 | 3.97 | - | - | - |
| 0 | 1.99 | 1.55 | 1.65 | 2.54 | 3.64 | 1.62 | 1.97 | 2.00 | 2.59 | 1.60 |
| Catalyst | Time Range (min) | Reaction Order | R2 | Rate Constant, K * |
|---|---|---|---|---|
| Ht | [0–3] | 2 | 0.9613 | 1.039 |
| [5–15] | 1 | 0.9999 | 0.136 | |
| Bentonite | [0–5] | 2 | 0.9909 | 0.266 |
| [5–15] | 2 | 0.9972 | 0.324 | |
| NaMt | [0–5] | 2 | 0.9889 | 0.251 |
| [5–15] | 2 | 0.9824 | 0.785 | |
| CaMt | [0–5] | 2 | 0.9562 | 0.158 |
| KMt | [0–5] | 2 | 0.9672 | 0.288 |
| [5–15] | 2 | 0.998 | 0.37 | |
| MgMt | [0–3] | 2 | 0.9566 | 0.36 |
| [5–15] | 1 | 0.9346 | 0.162 | |
| HMt-1 | [0–5] | 2 | 0.9448 | 0.195 |
| [5–15] | 2 | 0.9997 | 0.26 | |
| HMt- 4 | [5–15] | 2 | 0.9905 | 0.36 |
| HMt-8 | [0–5] | 2 | 0.9286 | 0.28 |
| [5–15] | 2 | 0.9999 | 0.251 | |
| HMt-15 | [0–5] | 2 | 0.9513 | 0.355 |
| [5–15] | 2 | 0.9971 | 0.234 | |
| HMt-24 | [0–5] | 2 | 0.941 | 0.192 |
| [5–15] | 1 | 0.9428 | 0.274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Baktaoui, M.; Hadj-Abdelkader, N.E.H.; Benghaffour, A.; Arus, V.-A.; Bennani-Daouadji, N.; Belkhadem, F.; Roy, R.; Azzouz, A. Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition. Molecules 2022, 27, 6502. https://doi.org/10.3390/molecules27196502
El Baktaoui M, Hadj-Abdelkader NEH, Benghaffour A, Arus V-A, Bennani-Daouadji N, Belkhadem F, Roy R, Azzouz A. Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition. Molecules. 2022; 27(19):6502. https://doi.org/10.3390/molecules27196502
Chicago/Turabian StyleEl Baktaoui, Meriem, Nour El Houda Hadj-Abdelkader, Amina Benghaffour, Vasilica-Alisa Arus, Nadia Bennani-Daouadji, Fatiha Belkhadem, René Roy, and Abdelkrim Azzouz. 2022. "Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition" Molecules 27, no. 19: 6502. https://doi.org/10.3390/molecules27196502
APA StyleEl Baktaoui, M., Hadj-Abdelkader, N. E. H., Benghaffour, A., Arus, V.-A., Bennani-Daouadji, N., Belkhadem, F., Roy, R., & Azzouz, A. (2022). Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition. Molecules, 27(19), 6502. https://doi.org/10.3390/molecules27196502








