H-Bonding Room Temperature Phosphorescence Materials via Facile Preparation for Water-Stimulated Photoluminescent Ink
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 4,4’-(9H-Carbazole-2,7-diyl)Dibenzoic Acid (DBAc-Cz)
3.3. Synthesis of 4,4’-(9H-Carbazole-2,7-diyl)Diphenol (DPOl-Cz)
3.4. Preparation of DT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Evans, R.C.; Douglas, P.; Winscom, C.J. Coordination complexes exhibiting room-temperature phosphorescence: Evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord. Chem. Rev. 2006, 250, 2093–2126. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, J.; Guo, H.; Sun, J.; Ji, S.; Wang, Z. Long-Lived Room-Temperature Near-IR Phosphorescence of BODIPY in a Visible-Light-Harvesting N^C^N PtII–Acetylide Complex with a Directly Metalated BODIPY Chromophore. Chem. Eur. J. 2012, 18, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, C.; Wang, T.; Zhou, C.; Du, J.; Wang, Z.; Xu, H.; Xie, T.; Bi, G.; Jiang, J.; et al. Versatile Room-Temperature-Phosphorescent Materials Prepared from N-Substituted Naphthalimides: Emission Enhancement and Chemical Conjugation. Angew. Chem. Int. Ed. 2016, 55, 9872–9876. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, F.; Han, C.; Xu, H.; Li, J.; Zhang, Z.; Deng, Z.; Ma, D.; Yan, P. Ternary Ambipolar Phosphine Oxide Hosts Based on Indirect Linkage for Highly Efficient Blue Electrophosphorescence: Towards High Triplet Energy, Low Driving Voltage and Stable Efficiencies. Adv. Mater. 2012, 24, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S. Recent Advances in Materials with Room-Temperature Phosphorescence: Photophysics for Triplet Exciton Stabilization. Adv. Opt. Mater. 2017, 5, 1700116. [Google Scholar] [CrossRef]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chen, G.; Peng, Q.; Yuan, W.Z.; Xie, Y.; Li, S.; Zhang, Y.; Tang, B.Z. Achieving Persistent Room Temperature Phosphorescence and Remarkable Mechanochromism from Pure Organic Luminogens. Adv. Mater. 2015, 27, 6195–6201. [Google Scholar] [CrossRef]
- Yang, J.; Zhen, X.; Wang, B.; Gao, X.; Ren, Z.; Wang, J.; Xie, Y.; Li, J.; Peng, Q.; Pu, K.; et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat. Commun. 2018, 9, 840. [Google Scholar] [CrossRef]
- Gan, N.; Shi, H.; An, Z.; Huang, W. Recent Advances in Polymer-Based Metal-Free Room-Temperature Phosphorescent Materials. Adv. Funct. Mater. 2018, 28, 1802657. [Google Scholar] [CrossRef]
- Hirata, S.; Totani, K.; Zhang, J.; Yamashita, T.; Kaji, H.; Marder, S.R.; Watanabe, T.; Adachi, C. Efficient persistent room temperature phosphorescence in organic amorphous materials under ambient conditions. Adv. Funct. Mater. 2013, 23, 3386–3397. [Google Scholar] [CrossRef]
- Tian, R.; Xu, S.-M.; Xu, Q.; Lu, C. Large-scale preparation for efficient polymer-based room-temperature phosphorescence via click chemistry. Sci. Adv. 2020, 6, 6107. [Google Scholar] [CrossRef] [PubMed]
- Notsuka, N.; Kabe, R.; Goushi, K.; Adachi, C. Confinement of Long-Lived Triplet Excitons in Organic Semiconducting Host–Guest Systems. Adv. Funct. Mater. 2017, 27, 1703902. [Google Scholar] [CrossRef]
- Qu, G.; Zhang, Y.; Ma, X. Recent progress on pure organic room temperature phosphorescence materials based on host-guest interactions. Chin. Chem. Lett. 2019, 30, 1809–1814. [Google Scholar] [CrossRef]
- Ma, X.-K.; Zhang, W.; Liu, Z.; Zhang, H.; Zhang, B.; Liu, Y. Supramolecular Pins with Ultralong Efficient Phosphorescence. Adv. Mater. 2021, 33, 2007476. [Google Scholar] [CrossRef]
- Kabe, R.; Adachi, C. Organic long persistent luminescence. Nature 2017, 550, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Ye, W.; Liang, X.; Lv, A.; Ma, H.; Singh, M.; Jia, W.; Shen, Z.; Guo, Y.; Gao, Y.; et al. Circularly Polarized Organic Room Temperature Phosphorescence from Amorphous Copolymers. J. Am. Chem. Soc. 2021, 143, 18527–18535. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Yang, J.; Fang, M.; Ding, D.; Tang, B.Z.; Li, Z. High Performance of Simple Organic Phosphorescence Host–Guest Materials and their Application in Time-Resolved Bioimaging. Adv. Mater. 2021, 33, 2007811. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Z.; Nie, X.; Huang, L.; Hui, M.; Sun, X.; Zhang, G. Thermochromic aggregation-induced dual phosphorescence via temperature-dependent sp3-linked donor-acceptor electronic coupling. Nat. Commun. 2021, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Dai, X.-Y.; Liu, Y. Ultrahigh Supramolecular Cascaded Room-Temperature Phosphorescence Capturing System. Angew. Chem. Int. Ed. 2021, 60, 27171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, L.; Li, X.; Zhao, L.; Wang, S.; Lu, Z.-H.; Ding, J.; Wang, L. An Electroactive Pure Organic Room-Temperature Phosphorescence Polymer Based on a Donor-Oxygen-Acceptor Geometry. Angew. Chem. Int. Ed. 2021, 60, 2455–2463. [Google Scholar] [CrossRef]
- Chen, X.-M.; Feng, W.-J.; Bisoyi, H.K.; Zhang, S.; Chen, X.; Yang, H.; Li, Q. Light-activated photodeformable supramolecular dissipative self-assemblies. Nat. Commun. 2022, 13, 3216. [Google Scholar] [CrossRef]
- Chen, X.; Bisoyi, H.K.; Chen, X.-F.; Chen, X.-M.; Zhang, S.; Tang, Y.; Zhu, G.; Yang, H.; Li, Q. Hierarchical self-assembly of an excitation-wavelength-dependent emissive fluorophore and cucurbiturils for secondary encryption. Matter 2022. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, X.; Wu, H.; Zhu, L.; Zhao, Y.; Tian, H. Molecular Engineering for Metal-Free Amorphous Materials with Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 11206–11216. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, C.; Wang, J.; Tian, H. Amorphous Pure Organic Polymers for Heavy-Atom-Free Efficient Room-Temperature Phosphorescence Emission. Angew. Chem. Int. Ed. 2018, 57, 10854–10858. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, S.; Ding, B.; Ma, X.; Tian, H. Highly Efficient Room-Temperature Phosphorescence Based on Single-Benzene Structure Molecules and Photoactivated Luminescence with Afterglow. Adv. Funct. Mater. 2021, 31, 2010659. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, L.; Zheng, X.; Wang, Z.; Yang, C.; Tang, H.; Qu, L.; Li, Y.; Zhao, Y. Ultraviolet irradiation-responsive dynamic ultralong organic phosphorescence in polymeric systems. Nat. Commun. 2021, 12, 2297. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Wang, Z.; Xue, P.; Wang, W.; Zhang, L.; Shi, Y.; Huang, W.; Chen, R. Stimuli-Responsive Deep-Blue Organic Ultralong Phosphorescence with Lifetime over 5 s for Reversible Water-Jet Anti-Counterfeiting Printing. Angew. Chem. Int. Ed. 2021, 60, 17094–17101. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Shi, H.; Tian, D.; Ma, H.; Cheng, Z.; Wu, Q.; Gu, M.; Huang, L.; An, Z.; Peng, Q.; et al. Enhancing Ultralong Organic Phosphorescence by Effective π-Type Halogen Bonding. Adv. Funct. Mater. 2018, 28, 1705045. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, D. Hydrogen-Bonded Two-Component Ionic Crystals Showing Enhanced Long-Lived Room-Temperature Phosphorescence via TADF-Assisted Förster Resonance Energy Transfer. Adv.. Funct. Mater. 2019, 29, 1807599. [Google Scholar] [CrossRef]
- Zhao, W.; Cheung, T.S.; Jiang, N.; Huang, W.; Lam, J.W.Y.; Zhang, X.; He, Z.; Tang, B.Z. Boosting the efficiency of organic persistent room-temperature phosphorescence by intramolecular triplet-triplet energy transfer. Nat. Commun. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Qian, C.; Ma, Z.; Fu, X.; Zhang, X.; Li, Z.; Jin, H.; Chen, M.; Jiang, H.; Jia, X.; Ma, Z. More than Carbazole Derivatives Activate Room Temperature Ultralong Organic Phosphorescence of Benzoindole Derivatives. Adv. Mater. 2022, 34, 2200544. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Lou, L.; Xu, T.; Zhao, J.; Hai, M.; Wang, D.; Cao, H.; He, W.; Yang, Z. Epoxy Vitrimer Based on Temperature-Responsive Pure Organic Room Temperature Phosphorescent Materials. ChemistrySelect 2022, 7, e202104149. [Google Scholar] [CrossRef]
- Xu, T.; Wu, P.; Lou, L.; Li, Y.; Wang, D.; Cao, H.; He, W.; Yang, Z. Vitrimer enhanced carbazole-based organic room-temperature phosphorescent materials. New J. Chem. 2022, 46, 276–281. [Google Scholar] [CrossRef]
- Rao, Y.V.S.; De Vos, D.E.; Jacobs, P.A. 1,5,7-Triazabicyclo[4.4.0]dec-5-ene Immobilized in MCM-41: A Strongly Basic Porous Catalyst. Angew. Chem. Int. Ed. Engl. 1997, 36, 2661–2663. [Google Scholar] [CrossRef]
- Do, T.; Baral, E.R.; Kim, J.G. Chemical recycling of poly(bisphenol A carbonate): 1,5,7-Triazabicyclo[4.4.0]-dec-5-ene catalyzed alcoholysis for highly efficient bisphenol A and organic carbonate recovery. Polymer 2018, 143, 106–114. [Google Scholar] [CrossRef]
- He, W.; Pan, G.; Yang, Z.; Zhao, D.; Niu, G.; Huang, W.; Yuan, X.; Guo, J.; Cao, H.; Yang, H. Wide Blue Phase Range in a Hydrogen-Bonded Self-Assembled Complex of Chiral Fluoro-Substituted Benzoic Acid and Pyridine Derivative. Adv. Mater. 2009, 21, 2050–2053. [Google Scholar] [CrossRef]
- Liang, N.; Kuwata, S.; Ishige, R.; Ando, S. Large-Stokes-shifted yellow photoluminescence emission from an imide and polyimides forming multiple intramolecular hydrogen bonds. Mater. Chem. Front. 2022, 6, 24–32. [Google Scholar] [CrossRef]
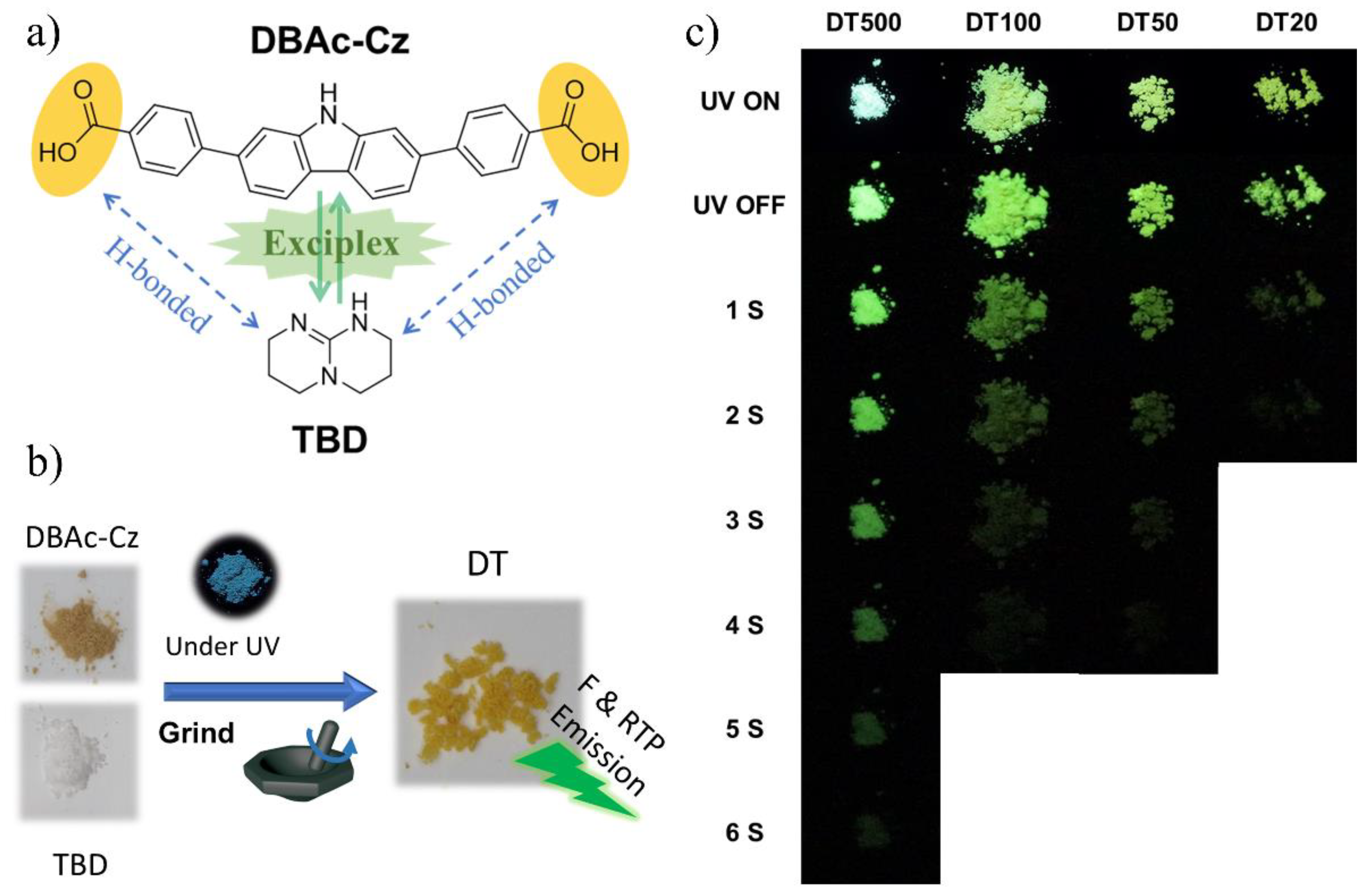

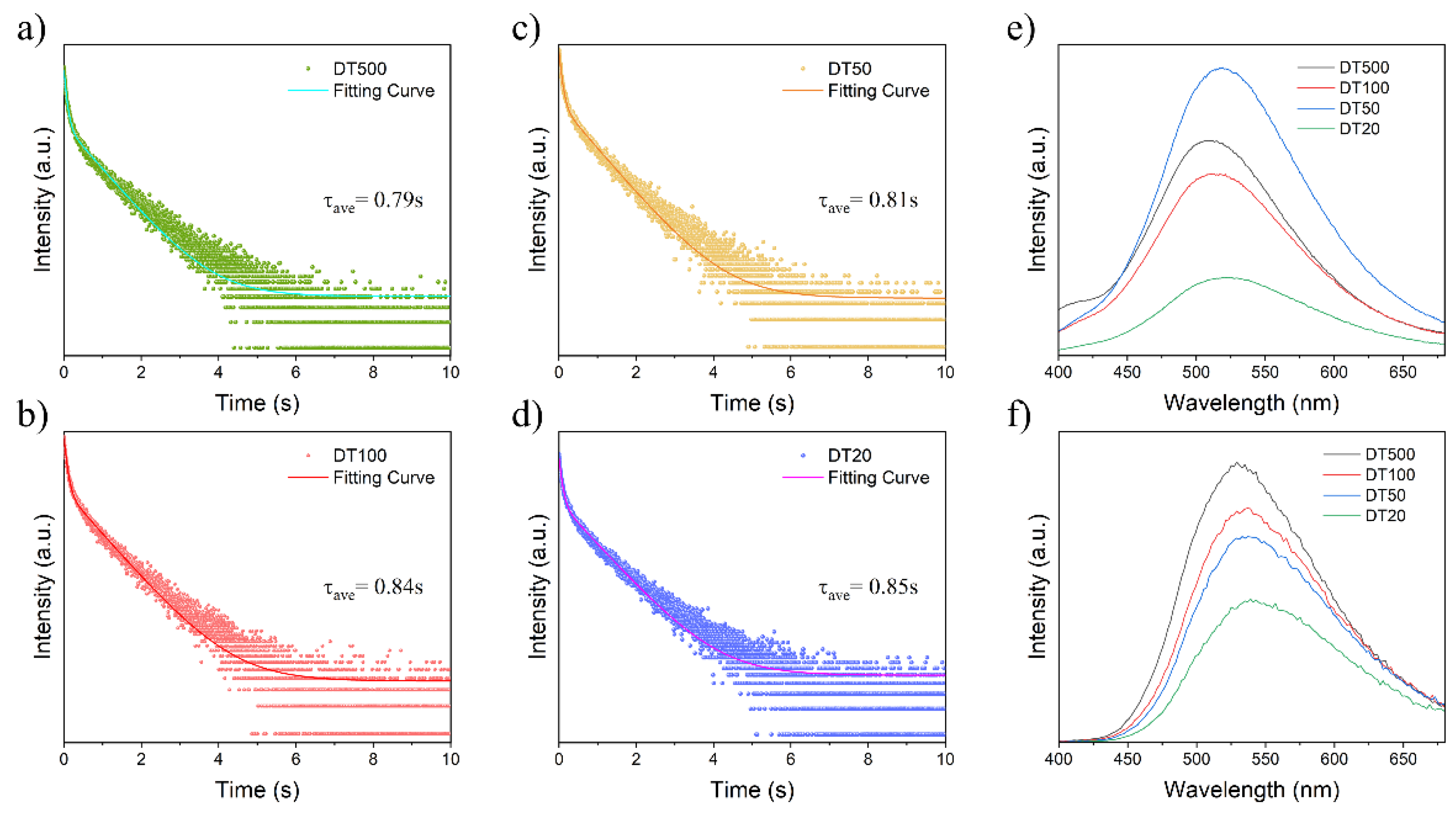


| Sample | Emf(max) (nm) | Emp(max) (nm) | τRTP(s) | Difference of Emp(max) and Emf(max) (nm) |
|---|---|---|---|---|
| DBAc-Cz | 461 | 528/572 (77 k) | / | 67/111 |
| DBAc-Cz (PVA) * | 414 | 519 | 0.52 | 105 |
| TBD | / | / | / | / |
| DT500 | 507 | 529 | 0.79 | 22 |
| DT100 | 511 | 537 | 0.81 | 26 |
| DT50 | 519 | 533 | 0.84 | 14 |
| DT20 | 525 | 539 | 0.85 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, L.; Xu, T.; Li, Y.; Zhang, C.; Wang, B.; Zhang, X.; Zhang, H.; Qiu, Y.; Yang, J.; Wang, D.; et al. H-Bonding Room Temperature Phosphorescence Materials via Facile Preparation for Water-Stimulated Photoluminescent Ink. Molecules 2022, 27, 6482. https://doi.org/10.3390/molecules27196482
Lou L, Xu T, Li Y, Zhang C, Wang B, Zhang X, Zhang H, Qiu Y, Yang J, Wang D, et al. H-Bonding Room Temperature Phosphorescence Materials via Facile Preparation for Water-Stimulated Photoluminescent Ink. Molecules. 2022; 27(19):6482. https://doi.org/10.3390/molecules27196482
Chicago/Turabian StyleLou, Lingyun, Tianqi Xu, Yuzhan Li, Changli Zhang, Bochun Wang, Xusheng Zhang, Hean Zhang, Yuting Qiu, Junyan Yang, Dong Wang, and et al. 2022. "H-Bonding Room Temperature Phosphorescence Materials via Facile Preparation for Water-Stimulated Photoluminescent Ink" Molecules 27, no. 19: 6482. https://doi.org/10.3390/molecules27196482
APA StyleLou, L., Xu, T., Li, Y., Zhang, C., Wang, B., Zhang, X., Zhang, H., Qiu, Y., Yang, J., Wang, D., Cao, H., He, W., & Yang, Z. (2022). H-Bonding Room Temperature Phosphorescence Materials via Facile Preparation for Water-Stimulated Photoluminescent Ink. Molecules, 27(19), 6482. https://doi.org/10.3390/molecules27196482







