The Effects of Drying Techniques on Phytochemical Contents and Biological Activities on Selected Bamboo Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. The Phytochemical Contents
2.1.1. Total Phenolic Content
2.1.2. Total Flavonoid Content
2.2. Antioxidant Activities
2.2.1. The 2,2-diphenyl-1-picrylhydrazyl Assay
2.2.2. The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Assay
2.2.3. The Ferric Reducing Antioxidant Power Assay
2.3. The Correlation between Phytochemical Contents and Antioxidant Capacities
2.4. The BSLA
2.5. Chemical Constituents
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Drying Process
3.4. Sample Extraction
3.5. Phytochemical Analysis
3.5.1. Determination of TPC
3.5.2. Determination of TFC
3.6. Antioxidant Analysis
3.6.1. Determination of DPPH
3.6.2. Determination of ABTS
3.6.3. Determination of FRAP
3.7. Determination of BSLA
3.8. LC-MS/MS Analysis
3.8.1. Sample Preparation
3.8.2. LC-ESI-QTOF-MS/MS Parameters
3.8.3. Data Processing
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Yuan, J.; Hao, L.-J.; Wu, G.; Wang, S.; Duan, J.; Xie, G.-Y.; Qin, M.-J. Effects of drying methods on the phytochemicals contents and antioxidant properties of Chrysanthemum flower heads harvested at two developmental stages. J. Funct. Foods 2015, 19, 786–795. [Google Scholar] [CrossRef]
- Nistor, O.V.; Seremet Ceclu, L.; Andronoiu, D.G.; Rudi, L.; Botez, E. Influence of different drying methods on the physicochemical properties of red beetroot (Beta vulgaris L. var. Cylindra). Food Chem. 2017, 236, 59–67. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, A.K. Drying kinetics and quality characteristics of beetroot slices under hot air followed by microwave finish drying. Afr. J. Agric. Res. 2014, 9, 1036–1044. [Google Scholar] [CrossRef]
- Mokhtarikhah, G.; Ebadi, M.T.; Ayyari, M. Qualitative changes of spearmint essential oil as affected by drying methods. Ind. Crops Prod. 2020, 153, 112492. [Google Scholar] [CrossRef]
- Lv, H.-F.; Ma, X.-X.; Zhang, B.; Chen, X.-F.; Liu, X.-M.; Fang, C.-H.; Fei, B.-H. Microwave-vacuum drying of round bamboo: A study of the physical properties. Constr. Build. Mater. 2019, 211, 44–51. [Google Scholar] [CrossRef]
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Wróblewska, K.B.; de Oliveira, D.C.S.; Grombone-Guaratini, M.T.; Moreno, P.R.H. Medicinal properties of bamboos. In Pharmacognosy—Medicinal Plants; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2019; pp. 159–176. [Google Scholar]
- Liu, M.H.; Ko, C.H.; Ma, N.; Tan, P.W.; Fu, W.M.; He, J.Y. Chemical profiles, antioxidant and anti-obesity effects of extract of Bambusa textilis McClure leaves. J. Funct. Foods 2016, 22, 533–546. [Google Scholar] [CrossRef]
- Lu, B.; Wu, X.; Shi, J.; Dong, Y.; Zhang, Y. Toxicology and safety of antioxidant of bamboo leaves. Part 2: Developmental toxicity test in rats with antioxidant of bamboo leaves. Food Chem. Toxicol. 2006, 44, 1739–1743. [Google Scholar] [CrossRef]
- Horn, T.; Häser, A. Bamboo tea: Reduction of taxonomic complexity and application of DNA diagnostics based on rbcL and matK sequence data. PeerJ 2016, 4, e2781. [Google Scholar] [CrossRef]
- Bhandari, S.; Tyagi, K.; Singh, B.; Goutam, U. Role of molecular markers to study genetic diversity in bamboo: A review. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 86–97. [Google Scholar]
- Yang, C.; Yi, L.; Dan, L.; Qian, Y.; Ming, J. Bamboo leaf flavones and tea polyphenols show a lipid-lowering effect in a rat model of hyperlipidemia. Drug Res. 2015, 65, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.-S.; Lee, H.A.; Lim, J.Y.; Kim, M.; Kwon, O.; Ko, H.-C.; Kim, S.-J.; Shin, J.-H.; Kim, Y. Sasa quelpaertensis leaf extract improves high fat diet-induced lipid abnormalities and regulation of lipid metabolism genes in rats. J. Med. Food 2014, 17, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Komatsu, K.; Takido, M.; Takeshita, K.; Kashiwagi, H.; Nagumo, S. Genetic profiling of Sasa species by analysis of chloroplast intron between rbcL and ORF106 and partial ORF106 regions. Biol. Pharm. Bull. 2007, 30, 1511–1515. [Google Scholar] [CrossRef]
- Singhal, P.; Satya, S.; Naik, S.N. Effect of different drying techniques on the nutritional, antioxidant and cyanogenic profile of bamboo shoots. Appl. Food Res. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Janjai, S.; Bala, B.K. Solar drying technology. Food Eng. Rev. 2012, 4, 16–54. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Meng, Q.; Fan, H.; Li, Y.; Zhang, L. Effect of drying methods on physico-chemical properties and antioxidant activity of Dendrobium officinale. J. Food Meas. Charact. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, Y.; Xu, G.; Gao, Q.; Gong, L.; Zhang, Y. Influence of harvest season and drying method on the antioxidant activity and active compounds of two bamboo grass leaves. J. Food Process. Preserv. 2014, 38, 1565–1576. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.G.; Mahdad, E.; Craker, L. Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chem. 2013, 141, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.P.; Melo, E.C.; Radünz, L.L. Influence of drying process on the quality of medicinal plants: A review. J. Med. Plant Res. 2011, 5, 7076–7084. [Google Scholar] [CrossRef]
- Soesanto, E. Antioxidant activity of extracts from Bambusa vulgaris and Gigantochloa apus Kurz bamboo shoots. Pak. J. Nutr. 2016, 15, 580–584. [Google Scholar] [CrossRef][Green Version]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A review on the effect of drying on antioxidant potential of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Mohd Ilham, A.; Vimala, S.; Abdull Rashih, A.; Rohana, S.; Jamaluddin, M.; Juliza, M. Antioxidant and antityrosinase properties of Malaysian bamboo leaf extracts. J. Trop. For. Sci. 2008, 20, 123–131. [Google Scholar]
- Van Hoyweghen, L.; De Beer, T.; Deforce, D.; Heyerick, A. Phenolic compounds and anti-oxidant capacity of twelve morphologically heterogeneous bamboo species. Phytochem. Anal. 2012, 23, 433–443. [Google Scholar] [CrossRef]
- Kozlowska, M.; Scibisz, I.; Przybyl, J.L.; Ziarno, M.; Zbikowska, A.; Majewska, E. Phenolic contents and antioxidant activity of extracts of selected fresh and dried herbal materials. Pol. J. Food Nutr. Sci. 2021, 71, 269–278. [Google Scholar] [CrossRef]
- Hihat, S.; Remini, H.; Madani, K. Effect of oven and microwave drying on phenolic compounds and antioxidant capacity of coriander leaves. Int. Food Res. J. 2017, 24, 503–509. [Google Scholar]
- Mudau, F.N.; Ngezimana, W. Effect of different drying methods on chemical composition and antimicrobial activity of bush tea (Athrixia phylicoides). Int. J. Agric. Biol. 2014, 16, 1011–1014. [Google Scholar]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Effects of four different drying methods on the carotenoid composition and antioxidant capacity of dried Gac peel. J. Sci. Food Agric. 2016, 97, 1656–1662. [Google Scholar] [CrossRef]
- Shaw, M.; Meda, V.; Tabil, L.; Opoku, A. Drying and color characteristics of coriander foliage using convective thin-layer and microwave drying. J. Microw. Power Electromagn. Energy 2007, 41, 59–68. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Gekas, V. Effect of drying method on the phenolic content and antioxidant capacity of spearmint. Czech J. Food Sci. 2013, 31, 509–513. [Google Scholar] [CrossRef]
- Kong, H.S.; Musa, K.H.; Abdullah Sani, N. Clinacanthus nutans (Belalai Gajah/Sabah Snake Grass): Antioxidant optimization on leaves and stems. AIP Conf. Proc. 2016, 1784, 030030. [Google Scholar] [CrossRef]
- Lasano, N.F.; Rahmat, A.; Ramli, N.S.; Abu Bakar, M.F. Effect of oven and microwave drying on polyphenols content and antioxidant capacity of herbal tea from Strobilanthes crispus leaves. Asian J. Pharm. Clin. Res. 2018, 11, 363–368. [Google Scholar] [CrossRef]
- Hu, C.; Xu, D.; Chen, H.; Yuan, K. Contents of the total flavonoids and the total phenols and antioxidant activities in the leaf from different species of Phyllostachys. Adv. Mater. Res. 2012, 343–344, 1103–1108. [Google Scholar] [CrossRef]
- Pande, H.; Kumar, B.; Varshney, V.K. HPLC-ESI-QTOF-MS analysis of phenolic compounds, antioxidant capacity and α-glucosidase inhibitory effect of Bambusa nutans leaves. Indian J. Chem. 2018, 57B, 988–996. [Google Scholar]
- Ouyang, W.; Lei, F.; Yang, Y.; Liu, L.; Li, Q.; Guo, A. Analysis of nutritional components of four bamboo leaves and antioxidant activity of flavonoid extracts in vitro. Nat. Prod. Res. Dev. 2019, 31, 1669–1674. [Google Scholar]
- Lu, Y.; Foo, L.Y. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, P.C.; Singh, A.; Rao, C.V. Brine shrimp bioassay of Pentapetes phoenicea Linn. and Ipomoea carnea Jacq. leaves. Der Pharm. Lett. 2013, 5, 162–167. [Google Scholar]
- Shawa, I.T.; Mponda, J.; Msefula, C.; Manda, H.; Gondwe, M.; Maliwichi-Nyirenda, C. Brine shrimp lethality and phytochemical determination of aqueous extracts of Senna singueana, Musa paradisiaca, and Ziziphus mucronata in Malawi. J. Basic Appl. Res. 2015, 1, 82–88. [Google Scholar]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Toxicity assessment of different solvent extracts of the medicinal plant, Phragmanthera capitata (Sprengel) Balle on brine shrimp (Artemia salina). Int. J. Pharmacol. 2016, 12, 701–710. [Google Scholar] [CrossRef]
- Artanti, N.; Firmansyah, T.; Darmawan, A. Bioactivities evaluation of Indonesian mistletoes (Dendrophthoe pentandra (L.) Miq.) leaves extracts. J. Appl. Pharm. Sci. 2012, 2, 24–27. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B. Traditional medicine-inspired evidence-based approaches to drug discovery. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 259–272. [Google Scholar]
- Martini, D.; Del Bo’, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef]
- George, S.E.; Ramalakshmi, K.; Rao, L.J.M. A perception on health benefits of coffee. Crit. Rev. Food Sci. Nutr. 2008, 48, 464–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.H.; Ergul, B.; Zhao, W. Caffeine’s antioxidant potency optically sensed with double-stranded DNA-encased single-walled carbon nanotubes. J. Phys. Chem. B 2015, 119, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Rathod, M.A.; Patel, D.; Das, A.; Tipparaju, S.R.; Shinde, S.S.; Anderson, R.F. Inhibition of radical-induced DNA strand breaks by water-soluble constituents of coffee: Phenolics and caffeine metabolites. Free Radic. Res. 2013, 47, 480–487. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A. Free radical scavenging activity of caffeine’s metabolites. Int. J. Quantum Chem. 2012, 112, 3472–3478. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Y.; Zhang, Z.; Qiu, L.; Zhai, H.; Gu, R.; Xie, Y. Total alkaloids from bamboo shoots and bamboo shoot shells of Pleioblastus amarus (Keng) Keng f. and their anti-inflammatory activities. Molecules 2019, 24, 2699. [Google Scholar] [CrossRef]
- Solmaz, V.; Kaya, M.; Uslu, F.B.; Atasoy, O.; Erbaş, O. Papaverine has therapeutic potential for sepsis-induced neuropathy in rats, possibly via the modulation of HMGB1-RAGE axis and its antioxidant prosperities. J. Investig. Surg. 2022, 35, 7–13. [Google Scholar] [CrossRef]
- Gremigni, P.; Hamblin, J.; Harris, D. Genotype x Environment Interactions and Lupin Alkaloids. In Proceedings of the 9th International Lupin Conference, Klink/Muritz, Germany, 20–24 June 2000. [Google Scholar]
- Sgambato, S.; Paolisso, G.; Passariello, N.; Varricchio, M.; D’Onofrio, F. Effect of sparteine sulphate upon basal and nutrient-induced insulin and glucagon secretion in normal man. Eur. J. Clin. Pharmacol. 1987, 32, 477–480. [Google Scholar] [CrossRef]
- Schmeller, T.; Wink, M. Utilization of alkaloids in modern medicine. In Alkaloids: Biochemistry, Ecology, and Medicinal Applications; Roberts, M.F., Wink, M., Eds.; Springer: Boston, MA, USA, 1998; pp. 435–459. [Google Scholar]
- de la Vega, R.; Gutierrez, M.P.; Sanz, C.; Calvo, R.; Robredo, L.M.; de la Cuadra, C.; Muzquiz, M. Bactericide-like effect of lupinus alkaloids. Ind. Crops Prod. 1996, 5, 141–148. [Google Scholar] [CrossRef]
- McDonough, J.H., Jr.; McMonagle, J.; Copeland, T.; Zoeffel, D.; Shih, T.-M. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch. Toxicol. 1999, 73, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Mishory, A.; Yaroslavsky, Y.; Bersudsky, Y.; Belmaker, R.H. Phenytoin as an antimanic anticonvulsant: A controlled study. Am. J. Psychiatry 2000, 157, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoropoulos, C.; Sotiriou, E.; Kotzadimitriou, D.; Drimala, P. At clinically relevant concentrations the anaesthetic/amnesic thiopental but not the anticonvulsant phenobarbital interferes with hippocampal sharp wave-ripple complexes. BMC Neurosci. 2007, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Antidepressant treatment of psychotic major depression: Potential role of the σ receptor. CNS Spectr. 2005, 10, 319–323. [Google Scholar] [CrossRef]
- Sikka, P.; Kaushik, S.; Kumar, G.; Kapoor, S.; Bindra, V.K.; Saxena, K.K. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J. Pharm. Bioallied Sci. 2011, 3, 412–416. [Google Scholar] [CrossRef]
- Wójcikowski, J.; Daniel, W.A. Distribution interactions between perazine and antidepressant drugs. In vivo studies. Pol. J. Pharmacol. 2000, 52, 449–457. [Google Scholar]
- Godeau, C.; Morin-Crini, N.; Staelens, J.-N.; Martel, B.; Rocchi, S.; Chanet, G.; Fourmentin, M.; Crini, G. Adsorption of a triazole antifungal agent, difenoconazole, on soils from a cereal farm: Protective effect of hemp felt. Environ. Technol. Innov. 2021, 22, 101394. [Google Scholar] [CrossRef]
- Firooz, A.; Nafisi, S.; Maibach, H.I. Novel drug delivery strategies for improving econazole antifungal action. Int. J. Pharm. 2015, 495, 599–607. [Google Scholar] [CrossRef]
- Husak, V.V.; Mosiichuk, N.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Acute exposure to the penconazole-containing fungicide Topas partially augments antioxidant potential in goldfish tissues. Comp. Biochem. Physiol. Part C 2017, 193, 1–8. [Google Scholar] [CrossRef]
- De Bruyne, P.; Christiaens, T.; Boussery, K.; Mehuys, E.; Van Winckel, M. Are antihistamines effective in children? A review of the evidence. Arch. Dis. Child. 2017, 102, 56–60. [Google Scholar] [CrossRef]
- Shah, U.; Joshi, G.; Sawant, K. Improvement in antihypertensive and antianginal effects of felodipine by enhanced absorption from PLGA nanoparticles optimized by factorial design. Mater. Sci. Eng. C 2014, 35, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.W.; Boldogh, I.; Popov, V.L.; Saini, S.S.; Chopra, A.K. Anti-inflammatory and antisecretory potential of histidine in Salmonella-challenged mouse small intestine. Lab. Investig. 1998, 78, 523–534. [Google Scholar] [PubMed]
- Elmaci, I.; Altinoz, M.A. Targeting the cellular schizophrenia. Likely employment of the antipsychotic agent pimozide in treatment of refractory cancers and glioblastoma. Crit. Rev. Oncol. Hematol. 2018, 128, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Noergaard, M.; Andersen, J.T.; Jimenez-Solem, E.; Christensen, M.B. Long term treatment with stimulant laxatives—Clinical evidence for effectiveness and safety? Scand. J. Gastroenterol. 2019, 54, 27–34. [Google Scholar] [CrossRef]
- Başpınar, Y.; Kotmakçı, M.; Öztürk, I. Antimicrobial activity of phytosphingosine nanoemulsions against bacteria and yeasts. Celal Bayar Univ. J. Sci. 2018, 14, 223–228. [Google Scholar] [CrossRef]
- Ghoniem, M.G.; Mohamed, M.A.; Eldin, G.M.G.; Errachid, A. Sensitive electrochemical strategy via the construction of functionalized carbon nanotubes/ionic liquid nanocomposite for the determination of anaesthetic drug cinchocaine. Measurement 2021, 185, 110071. [Google Scholar] [CrossRef]
- de Jong, J.P.; Voerman, J.S.A.; Leenen, P.J.M.; van der Sluijs-Gelling, A.J.; Ploemacher, R.E. Improved fixation of frozen lympho-haemopoietic tissue sections with hexazotized pararosaniline. Histochem. J. 1991, 23, 392–401. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Bukoye, B.B. Abortifacient potentials of the aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Contraception 2009, 80, 308–313. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Wang, S.; Mei, X.; Zhang, H.; Kan, J. Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis): Effect of drying procedures. Food Chem. 2019, 292, 281–293. [Google Scholar] [CrossRef]
- Neményi, A.; Stefanovitsné-Bányai, É.; Burján, S.S.; Pék, Z.; Hegedus, A.; Gyuricza, C.; Helyes, L. Seasonal variations in total antioxidant capacity and total phenolics content of leaves of Phyllostachys taxa using different extraction methods. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 43–50. [Google Scholar] [CrossRef][Green Version]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Hydroxyl radical and hypochlorous acid scavenging activity of small centaury (Centaurium erythraea) infusion. A comparative study with green tea (Camellia sinensis). Phytomedicine 2003, 10, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Umar, I.M.; Ismail, M. Antioxidant property enhancement of sweet potato flour under simulated gastrointestinal pH. Int. J. Mol. Sci. 2012, 13, 8987–8997. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid. -Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef]
- Russo, D.; Kenny, O.; Smyth, T.J.; Milella, L.; Hossain, M.B.; Diop, M.S.; Rai, D.K.; Brunton, N.P. Profiling of phytochemicals in tissues from Sclerocarya birrea by HPLC-MS and their link with antioxidant activity. ISRN Chromatogr. 2013, 2013, 283462. [Google Scholar] [CrossRef]
- Rajeh, M.A.B.; Zuraini, Z.; Sasidharan, S.; Latha, L.Y.; Amutha, S. Assessment of Euphorbia hirta L. leaf, flower, stem and root extracts for their antibacterial and antifungal activity and brine shrimp lethality. Molecules 2010, 15, 6008–6018. [Google Scholar] [CrossRef]
- Geethaa, S.; Thavamany, P.J.; Chiew, S.P.; Thong, O.M. Interference from ordinarily used solvents in the outcomes of Artemia salina lethality test. J. Adv. Pharm. Technol. Res. 2013, 4, 179–182. [Google Scholar] [CrossRef]
- Baravalia, Y.; Vaghasiya, Y.; Chanda, S. Brine shrimp cytotoxicity, anti-inflammatory and analgesic properties of Woodfordia fruticosa Kurz flowers. Iran. J. Pharm. Res. 2012, 11, 851–861. [Google Scholar]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]




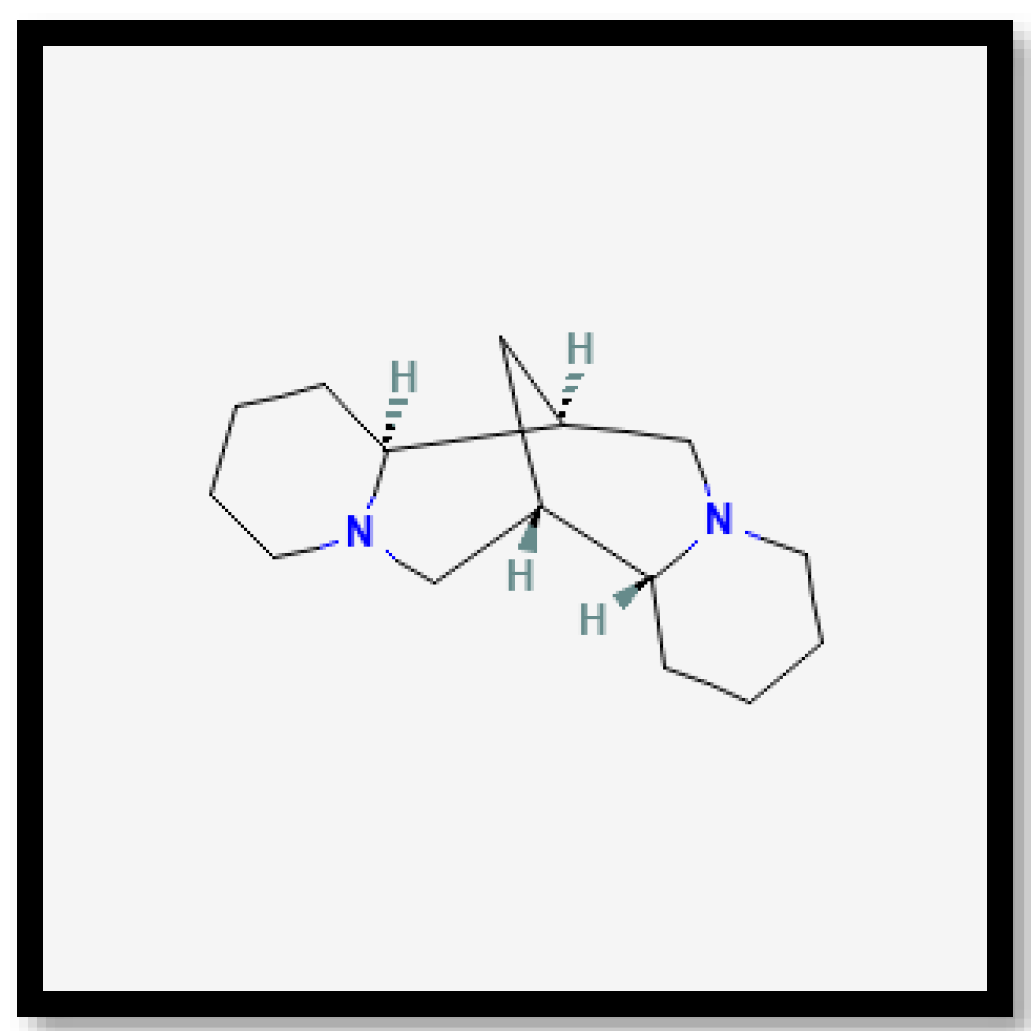
| Drying Methods | TPC 1 | TFC 2 |
|---|---|---|
| B. multiplex | ||
| Fresh 3 | 5.64 ± 0.09 b | 0.87 ± 0.01 f |
| Sun-drying | 5.09 ± 0.01 d | 1.13 ± 0.00 c |
| Shade-drying | 5.42 ± 0.02 c | 1.26 ± 0.00 b |
| Microwave-drying | 5.44 ± 0.05 c | 1.04 ± 0.01 d |
| Oven-drying | 5.18 ± 0.00 d | 0.89 ± 0.00 e |
| Freeze-drying | 5.74 ± 0.06 a | 1.62 ± 0.01 a |
| B. tuldoides | ||
| Fresh 3 | 5.26 ± 0.01 d | 1.65 ± 0.01 d |
| Sun-drying | 4.31 ± 0.03 f | 1.17 ± 0.00 f |
| Shade-drying | 4.92 ± 0.01 e | 1.41 ± 0.00 e |
| Microwave-drying | 5.91 ± 0.00 a | 2.06 ± 0.00 b |
| Oven-drying | 5.60 ± 0.04 c | 1.69 ± 0.02 c |
| Freeze-drying | 5.84 ± 0.01 b | 2.11 ± 0.00 a |
| B. vulgaris | ||
| Fresh 3 | 4.64 ± 0.05 e | 0.84 ± 0.00 d |
| Sun-drying | 4.24 ± 0.00 f | 0.81 ± 0.00 e |
| Shade-drying | 5.45 ± 0.00 d | 0.85 ± 0.00 d |
| Microwave-drying | 6.17 ± 0.04 a | 1.10 ± 0.01 a |
| Oven-drying | 5.77 ± 0.03 b | 0.94 ± 0.01 c |
| Freeze-drying | 5.58 ± 0.01 c | 0.96 ± 0.00 b |
| D. sublaevigata | ||
| Fresh 3 | 8.26 ± 0.05 b | 0.78 ± 0.00 b,c |
| Sun-drying | 7.38 ± 0.00 d | 0.78 ± 0.00 b |
| Shade-drying | 8.27 ± 0.09 b | 0.77 ± 0.00 c |
| Microwave-drying | 8.20 ± 0.02 b | 0.83 ± 0.00 a |
| Oven-drying | 7.91 ± 0.01 c | 0.84 ± 0.00 a |
| Freeze-drying | 12.59 ± 0.09 a | 0.84 ± 0.00 a |
| G. levis | ||
| Fresh 3 | 3.92 ± 0.00 d | 0.81 ± 0.01 c |
| Sun-drying | 2.69 ± 0.01 f | 0.77 ± 0.01 d |
| Shade-drying | 3.68 ± 0.02 e | 0.84 ± 0.00 b |
| Microwave-drying | 4.60 ± 0.03 b | 0.82 ± 0.01 c |
| Oven-drying | 4.29 ± 0.00 c | 0.84 ± 0.00 b |
| Freeze-drying | 4.78 ± 0.01 a | 0.87 ± 0.01 a |
| S. brachycladum | ||
| Fresh 3 | 4.34 ± 0.09 d | 1.85 ± 0.01 b |
| Sun-drying | 4.23 ± 0.01 e | 0.98 ± 0.00 f |
| Shade-drying | 4.34 ± 0.04 d | 1.31 ± 0.01 e |
| Microwave-drying | 5.01 ± 0.03 c | 1.70 ± 0.00 d |
| Oven-drying | 5.30 ± 0.09 b | 1.74 ± 0.00 c |
| Freeze-drying | 5.61 ± 0.01 a | 2.12 ± 0.01 a |
| Drying Methods | DPPH 1 | ABTS 2 | FRAP 3 |
|---|---|---|---|
| B. multiplex | |||
| Fresh 4 | 3.73 ± 0.00 e | 2.93 ± 0.01 e | 31.33 ± 0.05 d |
| Sun-drying | 4.09 ± 0.00 f | 2.77 ± 0.01 d | 22.39 ± 0.11 f |
| Shade-drying | 3.42 ± 0.00 b | 2.59 ± 0.01 b | 29.73 ± 0.07 e |
| Microwave-drying | 3.67 ± 0.01 d | 2.72 ± 0.01 c | 33.83 ± 0.10 b |
| Oven-drying | 3.49 ± 0.00 c | 2.50 ± 0.01 a | 32.06 ± 0.07 c |
| Freeze-drying | 3.20 ± 0.00 a | 2.73 ± 0.00 c | 36.65 ± 0.09 a |
| B. tuldoides | |||
| Fresh 4 | 3.54 ± 0.02 d | 3.07 ± 0.01 f | 27.68 ± 0.12 d |
| Sun-drying | 4.01 ± 0.00 f | 2.70 ± 0.00 e | 19.40 ± 0.11 f |
| Shade-drying | 3.82 ± 0.01 e | 2.60 ± 0.01 d | 25.87 ± 0.10 e |
| Microwave-drying | 3.37 ± 0.01 c | 2.29 ± 0.01 c | 35.98 ± 0.06 a |
| Oven-drying | 3.00 ± 0.02 b | 1.89 ± 0.01 a | 33.83 ± 0.01 c |
| Freeze-drying | 2.92 ± 0.01 a | 1.98 ± 0.00 b | 35.83 ± 0.05 b |
| B. vulgaris | |||
| Fresh 4 | 3.59 ± 0.01 c | 2.74 ± 0.00 d | 28.36 ± 0.14 d |
| Sun-drying | 4.14 ± 0.00 f | 2.94 ± 0.00 f | 15.86 ± 0.10 f |
| Shade-drying | 3.72 ± 0.00 e | 2.80 ± 0.01 e | 25.16 ± 0.13 e |
| Microwave-drying | 3.11 ± 0.00 a | 2.32 ± 0.00 c | 35.32 ± 0.15 a |
| Oven-drying | 3.37 ± 0.00 b | 2.01 ± 0.00 a | 33.47 ± 0.10 c |
| Freeze-drying | 3.63 ± 0.00 d | 2.13 ± 0.01 b | 34.74 ± 0.14 b |
| D. sublaevigata | |||
| Fresh 4 | 3.78 ± 0.00 d | 2.57 ± 0.00 c | 14.06 ± 0.10 e |
| Sun-drying | 4.33 ± 0.00 f | 2.76 ± 0.00 d | 6.40 ± 0.12 f |
| Shade-drying | 4.12 ± 0.00 e | 2.58 ± 0.01 c | 15.50 ± 0.01 d |
| Microwave-drying | 3.36 ± 0.00 c | 2.38 ± 0.01 b | 19.50 ± 0.12 c |
| Oven-drying | 3.16 ± 0.00 b | 2.22 ± 0.00 a | 24.60 ± 0.06 b |
| Freeze-drying | 3.05 ± 0.00 a | 2.38 ± 0.01 b | 31.23 ± 0.11 a |
| G. levis | |||
| Fresh 4 | 4.42 ± 0.01 f | 3.09 ± 0.00 d | 29.35 ± 0.14 c |
| Sun-drying | 4.36 ± 0.00 e | 3.47 ± 0.00 f | 18.26 ± 0.17 e |
| Shade-drying | 4.11 ± 0.01 d | 3.22 ± 0.01 e | 22.80 ± 0.09 d |
| Microwave-drying | 4.05 ± 0.00 c | 3.05 ± 0.01 c | 34.22 ± 0.11 b |
| Oven-drying | 3.37 ± 0.00 b | 2.47 ± 0.00 b | 34.85 ± 0.09 a |
| Freeze-drying | 3.23 ± 0.00 a | 2.44 ± 0.00 a | 35.02 ± 0.07 a |
| S. brachycladum | |||
| Fresh 4 | 3.49 ± 0.01 c | 2.41 ± 0.00 c | 27.40 ± 0.07 d |
| Sun-drying | 4.73 ± 0.02 f | 3.10 ± 0.01 e | 13.33 ± 0.03 e |
| Shade-drying | 4.45 ± 0.01 e | 3.33 ± 0.01 f | 13.18 ± 0.02 f |
| Microwave-drying | 3.71 ± 0.01 d | 2.36 ± 0.00 b | 30.22 ± 0.08 b |
| Oven-drying | 3.28 ± 0.01 b | 2.12 ± 0.01 a | 28.03 ± 0.06 c |
| Freeze-drying | 3.23 ± 0.00 a | 2.51 ± 0.00 d | 35.81 ± 0.09 a |
| Trolox 5 | 4.09 ± 0.00 | 4.55 ± 0.02 | – |
| Phytochemical | Antioxidant Capacity | |||||
|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | ||||
| R | p-Value | R | p-Value | R | p-Value | |
| TPC | −0.40 ** | 0.00 | −0.42 ** | 0.00 | −0.05 | 0.64 |
| TFC | −0.45 ** | 0.00 | −0.39 ** | 0.00 | 0.42 ** | 0.00 |
| Samples | Concentration (µg/mL) | % Mortality | LC50 (µg/mL) |
|---|---|---|---|
| K2Cr2O7 1 | 1000 | 100 | 11.23 |
| 100 | 33 | ||
| 10 | 33 | ||
| 1 | 27 | ||
| B. multiplex | 1000 | 17 | 3744.85 |
| 100 | 10 | ||
| 10 | 7 | ||
| 1 | 0 | ||
| B. tuldoides | 1000 | 20 | 2974.47 |
| 100 | 10 | ||
| 10 | 7 | ||
| 1 | 0 | ||
| B. vulgaris | 1000 | 17 | 3166.15 |
| 100 | 13 | ||
| 10 | 7 | ||
| 1 | 0 | ||
| D. sublaevigata | 1000 | 13 | 5668.14 |
| 100 | 10 | ||
| 10 | 7 | ||
| 1 | 0 | ||
| G. levis | 1000 | 27 | 1236.53 |
| 100 | 20 | ||
| 10 | 10 | ||
| 1 | 0 | ||
| S. brachycladum | 1000 | 20 | 2045.03 |
| 100 | 17 | ||
| 10 | 10 | ||
| 1 | 0 |
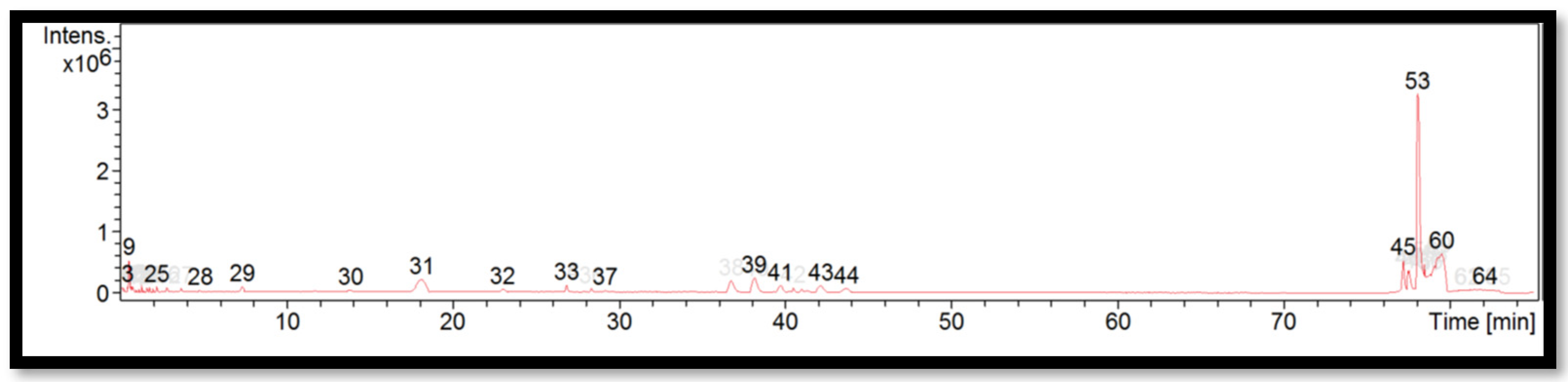
| Peak | RT (min) | Identified Compounds | Molecular Formula | Molecular Weight | m/z |
|---|---|---|---|---|---|
| 3 | 0.5 | Unidentified | – | 199.9663 | 200.9736 |
| 9 | 0.6 | L-Histidine | C6H9N3O2 | 155.0354 | 156.0427 |
| 25 | 2.2 | Unidentified | – | 445.2898 | 446.2971 |
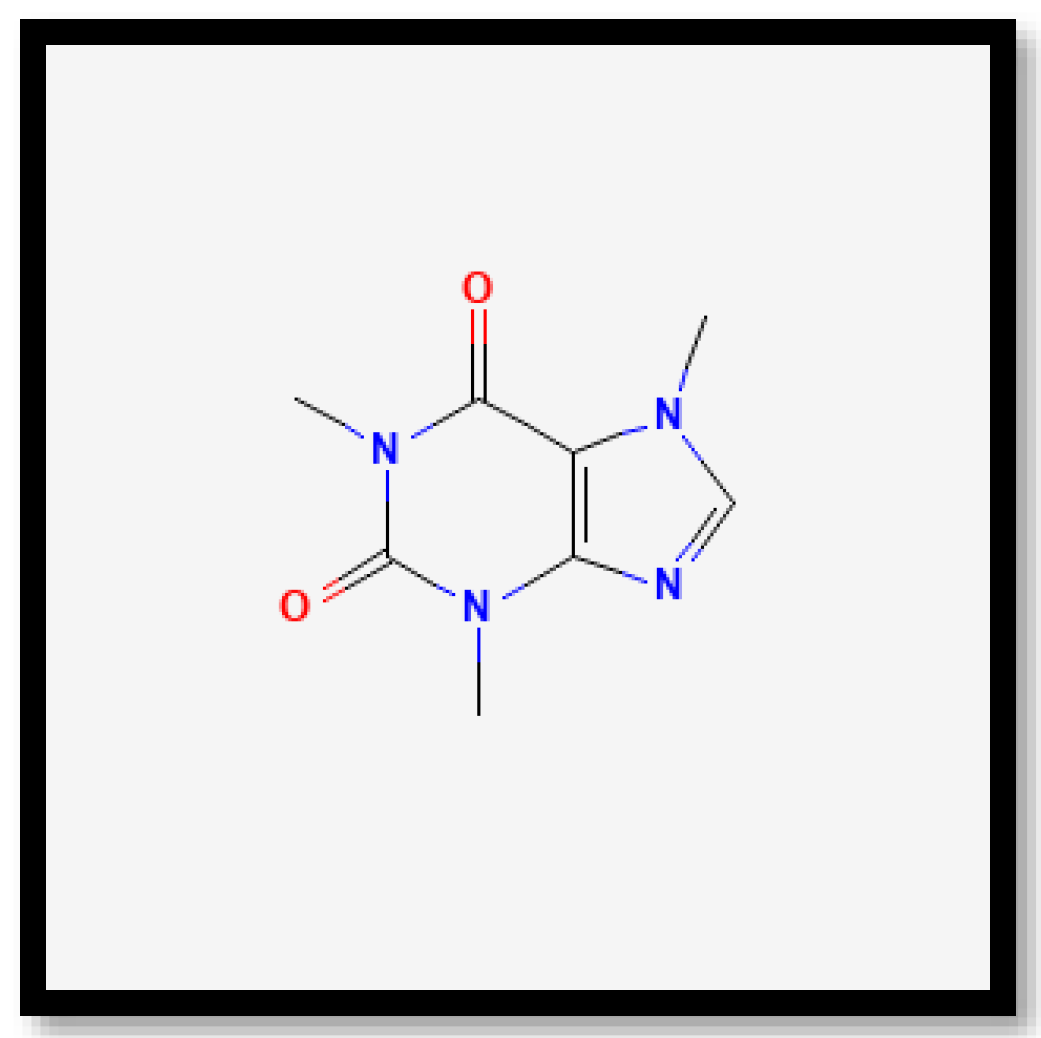
| 28 | 4.8 | Caffeine | C8H10N4O2 | 577.3688 | 578.3760 |
| 29 | 7.4 | Unidentified | – | 452.3372 | 453.3445 |
| 30 | 13.9 | Caffeine | C8H10N4O2 | 550.1329 | 551.1402 |
| 31 | 18.2 | Unidentified | – | 534.1379 | 535.1452 |
| 32 | 23.0 | Caffeine | C8H10N4O2 | 534.1378 | 535.1451 |
| 33 | 26.9 | Unidentified | – | 700.4868 | 701.4941 |
| 37 | 29.2 | Caffeine | C8H10N4O2 | 428.1840 | 429.1913 |
| 39 | 38.2 | Pararosaniline | C19H17N3 | 287.2837 | 288.2909 |
| 41 | 39.7 | Unidentified | – | 315.2784 | 316.2857 |
| 43 | 42.2 | Unidentified | – | 315.3145 | 316.3218 |
| 44 | 43.7 | Unidentified | – | 315.3144 | 316.3217 |
| 45 | 77.2 | Felodipine | C18H19Cl2NO4 | 337.3346 | 338.3419 |
| 53 | 78.0 | Felodipine | C18H19Cl2NO4 | 337.3365 | 338.3438 |
| 60 | 79.5 | Phytosphingosine | C18H39NO3 | 337.3346 | 338.3419 |
| 64 | 82.1 | Unidentified | – | 343.2721 | 344.2794 |
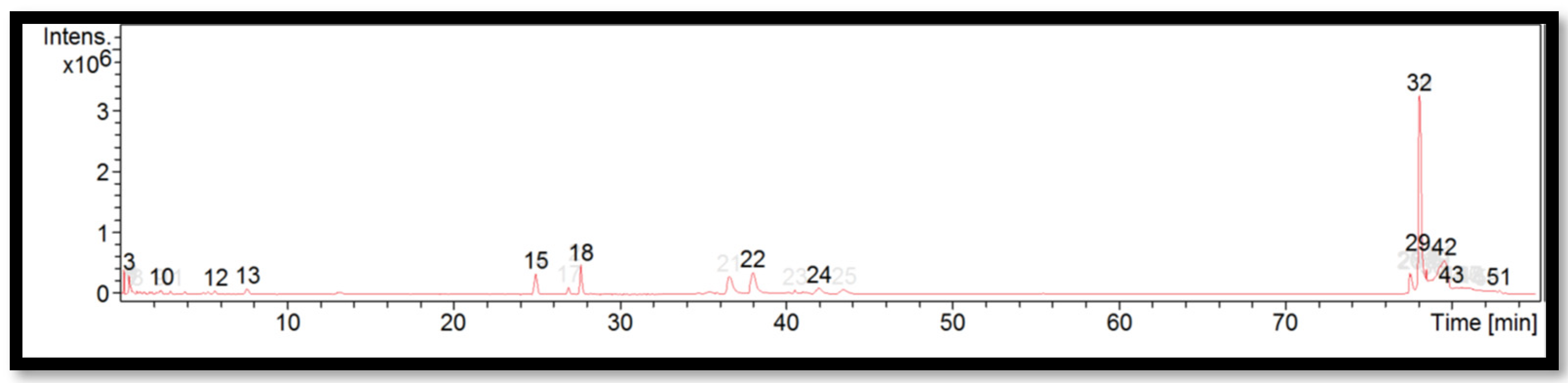
| Peak | RT (min) | Identified Compounds | Molecular Formula | Molecular Weight | m/z |
|---|---|---|---|---|---|
| 5 | 0.6 | Sparteine | C15H26N2 | 234.1583 | 235.1656 |
| 12 | 2.5 | PET-cGMP | C18H15N5O7PNa | 113.0840 | 114.0913 |
| 14 | 5.7 | Naloxone | C19H21NO4 | 327.2521 | 328.2594 |
| 15 | 7.6 | Unidentified | – | 452.3361 | 453.3434 |
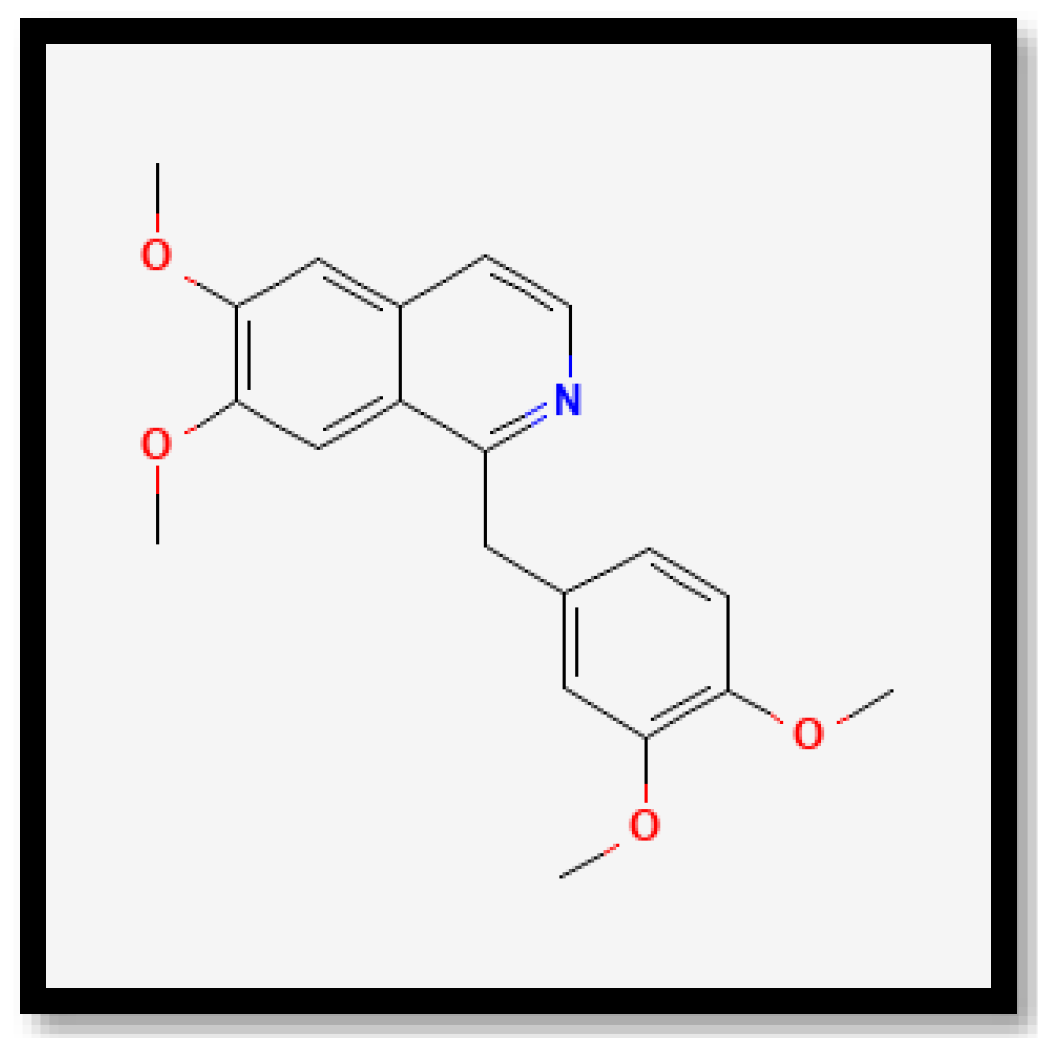
| 16 | 25.0 | Papaverine | C20H21NO4 | 678.5037 | 340.2591 |
| 22 | 27.7 | Thiopental | C11H18N2O2S | 791.5873 | 396.8009 |
| 24 | 38.0 | Cyproheptadine | C21H21N | 287.2827 | 288.2900 |
| 26 | 42.0 | Loprazolam | C23H21ClN6O3 | 315.3139 | 316.3211 |
| 28 | 77.5 | Difenoconazole | C19H17Cl2N3O3 | 309.3035 | 310.3108 |
| 31 | 78.0 | RP-8-pCPT-cGMPS | C16H14ClN5O6PS2Na | 311.3193 | 312.3265 |
| 35 | 78.0 | Felodipine | C18H19Cl2NO4 | 337.3367 | 338.3439 |
| 44 | 79.5 | Felodipine | C18H19Cl2NO4 | 337.3348 | 338.3421 |
| 49 | 81.2 | Unidentified | – | 337.3347 | 338.3419 |
| 52 | 82.8 | Unidentified | – | 225.9441 | 226.9514 |
| Peak | RT (min) | Identified Compounds | Molecular Formula | Molecular Weight | m/z |
|---|---|---|---|---|---|
| 3 | 0.5 | Econazole | C18H15Cl3N2O | 155.0349 | 156.0422 |
| 7 | 0.7 | Unidentified | – | 200.0322 | 201.0394 |
| 9 | 3.1 | Pimozide | C28H29F2N3O | 489.3156 | 490.3229 |
| 10 | 7.6 | Unidentified | – | 452.3377 | 453.3449 |
| 11 | 26.9 | Papaverine | C20H21NO4 | 678.5046 | 340.2596 |
| 15 | 37.9 | Cyproheptadine | C21H21N | 287.2832 | 288.2905 |
| 19 | 39.6 | Bisacodyl | C22H19NO4 | 361.1714 | 362.1787 |
| 25 | 41.9 | Loprazolam | C23H21ClN6O3 | 315.3143 | 316.3216 |
| 36 | 77.5 | Difenoconazole | C19H17Cl2N3O3 | 337.3351 | 338.3423 |
| 42 | 78.0 | Felodipine | C18H19Cl2NO4 | 320.3085 | 321.3158 |
| 51 | 79.5 | Felodipine | C18H19Cl2NO4 | 337.3352 | 338.3425 |
| 52 | 79.8 | Unidentified | – | 343.2730 | 344.2802 |
| 58 | 81.4 | Cinchocaine | C20H29N3O2 | 337.3349 | 338.3422 |
| Peak | RT (min) | Identified Compounds | Molecular Formula | Molecular Weight | m/z |
|---|---|---|---|---|---|
| 5 | 0.5 | Phenytoin | C15H12N2O2 | 155.0349 | 156.0421 |
| 12 | 2.4 | Perazine | C20H25N3S | 339.2523 | 340.2596 |
| 15 | 5.3 | Unidentified | – | 474.3177 | 475.3250 |
| 18 | 7.7 | Unidentified | – | 452.3365 | 453.3438 |
| 19 | 13.2 | Penconazole | C13H15Cl2N3 | 565.4204 | 566.4276 |
| 22 | 25.0 | Papaverine | C20H21NO4 | 678.5038 | 679.5111 |
| 24 | 27.0 | Papaverine | C20H21NO4 | 678.5026 | 340.2586 |
| 25 | 27.7 | Unidentified | – | 813.5703 | 814.5776 |
| 29 | 38.0 | Cyproheptadine | C21H21N | 287.2825 | 288.2897 |
| 31 | 42.0 | Unidentified | – | 315.3136 | 316.3209 |
| 36 | 78.0 | RP-8-pCPT-cGMPS | C16H14ClN5O6PS2Na | 311.3185 | 312.3258 |
| 40 | 78.0 | RP-8-pCPT-cGMPS | C16H14ClN5O6PS2Na | 320.3077 | 321.3149 |
| 49 | 79.5 | Felodipine | C18H19Cl2NO4 | 337.3339 | 338.3412 |
| 51 | 80.0 | Cinchocaine | C20H29N3O2 | 343.2714 | 344.2787 |
| 56 | 82.1 | Cinchocaine | C20H29N3O2 | 343.2717 | 344.2789 |
| Peak | RT (min) | Identified Compounds | Molecular Formula | Molecular Weight | m/z |
|---|---|---|---|---|---|
| 3 | 0.5 | L-Histidine | C6H9N3O2 | 155.0347 | 156.0420 |
| 10 | 2.5 | PET-cGMP | C18H15N5O7PNa | 113.0840 | 114.0913 |
| 12 | 5.7 | Naloxone | C19H21NO4 | 327.2530 | 328.2602 |
| 13 | 7.6 | Unidentified | – | 452.3369 | 453.3442 |
| 15 | 25.0 | Papaverine | C20H21NO4 | 678.5056 | 340.2601 |
| 18 | 27.7 | Unidentified | – | 813.5731 | 814.5803 |
| 22 | 38.0 | Cyproheptadine | C21H21N | 287.2833 | 288.2906 |
| 24 | 42.0 | Loprazolam | C23H21ClN6O3 | 315.3148 | 316.3221 |
| 29 | 78.0 | RP-8-pCPT-cGMPS | C16H14ClN5O6PS2Na | 311.3195 | 312.3268 |
| 32 | 78.0 | RP-8-pCPT-cGMPS | C16H14ClN5O6PS2Na | 674.6705 | 675.6778 |
| 42 | 79.5 | Felodipine | C18H19Cl2NO4 | 337.3351 | 338.3424 |
| 43 | 80.0 | Cinchocaine | C20H29N3O2 | 343.2728 | 344.2801 |
| 51 | 82.8 | Amphetamine | C9H13N | 225.9441 | 226.9513 |
| Peak | RT (min) | Identified Compounds | Molecular Formula | Molecular Weight | m/z |
|---|---|---|---|---|---|
| 3 | 0.5 | Unidentified | – | 155.0348 | 156.0421 |
| 7 | 2.5 | Amphetamine | C9H13N | 113.0840 | 114.0913 |
| 10 | 5.7 | Naloxone | C19H21NO4 | 327.2523 | 328.2596 |
| 11 | 7.6 | Unidentified | – | 452.3367 | 453.3440 |
| 15 | 25.0 | Perazine | C20H25N3S | 678.5043 | 340.2594 |
| 18 | 27.7 | Unidentified | – | 829.5351 | 415.7748 |
| 24 | 37.4 | Cyproheptadine | C21H21N | 287.2828 | 288.2901 |
| 25 | 41.3 | Unidentified | – | 315.3141 | 316.3214 |
| 27 | 77.5 | Difenoconazole | C19H17Cl2N3O3 | 309.3037 | 310.3110 |
| 32 | 78.0 | RP-8-pCPT-cGMPS | C16H14ClN5O6PS2Na | 311.3194 | 312.3267 |
| 35 | 78.0 | Felodipine | C18H19Cl2NO4 | 337.3365 | 338.3437 |
| 47 | 79.5 | Felodipine | C18H19Cl2NO4 | 337.3351 | 338.3423 |
| 52 | 81.4 | Unidentified | – | 343.2726 | 344.2799 |
| Chemical Compounds | Properties | References |
|---|---|---|
| Loprazolam | Anticonvulsant drugs | McDonough Jr et al. [65] |
| Phenytoin | Anticonvulsant drugs | Mishory et al. [66] |
| Thiopental | Anticonvulsant drugs | Papatheodoropoulos et al. [67] |
| Amphetamine | Antidepressant drugs | Stahl [68] |
| Naloxone | Antidepressant drugs | Sikka et al. [69] |
| Perazine | Antidepressant drugs | Wójcikowski and Daniel [70] |
| Difenoconazole | Antifungal drugs | Godeau et al. [71] |
| Econazole | Antifungal drugs | Firooz et al. [72] |
| Penconazole | Antifungal drugs | Husak et al. [73] |
| Cyproheptadine | Antihistamine drugs | De Bruyne et al. [74] |
| Felodipine | Antihypertensive drugs | Shah et al. [75] |
| L-Histidine | Anti-inflammatory drugs | Peterson et al. [76] |
| Pimozide | Antipsychotic drugs | Elmaci and Altinoz [77] |
| Bisacodyl | Stimulant laxative drugs | Noergaard et al. [78] |
| Phytosphingosine | Antimicrobial drugs | Başpınar et al. [79] |
| Cinchocaine | Anaesthetic drugs | Ghoniem et al. [80] |
| Pararosaniline | Dye agents | de Jong et al. [81] |
| Drying Methods | Drying Process |
|---|---|
| Sun-drying | Samples were exposed to sunlight for 1 week |
| Shade-drying | Samples were dried in room temperature at 22–25 °C and humidity levels between 30% and 50% for 1 week |
| Microwave-drying | Samples were put in a microwave dryer (Samsung, Seoul, Korea) at atmospheric pressure and 160 W power (three times, 2 min each time) |
| Oven-drying | Samples were put in an oven (Protech, Selangor, Malaysia) at 50 °C for 24 h |
| Freeze-drying | Samples were frozen at −80 °C for 48 h, and then put in a freeze-dryer (Labconco, Kansas City, MO, USA) for 24 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjamin, M.A.Z.; Ng, S.Y.; Saikim, F.H.; Rusdi, N.A. The Effects of Drying Techniques on Phytochemical Contents and Biological Activities on Selected Bamboo Leaves. Molecules 2022, 27, 6458. https://doi.org/10.3390/molecules27196458
Benjamin MAZ, Ng SY, Saikim FH, Rusdi NA. The Effects of Drying Techniques on Phytochemical Contents and Biological Activities on Selected Bamboo Leaves. Molecules. 2022; 27(19):6458. https://doi.org/10.3390/molecules27196458
Chicago/Turabian StyleBenjamin, Mohammad Amil Zulhilmi, Shean Yeaw Ng, Fiffy Hanisdah Saikim, and Nor Azizun Rusdi. 2022. "The Effects of Drying Techniques on Phytochemical Contents and Biological Activities on Selected Bamboo Leaves" Molecules 27, no. 19: 6458. https://doi.org/10.3390/molecules27196458
APA StyleBenjamin, M. A. Z., Ng, S. Y., Saikim, F. H., & Rusdi, N. A. (2022). The Effects of Drying Techniques on Phytochemical Contents and Biological Activities on Selected Bamboo Leaves. Molecules, 27(19), 6458. https://doi.org/10.3390/molecules27196458






