Facile Synthesis of NiCo2O4 Nanowire Arrays/Few-Layered Ti3C2-MXene Composite as Binder-Free Electrode for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
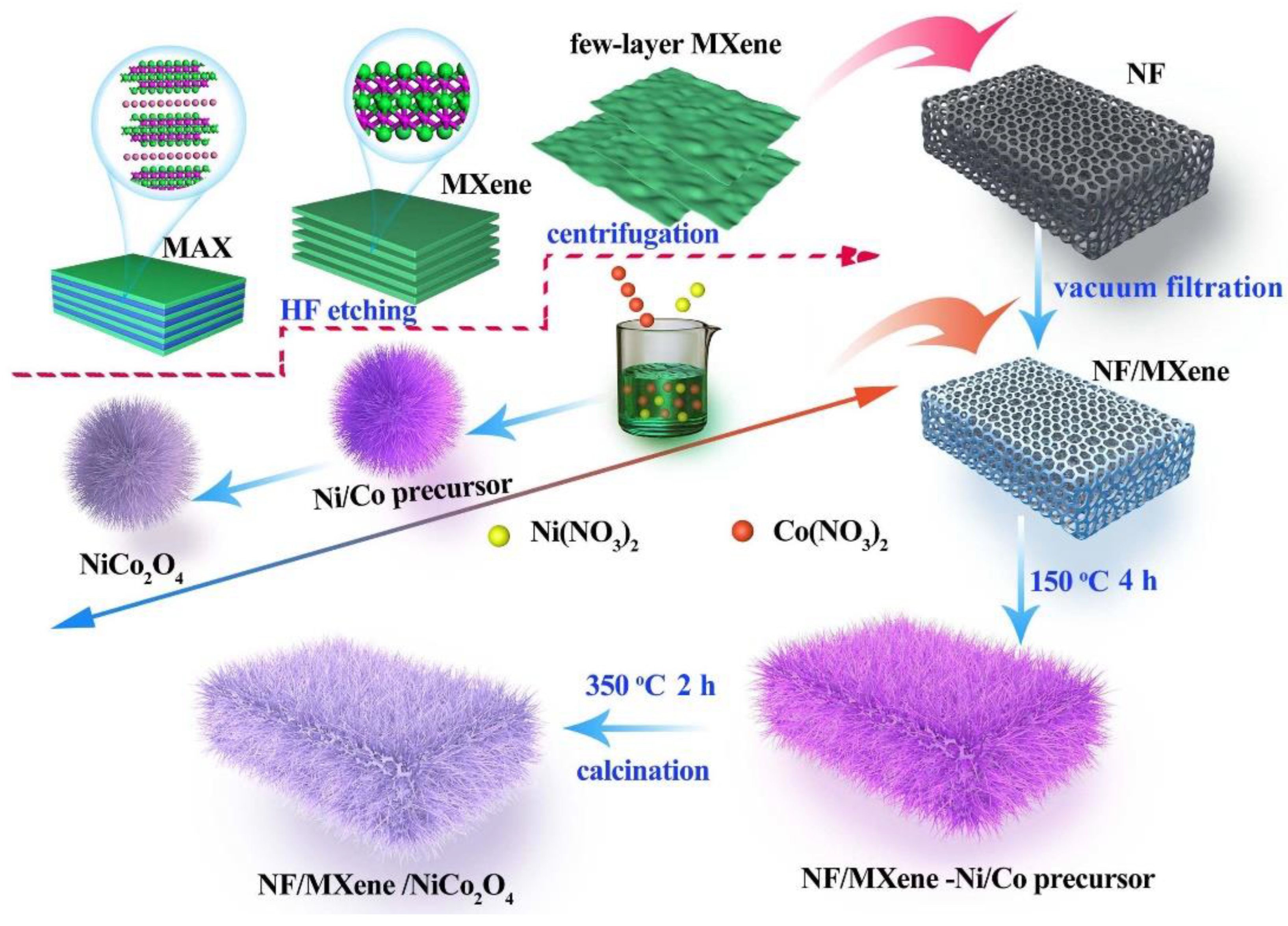
2.2. Synthesis of NiCo2O4/Ti3C2 Heterostructure
2.3. Instruments and Characterization
2.4. Fabrication of the NiCo2O4/Ti3C2//AC Asymmetrical Supercapacitor (ASC)
2.5. Electrochemical Measurements
3. Results and Discussion
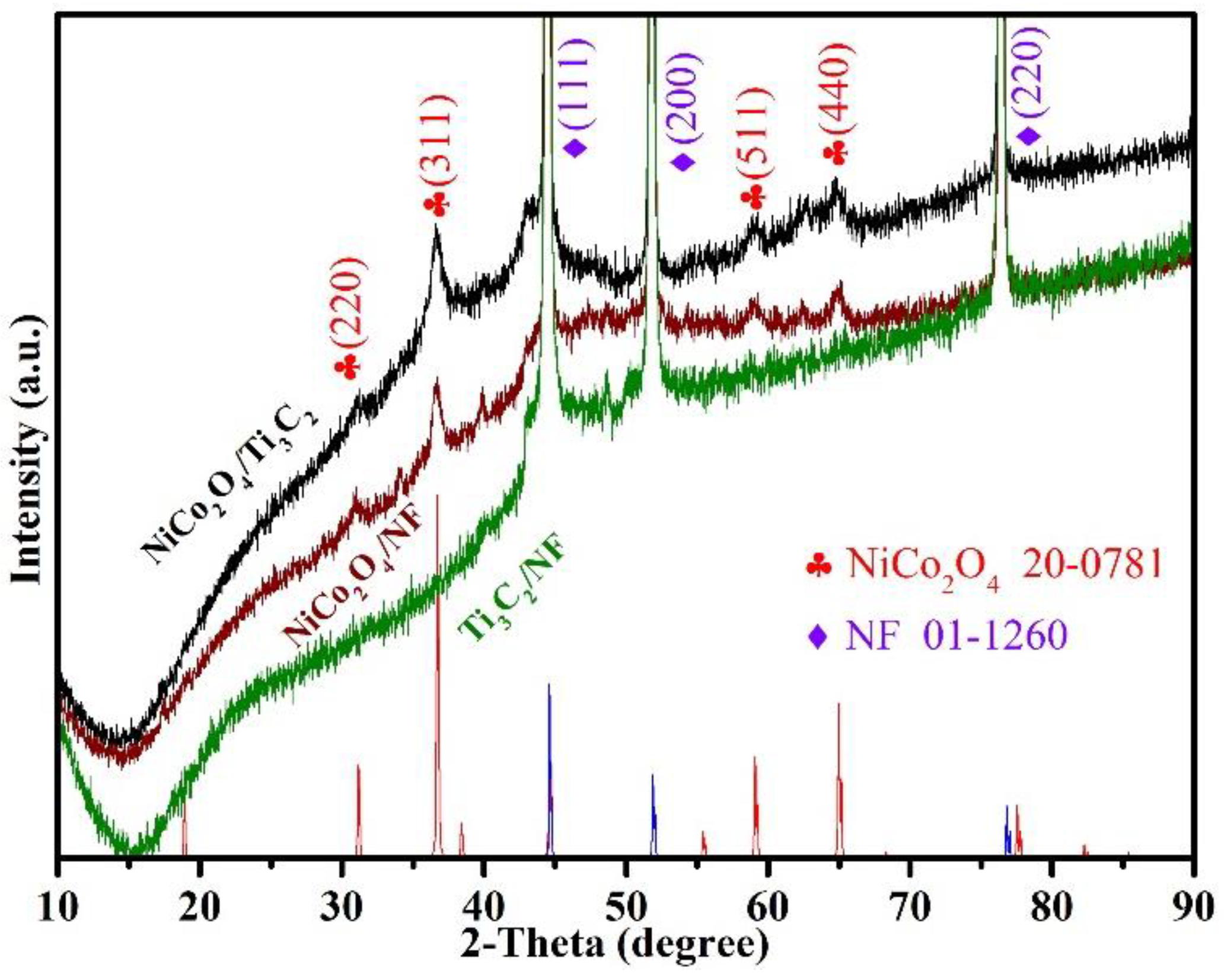
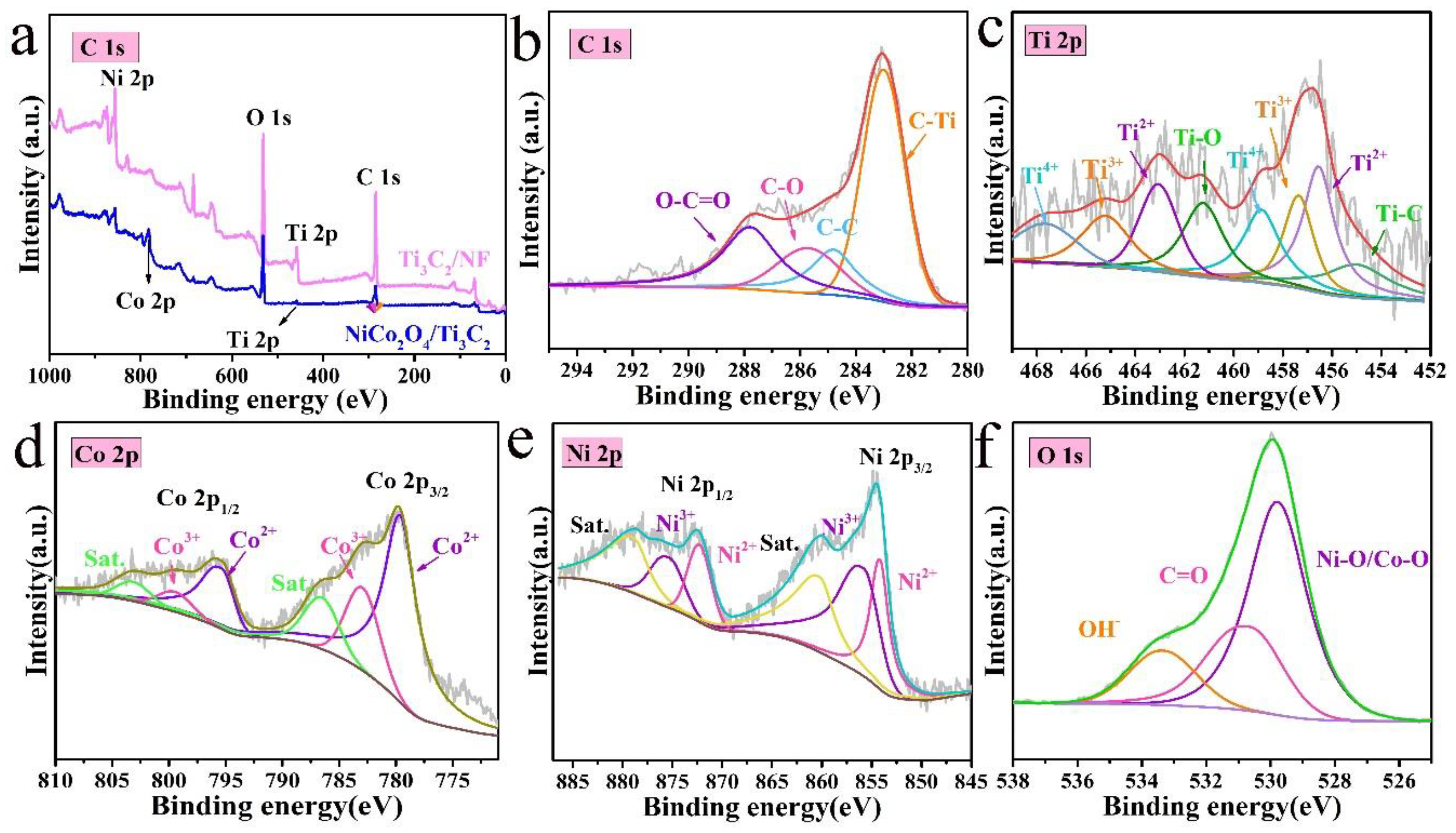
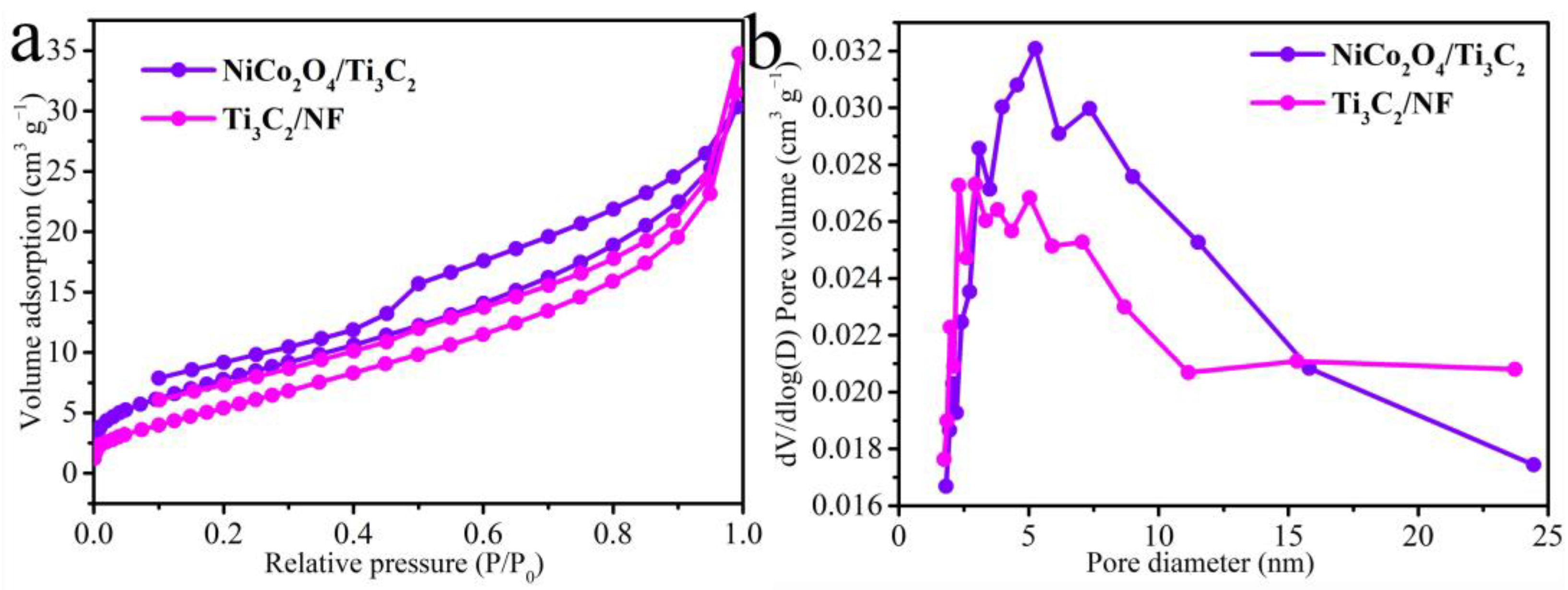
3.1. Structural and Morphological Characterization
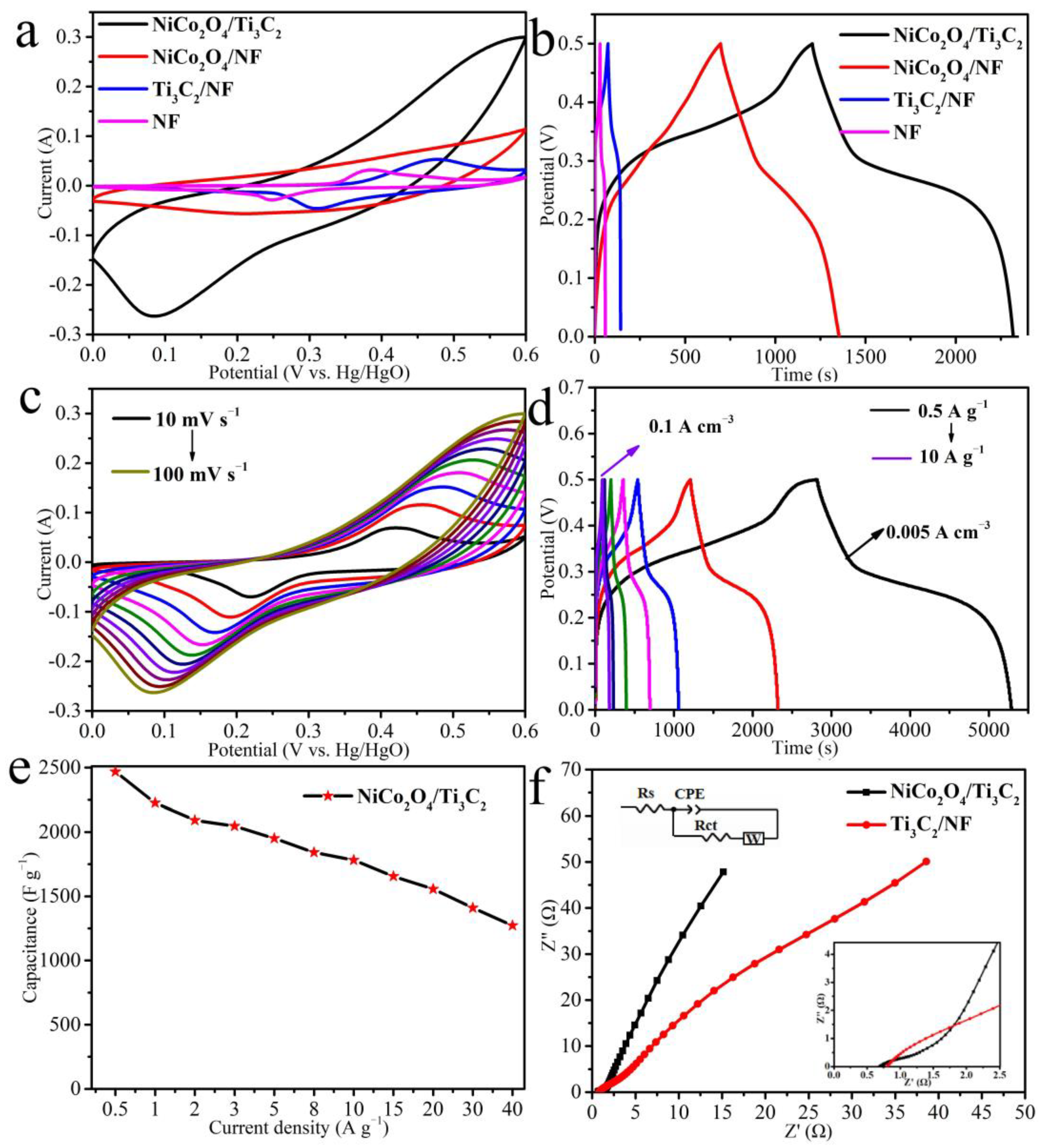
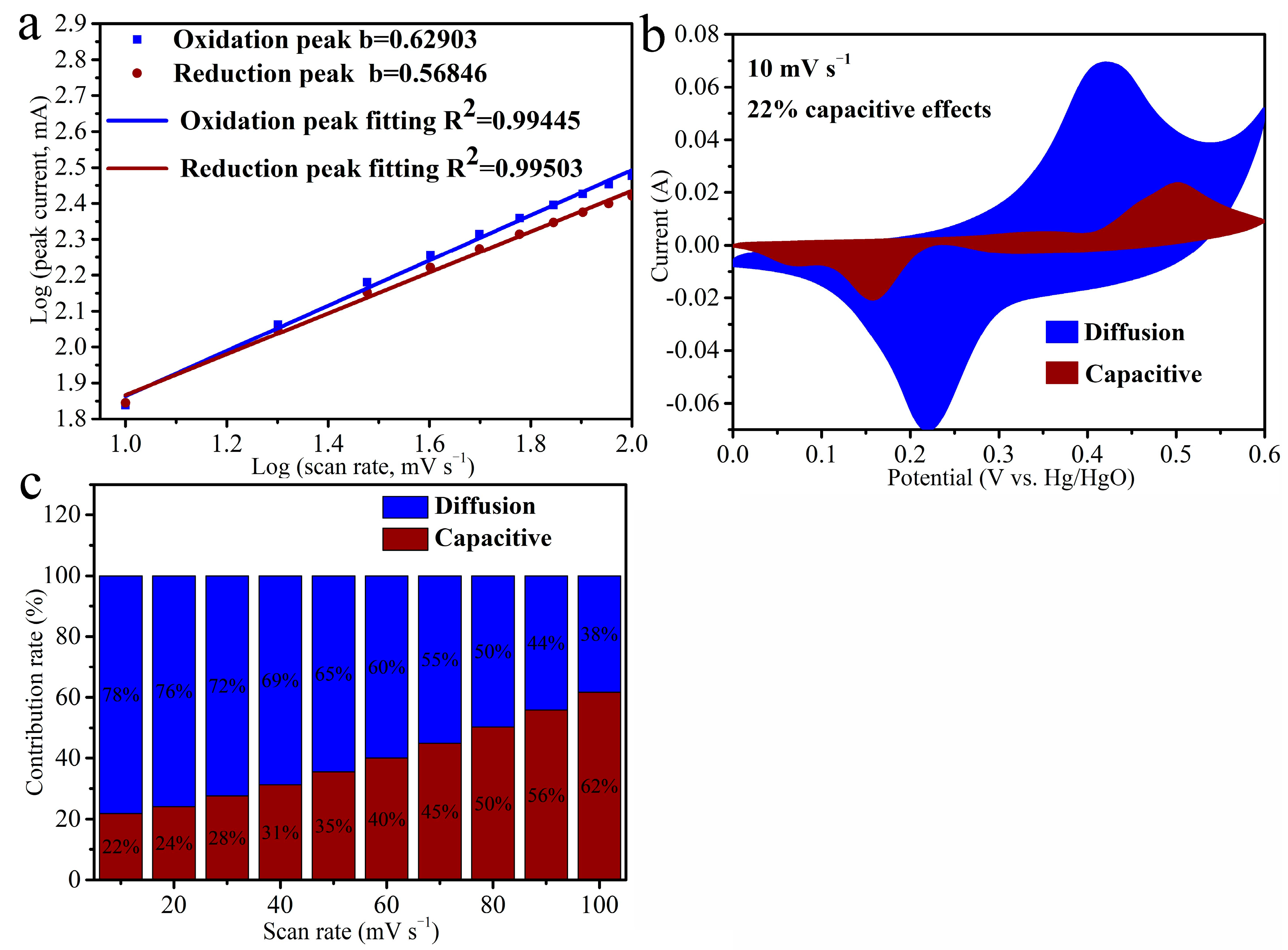
3.2. Electrochemical Performances of Samples
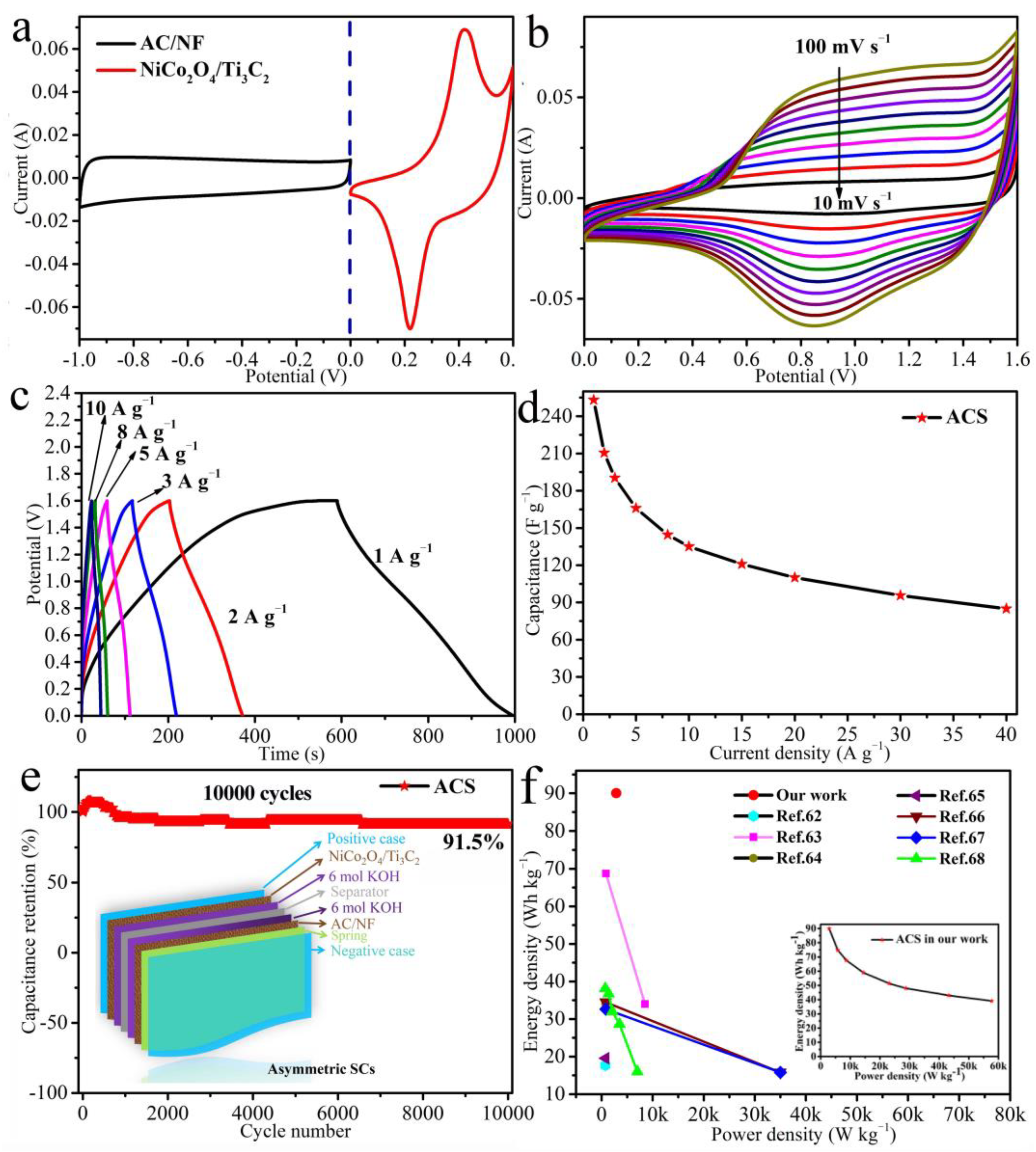
3.3. Electrochemical Performances of the ASC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sha, L.; Ye, K.; Wang, G.; Shao, J.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Hierarchical NiCo2O4 nanowire array supported on Ni foam for efficient urea electrooxidation in alkaline medium. J. Power Source 2019, 412, 265–271. [Google Scholar] [CrossRef]
- Hu, M.; Li, Z.; Hu, T.; Zhu, S.; Zhang, C.; Wang, X. High-capacitance mechanism for Ti3C2Tx MXene by in situ electrochemical Raman spectroscopy investigation. ACS Nano 2016, 10, 11344–11350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dou, H.; Wang, J.; Ding, B.; Xu, Y.; Chang, Z.; Hao, X. Three-dimensional porous MXene/layered double hydroxide composite for high performance supercapacitors. J. Power Source 2016, 327, 221–228. [Google Scholar] [CrossRef]
- Tao, B.; He, J.; Miao, F.; Zhang, Y. MnO2/NiCo2O4 loaded on nickel foam as a high-performance electrode for advanced asymmetric supercapacitor. Vacuum 2022, 195, 110668. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, Y.; Ning, J.; Hu, Y. Recent advances in the synthesis of non-carbon two-dimensional electrode materials for the aqueous electrolyte-based supercapacitors. Chin. Chem. Lett. 2021, 32, 3733–3752. [Google Scholar] [CrossRef]
- Yu, L.; Fan, Z.; Shao, Y.; Tian, Z.; Sun, J.; Liu, Z. Versatile N-doped MXene ink for printed electrochemical energy storage application. Adv. Energy Mater. 2019, 9, 1901839. [Google Scholar] [CrossRef]
- Liu, F.; Jin, S.; Xia, Q.; Zhou, A.; Fan, L. Research progress on construction and energy storage performance of MXene heterostructures. J. Energy Chem. 2021, 62, 220–242. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Hedhili, M.N.; Anjume, D.H.; Alshareef, H.N. Effect of postetch annealing gas composition on the structural and electrochemical properties of Ti2CTx MXene electrodes for supercapacitor applications. Chem. Mater. 2015, 27, 5314–5323. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile synthesis of crumpled nitrogen-doped MXene nanosheets as a new sulfur host for lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, J.; Zhang, X.; Insin, N.; Liu, R.; Qin, J. NiMn layered double hydroxide nanosheets in-situ anchored on Ti3C2 MXene via chemical bonds for superior supercapacitors. ACS Appl. Energy Mater. 2020, 3, 5949–5964. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Zalnezhad, E.; Hui, K.N.; Hui, K.S.; Ko, M.J. 3D hierarchical transition-metal sulfides deposited on MXene as binder-free electrode for high-performance supercapacitors. J. Ind. Eng. Chem. 2020, 82, 309–316. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Yuan, R.; Lu, J. Metal-organic framework derived hierarchical NiCo2O4 triangle nanosheet arrays@SiC nanowires network/carbon cloth for flexible hybrid supercapacitors. J. Mater. Sci. Technol. 2021, 81, 162–174. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Liu, A.; Ma, T. Electrostatic self-assembly of 2D delaminated MXene (Ti3C2) onto Ni foam with superior electrochemical performance for supercapacitor. Electrochim. Acta 2019, 305, 164–174. [Google Scholar] [CrossRef]
- Hu, M.; Hu, T.; Cheng, R.; Yang, J.; Cui, C.; Zhang, C.; Wang, X. MXene-coated silk-derived carbon cloth toward flexible electrode for supercapacitor application. J. Energy Chem. 2018, 27, 161–166. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, C.; Niu, D.; Zhu, J.; Wei, D.; Wang, C.; Wang, L.; Yang, L. Ultrathin N-doped Ti3C2-MXene decorated with NiCo2S4 nanosheets as advanced electrodes for supercapacitors. Appl. Surf. Sci. 2021, 539, 148272. [Google Scholar] [CrossRef]
- Wu, N.; Low, J.; Liu, T.; Yu, J.; Cao, S. Hierarchical hollow cages of Mn-Co layered double hydroxide as supercapacitor electrode materials. Appl. Surf. Sci. 2017, 413, 35–40. [Google Scholar] [CrossRef]
- Bhagwan, J.; Nagaraju, G.; Ramulu, B.; Sekhar, S.C.; Yu, J.S. Rapid synthesis of hexagonal NiCo2O4 nanostructures for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 299, 509–517. [Google Scholar] [CrossRef]
- Ma, L.; Shen, X.; Zhou, H.; Ji, Z.; Chen, K.; Zhu, G. High performance supercapacitor electrode materials based on porous NiCo2O4 hexagonal nanoplates/reduced graphene oxide composites. Chem. Eng. J. 2015, 262, 980–988. [Google Scholar] [CrossRef]
- Yu, J.; Yao, D.; Wu, Z.; Li, G.; Song, J.; Shen, H.; Yang, X.; Lei, W.; Wu, F.; Hao, Q. Construction of a high-performance three-dimensional structured NiCo2O4@PPy nanosheet array free-standing electrode for a hybrid supercapacitor. ACS Appl. Energy Mater. 2021, 4, 3093–3100. [Google Scholar] [CrossRef]
- Wei, S.; Wan, C.; Zhang, L.; Liu, X.; Tian, W.; Su, J.; Cheng, W.; Wu, Y. N-doped and oxygen vacancy-rich NiCo2O4 nanograss for supercapacitor electrode. Chem. Eng. J. 2022, 429, 132242. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, J.; Jin, J.; Yang, S.; Li, G. Flexible solid-state asymmetric supercapacitor with high energy density and ultralong lifetime based on hierarchical 3D electrode design. ACS Appl. Energy Mater. 2022, 5, 5830–5840. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Zhou, Y.; Liu, X. Design and characterization of monolayer Ti3C2 MXene/NiCo2O4 nanocones hybrid architecture for asymmetric supercapacitors. J. Electroanal. Chem. 2022, 923, 116787. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Wang, D.; Sun, M.; Sun, H. Exploration of metal/Ti3C2 MXene-derived composites as anode for high-performance zinc-ion supercapacitor. J. Power Source 2021, 506, 230197. [Google Scholar] [CrossRef]
- Oh, K.H.; Gund, G.S.; Park, H.S. Stabilizing NiCo2O4 hybrid architectures by reduced graphene oxide interlayers for improved cycling stability of hybrid supercapacitors. J. Mater. Chem. A 2018, 6, 22106–22114. [Google Scholar] [CrossRef]
- Qiu, H.; Chen, L.; Ito, Y.; Kang, J.; Guo, X.; Liu, P.; Kashani, H.; Hirata, A.; Fujita, T.; Chen, M. An ultrahigh volumetric capacitance of squeezable three-dimensional bicontinuous nanoporous graphene. Nanoscale 2016, 8, 18551–18557. [Google Scholar] [CrossRef]
- Wu, X.; Huang, B.; Wang, Q.; Wang, Y. High energy density of two-dimensional MXene/NiCo-LDHs interstratification assembly electrode: Understanding the role of interlayer ions and hydration. Chem. Eng. J. 2020, 380, 122456. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Xiang, Q.; Chen, R.; Wu, D.; Li, G.; Wang, L. A three-dimensional flower-like NiCo-layered double hydroxide grown on nickel foam with an MXene coating for enhanced oxygen evolution reaction electrocatalysis. RSC Adv. 2021, 11, 12392–12397. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Wen, Y.; Zhao, Y.; Zhang, Y.; Qin, H. Design of ZIF-67 MOF-derived Co3O4/NiCo2O4 nanosheets for supercapacitor electrode materials. J. Chem. Res. 2021, 45, 983–991. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, G.; Yuan, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar] [CrossRef]
- Sivakumar, P.; Vikraman, D.; Raj, C.J.; Hussain, S.; Park, J.; Kim, H.S.; Jung, H. Hierarchical NiCo/NiO/NiCo2O4 composite formation by solvothermal reaction as a potential electrode material for hydrogen evolutions and asymmetric supercapacitors. Int. J. Energy Res. 2021, 45, 19947–19961. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, J.; Liu, A.; Ma, T. 2D heterostructure comprised of Ni3S2/d-Ti3C2 supported on Ni foam as binder-free electrode for hybrid supercapacitor. J. Alloys Compd. 2020, 814, 152271. [Google Scholar] [CrossRef]
- Chang, B.; Guo, Y.; Wu, D.; Li, L.; Yang, B.; Wang, J. Plasmon-enabled N2 photofixation on partially reduced Ti3C2 MXene. Chem. Sci. 2021, 12, 11213–11224. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Feng, Y.; Peng, T.; Yuan, B. Sea urchin-shaped Fe2O3 coupled with 2D MXene nanosheets as negative electrode for high-performance asymmetric supercapacitors. Electrochim. Acta 2021, 381, 138245. [Google Scholar] [CrossRef]
- Wei, W.; Mi, L.; Cui, S.; Wang, B.; Chen, W. Carambola-like Ni@Ni1.5Co1.5S2 for use in high-performance supercapacitor devices design. ACS Sustain. Chem. Eng. 2015, 3, 2777–2785. [Google Scholar] [CrossRef]
- BoopathiRaja, R.; Parthibavarman, M. Desert rose like heterostructure of NiCo2O4/NF@PPy composite has high stability and excellent electrochemical performance for asymmetric super capacitor application. Electrochim. Acta 2020, 346, 136270. [Google Scholar] [CrossRef]
- Kumar, L.; Chauhan, H.; Yadav, N.; Hashmi, S.A.; Deka, S. Faster ion switching NiCo2O4 nanoparticle electrode-based supercapacitor device with high performances and long cycling stability. ACS Appl. Energy Mater. 2018, 1, 6999–7006. [Google Scholar] [CrossRef]
- Wang, S.; Zou, Y.; Xu, F.; Xiang, C.; Peng, H.; Zhang, J.; Sun, L. Morphological control and electrochemical performance of NiCo2O4@NiCo layered double hydroxide as an electrode for supercapacitors. J. Energy Storage 2021, 41, 102862. [Google Scholar] [CrossRef]
- Ma, Y.; Sheng, H.; Dou, W.; Su, Q.; Zhou, J.; Xie, E.; Lan, W. Fe2O3 nanoparticles anchored on the Ti3C2Tx MXene paper for flexible supercapacitors with ultrahigh volumetric capacitance. ACS Appl. Mater. Interfaces 2020, 12, 41410–41418. [Google Scholar] [CrossRef]
- Li, G.; Cai, H.; Li, X.; Zhang, J.; Zhang, D.; Yang, Y.; Xiong, J. Construction of hierarchical NiCo2O4@Ni-MOF hybrid arrays on carbon cloth as superior battery-type electrodes for flexible solid-state hybrid supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 37675–37684. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Xia, Q.; Yang, M.; Xia, H. Unique core–shell nanorod arrays with polyaniline deposited into mesoporous NiCo2O4 support for high-performance supercapacitor electrodes. ACS Appl. Mater. Interfaces 2019, 8, 6093–6100. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W. Hierarchical nanoflake-assembled flower-like NiCo double hydroxide@NiC2O4 microspheres for high-performance supercapacitor. Mater. Technol. 2019, 34, 571–580. [Google Scholar] [CrossRef]
- Dubal, D.P.; Abdel-Azeim, S.; Chodankar, N.R.; Han, Y.K. Molybdenum nitride nanocrystals anchored on phosphorus-incorporated carbon fabric as a negative electrode for high-performance asymmetric pseudocapacitor. iScience 2019, 16, 50–62. [Google Scholar] [CrossRef]
- Patil, A.M.; Yue, X.; Yoshida, A.; Li, S.; Hao, X.; Abudula, A.; Guan, G. Redox-ambitious route to boost energy and capacity retention of pouch type asymmetric solid-state supercapacitor fabricated with graphene oxide-based battery-type electrodes. Appl. Mater. Today 2020, 19, 100563. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Ruan, C.; Zhu, D.; Qi, J.; Meng, Q.; Wei, F.; Ren, Y.; Sui, Y.; Zhang, H. MXene-modulated CoNi2S4 dendrite as enhanced electrode for hybrid supercapacitors. Surf. Interfaces 2021, 25, 101274. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Cho, Y.R.; Yun, J.M.; Jung, Y.S.; Kwon, S.H.; Kim, K.H. Hierarchically layered nanocomposite electrodes formed by spray-injected MXene nanosheets for ultrahigh-performance flexible supercapacitors. Appl. Surf. Sci. 2021, 549, 149226. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, C.; Zhu, J.; Niu, D.; Wei, D.; Wang, C.; Wang, F.; Wang, L. Hierarchical materials constructed by 1D hollow nickel–cobalt sulfide nanotubes supported on 2D ultrathin MXenes nanosheets for high-performance supercapacitor. Ceram. Int. 2020, 46, 12200–12208. [Google Scholar] [CrossRef]
- Chen, J.; Ren, Y.; Zhang, H.; Qi, J.; Sui, Y.; Wei, F. Ni-Co-Fe layered double hydroxide coated on Ti3C2 MXene for high-performance asymmetric supercapacitor. Appl. Surf. Sci. 2021, 562, 150116. [Google Scholar] [CrossRef]
- Patil, A.M.; Kitiphatpiboon, N.; An, X.; Hao, X.; Li, S.; Hao, X.; Abudula, A.; Guan, G. Fabrication of a high-energy flexible all-solid-state supercapacitor using pseudocapacitive 2D-Ti3C2Tx-MXene and battery-type reduced graphene oxide/nickel–cobalt bimetal oxide electrode materials. ACS Appl. Mater. Interfaces 2020, 12, 52749–52762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ouyang, T.; Xiang, K.; Chen, J.; Zhang, Q.; Yi, Q.; Zhou, X.; Chen, H.; Zhang, X. Ni2CoS4 nanocubes anchored on nitrogen-doped ultra-thin hollow carbon spheres to achieve high-performance supercapacitor. Ionics 2022, 28, 415–422. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Demir, M.; Hu, X.; Liu, S.; Wang, L. Water chestnut shell-derived N/S-doped porous carbons and their applications in CO2 adsorption and supercapacitor. Fuel 2022, 326, 125119. [Google Scholar] [CrossRef]
- Jiao, Z.; Chen, Y.; Demir, M.; Du, M.; Gu, M.; Wang, C.; Zhang, X.; Deng, Y.; Wang, Z.; Wang, T.; et al. Vanadium-doped Co0.85Se nanowire arrays with high areal capacitance for hybrid supercapacitor electrodes. J Energy Storage 2022, 52, 104929. [Google Scholar] [CrossRef]
- Lei, N.; Qiao, Y.; Liu, G.; Xu, R.; Jiang, G.; Demir, m.; Ma, P. MnO2 modified perovskite oxide SrCo0.875Nb0.125O3 as supercapacitor electrode material. Mater. Chem. Phys. 2022, 288, 126389. [Google Scholar] [CrossRef]
- Xu, Z.; Younis, A.; Xu, H.; Li, S.; Chu, D. Improved super-capacitive performance of carbon foam supported CeOx nanoflowers by selective doping and UV irradiation. RSC Adv. 2014, 4, 35067–35071. [Google Scholar] [CrossRef]
- Xu, Z.; Younis, A.; Chu, D.; Ao, Z.; Xu, H.; Li, S. Electrodeposition of mesoporous Co3O4 nanosheets on carbon foam for high performance supercapacitors. J. Nanomater. 2014, 2014, 902730. [Google Scholar] [CrossRef]
- Liu, L.; Guan, T.; Fang, L.; Wu, F.; Lu, Y.; Luo, H.; Song, X.; Zhou, M.; Hu, B.; Wei, D.; et al. Self-supported 3D NiCo-LDH/Gr composite nanosheets array electrode for high-performance supercapacitor. J. Alloys Compd. 2018, 763, 926–934. [Google Scholar] [CrossRef]
- Luo, W.; Zeng, W.; Quan, H.; Pan, M.; Wang, Y.; Chen, D. Carbon dots decorated NiCo hydroxycarbonate hierarchical nanoarrays on carbon cloth with high areal capacitance as pseudocapacitor electrode. J. Alloys Compd. 2021, 868, 159048. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, J.; Yao, P.; Huang, J.; Sun, W.; Zhu, C.; Xu, J. A stretchable high performance all-in-one fiber supercapacitor. J. Power Source 2019, 440, 227150. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Guan, C.; Elshahawy, A.M.; Dong, Y.; Pennycook, S.J.; Wang, J. (Ni,Co)Se2/NiCo-LDH core/shell structural electrode with the cactus-like (Ni,Co)Se2 core for asymmetric supercapacitors. Small 2019, 15, 1803895. [Google Scholar]
- Liu, H.; Hu, R.; Qi, J.; Sui, Y.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Zhao, Y. A facile method for synthesizing NiS nanoflower grown on MXene (Ti3C2Tx) as positive electrodes for “supercapattery”. Electrochim. Acta 2020, 353, 136526. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Yun, J.M.; Lee, D.; Ryu, B.K.; Kim, K.H. Two-dimensional titanium carbide (MXene)-wrapped sisal-like NiCo2S4 as positive electrode for high-performance hybrid pouch-type asymmetric supercapacitor. Chem. Eng. J. 2019, 375, 121939. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Xu, M.; Wang, M.; Yang, F.; Wu, N.; Zhang, T.; Sun, L.; Yang, W. Zeolite-imidazole framework derived nickel-cobalt hydroxide on ultrathin MXene nanosheets for long life and high performance supercapacitance. J. Alloys Compd. 2022, 888, 161250. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Z.; Lu, B. Ultrafine Au nanoparticles decorated NiCo2O4 nanotubes as anode material for high-performance supercapacitor and lithium-ion battery applications. Nano Energy 2014, 7, 114–123. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Vinodgopal, K.; Neppolian, B. Development of nanohybrids based on porous spinel MCo2O4 (M = Zn, Cu, Ni and Mn)/reduced graphene oxide/carbon nanotube as promising electrodes for high performance energy storage devices. Appl. Surf. Sci. 2020, 513, 145781. [Google Scholar] [CrossRef]
- Wang, N.; Sun, B.; Zhao, P.; Yao, M.; Hu, W.; Komarneni, S. Electrodeposition preparation of NiCo2O4 mesoporous film on ultrafine nickel wire for flexible asymmetric supercapacitors. Chem. Eng. J. 2018, 345, 31–38. [Google Scholar] [CrossRef]
- Gong, J.; Yang, J.; Wang, J.; Lv, L.; Wang, W.; Pu, L.; Zhang, H.; Dai, Y. A dual NiCo metal-organic frameworks derived NiCo2S4 core-shell nanorod arrays as high-performance electrodes for asymmetric supercapacitors. Electrochim. Acta 2021, 374, 137794. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, S.; Ni, G.; Li, Q. Facile Synthesis of NiCo2O4 Nanowire Arrays/Few-Layered Ti3C2-MXene Composite as Binder-Free Electrode for High-Performance Supercapacitors. Molecules 2022, 27, 6452. https://doi.org/10.3390/molecules27196452
Li Y, Wang S, Ni G, Li Q. Facile Synthesis of NiCo2O4 Nanowire Arrays/Few-Layered Ti3C2-MXene Composite as Binder-Free Electrode for High-Performance Supercapacitors. Molecules. 2022; 27(19):6452. https://doi.org/10.3390/molecules27196452
Chicago/Turabian StyleLi, Yanhua, Shuhuan Wang, Guolong Ni, and Qun Li. 2022. "Facile Synthesis of NiCo2O4 Nanowire Arrays/Few-Layered Ti3C2-MXene Composite as Binder-Free Electrode for High-Performance Supercapacitors" Molecules 27, no. 19: 6452. https://doi.org/10.3390/molecules27196452
APA StyleLi, Y., Wang, S., Ni, G., & Li, Q. (2022). Facile Synthesis of NiCo2O4 Nanowire Arrays/Few-Layered Ti3C2-MXene Composite as Binder-Free Electrode for High-Performance Supercapacitors. Molecules, 27(19), 6452. https://doi.org/10.3390/molecules27196452




