Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces
Abstract
:1. Introduction
2. Experimental
2.1. Coating Preparation
2.2. Coating Characterization
2.3. Tensile Adhesion Tests
2.4. Surface Roughness and Wettability
2.5. Electrochemical Measurements
3. Results and Discussion
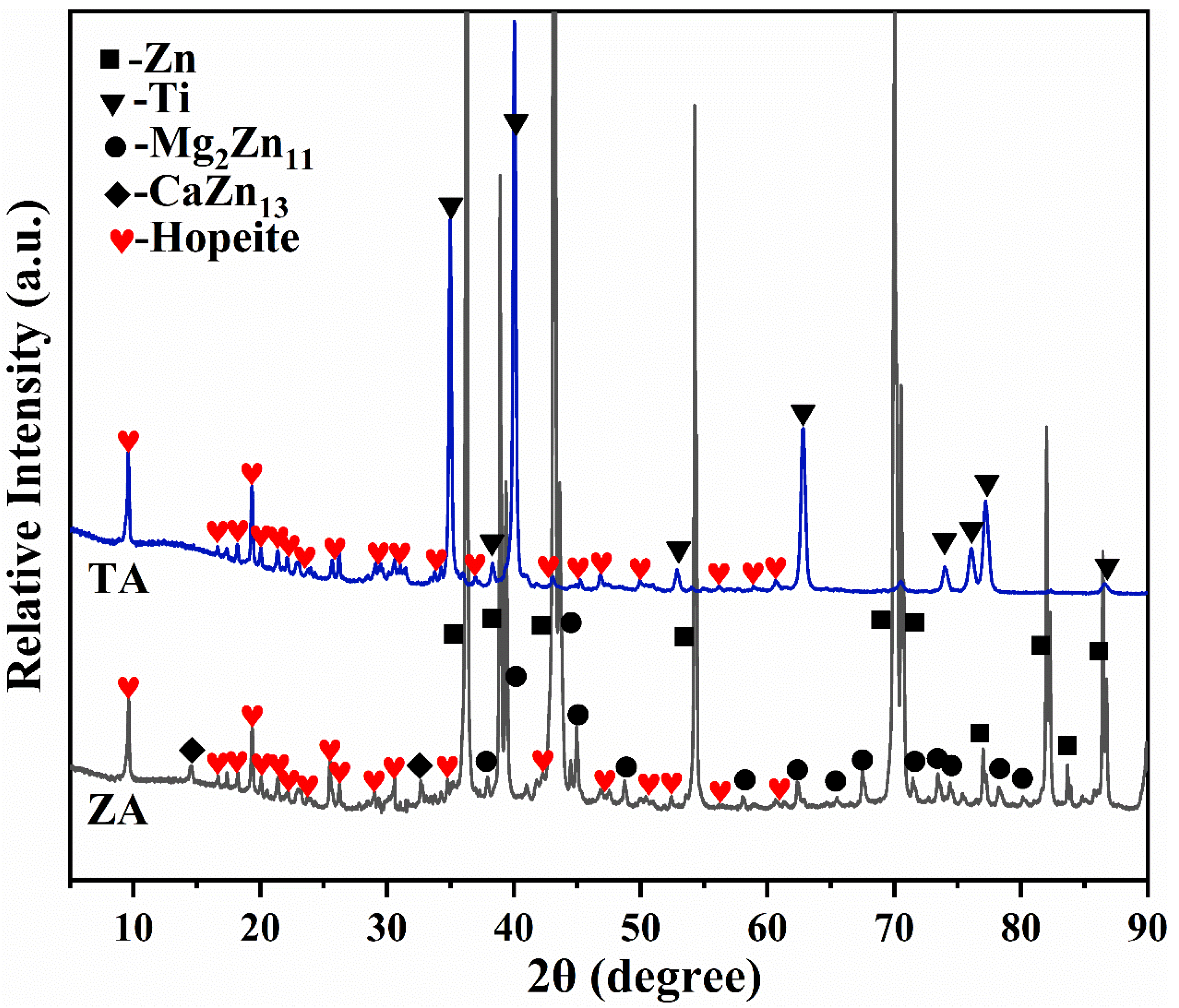
3.1. Phase Composition
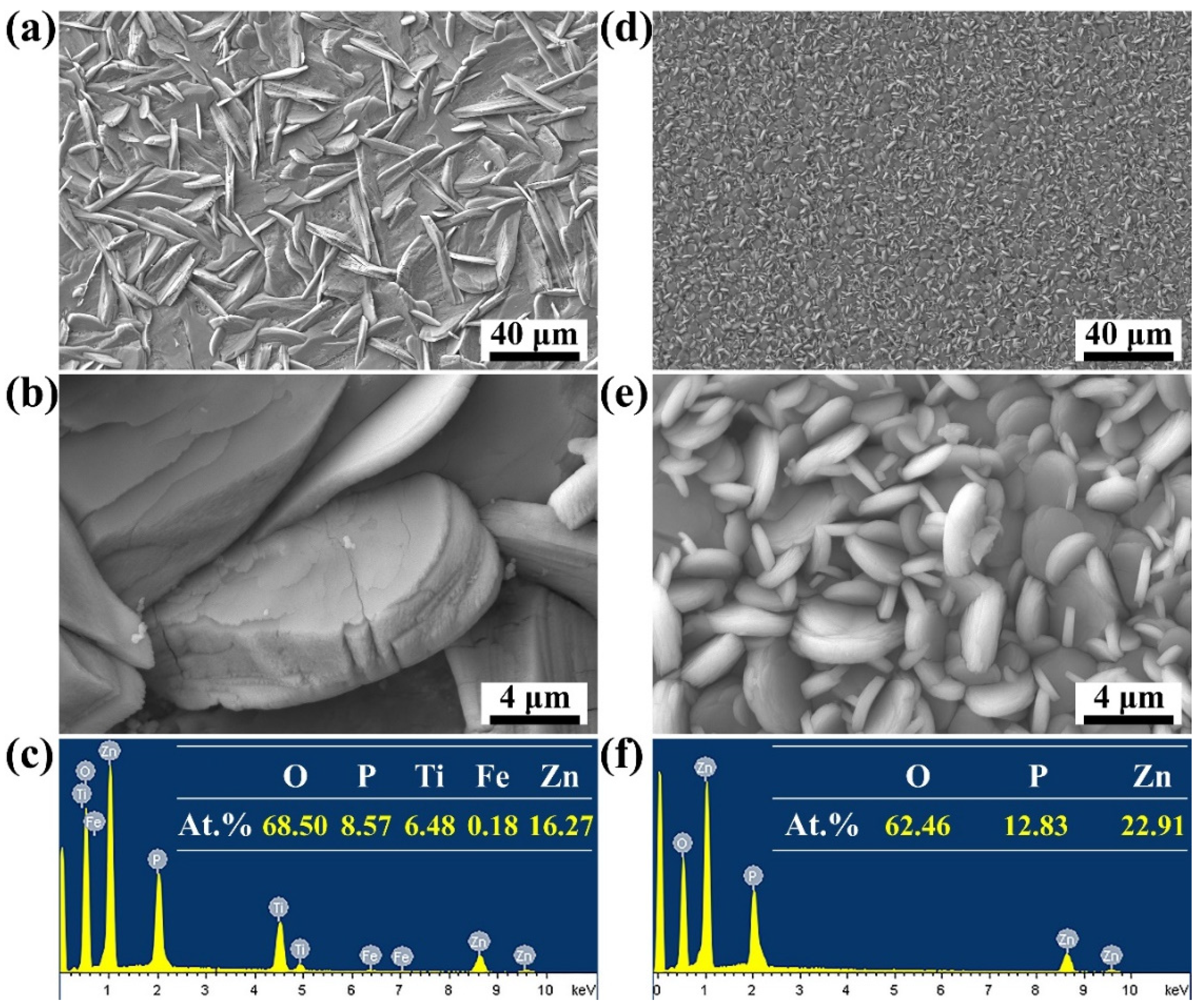
3.2. Microstructure of the Coatings
3.3. Coating Thickness and Bonding Strength
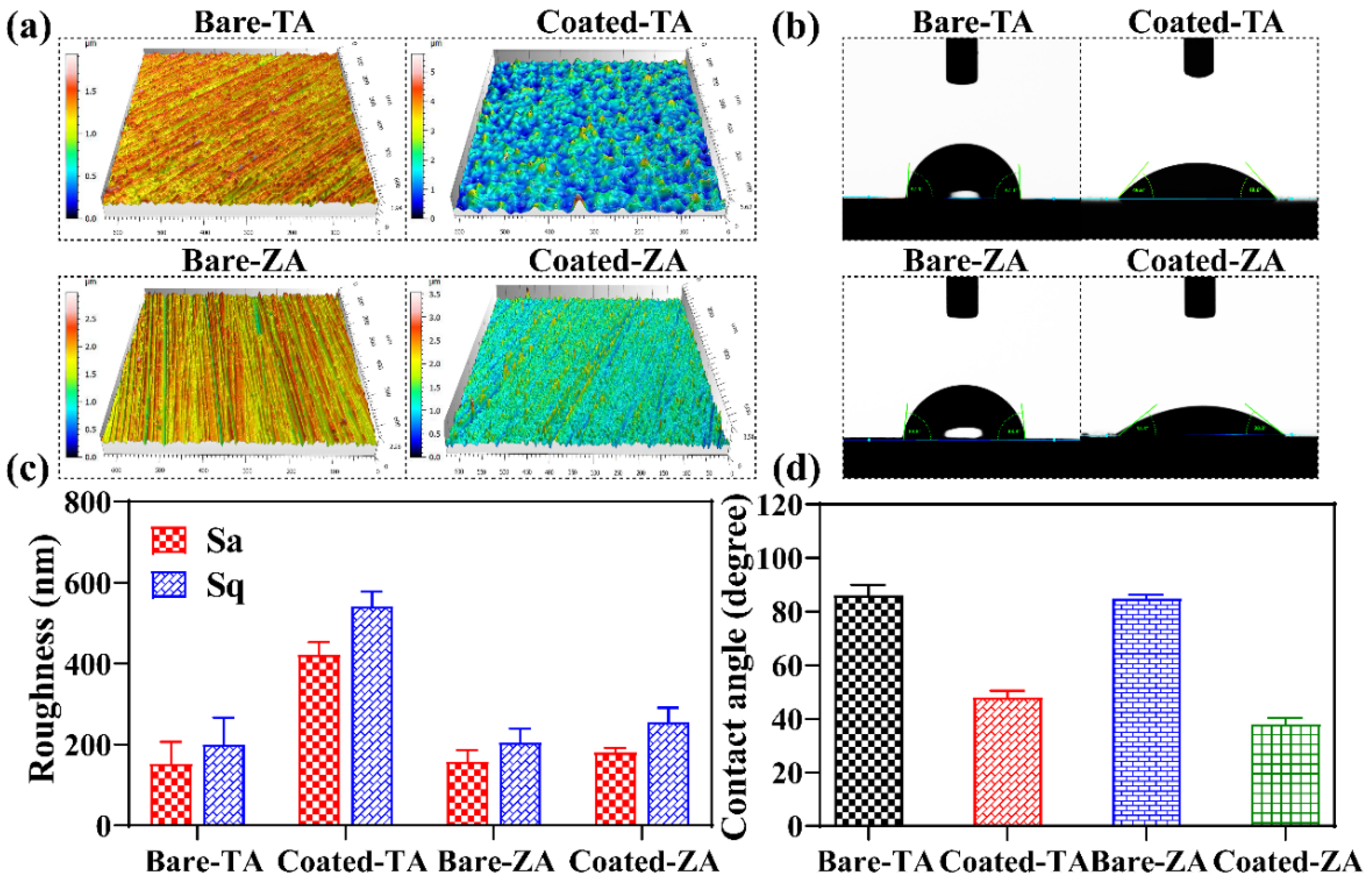
3.4. Surface Roughness and Wettability of the Coatings
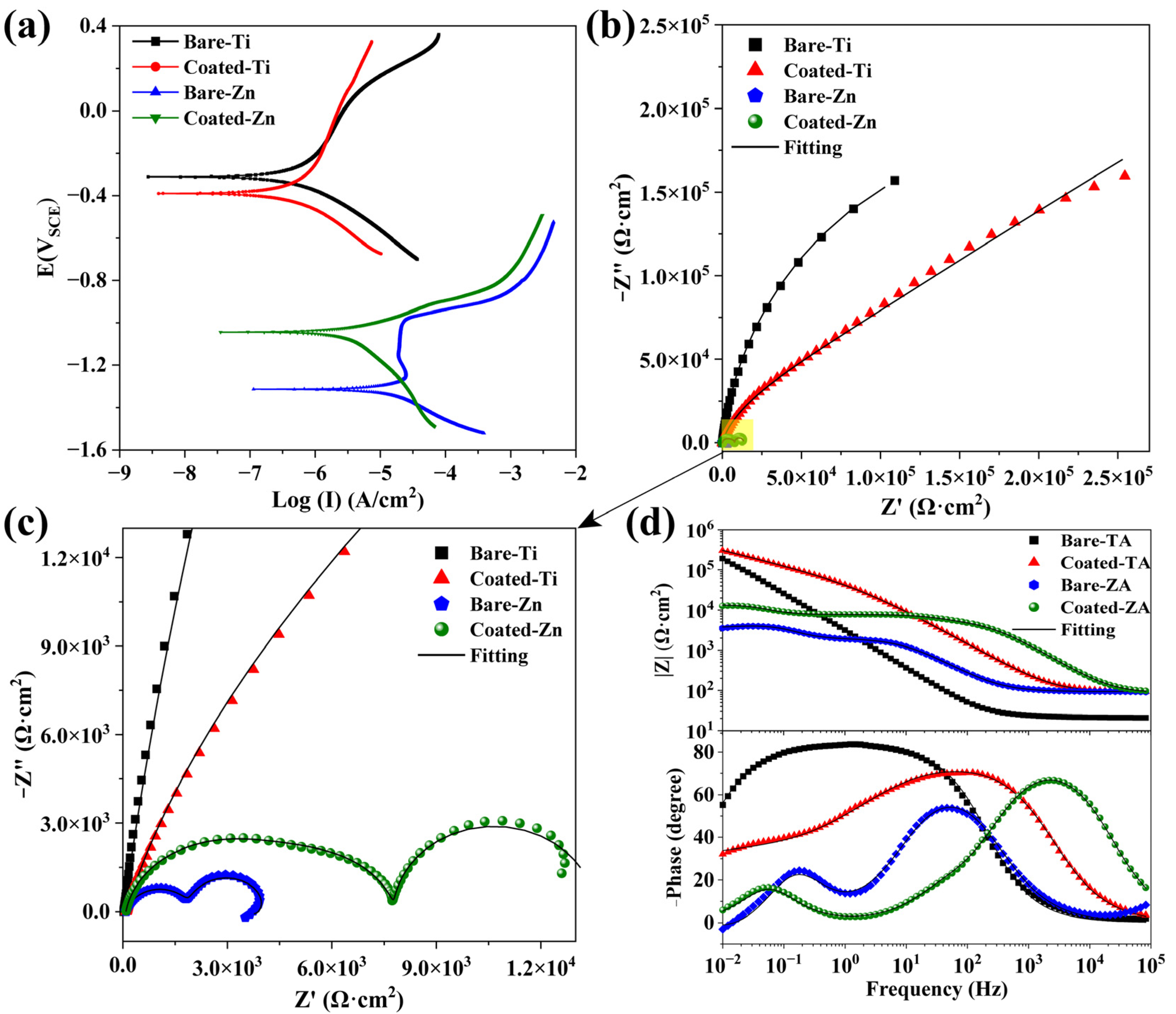
3.5. Corrosion Resistance
3.6. Formation Mechanism of the PCC Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, Y.; Li, W.L.; Zhang, C.; Wu, Z.Y.; Liu, J. Recent Developments of Biomaterials for Additive Manufacturing of Bone Scaffolds. Adv. Healthc. Mater. 2020, 9, 2000724. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiyan, S.; Khalil-Allafi, J.; Etminanfar, M.R.; Safavi, M.S.; Hosseini, M. Antibacterial activity and biocompatibility of Ag-coated Ti implants: Importance of surface modification parameters. Trans. IMF 2022, 100, 93–102. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, G.C.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Su, Y.C.; Yang, H.T.; Gao, J.L.; Qin, Y.X.; Zheng, Y.F.; Zhu, D.H. Interfacial Zinc Phosphate is the Key to Controlling Biocompatibility of Metallic Zinc Implants. Adv. Sci. 2019, 6, 1900112. [Google Scholar] [CrossRef]
- Yuan, Z.; He, Y.; Lin, C.A.C.A.; Liu, P.; Cai, K.Y. Antibacterial surface design of biomedical titanium materials for orthopedic applications. J. Mater. Sci. Technol. 2021, 78, 51–67. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Kasperkiewicz, K.; Major, R.; Sypien, A.; Kot, M.; Dyner, M.; Major, L.; Byrski, A.; Kopernik, M.; Lackner, J.M. Antibacterial Optimization of Highly Deformed Titanium Alloys for Spinal Implants. Molecules 2021, 26, 3145. [Google Scholar] [CrossRef]
- Hu, J.W.; Ding, Y.; Tao, B.L.; Yuan, Z.; Yang, Y.L.; Xu, K.; Li, X.; Liu, P.; Cai, K.Y. Surface modification of titanium substrate via combining photothermal therapy and quorum-sensing-inhibition strategy for improving osseointegration and treating biofilm-associated bacterial infection. Bioact. Mater. 2022, 18, 228–241. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Jia, B.; Yan, H.T.; Han, Y.; Wu, Q.; Dai, K.R.; Zheng, Y.F. Biodegradable ZnLiCa ternary alloys for critical-sized bone defect regeneration at load-bearing sites: In vitro and in vivo studies. Bioact. Mater. 2021, 6, 3999–4013. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y.C. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mintegui, C.; Cordoba, L.C.; Buxadera-Palomero, J.; Marquina, A.; Jimenez-Pique, E.; Ginebra, M.P.; Cortina, J.L.; Pegueroles, M. Zn-Mg and Zn-Cu alloys for stenting applications: From nanoscale mechanical characterization to in vitro degradation and biocompatibility. Bioact. Mater. 2021, 6, 4430–4446. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.J.; Liu, T.T.; Zhang, Z.Q.; Zhang, X.L.; Qiao, X.L.; Hu, Y.D.; Zhang, Q.Y.; Yang, Z.M. Preparation of chitosan-Zn2+ complex coating on polydopamine-modified TiO2 nanotubes to enhance osteoblast behaviors. Mater. Lett. 2021, 282, 128665. [Google Scholar] [CrossRef]
- Yang, H.T.; Qu, X.H.; Lin, W.J.; Chen, D.F.; Zhu, D.H.; Dai, K.R.; Zheng, Y.F. Enhanced Osseointegration of Zn-Mg Composites by Tuning the Release of Zn Ions with Sacrificial Mg-Rich Anode Design. ACS Biomater. Sci. Eng. 2019, 5, 453–467. [Google Scholar] [CrossRef]
- Su, Y.C.; Wang, K.; Gao, J.L.; Yang, Y.; Qin, Y.X.; Zheng, Y.F.; Zhu, D.H. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Savignac, P.; Menu, M.J.; Gressier, M.; Denat, B.; El Khadir, Y.; Manov, S.; Ansart, F. Improvement of Adhesion Properties and Corrosion Resistance of Sol-Gel Coating on Zinc. Molecules 2018, 23, 1079. [Google Scholar] [CrossRef]
- Zhao, D.W.; Liu, C.; Zuo, K.Q.; Su, P.; Li, L.B.; Xiao, G.Y.; Cheng, L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chem. Eng. J. 2021, 408, 127362. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.Y.; Liu, B.; Jiang, C.C.; Lu, Y.P. Influence of processing time on the phase, microstructure and electrochemical properties of hopeite coating on stainless steel by chemical conversion method. New J. Chem. 2015, 39, 5813–5822. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An innovative specimen configuration for the study of Mg corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- Vida, T.A.; Silva, C.A.P.; Lima, T.S.; Cheung, N.; Brito, C.; Garcia, A. Tailoring microstructure and microhardness of Zn-1wt.%Mg-(0.5wt.%Mn, 0.5wt.%Ca) alloys by solidification cooling rate. Trans. Nonferr. Metal. Soc. 2021, 31, 1031–1048. [Google Scholar] [CrossRef]
- Du, C.M.; Zuo, K.Q.; Wang, X.Y.; Huang, S.Y.; Liu, B.; Xiao, G.Y.; Lu, Y.P. Effect of reaction time on the microstructure and properties of in-situ hopeite chemical conversion coatings formed by self-corrosion on zinc alloy. J. Mater. Res. Technol. 2022, 18, 4445–4455. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.Y.; Chen, C.Z.; Lu, Y.P.; Geng, X.W. Hopeite and scholzite coatings formation on titanium via wet-chemical conversion with controlled temperature. Surf. Coat. Technol. 2020, 384, 125330. [Google Scholar] [CrossRef]
- Yao, J.H.; Wang, Y.; Wu, G.L.; Sun, M.; Wang, M.; Zhang, Q.L. Growth characteristics and properties of micro-arc oxidation coating on SLM-produced TC4 alloy for biomedical applications. Appl. Surf. Sci. 2019, 479, 727–737. [Google Scholar] [CrossRef]
- Zuo, K.Q.; Wang, L.L.; Wang, Z.H.; Yin, Y.X.; Du, C.M.; Liu, B.; Sun, L.Y.; Li, X.Y.; Xiao, G.Y.; Lu, Y.P. Zinc-Doping Induces Evolution of Biocompatible Strontium-Calcium-Phosphate Conversion Coating on Titanium to Improve Antibacterial Property. ACS Appl. Mater. Interfaces 2022, 14, 7690–7705. [Google Scholar] [CrossRef]
- Cai, S.X.; Wu, C.X.; Yang, W.G.; Liang, W.F.; Yu, H.B.; Liu, L.Q. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Lu, A.G.; Gao, Y.; Jin, T.; Luo, X.C.; Zeng, Q.R.; Shang, Z.T. Effects of surface roughness and texture on the bacterial adhesion on the bearing surface of bio-ceramic joint implants: An in vitro study. Ceram. Int. 2020, 46, 6550–6559. [Google Scholar] [CrossRef]
- Gu, Z.; Mao, P.; Gou, Y.F.; Chao, Y.; Xi, S.Q. Microstructure and properties of MgMoNbFeTi2Yx high entropy alloy coatings by laser cladding. Surf. Coat. Tech. 2020, 402, 126303. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.d.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, D.; Liang, L.X.; Cheng, S.C.; Cui, L.Y.; Li, S.Q.; Wang, Z.L.; Zeng, R.C. Corrosion resistance of Ca-P coating induced by layer-by-layer assembled polyvinylpyrrolidone/DNA multilayer on magnesium AZ31 alloy. Front. Mater. Sci. 2021, 15, 391–405. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chen, C.T.; Jiu, J.T.; Nagao, S.; Nogi, M.; Koga, H.; Zhang, H.; Zhang, G.; Suganuma, K. Electrochemical behavior of Zn-xSn high-temperature solder alloys in 0.5 M NaCl solution. J. Alloys Compd. 2017, 716, 231–239. [Google Scholar] [CrossRef]
- Yigit, O.; Ozdemir, N.; Dikici, B.; Kaseem, M. Surface Properties of Graphene Functionalized TiO2/nHA Hybrid Coatings Made on Ti6Al7Nb Alloys via Plasma Electrolytic Oxidation (PEO). Molecules 2021, 26, 3903. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Li, J.; Li, Q.Y.; Zhou, H.L.; Wang, G.M.; Peng, X.; Jin, W.H.; Yu, Z.T.; Chu, P.K.; Li, W. Titania-zinc phosphate/nanocrystalline zinc composite coatings for corrosion protection of biomedical WE43 magnesium alloy. Surf. Coat. Technol. 2021, 410, 126940. [Google Scholar] [CrossRef]
- Shishesaz, M.R.; Ghobadi, M.; Asadi, N.; Zarezadeh, A.; Saebnoori, E.; Amraei, H.; Schubert, J.; Chocholaty, O. Surface Pretreatments of AA5083 Aluminum Alloy with Enhanced Corrosion Protection for Cerium-Based Conversion Coatings Application: Combined Experimental and Computational Analysis. Molecules 2021, 26, 7413. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.Q.; Gong, Z.Y.; Xiao, G.Y.; Huang, S.Y.; Du, C.M.; Liu, B.; Zhang, D.S.; Lu, Y.P. Microstructural evolution of strontium-zinc-phosphate coating on titanium via changing Zn2+ concentration in phosphate solution for enhanced osteogenic activity. Surf. Coat. Technol. 2022, 433, 128143. [Google Scholar] [CrossRef]
- Qian, J.Y.; Chen, Y.Q.; Zhang, W.T.; Mo, X.S.; Zou, D.; Soliman, H.; Zhou, C.; Huang, N.; Sang, H.X.; Zeng, H.; et al. Micro/Nano-Structured Metal-Organic/Inorganic Hybrid Coatings on Biodegradable Zn for Osteogenic and Biocompatible Improvement. Adv. Mater. Interfaces 2022, 9, 2101852. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, I.; Sadrnezhaad, S.K. Hydroxyapatite based and anodic Titania nanotube biocomposite coatings: Fabrication, characterization and electrochemical behavior. Surf. Coat. Technol. 2016, 287, 67–75. [Google Scholar] [CrossRef]
- Duarte, L.T.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Preparation and characterization of biomimetically and electrochemically deposited hydroxyapatite coatings on micro-arc oxidized Ti-13Nb-13Zr. J. Mater. Sci.-Mater. Med. 2011, 22, 1663–1670. [Google Scholar] [CrossRef]
- Chen, L.Y.; Ma, X.M.; Ma, Z.; Lu, D.Z.; Hou, B.R. Study on microstructure and corrosion resistance of high-hardness Zn-1.0Cu-1.0Ti alloy. Mater. Charact. 2021, 178, 111283. [Google Scholar] [CrossRef]
- Kaabi Falahieh Asl, S.; Nemeth, S.; Tan, M.J. Mechanism of calcium phosphate deposition in a hydrothermal coating process. Surf. Coat. Technol. 2015, 270, 197–205. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.Y.; Jiang, C.C.; Zheng, Y.Z.; Wang, L.L.; Lu, Y.P. Formation initiation and structural changes of phosphate conversion coating on titanium induced by galvanic coupling and Fe2+ ions. RSC Adv. 2016, 6, 75365–75375. [Google Scholar] [CrossRef]


| Coating Type | Bath Composition | Concentration | Conditions |
|---|---|---|---|
| Phosphating | ZnO | 25 g/L | pH = 2.50 T = 25 °C t = 30 min |
| HNO3 | 30 mL/L | ||
| H3PO4 | 10 mL/L | ||
| NaClO3 | 2 g/L | ||
| Ca(NO3)2·4H2O | 5 g/L | ||
| C6H8O7·H2O | 5 g/L |
| Sample | Ecorr (V) | Icorr (×10−6, A/cm2) | βa (V·dec−1) | −βc (V·dec−1) | Rp (KΩ·cm2) | Pi (mm/Year) |
|---|---|---|---|---|---|---|
| Bare-TA | −0.29 ± 0.02 | 0.50 ± 0.04 | 0.24 ± 0.01 | 0.15 ± 0.01 | 83.33 ± 8.32 | 0.012 ± 0.003 |
| Coated-TA | −0.36 ± 0.02 | 0.41 ± 0.08 | 0.23 ± 0.03 | 0.16 ± 0.01 | 101.97 ± 24.81 | 0.009 ± 0.002 |
| Bare-ZA | −1.31 ± 0.01 | 43.46 ± 5.94 | 0.99 ± 0.17 | 0.19 ± 0.02 | 1.59 ± 0.13 | 0.993 ± 0.111 |
| Coated-ZA | −1.05 ± 0.02 | 4.64 ± 0.31 | 0.11 ± 0.01 | 0.28 ± 0.02 | 7.36 ± 0.88 | 0.106 ± 0.006 |
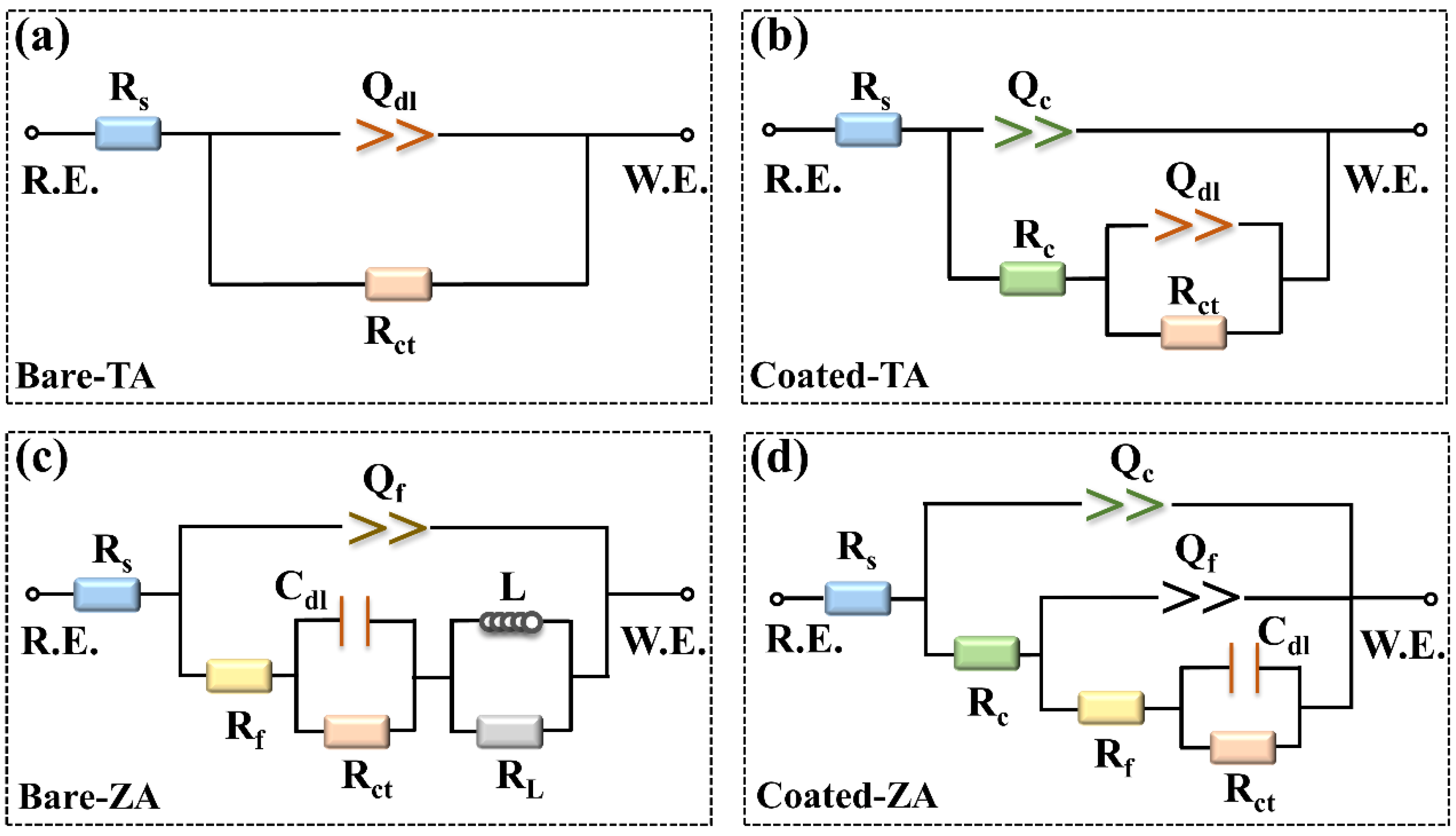
| Samples | Bare-TA | Coated-TA | Bare-ZA | Coated-ZA |
|---|---|---|---|---|
| Rs (Ω·cm2) | 83.19 ± 2.91 | 92.15 ± 3.51 | 94.20 ± 1.27 | 85.78 ± 2.50 |
| Qc (×10−6 Ω−1·cm−2·S−n) | - | 3.13 ± 0.11 | - | 0.31 ± 0.03 |
| nc | - | 0.88 ± 0.03 | - | 0.90 ± 0.01 |
| Rc (KΩ·cm2) | - | 35.68 ± 5.03 | - | 4.84 ± 0.62 |
| Qdl (×10−5 Ω−1·cm−2·S−n) | 6.01 ± 0.19 | 0.95 ± 0.02 | - | - |
| ndl | 0.92 ± 0.01 | 0.43 ± 0.05 | - | - |
| Qf (×10−5 Ω−1·cm−2·S−n) | - | - | 1.69 ± 0.02 | 0.47 ± 0.07 |
| nf | - | - | 0.87 ± 0.02 | 0.80 ± 0.07 |
| Rf (KΩ·cm2) | - | - | 1.02 ± 0.17 | 2.96 ± 0.67 |
| Cdl (10−4 F·cm−2) | - | - | 5.09 ± 1.42 | 5.93 ± 0.65 |
| Rct (KΩ·cm2) | 422.80 ± 30.97 | 4298.00 ± 9.90 | 2.30 ± 0.08 | 6.22 ± 0.71 |
| L (×103 H·cm−2) | - | - | 6.59 ± 0.46 | - |
| RL (KΩ·cm2) | - | - | 0.81 ± 0.08 | - |
| χ2 (10−3) | 1.00 ± 0.02 | 0.29 ± 0.04 | 1.17 ± 0.30 | 0.18 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Zuo, K.; Ma, Z.; Zhao, M.; Li, Y.; Tian, S.; Lu, Y.; Xiao, G. Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces. Molecules 2022, 27, 6434. https://doi.org/10.3390/molecules27196434
Du C, Zuo K, Ma Z, Zhao M, Li Y, Tian S, Lu Y, Xiao G. Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces. Molecules. 2022; 27(19):6434. https://doi.org/10.3390/molecules27196434
Chicago/Turabian StyleDu, Chunmiao, Kangqing Zuo, Zongliang Ma, Minru Zhao, Yibo Li, Shuai Tian, Yupeng Lu, and Guiyong Xiao. 2022. "Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces" Molecules 27, no. 19: 6434. https://doi.org/10.3390/molecules27196434
APA StyleDu, C., Zuo, K., Ma, Z., Zhao, M., Li, Y., Tian, S., Lu, Y., & Xiao, G. (2022). Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces. Molecules, 27(19), 6434. https://doi.org/10.3390/molecules27196434






