High Endogenously Synthesized N-3 Polyunsaturated Fatty Acids in Fat-1 Mice Attenuate High-Fat Diet-Induced Insulin Resistance by Inhibiting NLRP3 Inflammasome Activation via Akt/GSK-3β/TXNIP Pathway
Abstract
1. Introduction
2. Results
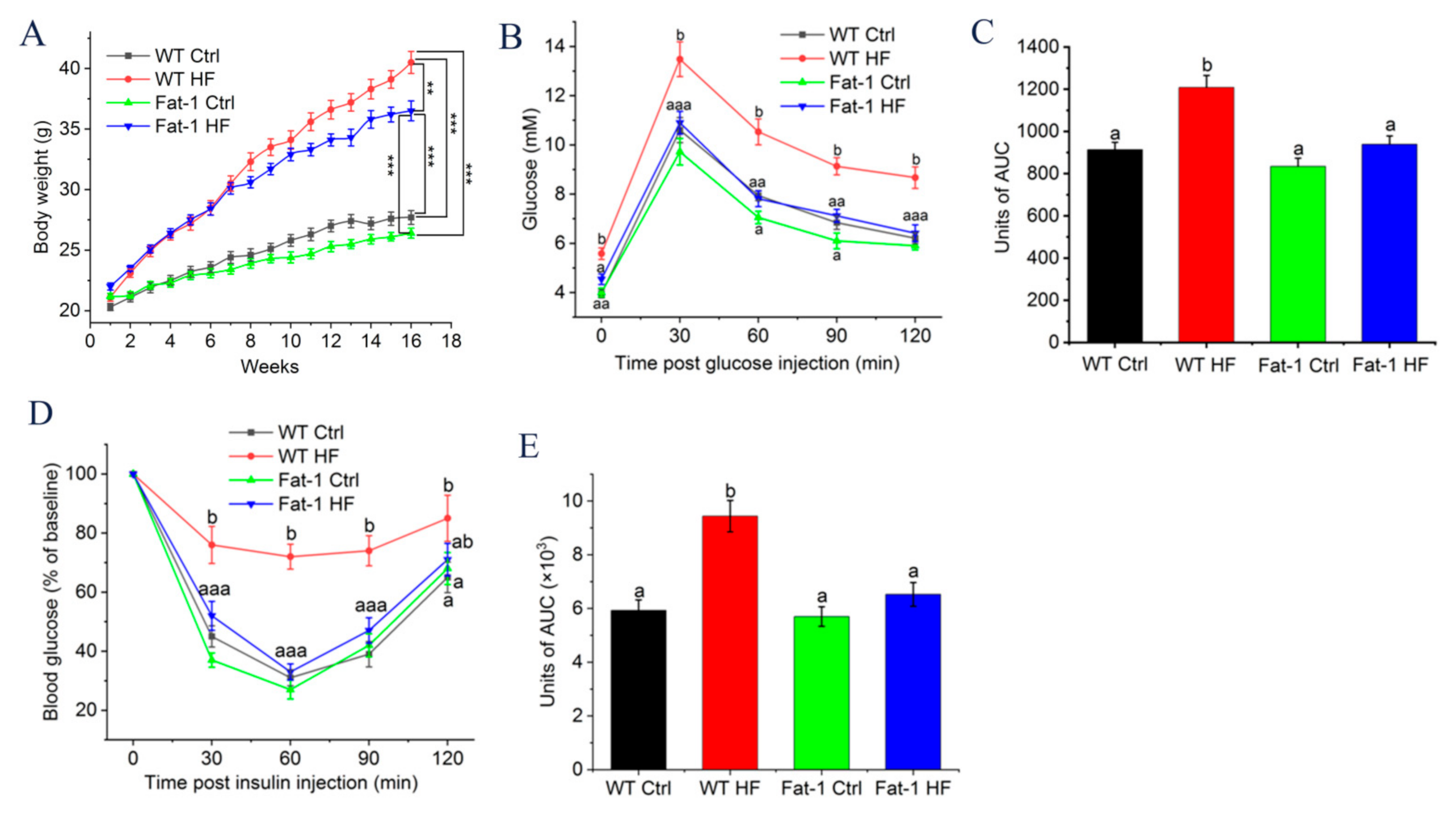
2.1. Fat-1 Trangenic Mice Exhibited Ameliorated Glucose Metabolism Disturbance Induced by HF Diet
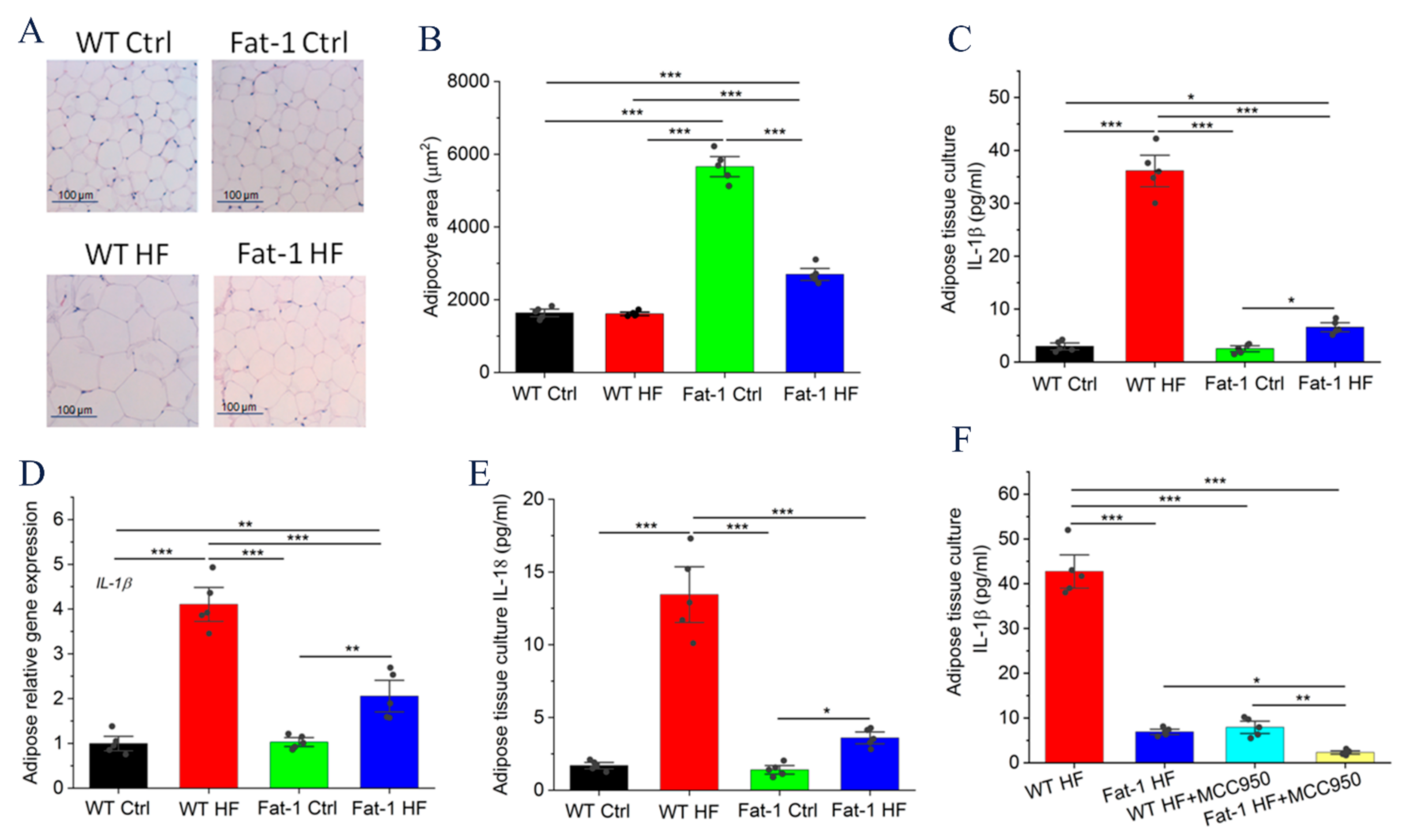
2.2. Fat-1 Mice Were Protected against HF Diet-Mediated IL-1β Secretion in Adipose Tissue
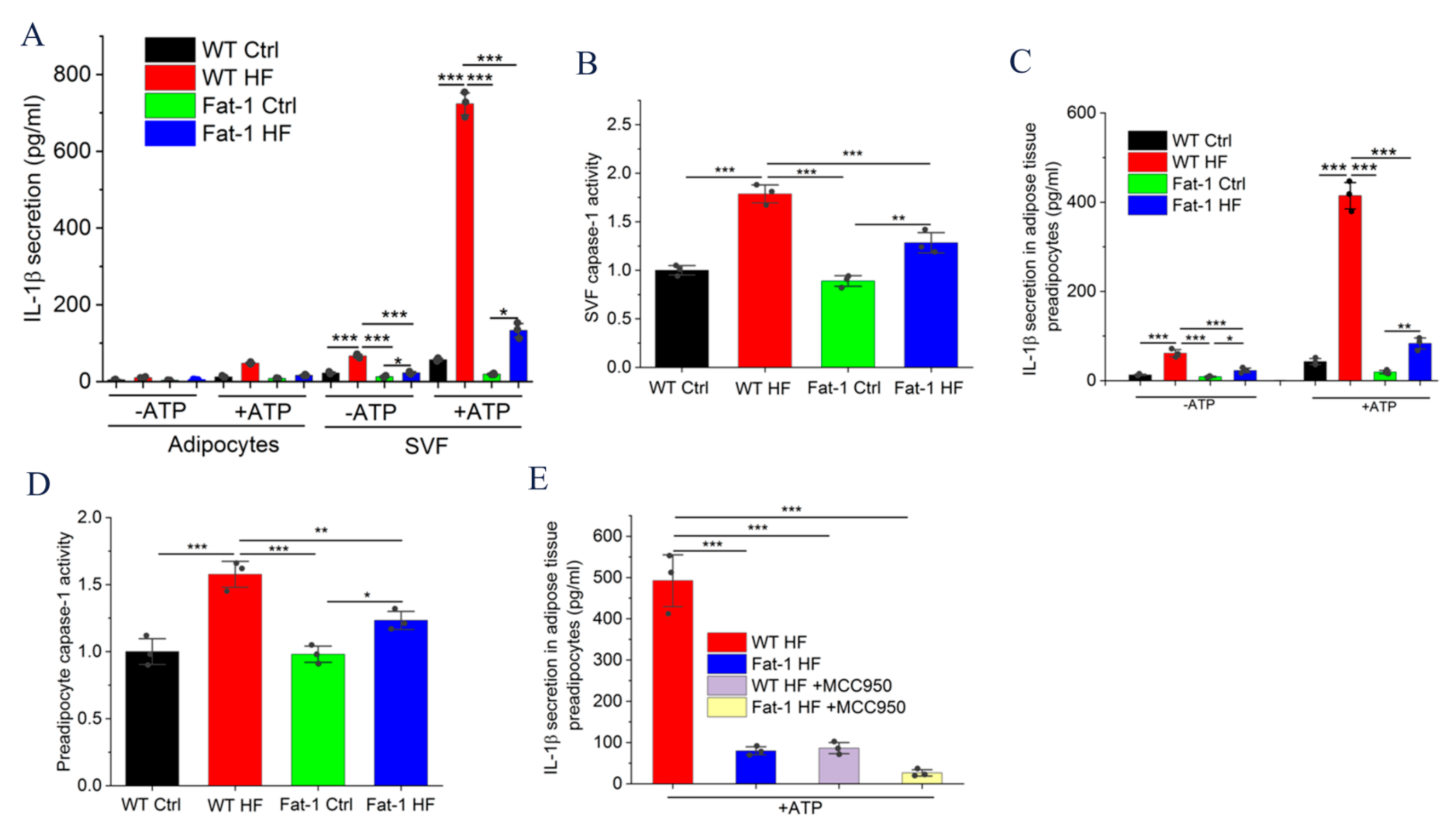
2.3. Fat-1 HF Mice Exhibited Attenuated NLRP3 Inflammasome Activity and IL-1β Secretion in Adipose Tissue Stromal Vascular Fraction (SVF) and Preadipocytes
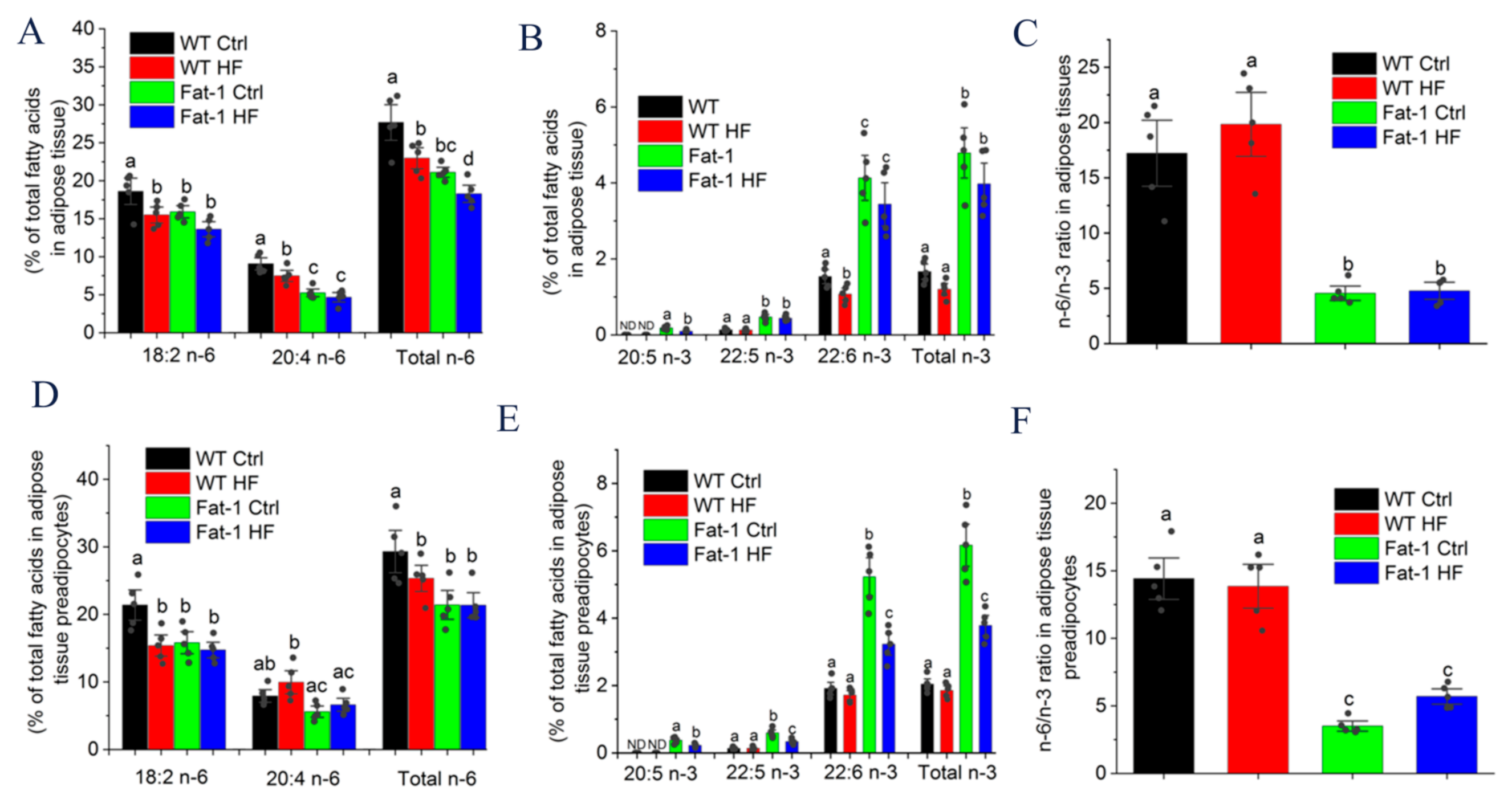
2.4. Major n-6 and n-3 Fatty Acid Composition and n-6/n-3 Ratio in Adipose Tissue and Preadipocytes
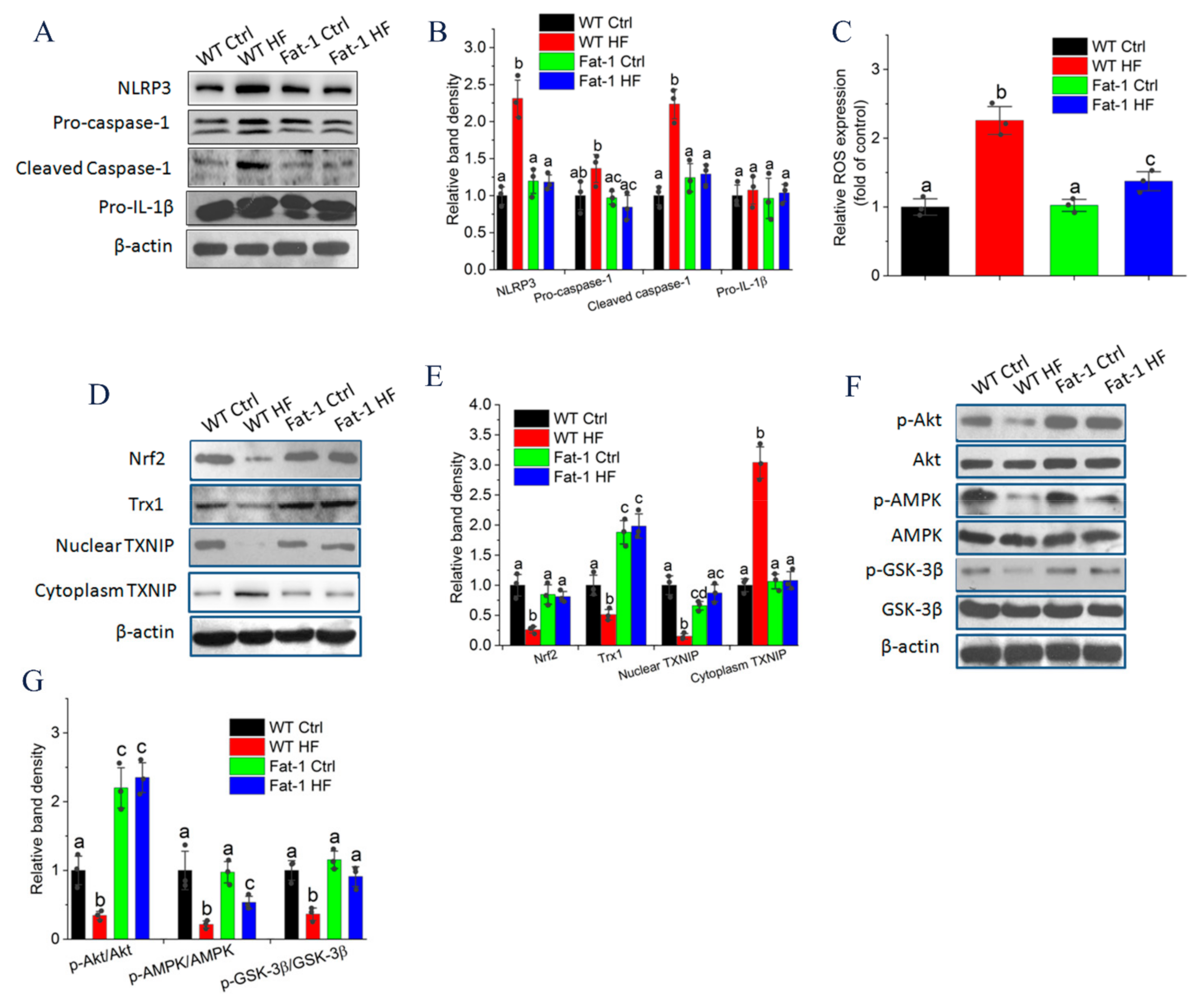
2.5. Endogenous n-3 PUFA Suppressed NLRP3 Inflammasome Signaling in Adipose Tissue
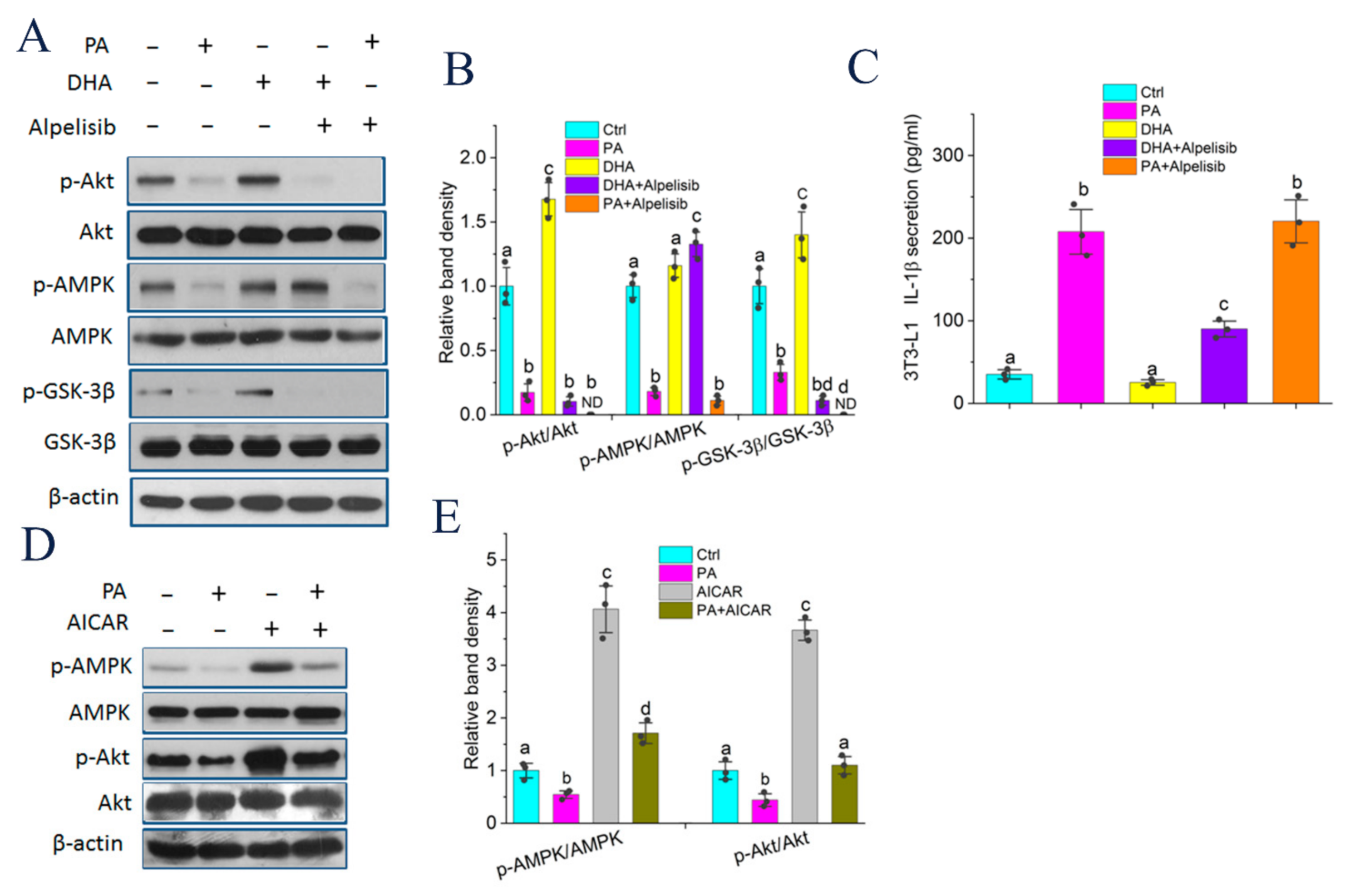
2.6. Endogenous n-3 PUFAs Antagonized HF Diet-Induced IL-1β Secretion via AMPK/Akt/GSK-3β/TXNIP Axis
2.7. Akt Phosphorylation Modulated by DHA and AMPK Controlled GSK-3β Activity and IL-1β Secretion
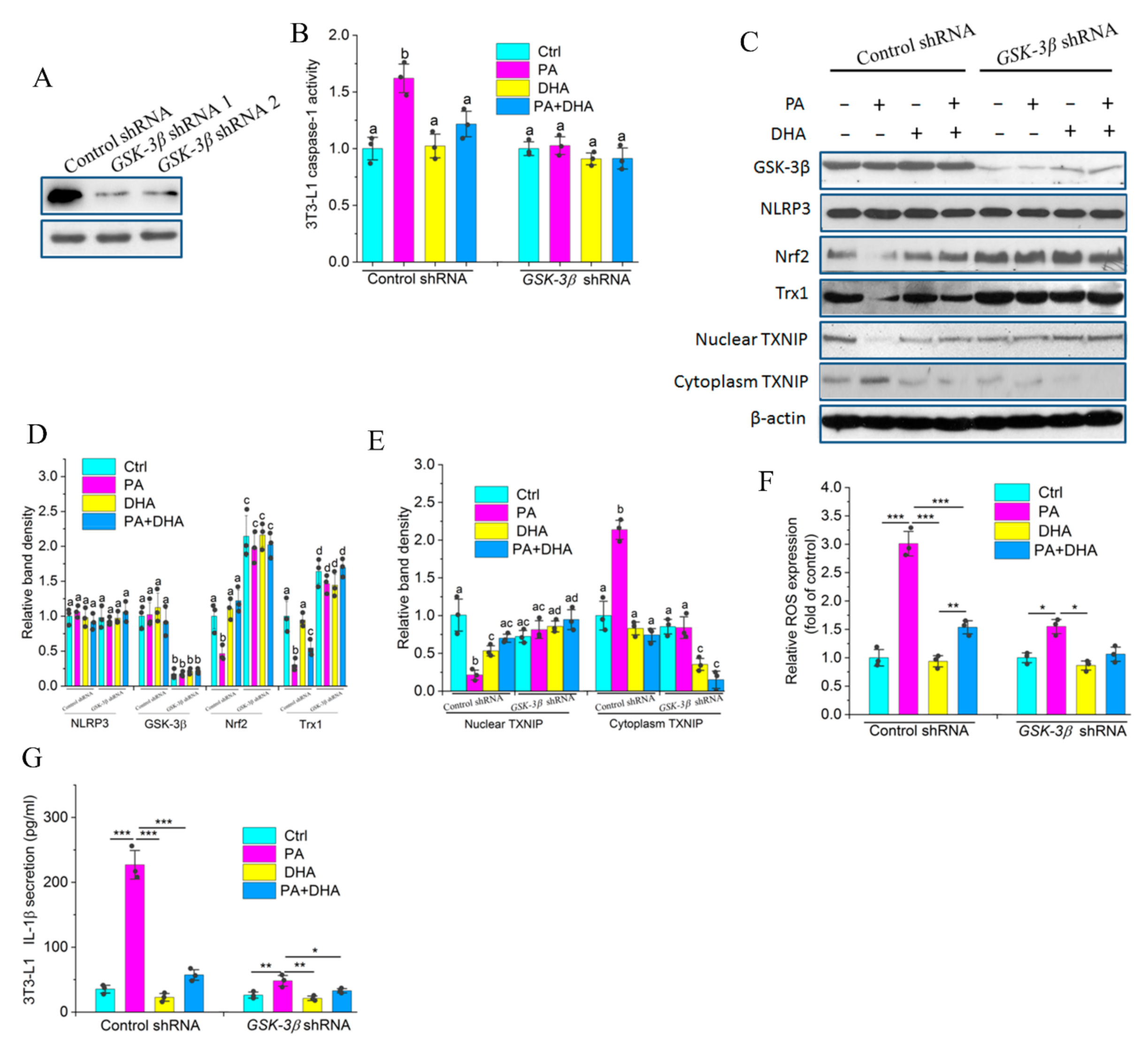
2.8. GSK-3β Is Indispensable for Impairing PA-Induced NLRP3 Activity by DHA
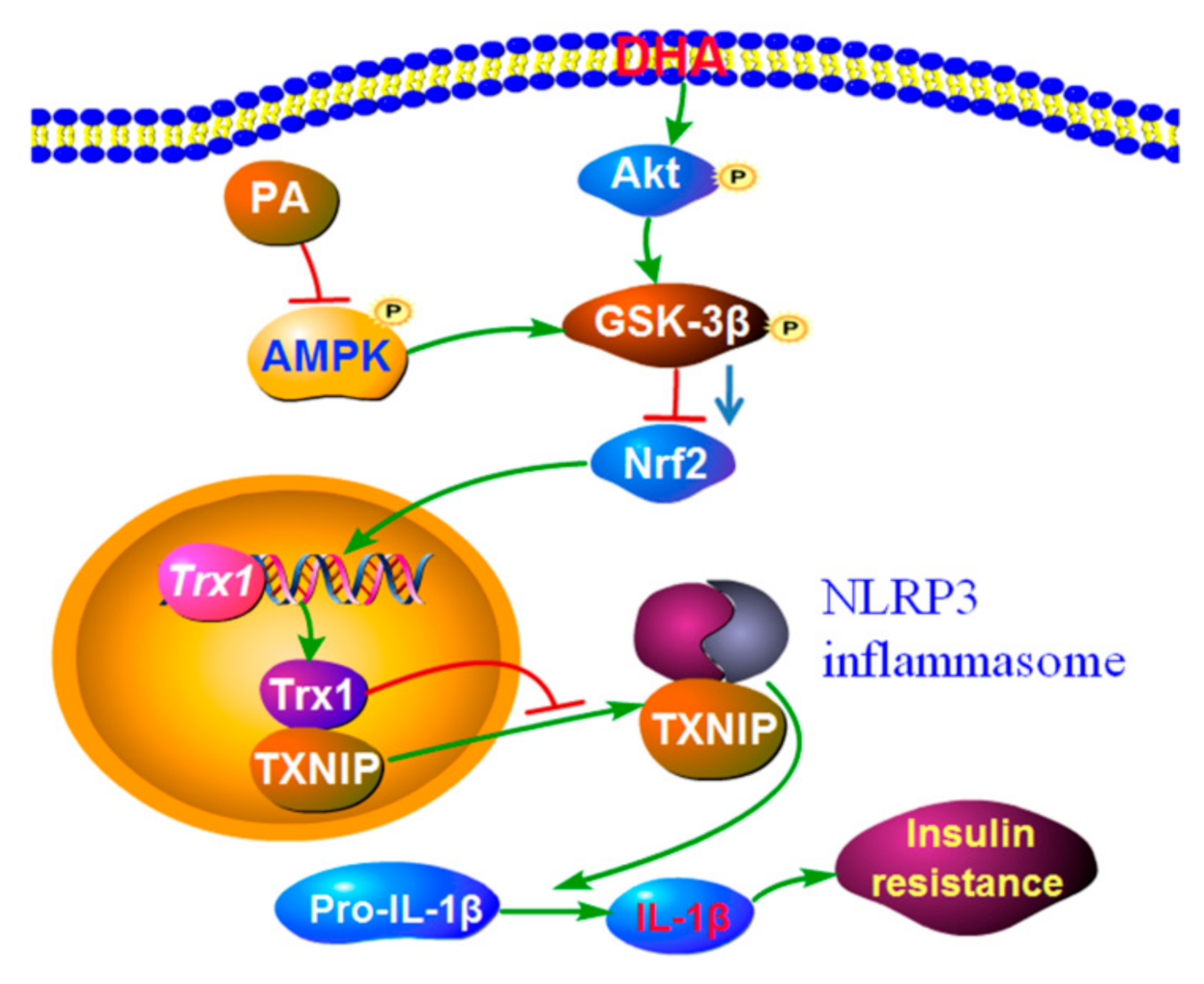
3. Discussion
4. Materials and Methods
4.1. Animals and Diet
4.2. Glucose and Insulin Tolerance Test
4.3. Tissue Culture
4.4. Stromal Vascular Fraction, Mature Adipocyte and Preadipocyte Fractionation and Culture
4.5. Cell Culture
4.6. Measurement of ROS of Adipose Tissue and Cultured 3T3-L1 Preadipocytes
4.7. Real-Time PCR Analysis
4.8. Transfection of GSK-3β shRNA
4.9. Western Blotting Analysis
4.10. Caspase-1 Activity Assay
4.11. The Fatty Acid Composition of Adipose Tissue and Adipose Tissue Preadipocytes
4.12. Histological Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Nov, O.; Kohl, A.; Lewis, E.C.; Bashan, N.; Dvir, I.; Ben-Shlomo, S.; Fishman, S.; Wueest, S.; Konrad, D.; Rudich, A. Interleukin-1beta may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology 2010, 151, 4247–4256. [Google Scholar] [CrossRef]
- Ghanbari, M.; Momen Maragheh, S.; Aghazadeh, A.; Mehrjuyan, S.R.; Hussen, B.M.; Abdoli Shadbad, M.; Dastmalchi, N.; Safaralizadeh, R. Interleukin-1 in obesity-related low-grade inflammation: From molecular mechanisms to therapeutic strategies. Int. Immunopharmacol. 2021, 96, 107765. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Wu, K.K.; Cheung, S.W.; Cheng, K.K. NLRP3 Inflammasome activation in adipose tissues and its implications on metabolic diseases. Int. J. Mol. Sci. 2020, 21, 4184. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef]
- Mirashrafi, S.; Borzoo-Isfahani, M.; Namjoo, I.; Kermani, M.; Moravejolahkami, A.R. A Mediterranean-type diet improved systemic inflammation in multiple sclerosis patients, as compared to the traditional Iranian diet: A single-center randomized controlled trial. Mediterr. J. Nutr. Meta. 2021, 14, 289–304. [Google Scholar] [CrossRef]
- Steele, C.C.; Steele, T.J.; Gwinner, M.K.; Rosenkranz, S.K.; Kirkpatrick, K. The relationship between dietary fat intake, impulsive choice, and metabolic health. Appetite 2021, 165, 105292. [Google Scholar] [CrossRef]
- Teng, K.T.; Yan, C.C.; Chang, L.F.; Nesaretnam, K. Obesity-induced inflammation: The link to insulin resistance and modulation by dietary fats. Nutr. J. 2014, 13, 12. [Google Scholar] [CrossRef]
- Frank, G.; Faustini-Fustini, M.; Mazzatenta, D.; Zoli, M.; Sciarretta, V.; Pasquini, E. Inclusion of n-3 polyunsaturated fatty acids into high-fat diets prevents adipose tissue inflammation in obese diabetic mice. Exp. Clin. Endocr. Diab. 2007, 115, 2–27. [Google Scholar] [CrossRef]
- Todoric, J.; Löffler, M.; Huber, J.; Bilban, M.; Reimers, M.; Kadl, A.; Zeyda, M.; Waldhäusl, W.; Stulnig, T.M. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n3 polyunsaturated fatty acids. Diabetologia 2006, 49, 2109–2119. [Google Scholar] [CrossRef]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef]
- Paschoal, V.A.; Vinolo, M.; Crisma, A.R.; Magdalon, J.; Curi, R. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid differentially modulate rat neutrophil function in vitro. Lipids 2013, 48, 93–103. [Google Scholar] [CrossRef]
- Han, L.; Lei, H.; Tian, Z.; Wang, X.; Cheng, D.; Wang, C. The immunomodulatory activity and mechanism of docosahexenoic acid (DHA) on immunosuppressive mice models. Food Funct. 2018, 9, 3254–3263. [Google Scholar] [CrossRef]
- Kang, J.X.; Wang, J.; Wu, L.; Kang, Z.B. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004, 427, 504. [Google Scholar] [CrossRef]
- Jia, Q.; Lupton, J.R.; Smith, R.; Weeks, B.R.; Callaway, E.; Davidson, L.A.; Kim, W.; Fan, Y.Y.; Yang, P.; Newman, R.A. Reduced Colitis-Associated Colon Cancer in Fat-1 (n-3 Fatty Acid Desaturase) Transgenic Mice. Cancer Res. 2008, 68, 3985–3991. [Google Scholar] [CrossRef]
- He, C.; Qu, X.; Cui, L.; Wang, J.; Kang, J.X. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA 2009, 106, 11370–11375. [Google Scholar] [CrossRef]
- Hardesty, J.E.; Warner, J.B.; Song, Y.L.; Rouchka, E.C.; Kirpich, I.A. Ileum Gene Expression in Response to Acute Systemic Inflammation in Mice Chronically Fed Ethanol: Beneficial Effects of Elevated Tissue n-3 PUFAs. Int. J. Mol. Sci. 2021, 22, 1582. [Google Scholar] [CrossRef]
- Hwang, T.W.; Kim, E.J.; Kim, D.B.; Jin, Y.J.; Kim, J.J. Fat-1 expression enhance hippocampal memory in scopolamine-induced amnesia. J. Nutr. Biochem. 2020, 82, 108394. [Google Scholar] [CrossRef]
- Romanatto, T.; Fiamoncini, J.; Wang, B.; Curi, R.; Kang, J.X. Elevated tissue omega-3 fatty acid status prevents age-related glucose intolerance in fat-1 transgenic mice. Biochim. Biophys. Acta 2014, 1842, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.Q.; Bellenger, S.; Massey, K.A.; Nicolaou, A.; Geissler, A.; Bidu, C.; Bonnotte, B.; Pierre, A.S.; Minville-Walz, M.; Rialland, M.; et al. Inhibition of the HER2 pathway by n-3 polyunsaturated fatty acids prevents breast cancer in fat-1 transgenic mice. J. Lipid Res. 2013, 54, 3453–3463. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Leuven, F.V. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Gollavilli, P.N.; Kanugula, A.K.; Koyyada, R.; Karnewar, S.; Neeli, P.K.; Kotamraju, S. AMPK inhibits MTDH expression via GSK3beta and SIRT1 activation: Potential role in triple negative breast cancer cell proliferation. FEBS J. 2015, 282, 3971–3985. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chuang, W.W.; Sun, Z. Phosphatidylinositol 3-Kinase/Akt Stimulates Androgen Pathway through GSK3β Inhibition and Nuclear β-Catenin Accumulation. J. Biol. Chem. 2002, 277, 30935–30941. [Google Scholar] [CrossRef] [PubMed]
- Sitar-Taut, A.V.; Coste, S.C.; Tarmure, S.; Orasan, O.H.; Cozma, A. Diabetes and Obesity—Cumulative or Complementary Effects On Adipokines, Inflammation, and Insulin Resistance. J. Clin. Med. 2020, 9, 2767. [Google Scholar] [CrossRef]
- De Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS. Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Oguszka, M.; Nawrocka, A.; Pierzchaa, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Donath, M.Y.; Mandrup-Poulsen, T. Role of IL-1β in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 314–321. [Google Scholar] [CrossRef]
- Eve, B.; Bastard, J.P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 305–311. [Google Scholar] [CrossRef]
- Van Asseldonk, E.J.; van Poppel, P.C.; Ballak, D.B.; Stienstra, R.; Netea, M.G.; Tack, C.J. One week treatment with the IL-1 receptor antagonist anakinra leads to a sustained improvement in insulin sensitivity in insulin resistant patients with type 1 diabetes mellitus. Clin. Immunol. 2015, 160, 155–162. [Google Scholar] [CrossRef]
- Bellenger, J.; Bellenger, S.; Bataille, A.; Massey, K.A.; Nicolaou, A.; Rialland, M.; Tessier, C.; Kang, J.X.; Narce, M. High Pancreatic n-3 Fatty Acids Prevent STZ-Induced Diabetes in Fat-1 Mice: Inflammatory Pathway Inhibition. Diabetes 2011, 60, 1090–1099. [Google Scholar] [CrossRef]
- Chung, S.; Lapoint, K.; Martinez, K.; Kennedy, A.; Boysen Sandberg, M.; McIntosh, M.K. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 2006, 147, 5340–5351. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef]
- Chen, B. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte 2015, 4, 149–152. [Google Scholar] [CrossRef]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Obes. Lipotoxicity 2017, 960, 327–343. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; Mcallan, L.; Nilaweera, K.N. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef]
- Wang, H.; Khor, T.O.; Saw, C.L.L.; Lin, W.; Wu, T.Y.; Huang, Y.; Kong, A.N.T. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol. Pharmaceutics 2010, 7, 2185–2193. [Google Scholar] [CrossRef]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Yin, H.; Zhang, L.; Feng, A.; Zhang, Q.X.; Lin, Y.; Bao, B.; Hernandez, L.L.; Shi, G.P.; et al. Functional Inactivation of Mast Cells Enhances Subcutaneous Adipose Tissue Browning in Mice. Cell Rep. 2019, 28, 792–803. [Google Scholar] [CrossRef]
- Koenen, T.B.; Stienstra, R.; Tits, L.J.V.; Graaf, J.D.; Stalenhoef, A.F.H.; Joosten, L.A.B.; Tack, C.J.; Netea, M.G. Hyperglycemia activates caspase-1 and TXNIP-Mediated IL-1β Transcription in Human Adipose Tissue. Diabetes 2011, 60, DB10–DB0266. [Google Scholar] [CrossRef]
- Liu, L.Y.; Jin, R.; Hao, J.Q.; Zeng, J.; Yin, D.; Yi, Y.M.; Zhu, M.M.; Mandal, A.; Hua, Y.; Ng, C.K.; et al. Consumption of the fish oil high-fat diet uncouples obesity and mammary tumor growth through induction of reactive oxygen species in protumor macrophages. Cancer Res. 2020, 80, 2564–2574. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Zhang, J.-J.; Cen, Y.; Yang, Y.; Wang, F.; Gu, K.-P.; Yang, H.-T.; Wang, Y.-Z.; Zou, Z.-Q. High Endogenously Synthesized N-3 Polyunsaturated Fatty Acids in Fat-1 Mice Attenuate High-Fat Diet-Induced Insulin Resistance by Inhibiting NLRP3 Inflammasome Activation via Akt/GSK-3β/TXNIP Pathway. Molecules 2022, 27, 6384. https://doi.org/10.3390/molecules27196384
Zhu P, Zhang J-J, Cen Y, Yang Y, Wang F, Gu K-P, Yang H-T, Wang Y-Z, Zou Z-Q. High Endogenously Synthesized N-3 Polyunsaturated Fatty Acids in Fat-1 Mice Attenuate High-Fat Diet-Induced Insulin Resistance by Inhibiting NLRP3 Inflammasome Activation via Akt/GSK-3β/TXNIP Pathway. Molecules. 2022; 27(19):6384. https://doi.org/10.3390/molecules27196384
Chicago/Turabian StyleZhu, Pan, Jin-Jie Zhang, Yi Cen, Yong Yang, Feng Wang, Kun-Peng Gu, Hai-Tao Yang, Yun-Zhi Wang, and Zu-Quan Zou. 2022. "High Endogenously Synthesized N-3 Polyunsaturated Fatty Acids in Fat-1 Mice Attenuate High-Fat Diet-Induced Insulin Resistance by Inhibiting NLRP3 Inflammasome Activation via Akt/GSK-3β/TXNIP Pathway" Molecules 27, no. 19: 6384. https://doi.org/10.3390/molecules27196384
APA StyleZhu, P., Zhang, J.-J., Cen, Y., Yang, Y., Wang, F., Gu, K.-P., Yang, H.-T., Wang, Y.-Z., & Zou, Z.-Q. (2022). High Endogenously Synthesized N-3 Polyunsaturated Fatty Acids in Fat-1 Mice Attenuate High-Fat Diet-Induced Insulin Resistance by Inhibiting NLRP3 Inflammasome Activation via Akt/GSK-3β/TXNIP Pathway. Molecules, 27(19), 6384. https://doi.org/10.3390/molecules27196384




