Enhancing Resistant Starch Content of High Amylose Rice Starch through Heat–Moisture Treatment for Industrial Application
Abstract
:1. Introduction
2. Results and Discussion
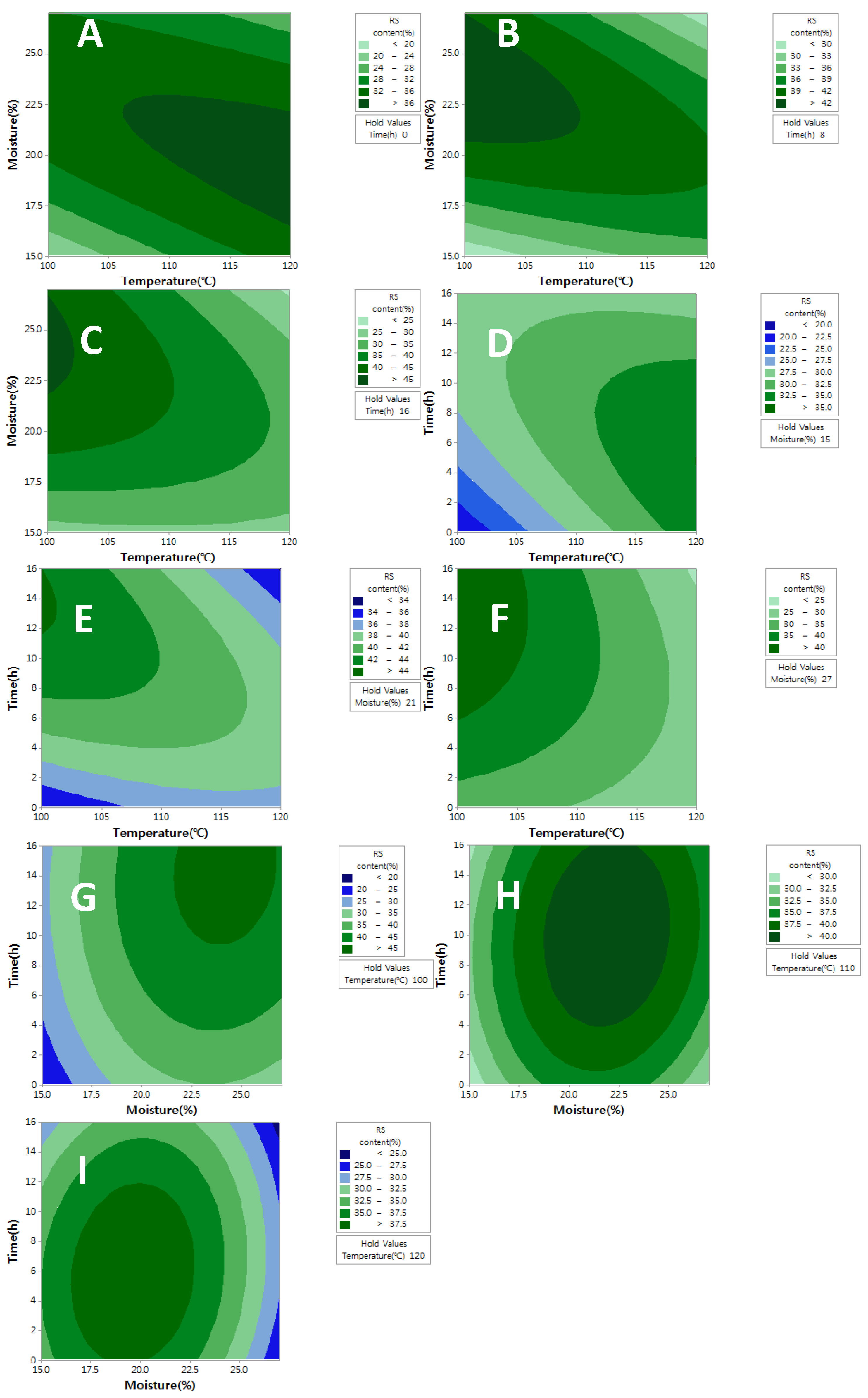
2.1. Optimization of HMT Conditions for Improving RS Content
2.2. Solubility and Swelling Power
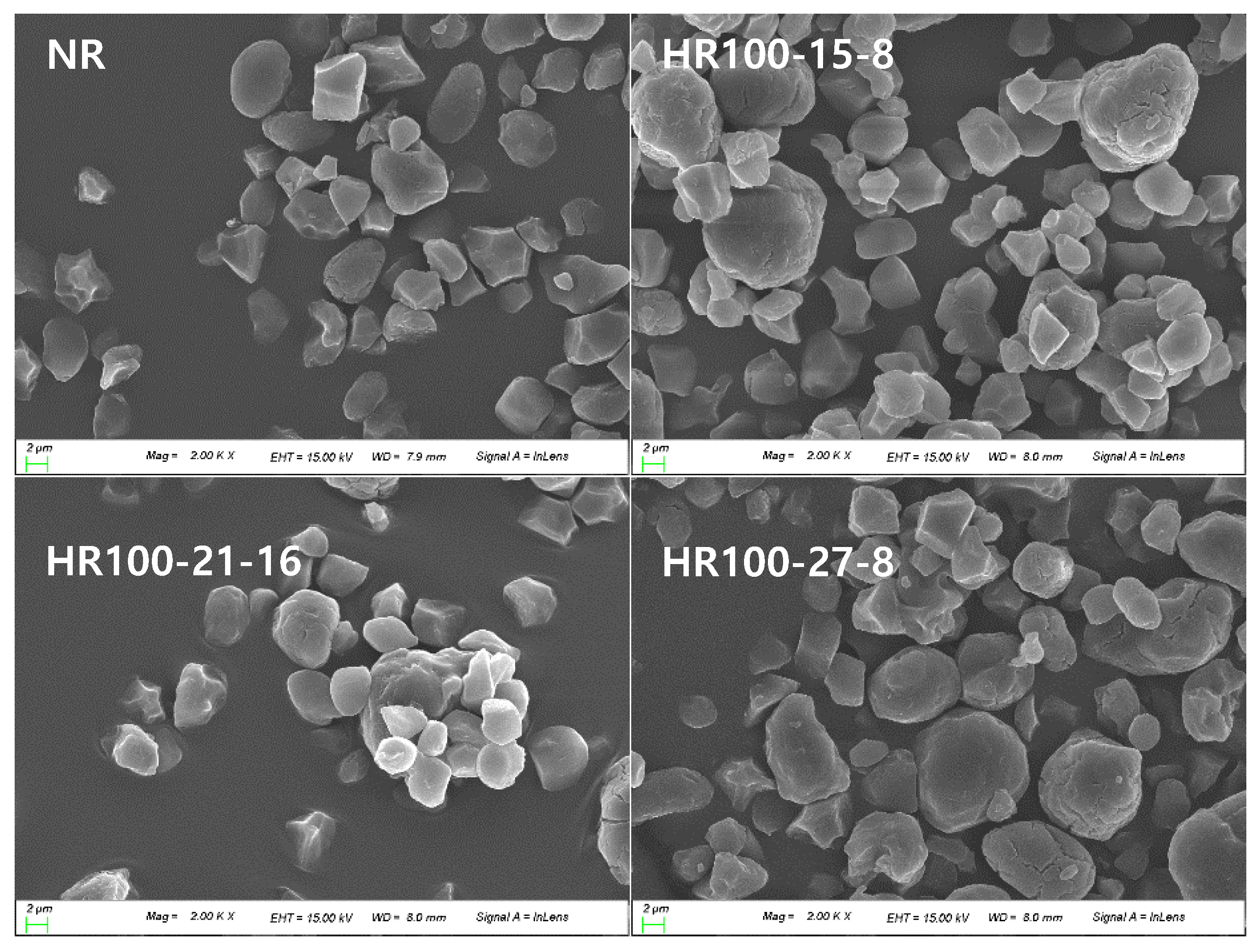
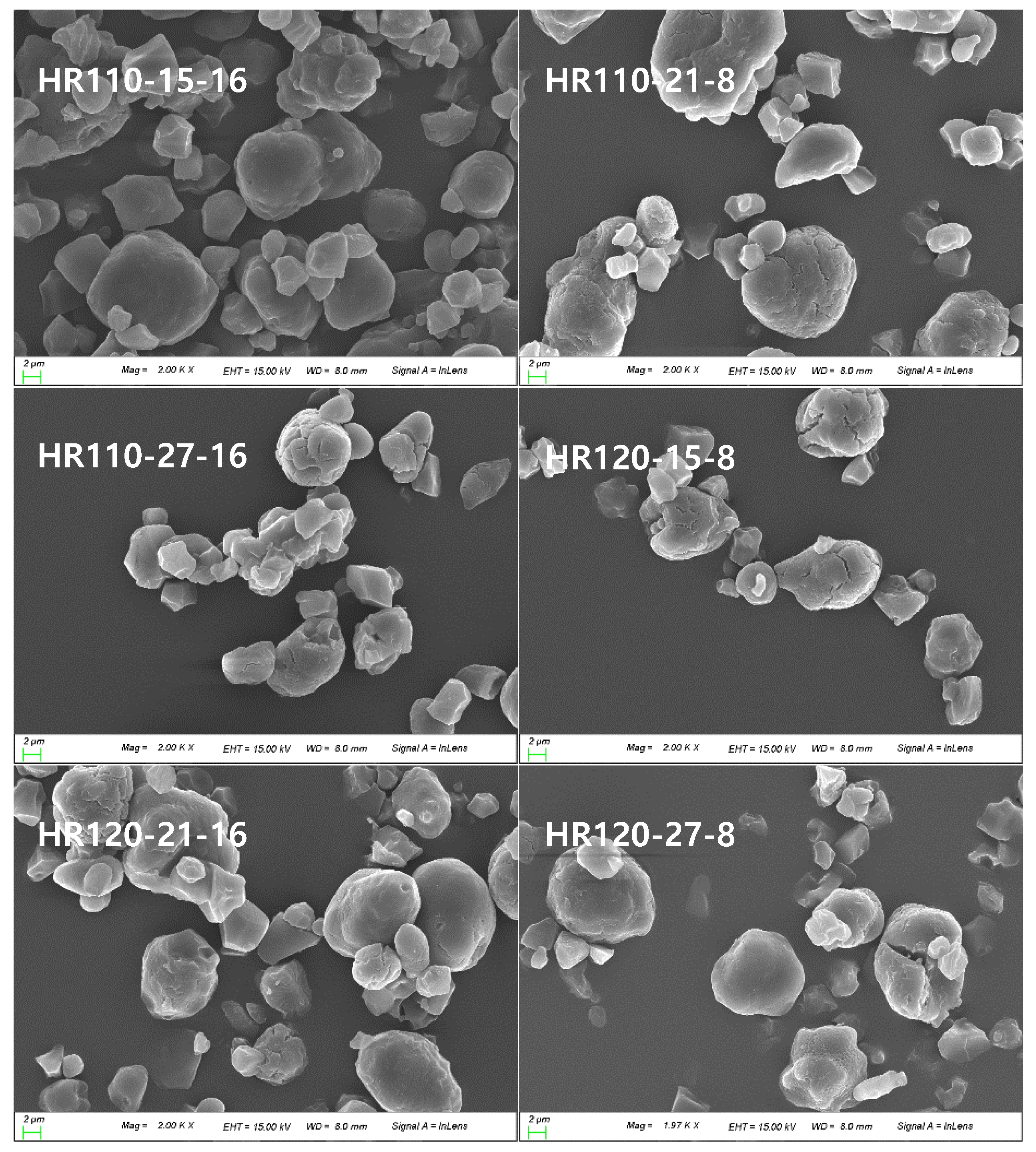
2.3. Scanning Electron Microscopy (SEM)
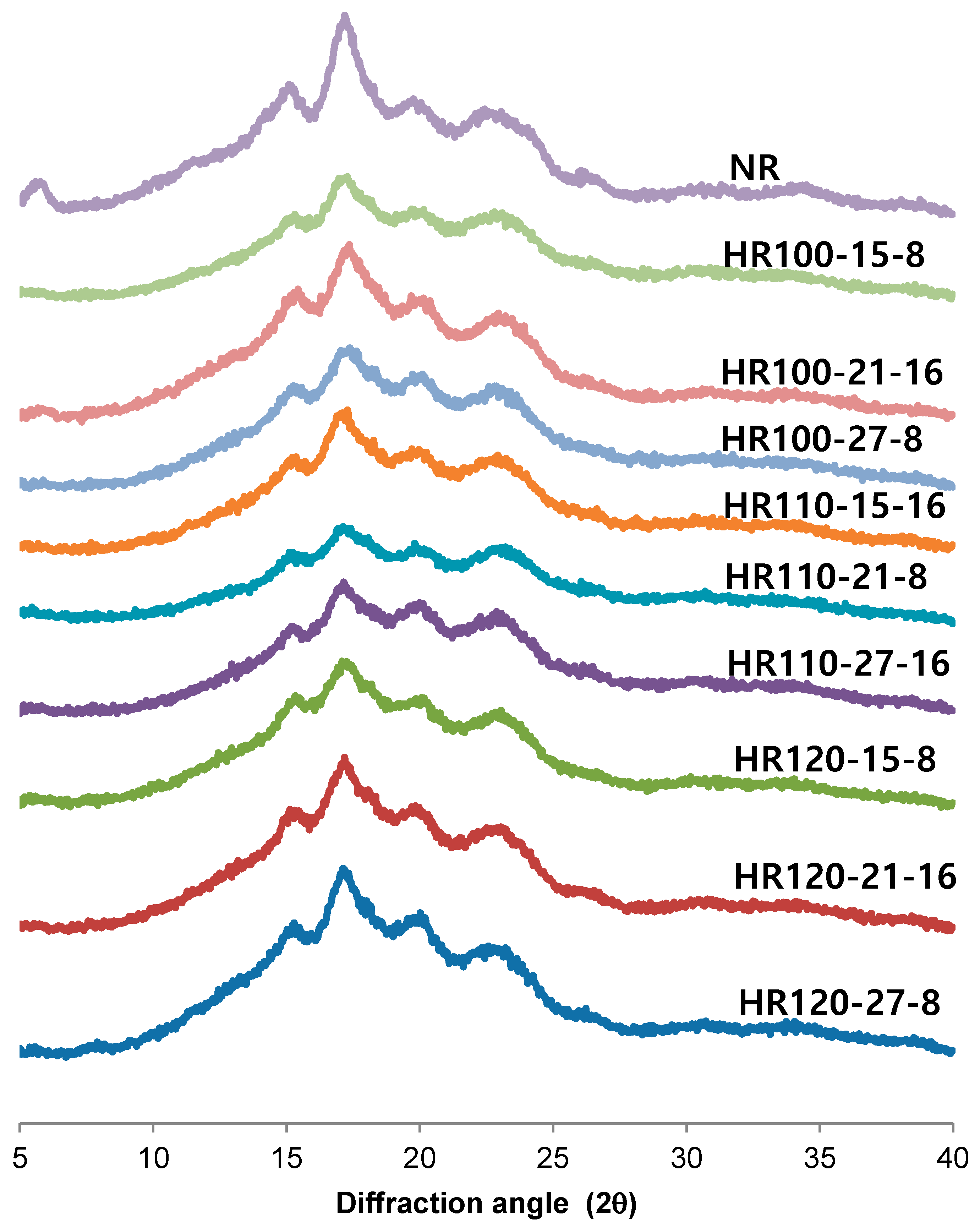
2.4. XRD
2.5. Thermal Analysis
3. Materials and Methods
3.1. Materials
3.2. HMT Experimental Design
3.3. Preparation of heat–moisture Treated Starches
3.4. Determination of RS Content
3.5. Swelling Power and Solubility
3.6. SEM
3.7. XRD Pattern
3.8. Thermal Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aschemann-Witzel, J.; Varela, P.; Peschel, A.O. Consumers’ categorization of food ingredients: Do consumers perceive them as ‘clean label’ producers expect? An exploration with projective mapping. Food Qual. Prefer. 2019, 71, 117–128. [Google Scholar] [CrossRef]
- Zavareze, D.Z.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Sindhu, R.; Devi, A.; Khatkar, B.S. Physicochemical, thermal and structural properties of heat moisture treated common buckwheat starches. J. Food Sci. Technol. 2019, 56, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; No, J.; Shin, M. Effects of heat-moisture treatment on functional properties of high amylose rice starches with different crystalline types. Korean J. Food Sci. Technol. 2020, 52, 31–39. [Google Scholar]
- Cindy, S.; Pieter, L.B. Starch in Food: Structures, Function and Applications, 2nd ed.; Woodhead Publishing: Cambridge, MA, USA, 2017; pp. 357–375. [Google Scholar]
- Chung, H.J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Shin, M. Development and applications of resistant starch. Food Ind. Nutr. 2004, 9, 1–9. [Google Scholar]
- Hung, P.V.; Vien, N.L.; Phi, N.T.L. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73. [Google Scholar] [CrossRef]
- Bao, J.; Zhou, X.; Hu, Y.; Zhang, Z. Resistant starch content and physicochemical properties of non-waxy rice starches modified by pullulanase, heat-moisture treatment, and citric acid. J. Cereal Sci. 2022, 105, 103472. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Chen, L.; Li, X.; Wang, J.; Xie, F. Insights into the multi-scale structure and digestibility of heat-moisture treated rice starch. Food Chem. 2018, 242, 323–329. [Google Scholar] [CrossRef]
- Zavareze, E.R.; Halal, S.L.M.E.; Santos, D.G.; Helbig, E.; Pereira, J.M.; Dias, A.R.G. Resistant starch and thermal, morphological and textural properties of heat-moisture treated rice starches with high-, medium- and low-amylose content. Starch/Särke 2012, 64, 45–54. [Google Scholar] [CrossRef]
- Shin, M.S.; Woo, K.S.; Seib, P.A. Supplementations of resistant starches to Asian noodles. Food Sci. Biotechnol. 2002, 11, 365–370. [Google Scholar]
- Huang, M.; Kim, J.S.; No, J.; Shin, M. Physicochemical properties and preparation of RS3 resistant starch prepared from high amylose dodamssal rice starch. Korean J. Food Cook. Sci. 2019, 35, 229–307. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Kim, J.M.; Song, J.Y.; Shin, M. Physicochemical properties of high amylose rice starches purified Korean cultivars. Starch/Särke 2010, 62, 262–268. [Google Scholar] [CrossRef]
- Zavareze, E.R.; Storck, C.R.; Castro, L.A.S.; Schirmer, M.A.; Dias, A.R.G. Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem. 2010, 121, 358–365. [Google Scholar] [CrossRef]
- Li, S.; Ward, R.; Gao, Q. Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus) starch. Food Hydrocoll. 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Kurakake, M.; Tachibana, Y.; Masaki, K.; Komaki, T. Adsorption of α-amylase on heat-moisture treated starch. J. Cereal Sci. 1996, 23, 163–168. [Google Scholar] [CrossRef]
- Kim, M.J.; Oh, S.G.; Chung, H.J. Impact of heat-moisture treatment applied to brown rice flour on the quality and digestibility characteristics of Korean rice cake. Food Sci Biotechnol. 2017, 26, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Piecyk, M.; Druzynska, A.; Oltarzewska, A.; Wolosiak, R.; Worobiej, E.; Ostrowska, L.E. Effect of hydrothermal modifications on properties and digestibility of grass pea starch. Int. J. Biol. Mactomol. 2018, 118, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.S. Studies on the hydrothermal modifications of new cocoyam (Xanthosoma sagittifolium) starch. Int. J. Biol. Macromol. 2005, 37, 268–277. [Google Scholar] [CrossRef]
- Hoover, R.; Manuel, H. Effect of heat-moisture treatment on the structure and physicochemical properties of normal maize, waxy maize, dull waxy maize and amylomaize V starches. J. Cereal Sci. 1996, 23, 153–162. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Lawal, O.S. Effect of annealing and heat moisture conditioning on the physicochemical characteristics of Bambarra groundnut (Voandzeia subterranea) starch. Nahrung-Food 2002, 46, 311–316. [Google Scholar] [CrossRef]
- Kulp, K.; Lorenz, K. Heat-moisture treatment of starches. I. Physicochemical properties. Cereal Chem. 1981, 58, 46–48. [Google Scholar]
- Ruiz, E.; Srikaeo, K.; Revilla, L.S. Effects of heat moisture treatment on physicochemical properties and starch digestibility of rice flours differing in amylose content. Food Appl. Biosci. J. 2018, 6, 140–153. [Google Scholar]
- Kang, H.J.; Hwang, I.K.; Kim, K.S.; Choi, H.C. Comparative structure and physicochemical properties of Ilpumbyeo, a high-quality japonica rice, and its mutant, Suweon 464. J. Agric. Food Chem. 2003, 51, 6598–6603. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Guo, D.; Huang, J.; Zhang, X.; Zhang, L.; Wei, C. Molecular structure and enzymatic hydrolysis properties of starches form high-amylose maize inbred lines and their hybrids. Food Hydrocoll. 2016, 58, 246–254. [Google Scholar] [CrossRef]
- Liu, C.; Song, M.; Hong, J.; Guan, E.; Brian, K.; Zheng, X. Effect of heat-moisture treatment on the structure and physicochemical properties of ball mill damaged starches from different botanical sources. Int. J. Bio. Mactromol. 2020, 156, 403–410. [Google Scholar] [CrossRef]
- Jeong, O.; Shin, M. Preparation and stability of resistant starch nanoparticles, using acid hydrolysis and cross-linking of waxy rice starch. Food Chem. 2018, 256, 77–84. [Google Scholar] [CrossRef]
- Lee, C.E.; No, J.; Shin, M. Physicochemical properties of resistant starch prepared from Singil rice starch. Korean J. Food Cook. Sci. 2018, 34, 626–634. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Schoch, T.J. Whole starches and modified starches. In Methods in Carbohydrate Chemistry; Whistler, R.L., Ed.; Academic Press: New York, NY, USA, 1964; pp. 106–108. [Google Scholar]
| Starch 1 | RS Content (%) | Solubility at 80 °C (%) | Swelling Power at 80 °C (g/g) |
|---|---|---|---|
| NR | 32.13 ± 0.49 d | 6.18 ± 1.33 ab | 8.48 ± 0.12 a |
| HR100-15-8 | 24.84 ± 0.64 f | 4.66 ± 0.14 cd | 6.17 ± 0.05 bc |
| HR100-21-16 | 49.00 ± 0.12 a | 6.70 ± 0.93 a | 6.56 ± 0.07 b |
| HR100-27-8 | 41.18 ± 0.04 b | 3.94 ± 0.08 de | 5.66 ± 0.06 d |
| HR110-15-16 | 26.58 ± 1.06 e | 4.64 ± 0.17 cd | 5.79 ± 0.01 cd |
| HR110-21-8 | 41.75 ± 0.42 b | 4.08 ± 0.34 cde | 5.40 ± 0.11 de |
| HR110-27-16 | 31.17 ± 0.79 d | 4.36 ± 0.62 cde | 5.44 ± 0.15 de |
| HR120-15-8 | 35.22 ± 0.38 c | 5.36 ± 0.28 bc | 6.36 ± 0.47 b |
| HR120-21-16 | 35.43 ± 1.74 c | 3.18 ± 0.08 e | 5.10 ± 0.15 ef |
| HR120-27-8 | 30.52 ± 0.85 d | 3.56 ± 0.00 de | 4.91 ± 0.09 f |
| Starch 1 | To (°C) | Tp (°C) | Tc (°C) | Tc−To (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| NR | 59.6 ± 0.6 e | 66.5 ± 0.3 i | 74.1 ± 1.1 e | 14.6 ± 0.5 d | 12.5 ± 1.5 a |
| HR100-15-8 | 76.3 ± 0.1 d | 83.5 ± 0.0 g | 91.6 ± 0.5 cd | 15.3 ± 0.6 d | 4.2 ± 0.0 f |
| HR100-21-16 | 75.6 ± 0.3 d | 82.3 ± 0.1 h | 89.6 ± 0.4 d | 14.0 ± 0.1 d | 5.2 ± 0.6 ef |
| HR100-27-8 | 80.6 ± 2.7 bc | 95.2 ± 1.3 a | 107.0 ± 0.1 a | 26.4 ± 2.6 abc | 8.9 ± 0.6 b |
| HR110-15-16 | 78.2 ± 0.1 cd | 90.6 ± 0.4 d | 106.6 ± 2.1 a | 28.5 ± 2.2 ab | 8.3 ± 0.0 bc |
| HR110-21-8 | 81.3 ± 0.3 b | 88.7 ± 0.1 e | 95.7 ± 0.8 c | 14.4 ± 0.6 d | 6.0 ± 0.1 de |
| HR110-27-16 | 80.5 ± 2.3 bc | 87.0 ± 0.1 f | 106.1 ± 0.6 a | 25.6 ± 1.7 bc | 6.8 ± 0.4 cd |
| HR120-15-8 | 77.1 ± 0.4 d | 93.7 ± 0.8 b | 108.0 ± 4.6 a | 30.9 ± 4.2 a | 8.4 ± 0.2 bc |
| HR120-21-16 | 82.9 ± 0.2 ab | 92.2 ± 0.1 c | 105.1 ± 3.7 a | 22.3 ± 3.9 c | 8.3 ± 0.1 bc |
| HR120-27-8 | 84.9 ± 1.2 a | 92.2 ± 0.4 c | 100.4 ± 0.6 b | 15.5 ± 0.6 d | 9.9 ± 0.6 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-S.; Chung, H.-J. Enhancing Resistant Starch Content of High Amylose Rice Starch through Heat–Moisture Treatment for Industrial Application. Molecules 2022, 27, 6375. https://doi.org/10.3390/molecules27196375
Lee C-S, Chung H-J. Enhancing Resistant Starch Content of High Amylose Rice Starch through Heat–Moisture Treatment for Industrial Application. Molecules. 2022; 27(19):6375. https://doi.org/10.3390/molecules27196375
Chicago/Turabian StyleLee, Chang-Seon, and Hyun-Jung Chung. 2022. "Enhancing Resistant Starch Content of High Amylose Rice Starch through Heat–Moisture Treatment for Industrial Application" Molecules 27, no. 19: 6375. https://doi.org/10.3390/molecules27196375
APA StyleLee, C.-S., & Chung, H.-J. (2022). Enhancing Resistant Starch Content of High Amylose Rice Starch through Heat–Moisture Treatment for Industrial Application. Molecules, 27(19), 6375. https://doi.org/10.3390/molecules27196375






