La3+’s Effect on the Surface (101) of Anatase for Methylene Blue Dye Removal, a DFT Study
Abstract
1. Introduction
2. Results
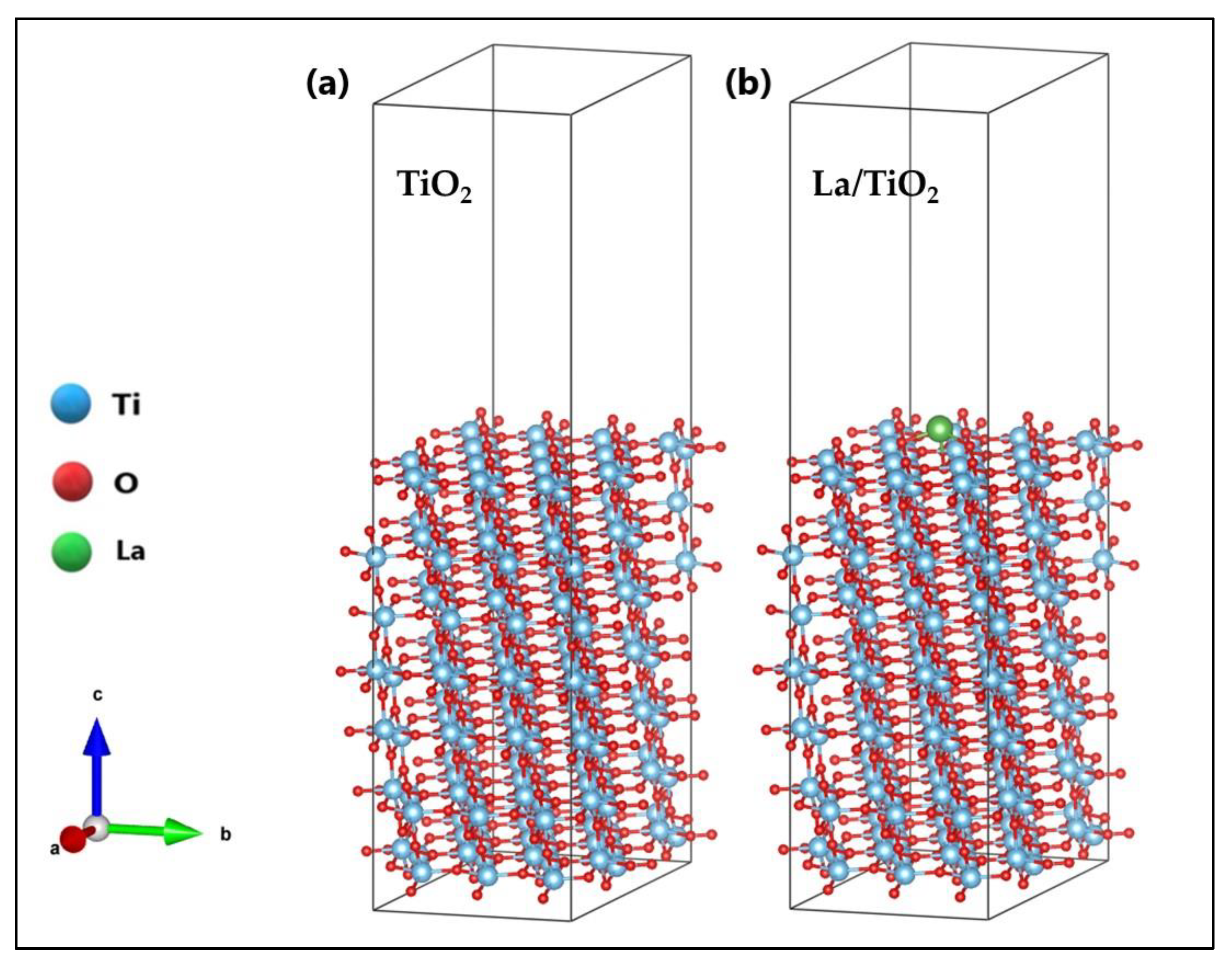
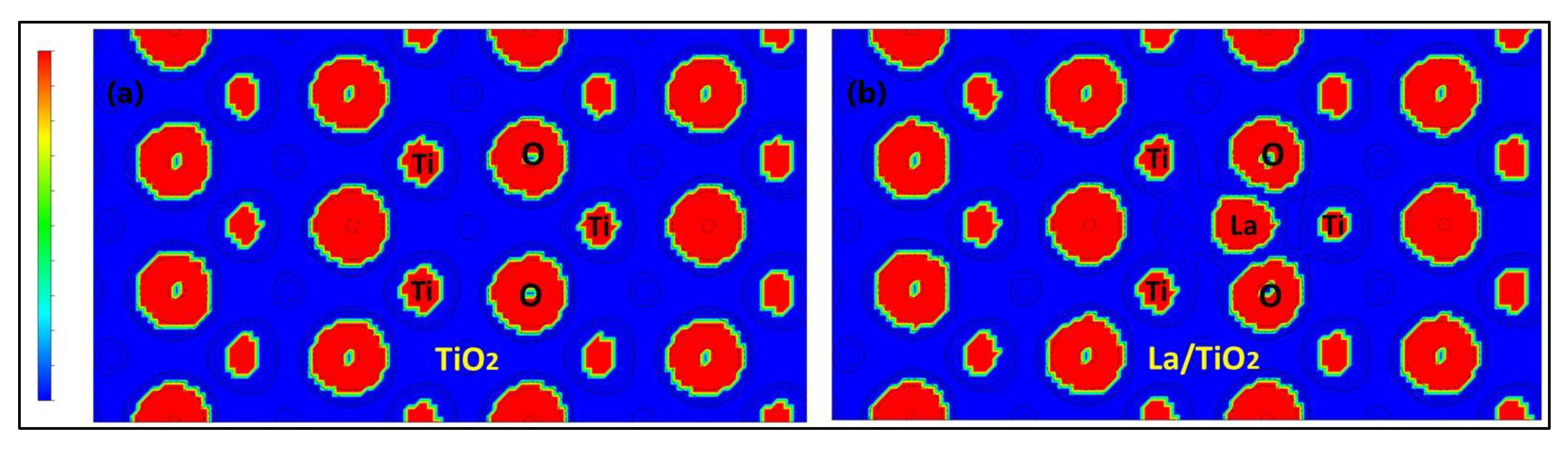
2.1. Optimization of La/TiO2
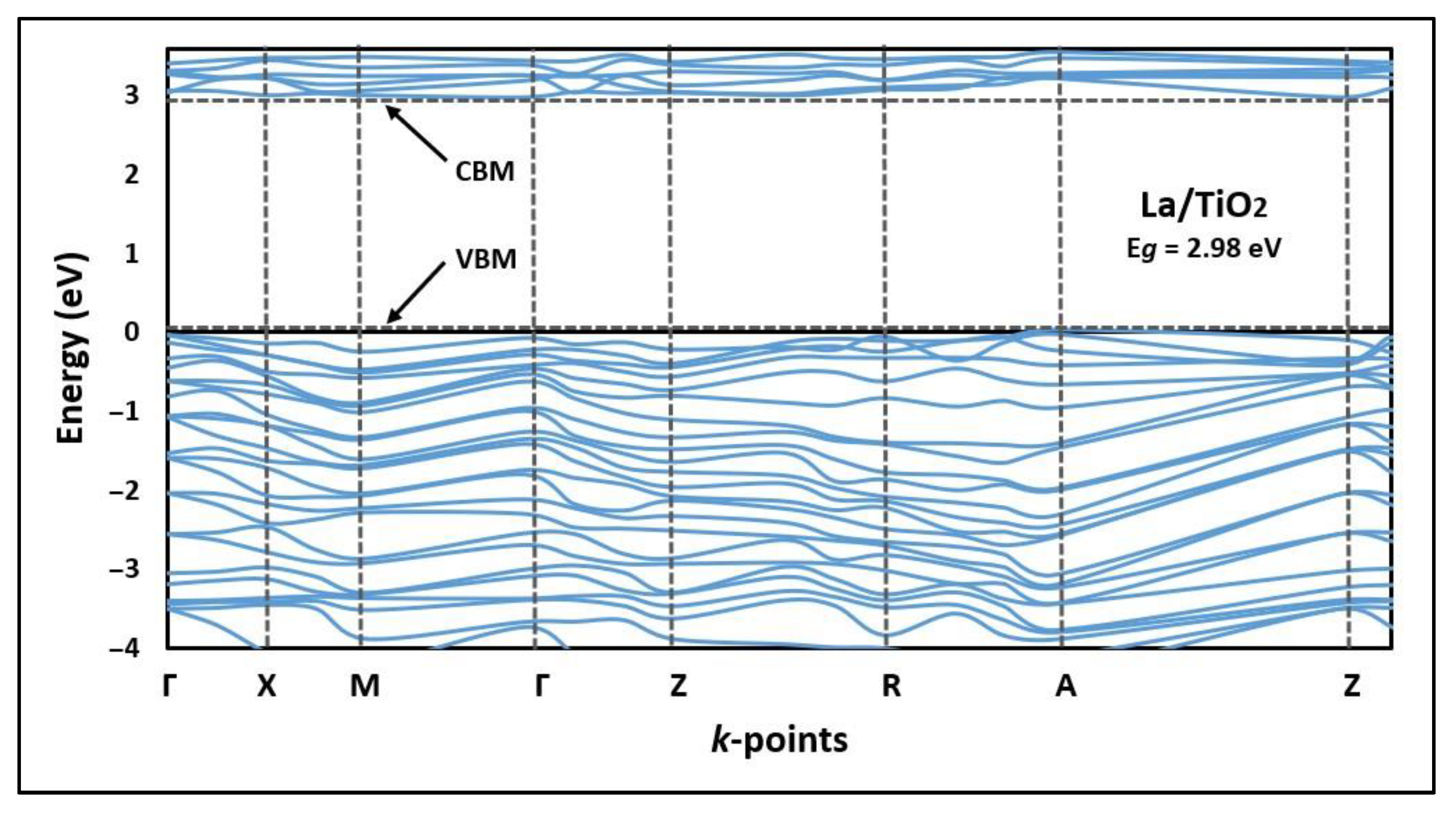
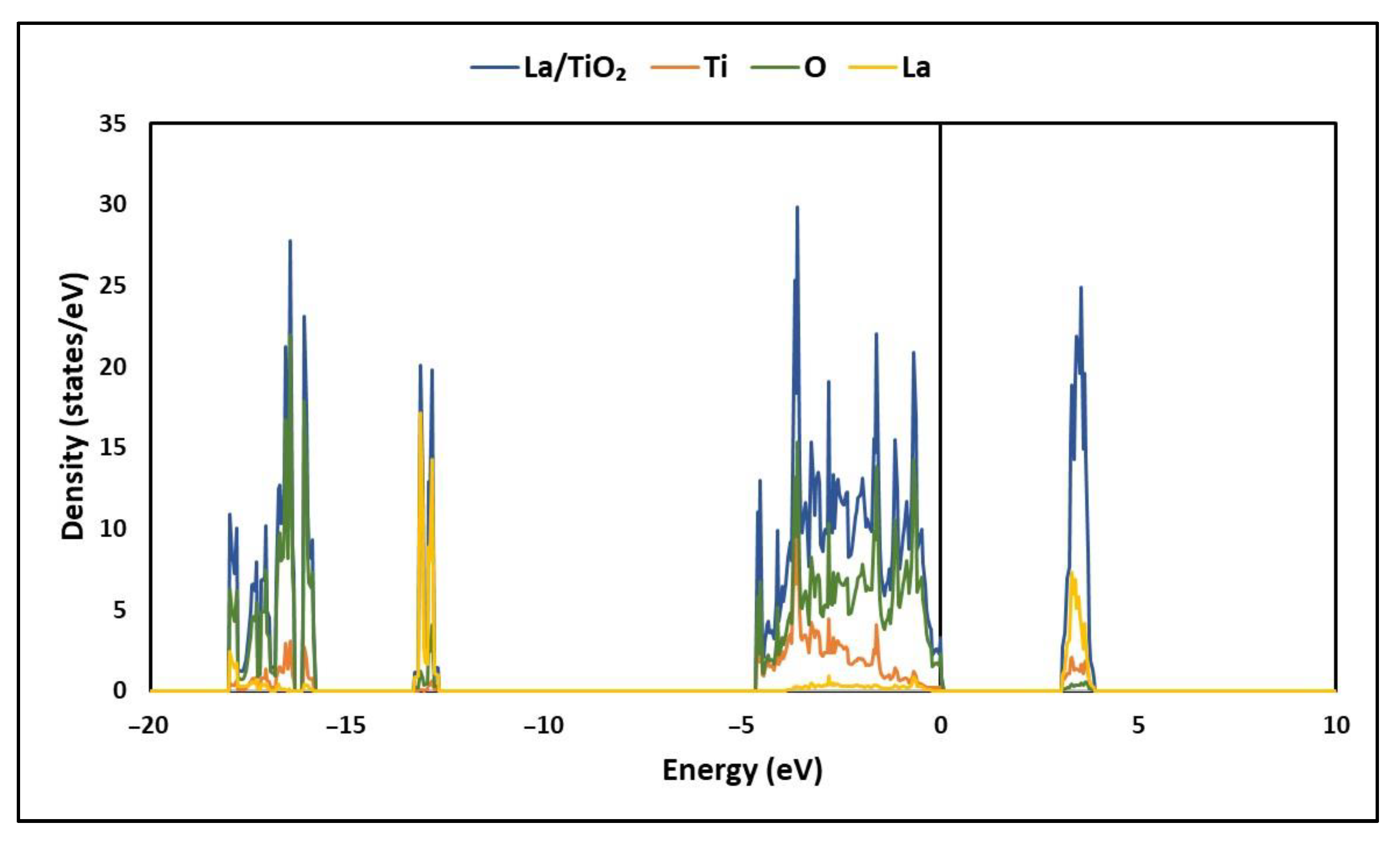
2.2. Electronic Structure of La/TiO2
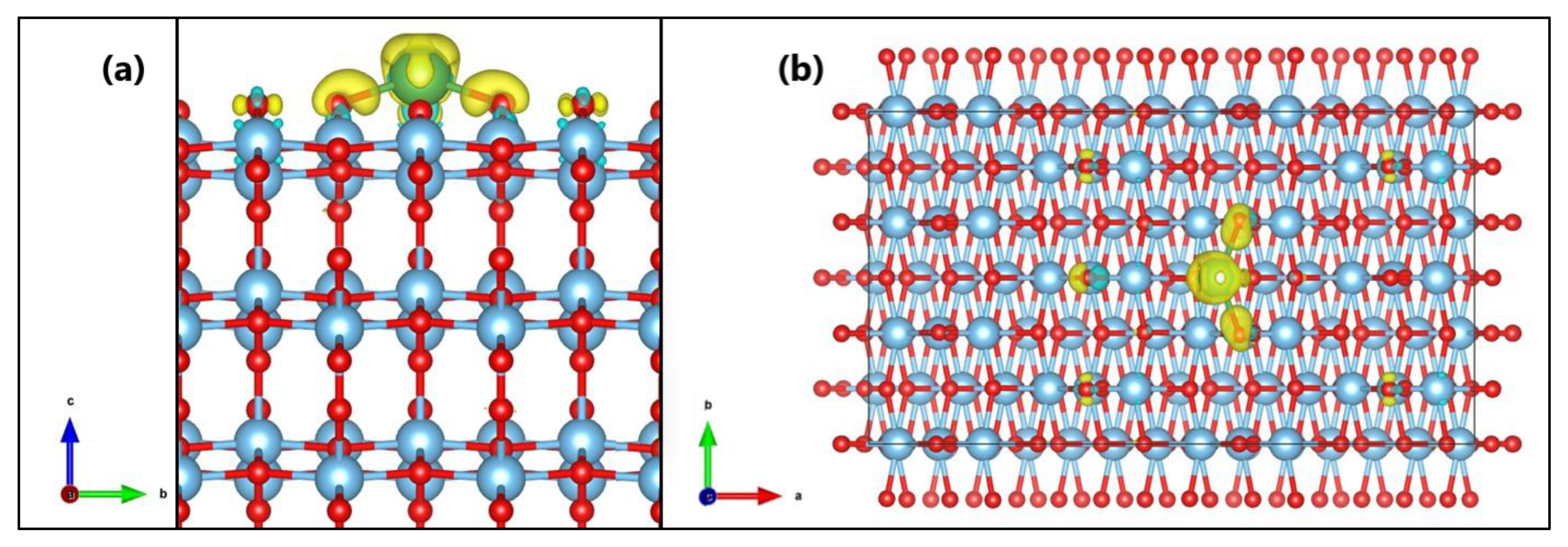
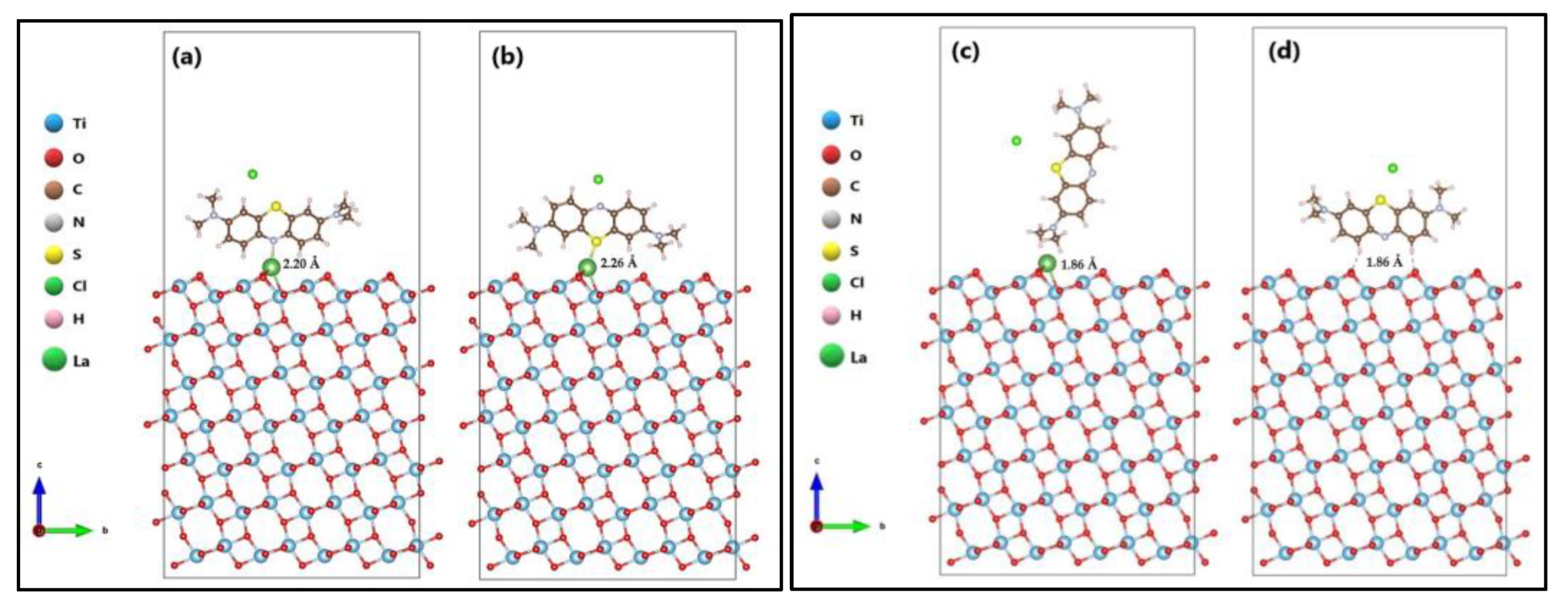
2.3. MB Adsorption on Surface (101) of TiO2 and La/TiO2
3. Discussion
3.1. Optimization of La/TiO2
3.2. Electronic Structure of La/TiO2
3.3. MB Adsorption on Surface (101) of TiO2 and La/TiO2
Proposed Photocatalytic Mechanism
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassani, F.; Liedl, G.L.; Wyder, P. Encyclopedia of Condensed Matter Physics; Elsevier Inc.: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Resnick, A. Introductory Quantum Mechanics with MATLAB® for Atoms, Molecules, Clusters and Nanocrystals; Taylor & Francis: Hoboken, NJ, USA, 2019; Volume 60. [Google Scholar]
- Koch, O.; Kreuzer, W.; Scrinzi, A. Approximation of the time-dependent electronic Schrödinger equation by MCTDHF. Appl. Math. Comput. 2006, 173, 960–976. [Google Scholar] [CrossRef]
- Buschow, K.; Cahn, R.; Flemings, M.; Ilschner, B.; Kramer, E.; Mahajan, S. The Science and Technology of Materials: An Introduction. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Liao, X.; Lu, R.; Xia, L.; Liu, Q.; Wang, H.; Zhao, K.; Wang, Z.; Zhao, Y. Density Functional Theory for Electrocatalysis. Energy Environ. Mater. 2021, 5, 157–185. [Google Scholar] [CrossRef]
- Al-Mahayni, H.; Wang, X.; Harvey, J.; Patience, G.S.; Seifitokaldani, A. Experimental methods in chemical engineering: Density functional theory. Can. J. Chem. Eng. 2021, 99, 1885–1911. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Capa, L.; Medina, F.; González, S. DFT Study of Methylene Blue Adsorption on ZnTiO3 and TiO2 Surfaces (101). Molecules 2021, 26, 3780. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Fierro, X.; Cuenca, G.; Ramón, J. The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study. Nanomaterials 2022, 12, 3137. [Google Scholar] [CrossRef]
- Xu, H.; Miao, B.; Zhang, M.; Chen, Y.; Wang, L. Mechanism of C–C and C–H bond cleavage in ethanol oxidation reaction on Cu2O(111): A DFT-D and DFT+U study. Phys. Chem. Chem. Phys. 2017, 19, 26210–26220. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Yang, Y.; Liu, F.; Yu, Y.; Yan, X. Molecular Mechanistic Nature of Elemental Mercury Oxidation by Surface Oxygens over the Co3O4 Catalyst. J. Phys. Chem. C 2020, 124, 4605–4612. [Google Scholar] [CrossRef]
- Abdollahizad, G.; Valadi, F.M.; Akbarzadeh, E.; Gholami, M.R. Adsorption Properties of Halloysite Modified Acrylamide/Quince Seeds-Based Hydrogel: Experimental and DFT Investigation. J. Polym. Environ. 2022, 1–14. [Google Scholar] [CrossRef]
- Sharifi, M.; Marjani, A.; Mahdavian, L.; Shamlouei, H.R. Density functional theory study of dyes removal from colored wastewater by a nano-composite of polysulfone/polyethylene glycol. J. Nanostructure Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Fan, X.; Wu, Z.; Wang, L.; Wang, C. Exploring the Origin of High Dechlorination Activity in Polar Materials M2B5O9Cl (M = Ca, Sr, Ba, Pb) with Built-In Electric Field. Chem. Mater. 2017, 29, 639–647. [Google Scholar] [CrossRef]
- Lei, Q.; Yang, S.; Ding, D.; Tan, J.; Liu, J.; Chen, R. Local-interaction-field-coupled semiconductor photocatalysis: Recent progress and future challenges. J. Mater. Chem. A 2021, 9, 2491–2525. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Solomon, S.; Daher, M.A.; Walker, G. Efficient removal of anionic and cationic dyes from aqueous systems using spent Yerba Mate “Ilex paraguariensis”. J. Taiwan Inst. Chem. Eng. 2018, 82, 144–155. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Afshariani, F.; Roosta, A. Experimental study and mathematical modeling of biosorption of methylene blue from aqueous solution in a packed bed of microalgae Scenedesmus. J. Clean. Prod. 2019, 225, 133–142. [Google Scholar] [CrossRef]
- Han, Q.; Wang, J.; Goodman, B.A.; Xie, J.; Liu, Z. High adsorption of methylene blue by activated carbon prepared from phosphoric acid treated eucalyptus residue. Powder Technol. 2020, 366, 239–248. [Google Scholar] [CrossRef]
- Moghazy, R.M.; Labena, A.; Husien, S. Eco-friendly complementary biosorption process of methylene blue using micro-sized dried biosorbents of two macro-algal species (Ulva fasciata and Sargassum dentifolium): Full factorial design, equilibrium, and kinetic studies. Int. J. Biol. Macromol. 2019, 134, 330–343. [Google Scholar] [CrossRef]
- Meza, C.L.; Kou, R.S.; Arroyo, T.C. Biosorción del colorante azul de metileno usando los cladodios de la tuna (Opuntia ficus indica). Rev. De La Soc. Química Del Perú 2020, 86, 231–245. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Vijayaraghavan, J.; Pushpa, T.B.; Basha, S.S.; Jegan, J. Isotherm, kinetics and mechanistic studies of methylene blue biosorption onto red seaweed Gracilaria corticata. Desalination Water Treat. 2016, 57, 13540–13548. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Stetsovych, O.; Todorovic, M.; Shimizu, T.K.; Moreno, C.; Ryan, J.; León, C.P.; Sagisaka, K.; Palomares, E.; Matolin, V.; Fujita, D.; et al. Atomic species identification at the (101) anatase surface by simultaneous scanning tunnelling and atomic force microscopy. Nat. Commun. 2015, 6, 7265. [Google Scholar] [CrossRef] [PubMed]
- Martsinovich, N.; Troisi, A. How TiO2 crystallographic surfaces influence charge injection rates from a chemisorbed dye sensitiser. Phys. Chem. Chem. Phys. 2012, 14, 13392–13401. [Google Scholar] [CrossRef] [PubMed]
- USGS. U.S. Geological Survey: Germanium Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/titanium-statistics-and-information (accessed on 27 August 2022).
- Liu, Z.; Zhong, X.; Liu, Y.; Rao, H.; Wei, H.; Hu, W.; Nie, X.; Liu, M. Adsorption and Mechanism of Glycine on the Anatase with Exposed (001) and (101) Facets. Minerals 2022, 12, 798. [Google Scholar] [CrossRef]
- Junior, A.B.B.; Espinosa, D.C.R.; Vaughan, J.; Tenório, J.A.S. Extraction of Rare-Earth Elements from Silicate-Based Ore through Hydrometallurgical Route. Metals 2022, 12, 1133. [Google Scholar] [CrossRef]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B Environ. 2017, 233, 301–317. [Google Scholar] [CrossRef]
- Ako, R.T.; Ekanayake, P.; Tan, A.L.; Young, D.J. La modified TiO2 photoanode and its effect on DSSC performance: A comparative study of doping and surface treatment on deep and surface charge trapping. Mater. Chem. Phys. 2016, 172, 105–112. [Google Scholar] [CrossRef]
- Colpani, G.L.; Santos, V.F.; Zeferino, R.C.F.; Zanetti, M.; de Mello, J.M.M.; Silva, L.L.; Padoin, N.; Moreira, R.D.F.P.M.; Fiori, M.A.; Soares, C. Propranolol hydrochloride degradation using La@TiO2 functionalized with CMCD. J. Rare Earths 2021, 40, 579–585. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, X.; Gao, G.; Gao, R.; Zhang, T.; Tian, M.; Su, H.; Wang, S. Understanding of photocatalytic partial oxidation of methanol to methyl formate on surface doped La(Ce) TiO2: Experiment and DFT calculation. J. Catal. 2022, 411, 31–40. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Wang, Y.-S.; Qin, Z.; Ke, S.-H. First-principles investigation of the ferroelectric, piezoelectric and nonlinear optical properties of LiNbO3-type ZnTiO3. Sci. Rep. 2019, 9, 17632. [Google Scholar] [CrossRef]
- Obodo, K.O.; Noto, L.L.; Mofokeng, S.J.; Ouma, C.N.M.; Braun, M.; Dhlamini, M.S. Influence of Tm, Ho and Er dopants on the properties of Yb activated ZnTiO3perovskite: A density functional theory insight. Mater. Res. Express 2018, 5, 106202. [Google Scholar] [CrossRef]
- Di Liberto, G.; Tosoni, S.; Pacchioni, G. Role of Heterojunction in Charge Carrier Separation in Coexposed Anatase (001)–(101) Surfaces. J. Phys. Chem. Lett. 2019, 10, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Fierro, X.; Pérez, S.G.; Jaramillo, X.; Cabello, F.M. Synthesis of the ZnTiO3/TiO2 Nanocomposite Supported in Ecuadorian Clays for the Adsorption and Photocatalytic Removal of Methylene Blue Dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Zenou, V.Y.; Bakardjieva, S. Microstructural analysis of undoped and moderately Sc-doped TiO2 anatase nanoparticles using Scherrer equation and Debye function analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Pan, Y.; Wen, M. Noble metals enhanced catalytic activity of anatase TiO2 for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 22055–22063. [Google Scholar] [CrossRef]
- Paterson, A.L.; Hanson, M.A.; Werner-Zwanziger, U.; Zwanziger, J.W. Relating 139La Quadrupolar Coupling Constants to Polyhedral Distortion in Crystalline Structures. J. Phys. Chem. C 2015, 119, 25508–25517. [Google Scholar] [CrossRef]
- Maldonado, F.; Villamagua, L.; Rivera, R. DFT Analysis of the Adsorption of Phenol on the Nonpolar () ZnO Surface. J. Phys. Chem. C 2019, 123, 12296–12304. [Google Scholar] [CrossRef]
- Hinuma, Y.; Pizzi, G.; Kumagai, Y.; Oba, F.; Tanaka, I. Band structure diagram paths based on crystallography. Comput. Mater. Sci. 2017, 128, 140–184. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, J.; Yang, Y.; Lu, Z.; Shi, W. Understanding electronic and optical properties of La and Mn co-doped anatase TiO2. Comput. Condens. Matter 2016, 6, 5–17. [Google Scholar] [CrossRef]
- Guo, W.; She, Z.; Yang, S.; Xue, H.; Zhang, X. Understanding the influence of Lu, La and Ga active elements on the bonding properties of Sn/SiO2 interfaces from first principle calculations. Ceram. Int. 2020, 46, 24737–24743. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.; Golub, P.; Manzhos, S. Stability of charges in titanium compounds and charge transfer to oxygen in titanium dioxide. J. Phys. Conf. Ser. 2018, 1136, 012017. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, W.; Wang, W.-C.; Shi, X.-Q. Ionicity of bonding in elemental solids. J. Phys. Commun. 2018, 2, 115009. [Google Scholar] [CrossRef]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
- Savin, P.-D.A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Zhang, L.; Ying, F.; Wu, W.; Hiberty, P.C.; Shaik, S. Topology of Electron Charge Density for Chemical Bonds from Valence Bond Theory: A Probe of Bonding Types. Chem.–A Eur. J. 2009, 15, 2979–2989. [Google Scholar] [CrossRef]
- Colpani, G.L.; Zanetti, M.; Zeferino, R.C.F.; Silva, L.L.; de Mello, J.M.M.; Riella, H.G.; Padoin, N.; Fiori, M.A.; Soares, C. Lanthanides Effects on TiO2 Photocatalysts. In Photocatalysts-Applications and Attributes; IntechOpen: London, UK, 2019. [Google Scholar]
- Wang, B.; Zhang, G.; Sun, Z.; Zheng, S.; Frost, R.L. A comparative study about the influence of metal ions (Ce, La and V) doping on the solar-light-induced photodegradation toward rhodamine B. J. Environ. Chem. Eng. 2015, 3, 1444–1451. [Google Scholar] [CrossRef]
- Wang, B.; de Godoi, F.C.; Zheng, S.; Gentle, I.R.; Li, C. Enhanced photocatalytic properties of reusable TiO 2 -loaded natural porous minerals in dye wastewater purification. Powder Technol. 2016, 302, 426–433. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Medina, F. La-Doped ZnTiO3/TiO2 Nanocomposite Supported on Ecuadorian Diatomaceous Earth as a Highly Efficient Photocatalyst Driven by Solar Light. Molecules 2021, 26, 6232. [Google Scholar] [CrossRef]
- Jin, M.; Nagaoka, Y.; Nishi, K.; Ogawa, K.; Nagahata, S.; Horikawa, T.; Katoh, M.; Tomida, T.; Hayashi, J. Adsorption properties and photocatalytic activity of TiO2 and La-doped TiO2. Adsorption 2008, 14, 257–263. [Google Scholar] [CrossRef]
- Han, M.; Dong, Z.; Liu, J.; Ren, G.; Ling, M.; Yang, X.; Zhang, L.; Xue, B.; Li, F. The role of lanthanum in improving the visible-light photocatalytic activity of TiO2 nanoparticles prepared by hydrothermal method. Appl. Phys. A 2020, 126, 950. [Google Scholar] [CrossRef]
- Prakash, J.; Samriti; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Coelho, L.L.; Hotza, D.; Estrella, A.S.; de Amorim, S.M.; Puma, G.L.; Moreira, R.D.F.P.M. Modulating the photocatalytic activity of TiO2 (P25) with lanthanum and graphene oxide. J. Photochem. Photobiol. A: Chem. 2019, 372, 1–10. [Google Scholar] [CrossRef]
- Priyanka, K.; Revathy, V.; Rosmin, P.; Thrivedu, B.; Elsa, K.; Nimmymol, J.; Balakrishna, K.; Varghese, T. Influence of La doping on structural and optical properties of TiO2 nanocrystals. Mater. Charact. 2016, 113, 144–151. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Tavakoli, A.; Ziarani, G.M. Ultrasonic-assisted synthesis of Ce doped cubic–hexagonal ZnTiO3 with highly efficient sonocatalytic activity. Ultrason. Sonochemistry 2016, 29, 258–269. [Google Scholar] [CrossRef]
- Khan, S.; Cho, H.; Kim, D.; Han, S.S.; Lee, K.H.; Cho, S.-H.; Song, T.; Choi, H. Defect engineering toward strong photocatalysis of Nb-doped anatase TiO2: Computational predictions and experimental verifications. Appl. Catal. B: Environ. 2017, 206, 520–530. [Google Scholar] [CrossRef]
- Meksi, M.; Turki, A.; Kochkar, H.; Bousselmi, L.; Guillard, C.; Berhault, G. The role of lanthanum in the enhancement of photocatalytic properties of TiO2 nanomaterials obtained by calcination of hydrogenotitanate nanotubes. Appl. Catal. B Environ. 2016, 181, 651–660. [Google Scholar] [CrossRef]
- Cherifi, K.; Cheknane, A.; Benghia, A.; Hilal, H.S.; Rahmoun, K.; Benyoucef, B.; Goumri-Said, S. Exploring N3 ruthenium dye adsorption onto ZnTiO3 (101) and (110) surfaces for dye sensitized solar cell applications: Full computational study. Mater. Today Energy 2019, 13, 109–118. [Google Scholar] [CrossRef]
- Tang, X.; Xue, Q.; Qi, X.; Cheng, C.; Yang, M.; Yang, T.; Chen, F.; Qiu, F.; Quan, X. DFT and experimental study on visible-light driven photocatalysis of rare-earth-doped TiO2. Vacuum 2022, 200, 110972. [Google Scholar] [CrossRef]
- Mulwa, W.M.; Ouma, C.N.; Onani, M.O.; Dejene, F.B. Energetic, electronic and optical properties of lanthanide doped TiO2: An ab initio LDA+U study. J. Solid State Chem. 2016, 237, 129–137. [Google Scholar] [CrossRef]
- Raghav, A.; Hongo, K.; Maezono, R.; Panda, E. Electronic structure and effective mass analysis of doped TiO2 (anatase) systems using DFT+U. Comput. Mater. Sci. 2022, 214, 111714. [Google Scholar] [CrossRef]
- Kumar, V.V.; Naresh, G.; Sudhakar, M.; Tardio, J.; Bhargava, S.K.; Venugopal, A. Role of Brønsted and Lewis acid sites on Ni/TiO2 catalyst for vapour phase hydrogenation of levulinic acid: Kinetic and mechanistic study. Appl. Catal. A Gen. 2015, 505, 217–223. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, X.; Huo, Y.; Tan, Y.; Zhang, C.; Zou, L. Construction of a Brönsted-Lewis solid acid catalyst La-PW-SiO2/SWCNTs based on electron withdrawing effect of La(III) on π bond of SWCNTs for biodiesel synthesis from esterification of oleic acid and methanol. Chin. J. Chem. Eng. 2022, 44, 351–362. [Google Scholar] [CrossRef]
- Li, H.; Feng, B. Visible-light-driven composite La2O3/TiO2 nanotube arrays: Synthesis and improved photocatalytic activity. Mater. Sci. Semicond. Process. 2016, 43, 55–59. [Google Scholar] [CrossRef]
- Khnifira, M.; El Hamidi, S.; Machrouhi, A.; Mahsoune, A.; Boumya, W.; Tounsadi, H.; Mahjoubi, F.Z.; Sadiq, M.; Barka, N.; Abdennouri, M. Theoretical and experimental study of the adsorption characteristics of Methylene Blue on titanium dioxide surface using DFT and Monte Carlo dynamic simulation. Desalination Water Treat. 2020, 190, 393–411. [Google Scholar] [CrossRef]
- Papanikolaou, K.G.; Stamatakis, M. Toward the accurate modeling of the kinetics of surface reactions using the kinetic Monte Carlo method. Front. Nanosci. 2020, 17, 95–125. [Google Scholar] [CrossRef]
- Chaker, H.; Ameur, N.; Saidi-Bendahou, K.; Djennas, M.; Fourmentin, S. Modeling and Box-Behnken design optimization of photocatalytic parameters for efficient removal of dye by lanthanum-doped mesoporous TiO2. J. Environ. Chem. Eng. 2020, 9, 104584. [Google Scholar] [CrossRef]
- Yu, J.; Li, D.; Zhu, L.; Xu, X. Application of ZnTiO3 in quantum-dot-sensitized solar cells and numerical simulations using first-principles theory. J. Alloys Compd. 2016, 681, 88–95. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Sujith, C.; Joseph, S.; Mathew, T.; Mathew, V. First-principles investigation of structural, electronic and optical properties of quasi-one-dimensional barium cadmium chalcogenides Ba2CdX3 (X = S, Se, Te) using HSE06 and GGA-PBE functionals. J. Phys. Chem. Solids 2022, 161, 110488. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L. Quantum Density Oscillations in an Inhomogeneous Electron Gas. Phys. Rev. 1965, 137, A1697–A1705. [Google Scholar] [CrossRef]
- German, E.; Faccio, R.; Mombrú, A.W. Comparison of standard DFT and Hubbard-DFT methods in structural and electronic properties of TiO2 polymorphs and H-titanate ultrathin sheets for DSSC application. Appl. Surf. Sci. 2018, 428, 118–123. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Hernández, K.; González, S. Cu(C3H3N3S3)3 Adsorption onto ZnTiO3/TiO2 for Coordination-Complex Sensitized Photochemical Applications. Materials 2022, 15, 3252. [Google Scholar] [CrossRef]
- Cherifi, K.; Cheknane, A.; Hilal, H.S.; Benghia, A.; Rahmoun, K.; Benyoucef, B. Investigation of triphenylamine-based sensitizer characteristics and adsorption behavior onto ZnTiO3 perovskite (1 0 1) surfaces for dye-sensitized solar cells using first-principle calculation. Chem. Phys. 2020, 530, 110595. [Google Scholar] [CrossRef]
- Nor, N.U.M.; Mazalan, E.; Risko, C.; Crocker, M.; Amin, N.A.S. Unveiling the structural, electronic, and optical effects of carbon-doping on multi-layer anatase TiO2 (1 0 1) and the impact on photocatalysis. Appl. Surf. Sci. 2022, 586, 152641. [Google Scholar] [CrossRef]
- Samanta, P.; English, N.J. Opto-electronic properties of stable blue photosensitisers on a TiO2 anatase-101 surface for efficient dye-sensitised solar cells. Chem. Phys. Lett. 2019, 731, 136624. [Google Scholar] [CrossRef]
- Chang, X.; Li, X.; Xue, Q. Sensing mechanism of acetone adsorption on charged ZnO and ZnSe surfaces: Insights from DFT calculations. Mater. Today Commun. 2022, 31, 103238. [Google Scholar] [CrossRef]
- Lai, W.; Zhang, K.; Shao, P.; Yang, L.; Ding, L.; Pavlostathis, S.G.; Shi, H.; Zou, L.; Liang, D.; Luo, X. Optimization of adsorption configuration by DFT calculation for design of adsorbent: A case study of palladium ion-imprinted polymers. J. Hazard. Mater. 2019, 379, 120791. [Google Scholar] [CrossRef] [PubMed]

| Atoms | Bond Length (Å) | Atoms | Angle (°) |
|---|---|---|---|
| La-O62 | 2.19 | La-O62-Ti87 | 92.95 |
| La-O62-Ti86 | 114.27 | ||
| La-O66 | 2.19 | La-O66-Ti143 | 92.96 |
| La-O66-Ti142 | 114.29 | ||
| La-O178 | 2.43 | La-O178-Ti143 | 90.30 |
| La-O178-Ti57 | 91.13 | ||
| La-O178-Ti87 | 91.97 |
| Position | Bond | ∆Eads (kJ/mol) | ∆Gseg (kJ/mol) |
|---|---|---|---|
| La/TiO2 (PN) | La-N | −270.14 | −58.19 |
| La/TiO2 (PS) | La-S | −112.75 | −57.00 |
| La/TiO2 (PV) | - | +78.35 | - |
| TiO2 (PH) | O-H | −95.30 | - |
| Absorption System | Atom | Total Electron (−e) before Adsorption | Total Electron (−e) after Adsorption | Transfer Charge (−e) |
|---|---|---|---|---|
| MB absorbed on La/TiO2 (PN) | N1(523) | 7.2142 | 7.6493 | −0.4351 |
| La1(505) | 9.2964 | 9.1019 | +0.1945 | |
| MB absorbed on TiO2 (PH) | H6(531) | 0.8447 | 0.8098 | +0.035 |
| H2(527) | 0.7363 | 0.7241 | +0.012 | |
| O66(234) | 7.1144 | 7.1951 | −0.0807 | |
| O59(227) | 7.1574 | 7.2155 | −0.0581 |
| Adsorbent | Bandgap Energy (eV) | Energy Difference (eV) | Method | Reference |
|---|---|---|---|---|
| TiO2 | 3.10 | 0.18 | Experimental | [52] |
| La/TiO2 | 2.92 | |||
| TiO2 (rutile) | 1.94 | 0.15 | CASTEP (GGA/PBE) | [64] |
| La/TiO2 (rutile) | 1.79 | |||
| TiO2 (anatase) | 3.20 | 0.70 | Quantum ESPRESSO (LDA + U) | [65] |
| La/TiO2 (anatase) | 2.50 | |||
| TiO2 (anatase) | 2.72 | 0.38 | VASP (GGA/PBE + U) | [66] |
| La/TiO2 (anatase) | 2.34 | |||
| TiO2 (anatase) | 3.21 | 0.23 | VASP (GGA/PBE + U) | [7] |
| La/TiO2 (anatase) | 2.98 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; Gaona, S.; Valarezo, E. La3+’s Effect on the Surface (101) of Anatase for Methylene Blue Dye Removal, a DFT Study. Molecules 2022, 27, 6370. https://doi.org/10.3390/molecules27196370
Jaramillo-Fierro X, Gaona S, Valarezo E. La3+’s Effect on the Surface (101) of Anatase for Methylene Blue Dye Removal, a DFT Study. Molecules. 2022; 27(19):6370. https://doi.org/10.3390/molecules27196370
Chicago/Turabian StyleJaramillo-Fierro, Ximena, Sneyder Gaona, and Eduardo Valarezo. 2022. "La3+’s Effect on the Surface (101) of Anatase for Methylene Blue Dye Removal, a DFT Study" Molecules 27, no. 19: 6370. https://doi.org/10.3390/molecules27196370
APA StyleJaramillo-Fierro, X., Gaona, S., & Valarezo, E. (2022). La3+’s Effect on the Surface (101) of Anatase for Methylene Blue Dye Removal, a DFT Study. Molecules, 27(19), 6370. https://doi.org/10.3390/molecules27196370









