High-Pressure Metal-Free Catalyzed One-Pot Two-Component Synthetic Approach for New 5-Arylazopyrazolo[3,4-b]Pyridine Derivatives
Abstract
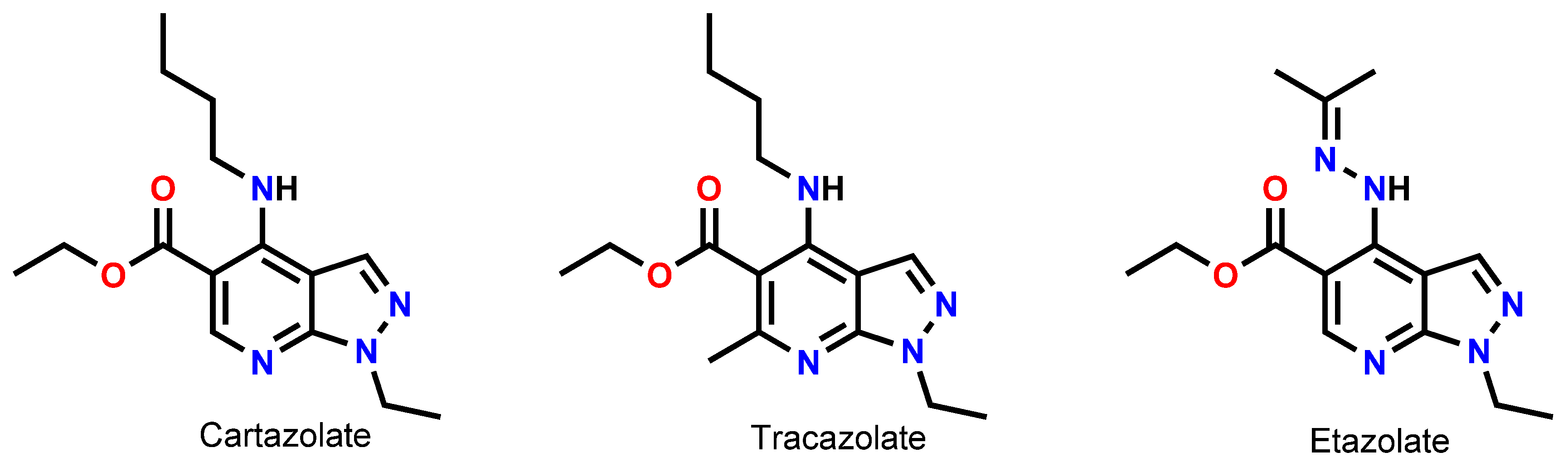
1. Introduction
2. Materials and Methods
2.1. General
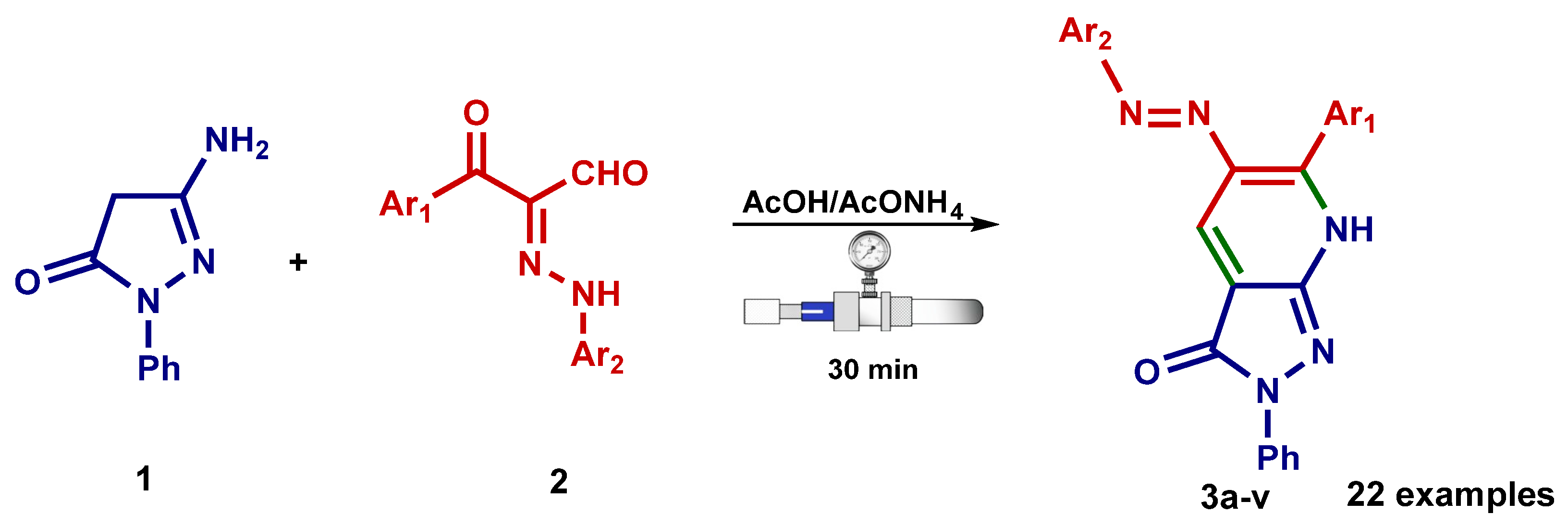
2.2. General Protocol for the Synthesis of Compounds 3a–v
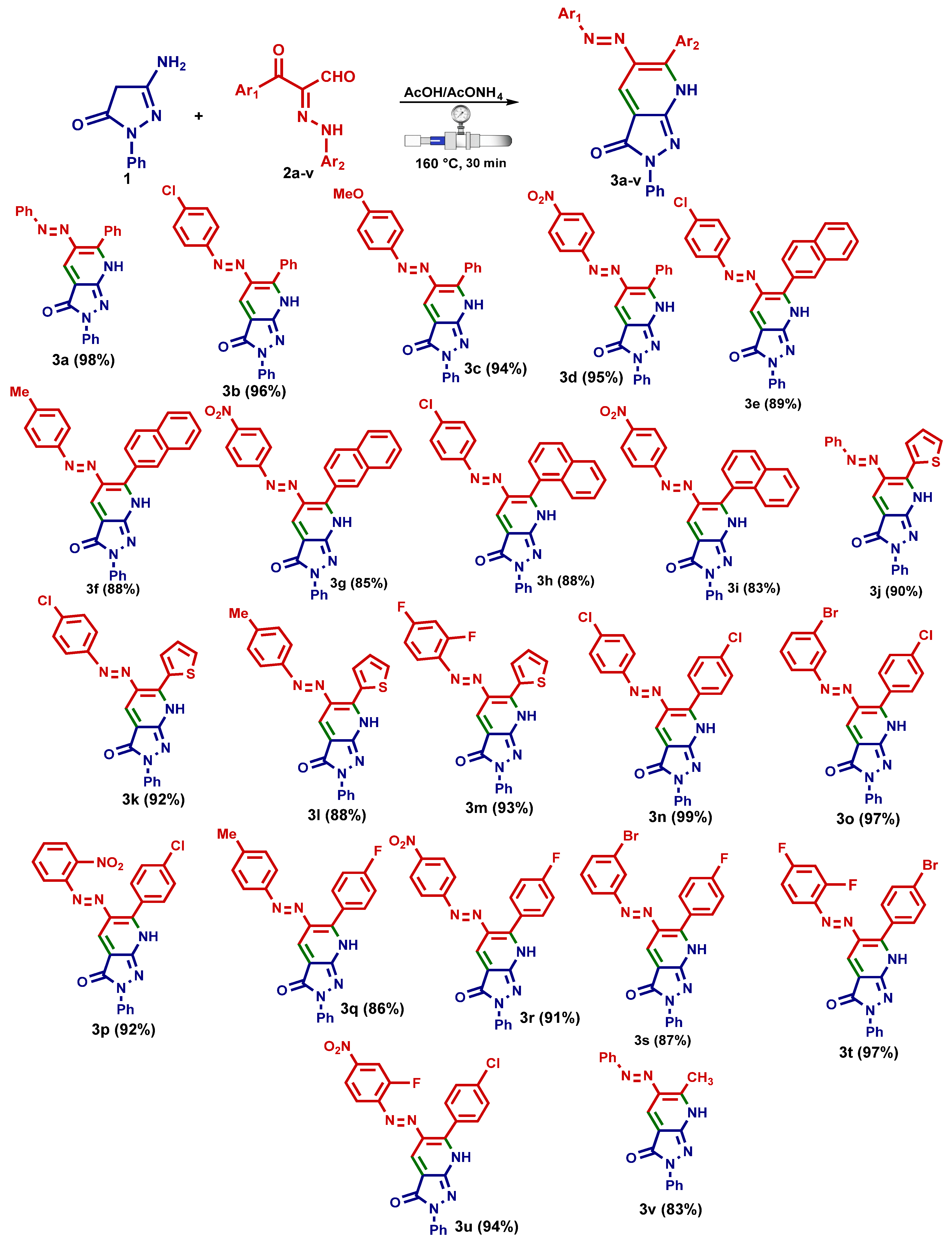
- (E)-2,6-Diphenyl-5-(phenyldiazenyl)-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3a). Yellow crystals [EtOH/dioxane mixture (1:3)], yield: 1.90 g (98%), m.p. 255–256 °C; FT-IR 𝜈/cm−1: 3105 (N-H), 1656 (C=O), 1606 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.27–7.59 (m, 9H, Ar-H), 7.74–7.99 (m, 6H, Ar-H), 8.52 (s, 1H, C-H4), 12.34 ppm (brs, 1H, NH); 13C NMR (TFA-d, 100 MHz): δ 114.74, 12571, 126.29, 130.96, 131.47, 131.69, 132.27, 133.00, 133.23, 134.61, 135.32, 135.81, 136.00, 141.85, 146.39, 153.04, 159.07, 159.33 ppm; MS (EI) m/z (%): 392 (M+ + 1, 18.04), 391 (M+, 69.57), 390 (M+ − 1, 100); HRMS (EI) m/z: [M]+ calcd for C24H17N5O 391.1428, found 391.1428.
- (E)-5-[(4-Chlorophenyl)diazenyl]-2,6-diphenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3b). Pale yellow crystals (dioxane), yield: 2.00 g (96%), m.p. 283–284 °C; FT-IR 𝜈/cm−1: 3106 (N-H), 1650 (C=O), 1620 (C=N); 1H NMR (TFA-d, 600 MHz): δ 8.19 (d, J = 8.4 Hz, 2H, Ar-H), 8.31–8.36 (m, 3H, Ar-H), 8.42–8.46 (m, 4H, Ar-H), 8.51–8.53 (m, 3H, Ar-H), 8.60 (d, J = 8.4 Hz, 2H, Ar-H), 10.18 ppm (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 115.00, 126.97, 12765, 131.83, 132.07, 132.54, 132.92, 133.57, 133.80, 135.32, 136.28, 136.44, 142.90, 143.31, 147.49, 153.36, 159.33, 159.68 ppm; MS (EI) m/z (%): 427 (M+ + 2, 25.31), 426 (M+ + 1, 52.13), 425 (M+, 76.98), 424 (M+ − 1, 100); HRMS (EI) m/z: [M]+ calcd for C24H16ClN5O (M+) 425.1038, found 425.1038.
- (E)-5-[(4-Methoxyphenyl)diazinyl]-2,6-diphenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3c). Yellow crystals [EtOH/dioxane mixture (1:2)], yield: 1.95 g (94%), m.p. 273–274 °C; FT-IR 𝜈/cm−1: 3116 (N-H), 1655 (C=O), 1602 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 3.82 (s, 3H, OCH3), 7.08 (d, J = 9.0 Hz, 2H, Ar-H), 7.27 (t, J = 7.2 Hz, 1H, Ar-H), 7.51 (t, J = 7.8 Hz, 2H, Ar-H), 7.54–7.56 (m, 3H, Ar-H), 7.71 (d, J = 7.8 Hz, 2H, Ar-H), 7.78 (d, J = 9.0 Hz, 2H, Ar-H), 7.98 (d, J = 7.2 Hz, 2H, Ar-H), 8.45 (s, 1H, C-H4), 12.30 ppm (brs, 1H, NH); 13C NMR (TFA-d, 100 MHz): δ 58.39 (OCH3), 113.77, 119.43, 126.38, 130.11, 130.75, 132.09, 132.41, 133.46, 133.56, 136.09, 136.46, 136.77, 137.03, 141.19, 146.86, 157.89, 160.54, 170.93 ppm; MS (EI) m/z (%): 422 (M+ + 1, 27.18), 421 (M+, 96.22), 420 (M+ − 1, 100); HRMS (EI) m/z: [M]+ calcd for C25H19N5O2 (M+) 421.1533, found 421.1533.
- (E)-5-[(4-Nitrophenyl)diazinyl]-2,6-diphenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3d). Orange crystals [EtOH/DMF mixture (1:2)], yield: 2.05 g (95%), m.p. 299–300 °C; FT-IR 𝜈/cm−1: 3103 (N-H), 1659 (C=O), 1607 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.27 (t, J = 7.2 Hz, 1H, Ar-H), 7.51 (t, J = 7.8 Hz, 2H, Ar-H), 7.57–7.62 (m, 3H, Ar-H), 7.81 (d, J = 7.2 Hz, 2H, Ar-H), 7.87 (d, J = 8.4 Hz, 2H, Ar-H), 8.02 (d, J = 8.4 Hz, 2H, Ar-H), 8.37 (d, J = 7.8 Hz, 2H, Ar-H), 8.59 (s, 1H, C-H4), 12.33 ppm (brs, 1H, NH); 13C NMR (TFA-d, 100 MHz): δ 114.24, 126.56, 126.82, 127.42, 131.24, 131.71, 132.51, 133.28, 133.44, 134.96, 135.62, 136.17, 142.31, 147.04, 151.78, 157.88, 159.22, 159.79 ppm; MS (EI) m/z (%): 437 (M+ + 1, 28.19), 436 (M+, 92.89), 435 (M+ − 1, 100); HRMS (EI) m/z: [M]+ calcd for C24H16N6O3 (M+) 436.1278, found 436.1278.
- (E)-5-[(4-Chlorophenyl)diazinyl]-6-(naphthalen-2-yl)-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3e). Pale orange crystals [EtOH/DMF mixture (1:3)], yield: 2.10 g (89%), m.p. 289–290 °C; FT-IR 𝜈/cm−1: 3111 (NH), 1653 (C=O), 1621 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.28 (t, J = 7.2 Hz, 1H, Ar-H), 7.53 (t, J = 7.8 Hz, 2H, Ar-H), 7.60–7.74 (m, 6H, Ar-H), 7.93–8.09 (m, 6H, Ar-H), 8.38 (s, 1H, Ar-H), 8.59 (s, 1H, C-H4), 12.57 ppm (brs, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 113.98, 126.49, 127.36, 128.32, 128.52, 130.40, 130.68, 131.57, 131.80, 132.25, 132.52, 132.65, 133.30, 134.59, 135.31, 135.81, 135.87, 138.06, 142.50, 142.56, 147.02, 152.71, 158.64, 159.05 ppm; MS (EI) m/z (%): 477 (M+ + 2, 34.11), 476 (M+ + 1, 52.35), 475 (M+, 100.00), 474 (M+ − 1, 71.98); HRMS (EI) m/z: [M]+ calcd for C28H18ClN5O (M+) 475.1194, found 475.1195.
- (E)-6-(Naphthalen-2-yl)-2-phenyl-5-(p-tolyldiazenyl)-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3f). Orange crystals [EtOH/DMF mixture (1:2)], yield: 2.00 g (88%), m.p. 284–285 °C; FT-IR 𝜈/cm−1: 3116 (NH), 1656 (C=O), 1605 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 2.36 (s, 3H, CH3), 7.29 (t, J = 7.8 Hz, 1H, Ar-H), 7.34 (d, J = 7.8 Hz, 2H, Ar-H), 7.53 (t, J = 7.8 Hz, 2H, Ar-H), 7.61–7.68 (m, 4H, Ar-H), 7.95 (d, J = 7.8 Hz, 1H, Ar-H), 8.01–8.09 (m, 5H, Ar-H), 8.38 (s, 1H, Ar-H), 8.55 (s, 1H, C-H4), 12.42 ppm (brs, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 22.68 (CH3), 115.00, 125.88, 126.30, 128.20, 128.40, 130.36, 130.70, 131.63, 131.91, 132.57, 132.64, 133.06, 133.10, 133.41, 134.23, 135.26, 136.15, 138.16, 140.78, 146.82, 149.10, 150.03, 159.45, 159.57 ppm; MS (EI) m/z (%): 456 (M+ + 1, 29.67), 455 (M+, 100.00), 454 (M+ − 1, 72.86); HRMS (EI) m/z: [M]+ calcd for C29H21N5O (M+) 455.1741, found 455.1740.
- (E)-6-(Naphthalen-2-yl)-5-[(4-nitrophenyl)diazinyl]-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3g). Deep orange crystals [EtOH/DMF mixture (1:3)], yield: 2.05 g (85%), m.p. above 300 °C; FT-IR 𝜈/cm−1: 3108 (NH), 1654 (C=O), 1622 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.27 (t, J = 7.8 Hz, 1H, Ar-H), 7.52 (t, J = 7.8 Hz, 2H, Ar-H), 7.60–7.69 (m, 4H, Ar-H), 7.88 (d, J = 7.8 Hz, 1H, Ar-H), 7.94–8.06 (m, 4H, Ar-H), 8.10 (d, J = 7.8 Hz, 2H, Ar-H), 8.36 (d, J = 8.4 Hz, 1H, Ar-H), 8.42 (s, 1H, Ar-H), 8.65 (s, 1H, C-H4), 11.99 ppm (brs, 1H, NH); 13C NMR (TFA-d, 100 MHz): δ 114.13, 126.98, 127.16, 127.28, 127.92, 128.81, 129.04, 129.83, 130.82, 131.10, 131.97, 132.25, 133.02, 135.09, 135.74, 136.20, 136.51, 138.47, 142.67, 147.85, 152.21, 158.34, 159.40, 160.19 ppm; MS (EI) m/z (%): 487 (M+ + 1, 29.98), 486 (M+, 100.00), 485 (M+ − 1, 66.18); HRMS (EI) m/z: [M]+ calcd for C28H18N6O3 (M+) 486.1435, found 486.1435.
- (E)-5-[(4-Chlorophenyl)diazinyl]-6-(naphthalen-1-yl)-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3h). Orange crystals [EtOH/DMF mixture (1:3)], yield: 2.10 g (88%), m.p. 277–278 °C; FT-IR 𝜈/cm−1: 3117 (NH), 1659 (C=O), 1611 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.25–7.27 (m, 3H, Ar-H), 7.42 (d, J = 8.4 Hz, 2H, Ar-H), 7.47 (t, J = 7.2 Hz, 1H, Ar-H), 7.51 (t, J = 7.8 Hz, 2H, Ar-H), 7.68–7.74 (m, 3H, Ar-H), 8.06–8.08 (m, 3H, Ar-H), 8.16 (d, J = 7.8 Hz, 2H, Ar-H), 8.63 (s, 1H, C-H4), 12.52 ppm (brs, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 115.23, 125.98, 126.37, 126.73, 126.83, 128.73, 129.46, 130.67, 131.12, 131.52, 132.07, 132.30, 133.12, 133.46, 134.20, 135.00, 135.98, 136.00, 142.12, 143.86, 146.54, 152.40, 158.81, 159.28 ppm; MS (EI) m/z (%): 477 (M+ + 2, 28.97), 476 (M+ + 1, 52.00), 475 (M+, 94.87), 474 (M+ − 1, 100.00); HRMS (EI) m/z: [M]+ calcd for C28H18ClN5O (M+) 475.1194, found 475.1194.
- (E)-6-(Naphthalen-1-yl)-5-[(4-nitrophenyl)diazinyl]-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3i). Orange crystals [EtOH/DMF mixture (1:3)], yield: 2.00 g (83%), m.p. 275–276 °C; FT-IR 𝜈/cm−1: 3112 (NH), 1660 (C=O), 1618 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.26 (t, J = 7.2 Hz, 1H, Ar-H), 7.38 (d, J = 9.0 Hz, 2H, Ar-H), 7.47–7.51 (m, 3H, Ar-H), 7.58 (t, J = 7.8 Hz, 1H, Ar-H), 7.69–7.74 (m, 2H, Ar-H), 7.77 (d, J = 7.8 Hz, 1H, Ar-H), 8.05 (d, J = 7.8 Hz, 2H, Ar-H), 8.08 (d, J = 7.8 Hz, 1H, Ar-H), 7.15–7.18 (m, 3H, Ar-H), 8.68 (s, 1H, C-H4), 12.28 ppm (brs, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 115.07, 126.54, 12671, 126.90, 127.16, 127.23, 129.10, 129.19, 129.81, 131.00, 131.52, 132.27, 132.53, 132.60, 133.46, 133.90, 134.28, 136.37, 143.54, 147.26, 151.63, 157.86, 159.31, 160.26 ppm; MS (EI) m/z (%): 487 (M+ + 1, 26.02), 486 (M+, 100.00), 485 (M+ − 1, 97.84); HRMS (EI) m/z: [M]+ calcd for C28H18N6O3 (M+) 486.1435, found 486.1435.
- (E)-2-Phenyl-5-(phenyldiazenyl)-6-(thiophen-2-yl)-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3j). Pale yellow crystals [EtOH/dioxane mixture (1:2)], yield: 1.75 g (90%), m.p. 256–257 °C; FT-IR 𝜈/cm−1: 3112 (NH), 1656 (C=O), 1618 (C=N); 1H NMR (TFA-d, 600 MHz): δ 7.33 (t, J = 5.4 Hz, 1H, thiophene-H), 7.45–7.50 (m, 6H, Ar-H), 7.57 (d, J = 7.8 Hz, 2H, Ar-H), 7.92 (d, J = 7.8 Hz, 2H, Ar-H), 8.07 (d, J = 5.4 Hz, 1H, thiophene-H), 8.28 (d, J = 5.4 Hz, 1H, thiophene-H), 9.15 ppm (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 111.76, 123.26, 124.85, 125.94, 126.48, 131.27, 131.71, 132.14, 132.75, 135.53, 135.90, 136.61, 139.61, 144.99, 146.45, 150.75, 153.13, 158.53 ppm; MS (EI) m/z (%): 398 (M+ + 1, 4.95), 397 (M+, 15.81), 396 (M+ − 1, 15.34); HRMS (EI) m/z: [M]+ calcd for C22H15N5OS (M+) 397.0992, found 397.0992.
- (E)-5-[(4-Chlorophenyl)diazinyl]-2-phenyl-6-(thiophen-2-yl)-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3k). Yellow crystals (dioxane), yield: 2.00 g (92%), m.p. 265–266 °C; FT-IR 𝜈/cm−1: 3114 (NH), 1659 (C=O), 1622 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.23 (t, J = 4.8 Hz, 1H, thiophene-H), 7.27 (t, J = 7.8 Hz, 1H, Ar-H), 7.50 (t, J = 7.8 Hz, 2H, Ar-H), 7.64 (d, J = 8.4 Hz, 2H, Ar-H), 7.89–7.92 (m, 5H, Ar-H), 8.07 (d, J = 4.8 Hz, 1H, thiophene-H), 8.30 (s, 1H, C-H4), 12.32 ppm (brs, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 112.24, 126.49, 128.28, 131.74, 131.92, 132.48, 132.69, 133.19, 133.55, 136.44, 136.99, 140.84, 143.02, 144.52, 147.59, 150.99, 153.29, 159.19 ppm; MS (EI) m/z (%): 433 (M+ + 2, 7.14), 432 (M+ + 1, 9.97), 431 (M+, 18.21), 430 (M+ − 1, 14.06); HRMS (EI) m/z: [M]+ calcd for C22H14ClN5OS (M+) 431.0602, found 431.0601.
- (E)-2-Phenyl-6-(thiophen-2-yl)-5-(p-tolyldiazenyl)-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3l). Yellow crystals (dioxane), yield: 2.05 g (88%), m.p. 263–264 °C; FT-IR 𝜈/cm−1: 3112 (NH), 1660 (C=O), 1618 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 2.44 (s, 3H, CH3), 7.28–7.31 (m, 2H, Ar-H), 7.47 (d, J = 7.8 Hz, 2H, Ar-H), 7.53 (t, J = 7.8 Hz, 2H, Ar-H), 7.91–7.94 (m, 4H, Ar-H), 7.96 (d, J = 4.8 Hz, 1H, thiophene-H), 8.15 (d, J = 4.8 Hz, 1H, thiophene-H), 8.39 (s, 1H, C-H4), 12.21 ppm (brs, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 22.40 (CH3), 111.96, 125.76, 126.63, 131.10, 131.41, 132.20, 132.69, 135.82, 136.66, 139.19, 145.17, 146.49, 148.78, 150.52, 150.78, 158.47 ppm; MS (EI) m/z (%): 412 (M+ + 1, 5.61), 411 (M+, 18.17), 410 (M+ − 1, 15.01); HRMS (EI) m/z: [M]+ calcd for C23H17N5OS (M+) 411.1148, found 411.1149.
- (E)-5-[(2,4-Difluorophenyl)diazinyl]-2-phenyl-6-(thiophen-2-yl)-2,7-dihydro-3H-pyrazolo- [3,4-b]pyridin-3-one (3m). Orange crystals [EtOH/DMF mixture (1:2)], yield: 2.00 g (93%), m.p. 272–273 °C; FT-IR 𝜈/cm−1: 3110 (NH), 1650 (C=O), 1607 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.28–7.34 (m, 3H, Ar-H), 7.52 (t, J = 8.4 Hz, 2H, Ar-H), 7.62 (t, J = 8.4 Hz, 1H, Ar-H), 7.90–7.93 (m, 3H, Ar-H), 7.97 (d, J = 4.8 Hz, 1H, thiophene-H), 8.13 (d, J = 4.8 Hz, 1H, thiophene-H), 8.34 (s, 1H, C-H4), 12.45 ppm (brs, 1H, NH); 13C NMR (DMSO-d6, 150 MHz): δ (105.60, 105.77, 105.94) (t, 2JCF = 25.5 Hz), 109.60, (112.71, 112.86)] (dd, 2JCF = 22.5 Hz), 119.41, (120.15, 120.22) (d, 3JCF = 10.5 Hz), 121.14, 125.38, 128.27, 129.07, 132.60, 133.80, 137.01, 137.27, 139.30, 158.52, 159.03, 160.79, (163.28, 164.95 ppm) (d, 1JCF = 250.5 Hz); MS (EI) m/z (%): 434 (M+ + 1, 5.34), 433 (M+, 18.45), 432 (M+ − 1, 15.23); HRMS (EI) m/z: [M]+ calcd for C22H13F2N5OS (M+) 433.0803, found 433.0803.
- (E)-6-(4-Chlorophenyl)-5-[(4-chlorophenyl)diazinyl]-2-phenyl-2,7-dihydro-3H-pyrazolo- [3,4-b]pyridin-3-one (3n). Yellow crystals (dioxane), yield: 2.25 g (99%), m.p. above 300 °C; FT-IR 𝜈/cm−1: 3114 (NH), 1651 (C=O), 1622 (C=N); 1H NMR (TFA-d, 600 MHz): δ 8.41 (d, J = 9.0 Hz, 2H, Ar-H), 8.53–8.57 (m, 3H, Ar-H), 8.62–8.66 (m, 4H, Ar-H), 8.74–8.77 (m, 4H, Ar-H), 10.42 ppm (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 126.76, 127.47, 127.74, 129.78, 132.27, 132.35, 132.73, 133.54, 134.84, 135.10, 135.80, 142.65, 142.77, 143.87, 147.10, 152.85, 157.85, 159.04 ppm; MS (EI) m/z (%): 461 (M+ + 2, 66.28), 460 (M+ + 1, 92.93), 459 (M+, 100.00), 458 (M+ − 1, 98.02); HRMS (EI) m/z: [M]+ calcd for C24H15Cl2N5O (M+) 459.0648, found 459.0647.
- (E)-5-[(3-Bromophenyl)diazenyl]-6-(4-chlorophenyl)-2-phenyl-2,7-dihydro-3H-pyrazolo- [3,4-b]pyridin-3-one (3o). Orange crystals (dioxane), yield: 2.40 g (97%), m.p. above 300 °C; FT-IR 𝜈/cm−1: 3111 (NH), 1650 (C=O), 1621 (C=N); 1H NMR (TFA-d, 600 MHz): δ 7.23 (t, J = 7.8 Hz, 1H, Ar-H), 7.42–7.48 (m, 4H, Ar-H), 7.53–7.57 (m, 4H, Ar-H), 7.66–7.70 (m, 3H, Ar-H), 7.74 (s, 1H, Ar-H), 9.28 ppm (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 114.32, 125.57, 126.07, 126.26, 127.53, 129.37, 131.95, 132.36, 132.96, 133.08, 134.53, 134.74, 135.43, 138.11, 142.09, 143.59, 146.75, 155.01, 157.38, 158.89 ppm; MS (EI) m/z (%): 505 (M+ + 2, 89.91), 504 (M+ + 1, 100.00), 503 (M+, 61.42), 502 (M+ − 1, 55.03); HRMS (EI) m/z: [M]+ calcd for C24H15BrClN5O (M+) 503.0143, found 503.0144.
- (E)-6-(4-Chlorophenyl)-5-[(2-nitrophenyl)diazinyl]-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3p). Orange crystals [EtOH/DMF mixture (1:3)], yield: 2.15 g (92%), m.p. 296–297 °C; FT-IR 𝜈/cm−1: 3106 (NH), 1662 (C=O), 1626 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.28 (t, J = 7.8 Hz, 1H, Ar-H), 7.50–7.52 (m, 3H, Ar-H), 7.61 (dd, J = 1.8, 8.4 Hz, 2H, Ar-H), 7.69 (t, J = 7.8 Hz, 1H, Ar-H), 7.79 (t, J = 7.8 Hz, 1H, Ar-H), 7.83 (d, J = 8.4 Hz, 2H, Ar-H), 7.99 (d, J = 8.4 Hz, 2H, Ar-H), 8.04 (d, J = 7.8 Hz, 1H, Ar-H), 8.37 (s, 1H, C-H4), 12.34 ppm (brs, 1H, NH); 13C NMR (DMSO-d6, 150 MHz): δ 118.12, 119.23, 122.20, 123.58, 124.93, 127.48, 128.51, 130.64, 132.28, 132.97, 133.77, 134.73, 137.23, 138.58, 140.62, 143.79, 146.80, 152.79, 158.36, 158.66 ppm; MS (EI) m/z (%): 472 (M+ + 2, 10.04), 471 (M+ + 1, 13.45), 470 (M+, 27.09), 469 (M+ − 1, 21.27); HRMS (EI) m/z: [M]+ calcd for C24H15ClN6O3 (M+) 470.0889, found 470.0887.
- (E)-6-(4-Fluorophenyl)-2-phenyl-5-(p-tolyldiazenyl)-2,7-dihydro-3H-pyrazolo[3,4-b] pyridin-3-one (3q). Yellow crystals (dioxane), yield: 1.80 g (86%), m.p. above 300 °C; FT-IR 𝜈/cm−1: 3114 (NH), 1651 (C=O), 1622 (C=N); 1H NMR (TFA-d, 600 MHz): δ 2.43 (s, 3H, CH3), 7.35–7.40 (m, 4H, Ar-H), 7.57–8.62 (m, 3H, Ar-H), 7.69 (d, J = 8.4 Hz, 2H, Ar-H), 7.77 (d, J = 8.4 Hz, 2H, Ar-H), 7.90–7.92 (m, 2H, Ar-H), 9.46 (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 22.29 (CH3), (118.91, 119.07) (d, 2JCF = 24.0 Hz), (125.34, 125.36) (d, 4JCF = 3.0 Hz), 126.87, 127.27, 128.26, 132.36, 132.42, (135.80, 135.87) (d, 3JCF = 10.5 Hz), 139.46, 140.78, 150.04, 150.28, 153.31, 153.45, 154.17, 155.53, 168.02, (168.18, 169.90 ppm) (d, 1JCF = 258.0 Hz); MS (EI) m/z (%): 424 (M+ + 1, 29.05), 423 (M+, 100.00), 422 (M+ − 1, 89.92); HRMS (EI) m/z: [M]+ calcd for C25H18FN5O (M+) 423.1490, found 423.1490.
- (E)-6-(4-Fluorophenyl)-5-[(4-nitrophenyl)diazinyl]-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3r). Orange crystals [EtOH/DMF mixture (1:3)], yield: 2.05 g (91%), m.p. above 300 °C; FT-IR 𝜈/cm−1: 3112 (NH), 1662 (C=O), 1604 (C=N); 1H NMR (TFA-d, 600 MHz): δ 7.26 (t, J = 8.4 Hz, 2H, Ar-H), 7.45–7.49 (m, 3H, Ar-H), 7.56 (d, J = 7.8 Hz, 2H, Ar-H), 7.77–7.80 (m, 2H, Ar-H), 7.91 (d, J = 9.0 Hz, 2H, Ar-H), 8.29 (d, J = 9.0 Hz, 2H, Ar-H), 9.43 (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 114.15, (118.86, 119.01) (d, 2JCF = 22.5 Hz), 126.35, 126.58, 127.16, 127.26, 132.32, 133.14, 134.83, 135.35, (135.81, 135.88) (d, 3JCF = 10.5 Hz), 142.02, 146.87, 151.65, 157.69, 158.36, 159.08, (167.97, 169.68 ppm) (d, 1JCF = 256.5 Hz); MS (EI) m/z (%): 455 (M+ + 1, 32.41), 454 (M+, 100.00), 422 (M+ − 1, 89.92); HRMS (EI) m/z: [M]+ calcd for C24H15FN6O3 (M+) 454.1184, found 454.1184.
- (E)-5-[(3-Bromophenyl)diazinyl]-6-(4-fluorophenyl)-2-phenyl-2,7-dihydro-3H-pyrazolo- [3,4-b]pyridin-3-one (3s). Deep orange crystals [dioxane/DMF mixture (1:1)], yield: 2.10 g (87%), m.p. 285–286 °C; FT-IR 𝜈/cm−1: 3112 (NH), 1650 (C=O), 1622 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 7.26 (t, J = 7.2 Hz, 1H, Ar-H), 7.39 (t, J = 8.4 Hz, 2H, Ar-H), 7.48–7.51 (m, 3H, Ar-H), 7.66 (d, J = 7.2 Hz, 1H, Ar-H), 7.72 (d, J = 7.2 Hz, 1H, Ar-H), 7.77 (s, 1H, Ar-H), 7.82–7.96 (m, 4H, Ar-H), 8.46 (s, 1H, C-H4), 12.75 ppm (brs, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 114.13, (118.70, 118.85) (d, 2JCF = 22.5 Hz), 125.40, 125.96, 126.25, 127.25, 132.20, 132.68, 133.03, 134.72, 135.03, (135.70, 135.76) (d, 3JCF = 9.0 Hz), 138.04, 142.25, 146.41, 152.56, 154.87, 157.45, 158.94, (167.85, 169.56 ppm) (d, 1JCF = 256.5 Hz); MS (EI) m/z (%): 489 (M+ + 2, 72.19), 488 (M+ + 1, 83.04), 487 (M+, 71.43), 486 (M+ − 1, 62.35); H HRMS (EI) m/z: [M]+ calcd for C24H15BrFN5O (M+) 487.0439, found 487.0438.
- (E)-6-(4-Bromophenyl)-5-[(2,4-difluorophenyl)diazinyl]-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3t). Orange crystals (DMF), yield: 2.40 g (97%), m.p. above 300 °C; FT-IR 𝜈/cm−1: 3112 (NH), 1655 (C=O), 1610 (C=N); 1H NMR (TFA-d, 600 MHz): δ 6.92 (td, J = 9.0, 4.5 Hz, 1H, Ar-H), 7.01 (td, J = 9.0, 4.5 Hz, 1H, Ar-H), 7.57–7.67 (m, 4H, Ar-H), 7.69 (d, J = 7.2 Hz, 2H, Ar-H), 7.73 (d, J = 8.4, Hz, 2H, Ar-H), 8.84 (d, J = 8.4, Hz, 2H, Ar-H), 9.49 (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ (107.36, 107.53, 107.69) (t, 2JCF = 25.5 Hz), 114.37, (114.50, 114.53) (d, 4JCF = 4.5 Hz), (122.15, 122.23) (d, 3JCF = 12.0 Hz), 126.34, 129.77, 131.56, 132.31, 133.14, 134.37, 134.91, 135.13, 135.18, (139.40, 139.43, 139.46) (t, 4JCF = 4.5 Hz), 142.87, 146.59, 157.32, 158.89, [(163.00, 163.09), (164.76, 164.85)] (dd, 1JCF = 13.5, 264.0 Hz), [(168.27, 168.35), (170.00, 170.08 ppm)] (dd, 1JCF = 12.0, 259.5 Hz); MS (EI) m/z (%): 507 (M+ + 2, 85.00), 506 (M+ + 1, 100.00), 505 (M+, 83.57), 504 (M+ − 1, 80.76); HRMS (EI) m/z: [M]+ calcd for C24H14BrF2N5O (M+) 505.0344, found 505.0351.
- (E)-6-(4-Chlorophenyl)-5-[(2-fluoro-5-nitrophenyl)diazinyl]-2-phenyl-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3u). Orange crystals (DMF), yield: 2.25 g (94%), m.p. above 300 °C; FT-IR 𝜈/cm−1: 3117 (NH), 1656 (C=O), 1622 (C=N); 1H NMR (TFA-d, 600 MHz): δ 6.91–7.73 (m, 9H, Ar-H), 9.66–9.98 (m, 3H, Ar-H), 10.61 (s, 1H, C-H4), (exchanged proton with TFA, 1H, NH); 13C NMR (TFA-d, 150 MHz): δ 114.22, 117.38, (121.76, 121.92) (d, 2JCF = 24.0 Hz), 127.13, 130.02, (131.71, 131.79) (d, 3JCF = 12.0 Hz), 132.66, 133.01, 133.92, 135.12, 135.47, 136.35, 142.43, (142.89, 142.96) (d, 3JCF = 10.5 Hz), 144.31, 147.20, 147.89, 159.05, 159.35, (166.23, 168.04 ppm) (d, 1JCF = 271.5 Hz); MS (EI) m/z (%): 490 (M+ + 2, 36.42), 489 (M+ + 1, 57.98), 488 (M+, 100.00), 487 (M+ − 1, 89.83); HRMS (EI) m/z: [M]+ calcd for C24H14ClFN6O3 (M+) 488.0794, found 488.0796.
- (E)-6-Methyl-2-phenyl-5-(phenyldiazenyl)-2,7-dihydro-3H-pyrazolo[3,4-b]pyridin-3-one (3v). Pale yellow [EtOH/dioxane mixture (1:1)], yield: 1.35 g (83%), m.p. 283–284 °C; FT-IR 𝜈/cm−1: 3122 (NH), 1661 (C=O), 1620 (C=N); 1H NMR (DMSO-d6, 600 MHz): δ 2.89 (s, 3H, CH3), 7.18 (t, J = 7.8 Hz, 1H, Ar-H), 7.44 (t, J = 7.8 Hz, 2H, Ar-H), 7.49 (t, J = 7.8 Hz, 1H, Ar-H), 7.56 (t, J = 7.8 Hz, 2H, Ar-H), 7.84 (d, J = 7.8 Hz, 2H, Ar-H), 8.07 (d, J = 7.8 Hz, 2H, Ar-H), 8.42 (s, 1H, C-H4), 13.94 ppm (s, 1H, NH); 13C NMR (DMSO-d6, 150 MHz): δ 17.74 (CH3), 119.20, 122.75, 124.14, 124.89, 129.24, 129.77, 130.95, 135.71, 139.62, 152.71, 157.89, 160.03 ppm; MS (EI) m/z (%): 330 (M+ + 1, 23.91), 329 (M+, 100.00), 328 (M+ − 1, 8.23); HRMS (EI) m/z: [M]+ calcd for C19H15N5O (M+) 329.1271, found 329.1271.
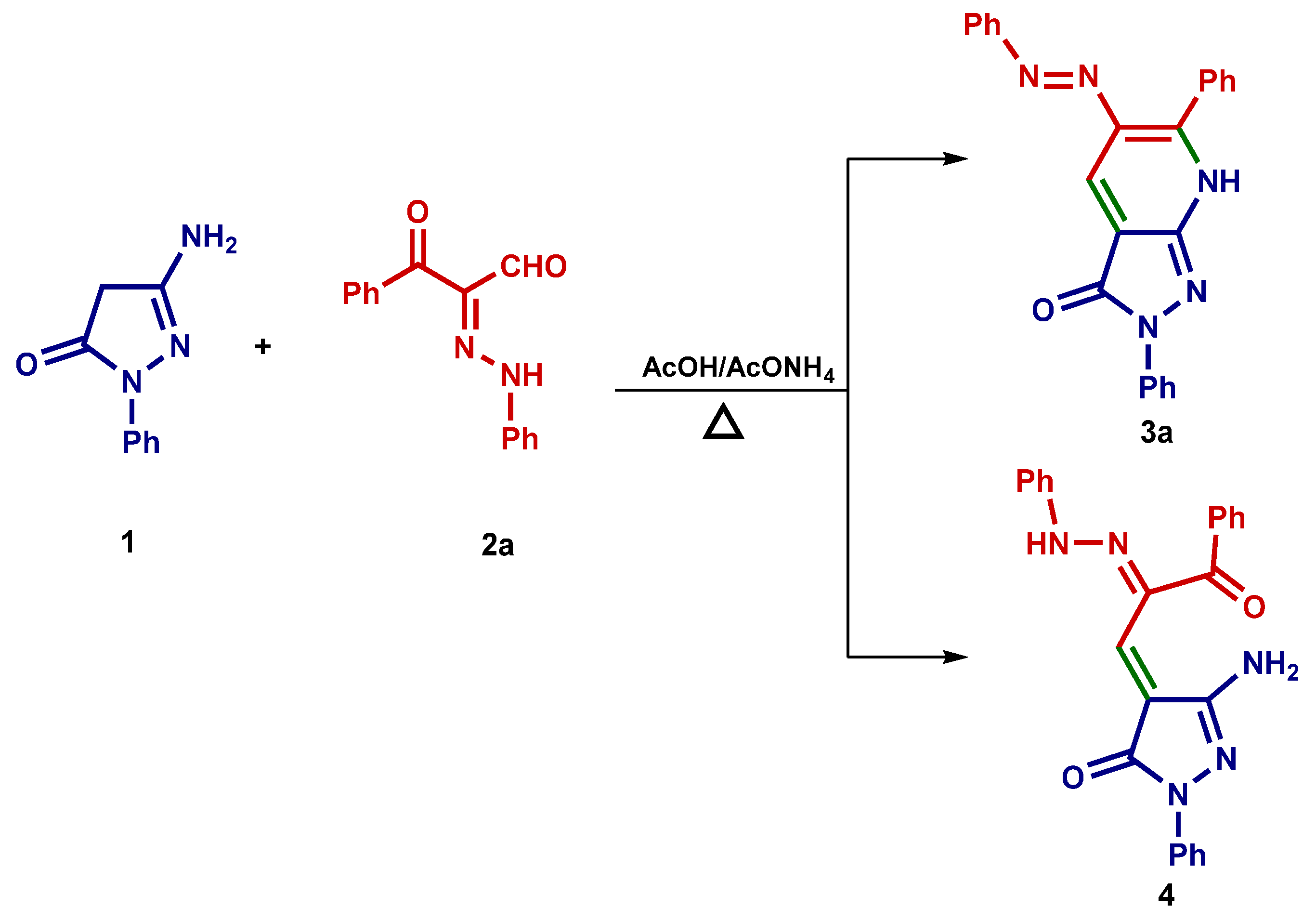
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Malick, J.; Salama, A.; Goldberg, M. Pharmacology of pyrazolopyridines. Pharmacol. Biochem. Behav. 1985, 23, 675–680. [Google Scholar] [CrossRef]
- Boerrigter, G.; Burnett, J.C. Nitric Oxide-Independent Stimulation of Soluble Guanylate Cyclase with BAY 41-2272 in Cardiovascular Disease. Cardiovasc. Drug Rev. 2007, 25, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, Y.S.; Larson, S.B.; Willis, R.C.; Robins, R.K.; Revankar, G.R. Synthesis and biological evaluation of certain C-4 substituted pyrazolo[3,4-b]pyridine nucleosides. J. Med. Chem. 1989, 32, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Umar, T.; Shalini, S.; Raza, K.; Gusain, S.; Kumar, J.; Seth, P.; Tiwari, M.; Hoda, N. A multifunctional therapeutic approach: Synthesis, biological evaluation, crystal structure and molecular docking of diversified 1H-pyrazolo[3,4-b]pyridine derivatives against Alzheimer’s disease. Eur. J. Med. Chem. 2019, 175, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, W.; Lin, F.; Lu, X.; Chen, W.; Li, X.; Zhou, X.; Su, R.; Wang, L.; Zheng, Z.; et al. Synthesis and biological evaluation of pyrazolo[3,4-b]pyridine-3-yl pyrimidine derivatives as sGC stimulators for the treatment of pulmonary hypertension. Eur. J. Med. Chem. 2019, 173, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, M.; Hu, L.; Tian, X.; He, X.; Zhao, C.; Li, Y.; Li, Q.; Li, X. Novel Pyrazolo[3,4-b] Pyridine Derivative (HLQ2g) Attenuates Hypoxic Pulmonary Hypertension via Restoring cGKI Expression and BMP Signaling Pathway. Front. Pharmacol. 2021, 12, 691405. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.; Shaban, A.; Nassar, I.; El-Kady, D.; Ismail, N.; Mahmoud, S.; Awad, H.; El-Sayed, W. Discovery of New Pyrazolopyridine, Furopyridine, and Pyridine Derivatives as CDK2 Inhibitors: Design, Synthesis, Docking Studies, and Anti-Proliferative Activity. Molecules 2021, 26, 3923. [Google Scholar] [CrossRef]
- Nam, Y.; Hwang, D.; Kim, N.; Seo, H.-S.; Selim, K.B.; Sim, T. Identification of 1H-pyrazolo[3,4-b]pyridine derivatives as potent ALK-L1196M inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Supe, L.; Afzal, S.; Mahmood, A.; Ejaz, S.A.; Hein, M.; Iaroshenko, V.O.; Villinger, A.; Lecka, J.; Sévigny, J.; Iqbal, J.; et al. Deazapurine Analogues Bearing a 1H-Pyrazolo[3,4-b]pyridin-3(2H)-one Core: Synthesis and Biological Activity. Eur. J. Org. Chem. 2018, 2018, 2629–2644. [Google Scholar] [CrossRef]
- El-Gohary, N.; Gabr, M.; Shaaban, M. Synthesis, molecular modeling and biological evaluation of new pyrazolo[3,4-b]pyridine analogs as potential antimicrobial, antiquorum-sensing and anticancer agents. Bioorganic Chem. 2019, 89, 102976. [Google Scholar] [CrossRef]
- Xing, Y.; Zuo, J.; Krogstad, P.; Jung, M.E. Synthesis and Structure–Activity Relationship (SAR) Studies of Novel Pyrazolopyridine Derivatives as Inhibitors of Enterovirus Replication. J. Med. Chem. 2018, 61, 1688–1703. [Google Scholar] [CrossRef]
- Hawas, S.S.; El-Gohary, N.S.; Gabr, M.T.; Shaaban, M.I.; El-Ashmawy, M.B. Synthesis, molecular docking, antimicrobial, antiquorum-sensing and antiproliferative activities of new series of pyrazolo[3,4-b]pyridine analogs. Synth. Commun. 2019, 49, 2466–2487. [Google Scholar] [CrossRef]
- Eissa, I.H.; El-Naggar, A.M.; El-Hashash, M.A. Design, synthesis, molecular modeling and biological evaluation of novel 1H-pyrazolo[3,4-b]pyridine derivatives as potential anticancer agents. Bioorgan. Chem. 2016, 67, 43–56. [Google Scholar] [CrossRef]
- Bilavendran, J.D.; Manikandan, A.; Thangarasu, P.; Sivakumar, K. Synthesis and discovery of pyrazolo-pyridine analogs as inflammation medications through pro- and anti-inflammatory cytokine and COX-2 inhibition assessments. Bioorgan. Chem. 2020, 94, 103484. [Google Scholar] [CrossRef] [PubMed]
- Fichez, J.; Soulie, C.; Le Corre, L.; Sayon, S.; Priet, S.; Alvarez, K.; Delelis, O.; Gizzi, P.; Prestat, G.; Gravier-Pelletier, C.; et al. Discovery, SAR study and ADME properties of methyl 4-amino-3-cyano-1-(2-benzyloxyphenyl)-1H-pyrazole-5-carboxylate as an HIV-1 replication inhibitor. RSC Med. Chem. 2020, 11, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.A. Synthesis and Antioxidant Evaluation of Some New Pyrazolopyridine Derivatives. Arch. der Pharm. 2011, 345, 155–162. [Google Scholar] [CrossRef]
- Gudmundsson, K.S.; Johns, B.A.; Wang, Z.; Turner, E.M.; Allen, S.H.; Freeman, G.A.; Boyd, F.L.; Sexton, C.J.; Selleseth, D.W.; Moniri, K.R.; et al. Synthesis of novel substituted 2-phenylpyrazolopyridines with potent activity against herpesviruses. Bioorgan. Med. Chem. 2005, 13, 5346–5361. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Behbehani, H. The first Q-Tube based high-pressure synthesis of anti-cancer active thiazolo[4,5-c]pyridazines via the [4 + 2] cyclocondensation of 3-oxo-2-arylhydrazonopropanals with 4-thiazolidinones. Sci. Rep. 2020, 10, 6492. [Google Scholar] [CrossRef] [PubMed]
- Nacca, F.G.; Merlino, O.; Mangiavacchi, F.; Krasowska, D.; Santi, C.; Sancineto, L. The Q-tube System, A Nonconventional Technology for Green Chemistry Practitioners. Curr. Green Chem. 2017, 4, 58–66. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. Facile Ecofriendly Synthesis of Monastrol and Its Structural Isomers via Biginelli Reaction. ACS Sustain. Chem. Eng. 2014, 2, 1228–1233. [Google Scholar] [CrossRef]
- Almarzouq, D.S.; Zaky, O.S.; Alnajjar, A.A.; Sadek, K.U. Pressure as effective green technology for synthesis of polyfunctionally substituted heteroaromatics: Synthesis of a variety of pyrazolo[1,5-a]pyrimidines. Eur. J. Chem. 2016, 7, 347–351. [Google Scholar] [CrossRef]
- Costanzo, P.; Calandruccio, C.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. First multicomponent reaction exploiting glycerol carbonate synthesis. J. Clean. Prod. 2018, 202, 504–509. [Google Scholar] [CrossRef]
- Alzaydi, K.M.; Abojabal, N.S.; Elnagdi, M.H. Multicomponent reactions in Q-Tubes™: One-pot synthesis of benzo[c]chromen-6-one and phenanthridin-6(5H)-one derivatives in a four-component reaction. Tetrahedron Lett. 2016, 57, 3596–3599. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Behbehani, H. TFA-catalyzed Q-Tube Reactor-Assisted Strategy for the Synthesis of Pyrido[1,2-b][1,2,4]triazine and Pyrido[1′,2′:2,3][1,2,4]triazino[5,6-b]indole Derivatives. ACS Omega 2021, 6, 16086–16099. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Behbehani, H. Palladium-Catalyzed Q-Tube-Assisted Protocol for Synthesizing Diaza-dibenzo[a,e]azulene and Diaza-benzo[a]fluorene Derivatives via O2 Acid-Promoted Cross-Dehydrogenative Coupling. J. Org. Chem. 2020, 85, 15368–15381. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Behbehani, H.; Mostafa, N.S. Scalable Sonochemical Synthetic Strategy for Pyrazolo[1,5-a]pyridine Derivatives: First Catalyst-Free Concerted [3 + 2] Cycloaddition of Alkyne and Alkene Derivatives to 2-Imino-1H-pyridin-1-amines. ACS Omega 2019, 4, 7182–7193. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, H.; Dawood, K.M.; Aryan, F.A.; Ibrahim, H.M. Green Protocol for the Novel Synthesis of Thiochromeno[4,3-b]pyridine and Chromeno[4,3-b]pyridine Derivatives Utilizing a High-Pressure System. ACS Omega 2021, 6, 34065–34074. [Google Scholar] [CrossRef]
- Behbehani, H.; Aryan, F.A.; Dawood, K.M.; Ibrahim, H.M. High pressure assisted synthetic approach for novel 6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-b]pyridine and 5,6-dihydrobenzo[h]quinoline derivatives and their assessment as anticancer agents. Sci. Rep. 2020, 10, 21691. [Google Scholar] [CrossRef]
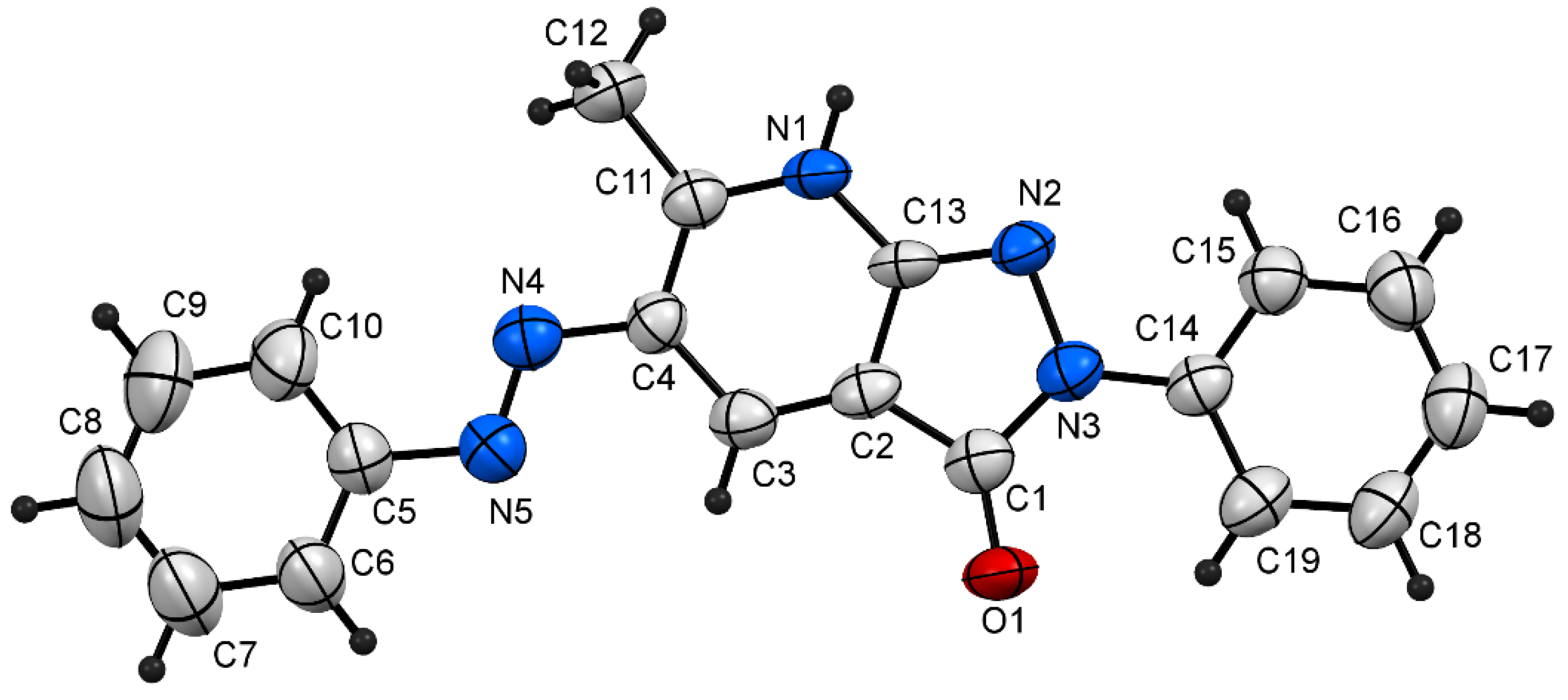
- Crystallographic Data for 3v (ref. CCDC 2188305) Can Be Obtained on Request from the Director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 1 September 2022).

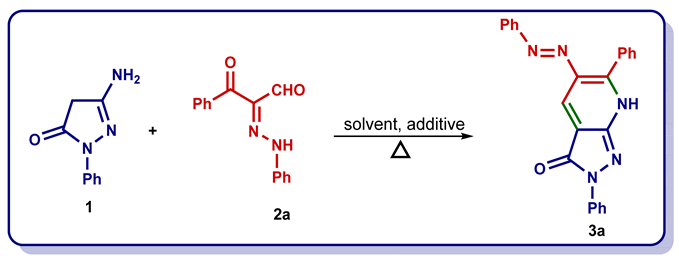
| Entry | Solvent | Additive (Equiv) | Temp. (°C) | Time | Yield (%) |
|---|---|---|---|---|---|
| 1 a | EtOH | NH4OAc or NaOAc | 120 | 12 h | 0 |
| 2 a | CH3CN | NH4OAc or NaOAc | 120 | 12 h | 0 |
| 3 a | 1,4-dioxane | NH4OAc or NaOAc | 140 | 12 h | 0 |
| 4 a | propanol | NH4OAc or NaOAc | 130 | 12 h | 0 |
| 5 a | DMF | NH4OAc or NaOAc | 140 | 6h | ≈14 |
| 6 a | AcOH | NH4OAc | 140 | 6 h | 45 |
| 7 a | AcOH | NaOAc | 140 | 6 h | 30 |
| 8 b | AcOH | NH4OAc | 140 | 30 min | 66 |
| 9 c | AcOH | NH4OAc | 140 | 30 min | 85 |
| 10 c | AcOH | NH4OAc | 150 | 30 min | 92 |
| 11 c | AcOH | NH4OAc | 155 | 30 min | 96 |
| 12 c | AcOH | NH4OAc | 160 | 30 min | 98 |
| 13 c | AcOH | NH4OAc | 165 | 30 min | 98 |
| Bond | Bond length (Å) | Bond | Bond Angle (o) |
|---|---|---|---|
| C5-C10 | 1.380 (9) | C7-C8-C9 | 119.5 (6) |
| C5-N5 | 1.444 (6) | C6-C5-C10 | 120.5 (5) |
| N4-C4 | 1.409 (6) | C3-C4-C11 | 121.0 (4) |
| N4-N5 | 1.258 (6) | N5-N4-C4 | 114.7 (5) |
| C3-C4 | 1.404 (8) | N4-N5-C5 | 112.3 (5) |
| N1-C11 | 1.353 (6) | C11-N1-C13 | 122.2 (4) |
| N2-N3 | 1.424 (6) | C2-C3-C4 | 118.7 (5) |
| C1-O1 | 1.238 (7) | N3-N2-C14 | 116.6 (4) |
| N3-C14 | 1.412 (6) | O1-C1-C2 | 131.9 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayl, A.A.; Ibrahim, H.M.; Dawood, K.M.; Arafa, W.A.A.; Abd-Elhamid, A.I.; Ahmed, I.M.; Abdelgawad, M.A.; Ali, H.M.; Alsohaimi, I.H.; Aly, A.A.; et al. High-Pressure Metal-Free Catalyzed One-Pot Two-Component Synthetic Approach for New 5-Arylazopyrazolo[3,4-b]Pyridine Derivatives. Molecules 2022, 27, 6369. https://doi.org/10.3390/molecules27196369
Nayl AA, Ibrahim HM, Dawood KM, Arafa WAA, Abd-Elhamid AI, Ahmed IM, Abdelgawad MA, Ali HM, Alsohaimi IH, Aly AA, et al. High-Pressure Metal-Free Catalyzed One-Pot Two-Component Synthetic Approach for New 5-Arylazopyrazolo[3,4-b]Pyridine Derivatives. Molecules. 2022; 27(19):6369. https://doi.org/10.3390/molecules27196369
Chicago/Turabian StyleNayl, AbdElAziz A., Hamada Mohamed Ibrahim, Kamal M. Dawood, Wael A. A. Arafa, Ahmed I. Abd-Elhamid, Ismail M. Ahmed, Mohamed A. Abdelgawad, Hazim M. Ali, Ibrahim Hotan Alsohaimi, Ashraf A. Aly, and et al. 2022. "High-Pressure Metal-Free Catalyzed One-Pot Two-Component Synthetic Approach for New 5-Arylazopyrazolo[3,4-b]Pyridine Derivatives" Molecules 27, no. 19: 6369. https://doi.org/10.3390/molecules27196369
APA StyleNayl, A. A., Ibrahim, H. M., Dawood, K. M., Arafa, W. A. A., Abd-Elhamid, A. I., Ahmed, I. M., Abdelgawad, M. A., Ali, H. M., Alsohaimi, I. H., Aly, A. A., Bräse, S., & Mourad, A. K. (2022). High-Pressure Metal-Free Catalyzed One-Pot Two-Component Synthetic Approach for New 5-Arylazopyrazolo[3,4-b]Pyridine Derivatives. Molecules, 27(19), 6369. https://doi.org/10.3390/molecules27196369








