Improving the Efficiency of Organic Solar Cells with Methionine as Electron Transport Layer
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hou, J.; Ingans, O.; Friend, R.H.; Feng, G. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zhan, X. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Zheng, Z.; Yao, H.; Wolfgang, T.; Hopper, T.R.; Chen, S.; Li, S.; Jing, L.; Chen, S.; Zhang, J. Design rules for minimizing voltage losses in high-efficiency organic solar cells. Nat. Mater. 2018, 17, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, X.; Xu, C.; Xu, W.; Jeong, S.Y.; Woo, H.Y.; Zhou, Z.; Zhang, X.; Zhang, F. Boosted Efficiency Over 18.1% of Polymer Solar Cells by Employing Large Extinction Coefficients Material as the Third Component. Macromol. Rapid Commun. 2022, 43, e2200345. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jin, K.; Xiao, Z.; Zhao, Z.; Yan, Y.; Zhu, X.; Li, X.; Zhou, Z.; Jeong, S.Y.; Ding, L.; et al. Efficient Semitransparent Layer-by-Layer Organic Photovoltaics via Optimizing Wide Bandgap and Narrow Absorption Polymer Layer Thickness. Solar RRL 2022, 6, 2200308. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, X.; Ma, X.; Zhou, H.; Li, X.; Jeong, S.Y.; Woo, H.Y.; Zhou, Z.; Sun, Q.; Zhang, F. Achieving 15.81% and 15.29% efficiency of all-polymer solar cells based on layer-by-layer and bulk heterojunction structures. J. Mater. Chem. A 2022, 10, 13492–13499. [Google Scholar] [CrossRef]
- He, C.; Pan, Y.; Ouyang, Y.; Shen, Q.; Gao, Y.; Yan, K.; Fang, J.; Chen, Y.; Ma, C.-Q.; Min, J. Manipulating the D: A interfacial energetics and intermolecular packing for 19.2% efficiency organic photovoltaics. Energy Environ. Sci. 2022, 15, 2537–2544. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, B.; Yang, B.; Yao, H.; Li, S.; Hou, J. A novel pH neutral self-doped polymer for anode interfacial layer in efficient polymer solar cells. Macromolecules 2016, 49, 8126–8133. [Google Scholar] [CrossRef]
- Mai, C.K.; Zhou, H.; Zhang, Y.; Henson, Z.B.; Nguyen, T.Q.; Heeger, A.J.; Bazan, G.C. Facile doping of anionic narrow-band-gap conjugated polyelectrolytes during dialysis. Angew. Chem. Int. Ed. 2013, 52, 12874–12878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Mai, C.K.; Collins, S.D.; Nguyen, T.Q.; Bazan, G.C.; Heeger, A.J. Conductive conjugated polyelectrolyte as hole-transporting layer for organic bulk heterojunction solar cells. Adv. Mater. 2014, 26, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, M.; Duan, C.; Hu, X.; Huang, L.; Peng, J.; Huang, F.; Gong, X. Inverted polymer solar cells with 8.4% efficiency by conjugated polyelectrolyte. Energy Environ. Sci. 2012, 5, 8208. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of polymer solar cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef] [PubMed]
- Hau, S.K.; Yip, H.L.; Jen, A.K.Y. A review on the development of the inverted polymer solar cell architecture. Polym. Rev. 2010, 50, 474–510. [Google Scholar] [CrossRef]
- Zhang, K.; Zhong, C.; Liu, S.; Mu, C.; Li, Z.; Yan, H.; Huang, F.; Cao, Y. Highly efficient inverted polymer solar cells based on a cross-linkable water-/alcohol-soluble conjugated polymer interlayer. ACS Appl. Mater. Inter. 2014, 6, 10429–10435. [Google Scholar] [CrossRef]
- Liu, Y.; Page, Z.; Ferdous, S.; Liu, F.; Kim, P.; Emrick, T.; Russell, T. Organic photovoltaics: Dual functional zwitterionic fullerene interlayer for efficient inverted polymer solar cells. Adv. Energy Mater. 2015, 5, 1500405. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Meng, T.; Yi, C.; Gong, X. Inverted organic photovoltaic cells. Chem. Chem. Soc. Rev. 2016, 45, 2937–2975. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J. A universal method to produce low-work function electrodes for organic electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Herbst, S.; Li, Q.; Yu, C.; Jiang, X.; Dong, H.; Li, F.; Liu, L.; Würthner, F.; et al. Aqueous Solution Processed Photoconductive Cathode Interlayer for High Performance Polymer Solar Cells with Thick Interlayer and Thick Active Layer. Adv. Mater. 2016, 28, 7521–7526. [Google Scholar]
- Xu, B.; Hou, J. Solution-Processable Conjugated Polymers as Anode Interfacial Layer Materials for Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1800022. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, B.; Xie, C.; Ma, Y.; Wang, J.; Liu, M.; Yang, K.; Xu, Y.; Zhang, J.; Li, W.; et al. Highly sensitive, sub-microsecond polymer photodetectors for blood oxygen saturation testing. Sci. China Chem. 2021, 64, 1302–1309. [Google Scholar] [CrossRef]
- Liu, M.; Fan, Q.; Yang, K.; Zhao, Z.; Zhao, X.; Zhou, Z.; Zhang, J.; Lin, F.; Jen, A.K.Y.; Zhang, F. Broadband photomultiplication-type polymer photodetectors and its application in light-controlled circuit. Sci. China Chem. 2022, 65, 1642–1649. [Google Scholar] [CrossRef]
- Huang, Z.; Ouyang, D.; Shih, C.J.; Yang, B.; Choy, W.C.H. Solution-Processed Ternary Oxides as Carrier Transport/Injection Layers in Optoelectronics. Adv. Energy Mater. 2020, 10, 1900903. [Google Scholar] [CrossRef]
- Bi, S.; Leng, X.; Li, Y.; Zheng, Z.; Zhang, X.; Zhang, Y.; Zhou, H. Interfacial modification in organic and perovskite solar cells. Adv. Mater. 2019, 31, 1805708. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Usman, K.; Fang, J.J. Small molecule interlayers in organic solar cells. Adv. Energy Mater. 2018, 8, 1702730. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Huang, Y.; Liu, J.; Liu, K.; Wang, Z.; Zhao, C.; Qu, S.; Wang, Z. Engineering the photoelectrochemical behaviors of ZnO for efficient solar water splitting. J. Semicond. 2020, 41, 091702. [Google Scholar] [CrossRef]
- Xu, G.; Chen, L.; Lei, H.; Liao, Z.; Yi, N.; Liu, J.; Chen, Y. A novel alkylsilyl-fused copolymer-based non-fullerene solar cell with over 12% efficiency. J. Mater. Chem. A 2019, 7, 4145–4152. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Chen, L.; Liao, X.; Xie, Q.; Cui, Y.; Huang, B.; Yang, C.; Chen, Y. Non-halogenated-solvent-processed highly efficient organic solar cells with a record open circuit voltage enabled by noncovalently locked novel polymer donors. J. Mater. Chem. A 2019, 7, 27394–27402. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, S.; Wang, J.; Zhang, J.; Zhang, D.; Zhang, Y.; Wei, Z.; Tang, Z.; Hou, J.; Zhou, H.J.; et al. Exquisite modulation of ZnO nanoparticle electron transporting layer for high-performance fullerene-free organic solar cell with inverted structure. J. Mater. Chem. A 2019, 7, 3570–3576. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue Luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Liao, S.H.; Jhuo, H.J.; Cheng, Y.S.; Chen, S.A. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, Q.; Jiang, L.; Cao, G.; Science, E. ZnO cathode buffer layers for inverted polymer solar cells. Energy Environ. Sci. 2015, 8, 3442–3476. [Google Scholar] [CrossRef]
- Pali, L.S.; Gupta, S.K.; Garg, A. Organic solar cells on Al electroded opaque substrates: Assessing the need of ZnO as electron transport layer. Sol. Energy 2018, 160, 396–403. [Google Scholar] [CrossRef]
- Yu, W.; Huang, L.; Yang, D.; Fu, P.; Zhou, L.; Zhang, J.; Li, C. Efficiency exceeding 10% for inverted polymer solar cells with a ZnO/ionic liquid combined cathode interfacial layer. J. Mater. Chem. A 2015, 3, 10660–10665. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Chen, Z.; Liu, Y. Adenine-based polymer modified zinc oxide for efficient inverted organic solar cells. J. Mater. Chem. C 2021, 9, 11851–11858. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, Z.; Liu, B.; He, Z.; Zou, J.; Wang, L.; Wang, J.; Peng, J.; Cao, Y. Coffee-ring-free quantum dot thin film using inkjet printing from a mixed-solvent system on modified ZnO transport layer for light-emitting devices. ACS Appl. Mater. Interfaces 2016, 8, 26162–26168. [Google Scholar] [CrossRef] [PubMed]
- Nian, L.; Zhang, W.; Wu, S.; Qin, L.; Liu, L.; Xie, Z.; Wu, H.; Ma, Y. Perylene bisimide as a promising zinc oxide surface modifier: Enhanced interfacial combination for highly efficient inverted polymer solar cells. ACS Appl. Mater. Interfaces 2015, 7, 25821–25827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.; Yi, J.; Zuo, C.; Gong, Y.; Liu, Y.; Lai, W.-Y.; Huang, W. Improving the exciton dissociation of polymer/fullerene interfaces with a minimal loading amount of energy cascading molecular dopant. J. Mater. Chem. A 2018, 6, 15977–15984. [Google Scholar] [CrossRef]
- Dagar, J.; Scavia, G.; Scarselli, M.; Destri, S.; De Crescenzi, M.; Brown, T.M. Coating ZnO nanoparticle films with DNA nanolayers for enhancing the electron extracting properties and performance of polymer solar cells. Nanoscale 2017, 9, 19031–19038. [Google Scholar] [CrossRef]
- Ahmad, N.; Yanxun, L.; Zhang, X.; Wang, B.; Zhang, Y.; Zhou, H. A biopolymeric buffer layer improves device efficiency and stability in inverted organic solar cells. J. Mater. Chem. C 2020, 8, 15795–15803. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Islam, A.; Zheng, Q.; Li, J.; Ji, W.; Chen, L.; Ouyang, X. From Straw to Device Interface: Carboxymethyl-Cellulose-Based Modified Interlayer for Enhanced Power Conversion Efficiency of Organic Solar Cells. Adv. Sci. 2020, 7, 1902269. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, F.; Wang, J.; An, Q.; Zhao, C.; Li, L.; Teng, F.; Hu, B. A two-step strategy to clarify the roles of a solution processed PFN interfacial layer in highly efficient polymer solar cells. J. Mater. Chem. A 2015, 3, 18432–18441. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Gao, Z.; Zhang, C.; Yu, J.; Wang, J.; Ma, X.; Hu, H.; Yin, H.; Zhang, F. Natural biomaterial sarcosine as an interfacial layer enables inverted organic solar cells exhibiting over 16.4% efficiency. Nanoscale 2021, 13, 11128–11137. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Jahandar, M.; Prasetio, A.; Kim, J.M.; Kim, J.H.; Kim, S.; Lim, D.C. Multi-dimensional interfacial engineering for a practical large-area transparent flexible organic photovoltaics. Chem. Eng. J. 2021, 419, 129672. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q.; Chen, S.C.; Cai, D.; Ma, Y. Controllable ZnMgO electron-transporting layers for long-term stable organic solar cells with 8.06% efficiency after one-year storage. Adv. Energy Mater. 2016, 6, 1501493. [Google Scholar] [CrossRef]
- Seitkhan, A.; Neophytou, M.; Kirkus, M.; Abou-Hamad, E.; Hedhili, M.N.; Yengel, E.; Firdaus, Y.; Faber, H.; Lin, Y.; Tsetseris, L. Use of the Phen-NaDPO: Sn(SCN)2 blend as electron transport layer results to consistent efficiency improvements in organic and hybrid perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1905810. [Google Scholar] [CrossRef]
- Lin, Y.; Magomedov, A.; Firdaus, Y.; Kaltsas, D.; El-Labban, A.; Faber, H.; Naphade, D.R.; Yengel, E.; Zheng, X.; Yarali, E. 18.4% Organic Solar Cells Using a High Ionization Energy Self-Assembled Monolayer as Hole-Extraction Interlayer. ChemSusChem 2021, 14, 3569–3578. [Google Scholar] [CrossRef]
- Bulliard, X.; Ihn, S.G.; Yun, S.; Kim, Y.; Choi, D.; Choi, J.Y.; Kim, M.; Sim, M.; Park, J.H.; Choi, W. Enhanced performance in polymer solar cells by surface energy control. Adv. Funct. Mater. 2010, 20, 4381–4387. [Google Scholar] [CrossRef]
- Hong, L.; Yao, H.; Cui, Y.; Yu, R.; Lin, Y.W.; Chen, T.W.; Xu, Y.; Qin, J.; Hsu, C.S.; Ge, Z. Simultaneous improvement of efficiency and stability of organic photovoltaic cells by using a cross-linkable fullerene derivative. Small 2021, 17, 2101133. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Dong, H.; Pan, W.; Liu, B.; Chen, X.; Huang, R.; Li, Z.; Li, F.; Luo, Q.; Zhang, J.; et al. Interfaces, An Efficiency of 16.46% and a T 80 Lifetime of Over 4000 h for the PM6: Y6 Inverted Organic Solar Cells Enabled by Surface Acid Treatment of the Zinc Oxide Electron Transporting Layer. ACS Appl. Mater. Interfaces 2021, 13, 17869–17881. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Cheng, P.; Yang, Y.; Li, G.; Yang, Y. High-performance organic bulk-heterojunction solar cells based on multiple-donor or multiple-acceptor components. Adv. Mater. 2018, 30, 1705706. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, M.; Liu, C.; Li, Z.; Fu, D.; Guo, W. Realizing efficiency improvement of polymer solar cells by using multi-functional cascade electron transport layers. Org. Electron. 2020, 76, 105482. [Google Scholar] [CrossRef]
- An, Q.S.; Zhang, F.J.; Sun, Q.Q.; Zhang, M.; Zhang, J.; Tang, W.H.; Yin, X.X.; Deng, Z.B. Efficient organic ternary solar cells with the third component as energy acceptor. Nano Energy 2016, 26, 180–191. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.J.; An, Q.S.; Sun, Q.Q.; Wang, W.B.; Zhang, J.; Tang, W.H. Highly efficient ternary polymer solar cells by optimizing photon harvesting and charge carrier transport. Nano Energy 2016, 22, 241–254. [Google Scholar] [CrossRef]
- Brütting, W.; Berleb, S.; Mückl, A.G. Device physics of organic light-emitting diodes based on molecular materials. Org. Electron. 2001, 2, 1–36. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, H.; Xu, Y.; Xu, W.; Zhou, J.; Zhang, T.; Ma, X.; Wang, J.; Zhang, F.; Sun, Q. Highly efficient inverted organic solar cells with natural biomaterial histidine as electron transport layer. Org. Electron. 2022, 106, 106538. [Google Scholar] [CrossRef]
- Duan, L.; Sang, B.; He, M.; Zhang, Y.; Hossain, M.A.; Rahaman, M.H.; Wei, Q.; Zou, Y.; Uddin, A.; Hoex, B. Interface modification enabled by atomic layer deposited ultra-thin titanium oxide for high-efficiency and semitransparent organic solar cells. Solar RRL 2020, 4, 2000497. [Google Scholar] [CrossRef]
- You, J.B.; Chen, C.C.; Dou, L.T.; Murase, S.; Duan, H.S.; Hawks, S.A.; Xu, T.; Son, H.J.; Yu, L.P.; Li, G.; et al. Metal oxide nanoparticles as an electron-transport layer in high-performance and stable inverted polymer solar cells. Adv. Mater. 2012, 24, 5267–5272. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Guo, X.; Wang, Z.Y.; Li, W.B.; Guo, B.; Ma, W.; Zhang, M.J.; Li, Y.F. 10.8% efficiency polymer solar cells based on PTB7-Th and PC71BM via binary solvent additives treatment. Adv. Funct. Mater. 2016, 26, 6635–6640. [Google Scholar] [CrossRef]
- Huang, S.; Kang, B.N.; Duan, L.; Zhang, D.D. Highly efficient inverted polymer solar cells by using solution processed MgO/ZnO composite interfacial layers. J. Colloid Interface Sci. 2021, 583, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhang, Z.; Su, Z.; Zhang, X.; Zhang, J. 16.5% efficiency ternary organic photovoltaics with two polymer donors by optimizing molecular arrangement and phase separation. Nano Energy 2020, 69, 104447. [Google Scholar] [CrossRef]
- Wang, X.K.; Zhang, L.F.; Hu, L.; Xie, Z.J.; Mao, H.D.; Tan, L.C.; Zhang, Y.D.; Chen, Y.W. High-efficiency (16.93%) pseudo-planar heterojunction organic solar cells enabled by binary additives strategy. Adv. Funct. Mater. 2021, 31, 2102291. [Google Scholar] [CrossRef]
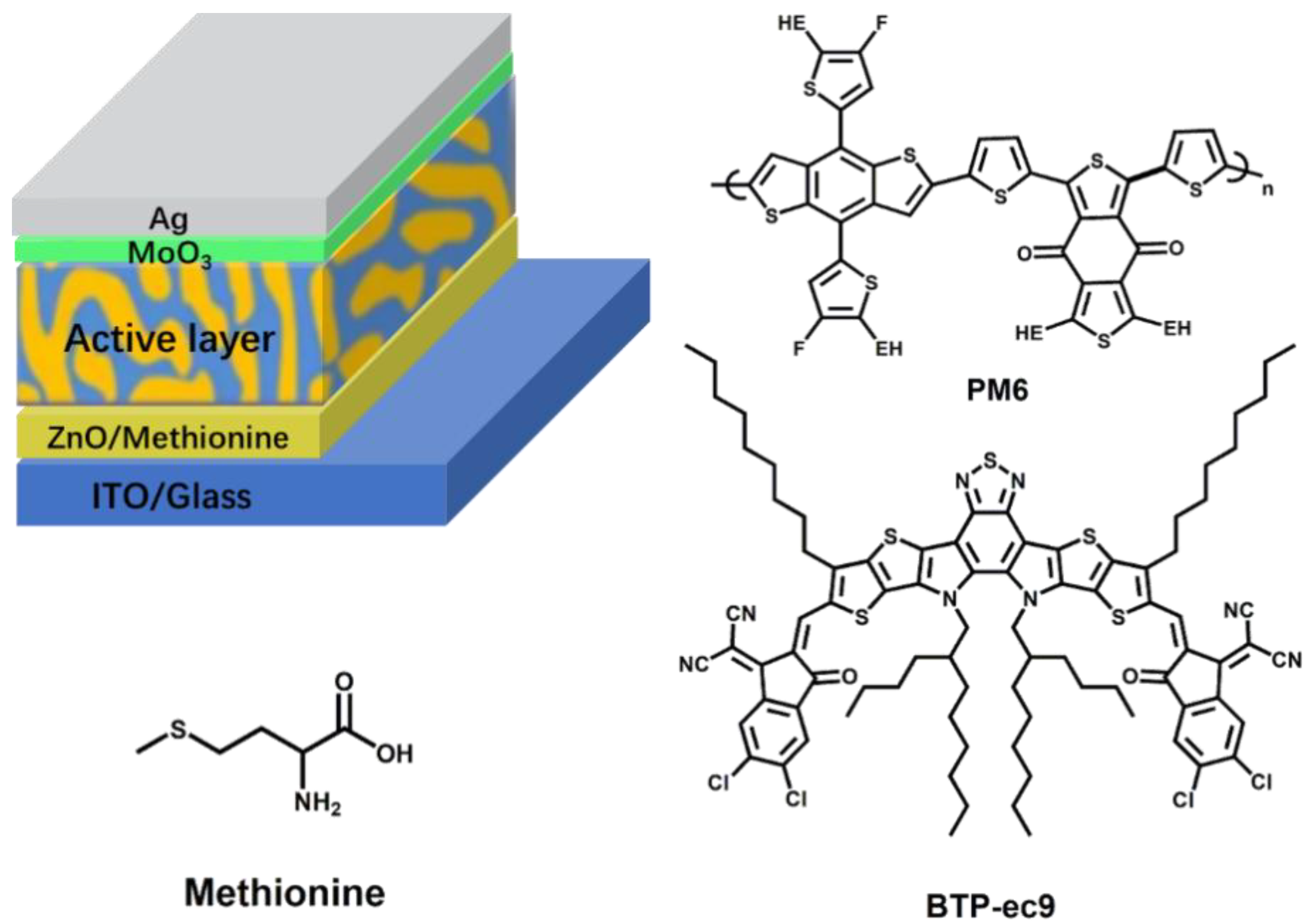
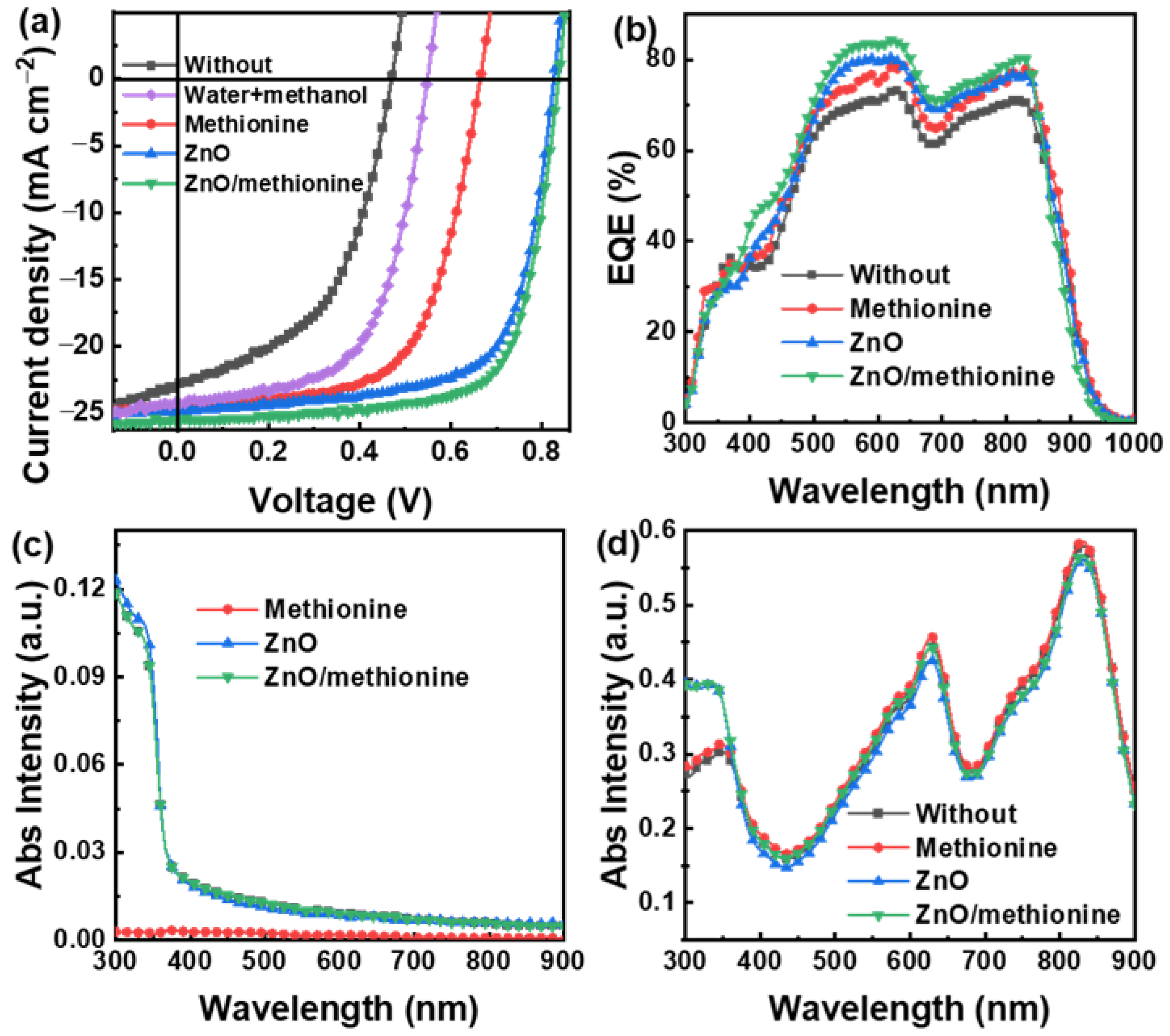
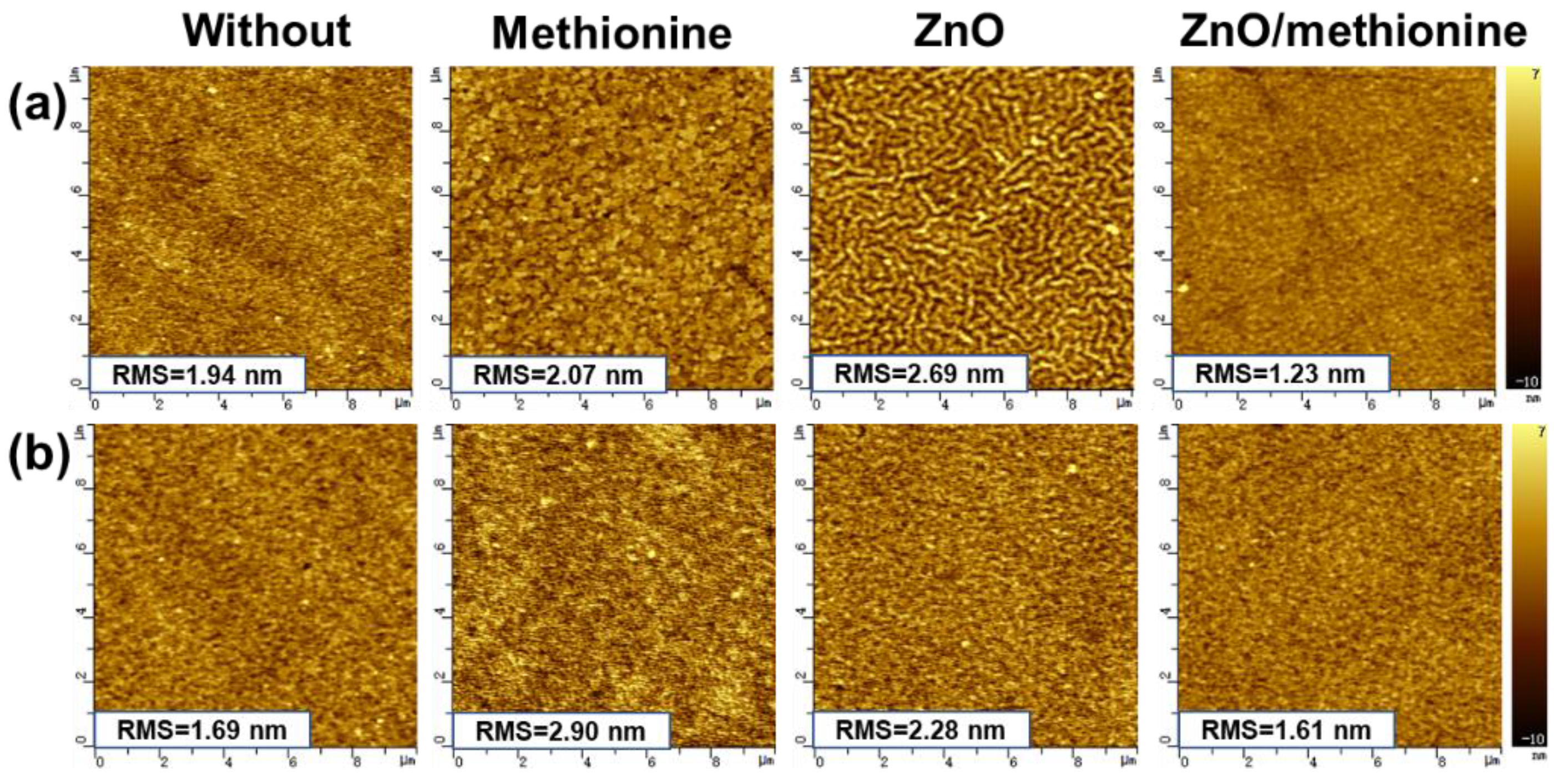

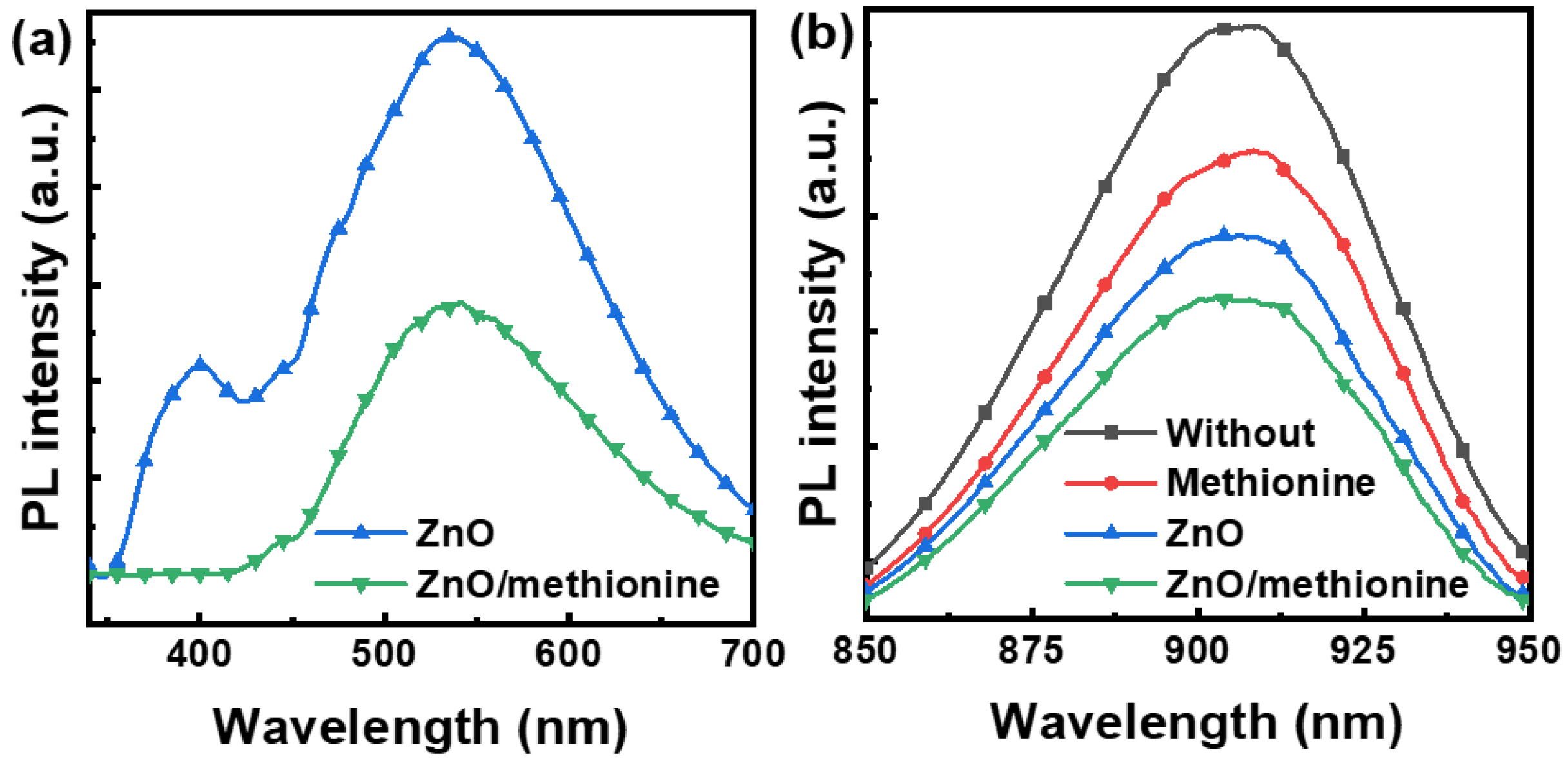
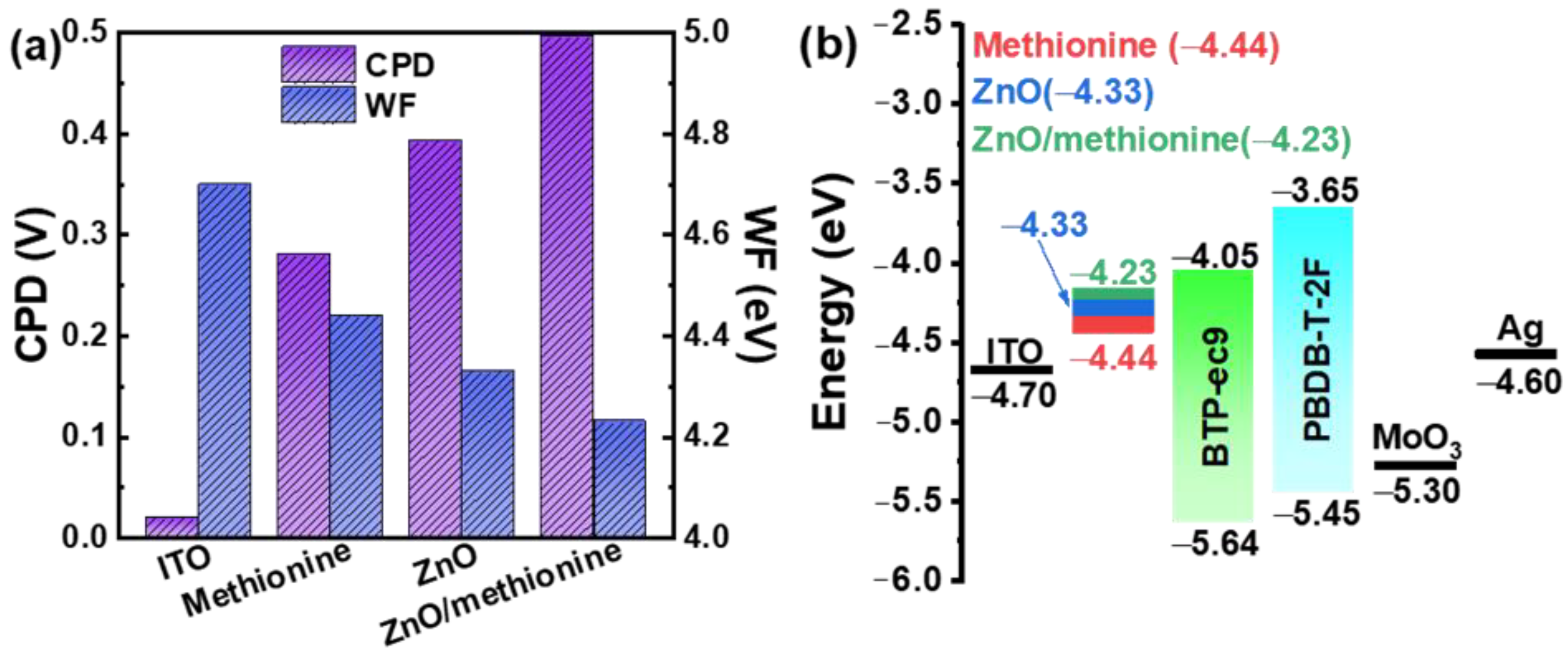
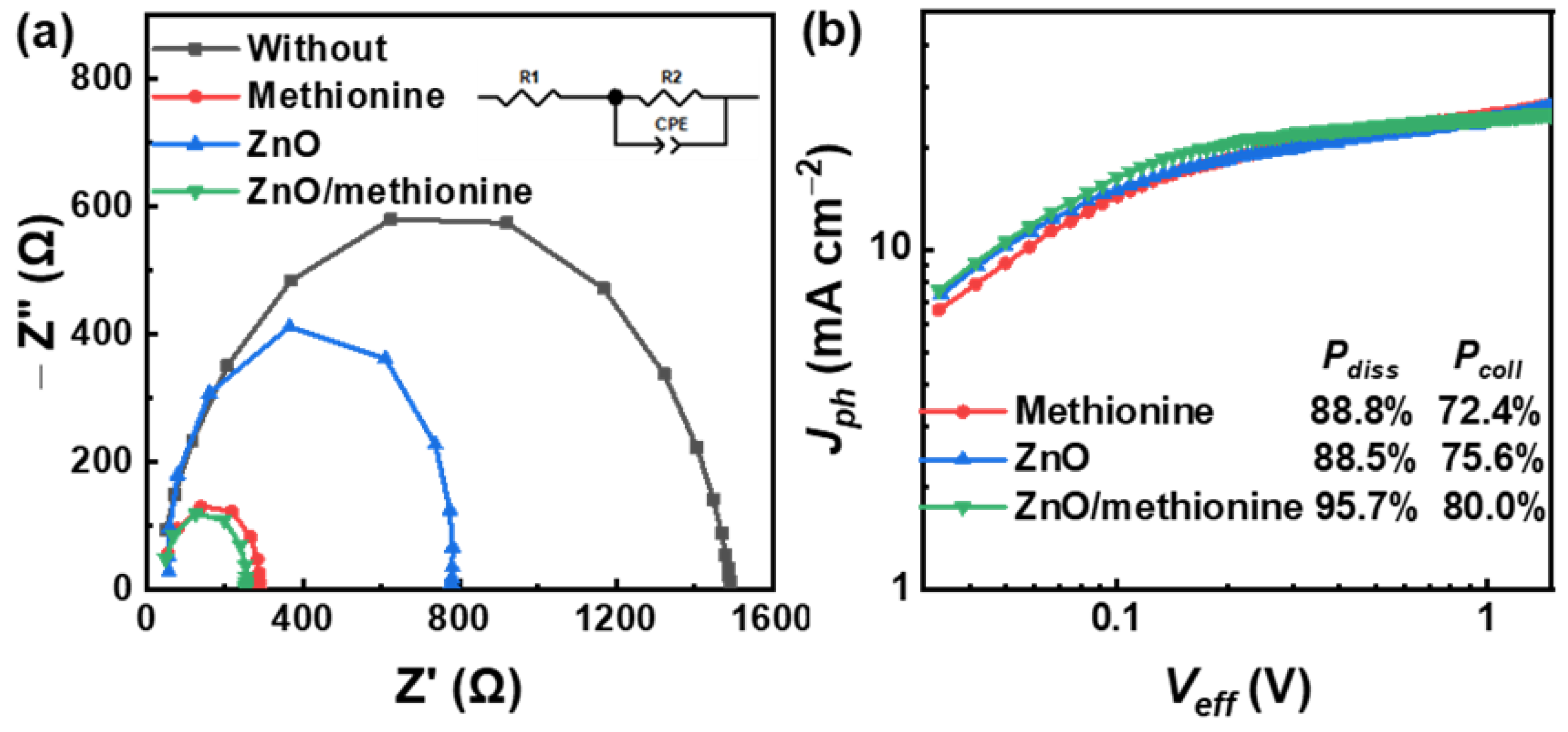
| ETL | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Without | 23.25 | 0.47 | 49.88 | 5.45 |
| Water+methanol | 24.16 | 0.55 | 60.40 | 8.02 |
| Methionine solution | 24.43 | 0.67 | 63.16 | 10.33 |
| ZnO | 24.79 | 0.83 | 69.26 | 14.25 |
| ZnO/methionine solution | 25.52 | 0.84 | 71.58 | 15.34 |
| ITO/ETLs | (o) | (o) | Surface Energy |
|---|---|---|---|
| (mN m−1) | |||
| Without | 33.33 | 31.51 | 63.76 |
| Methionine solution | 66.67 | 49.15 | 39.07 |
| ZnO | 49.31 | 39.12 | 51.65 |
| ZnO/methionine solution | 73.10 | 69.50 | 36.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhou, H.; Duan, P.; Shan, B.; Xu, W.; Wang, J.; Liu, M.; Zhang, F.; Sun, Q. Improving the Efficiency of Organic Solar Cells with Methionine as Electron Transport Layer. Molecules 2022, 27, 6363. https://doi.org/10.3390/molecules27196363
Xu Y, Zhou H, Duan P, Shan B, Xu W, Wang J, Liu M, Zhang F, Sun Q. Improving the Efficiency of Organic Solar Cells with Methionine as Electron Transport Layer. Molecules. 2022; 27(19):6363. https://doi.org/10.3390/molecules27196363
Chicago/Turabian StyleXu, Yujie, Hang Zhou, Pengyi Duan, Baojie Shan, Wenjing Xu, Jian Wang, Mei Liu, Fujun Zhang, and Qianqian Sun. 2022. "Improving the Efficiency of Organic Solar Cells with Methionine as Electron Transport Layer" Molecules 27, no. 19: 6363. https://doi.org/10.3390/molecules27196363
APA StyleXu, Y., Zhou, H., Duan, P., Shan, B., Xu, W., Wang, J., Liu, M., Zhang, F., & Sun, Q. (2022). Improving the Efficiency of Organic Solar Cells with Methionine as Electron Transport Layer. Molecules, 27(19), 6363. https://doi.org/10.3390/molecules27196363








