Phytochemical Composition, Antibacterial, Antioxidant and Antidiabetic Potentials of Cydonia oblonga Bark
Abstract
1. Introduction
2. Results
2.1. Phytochemical Evaluation
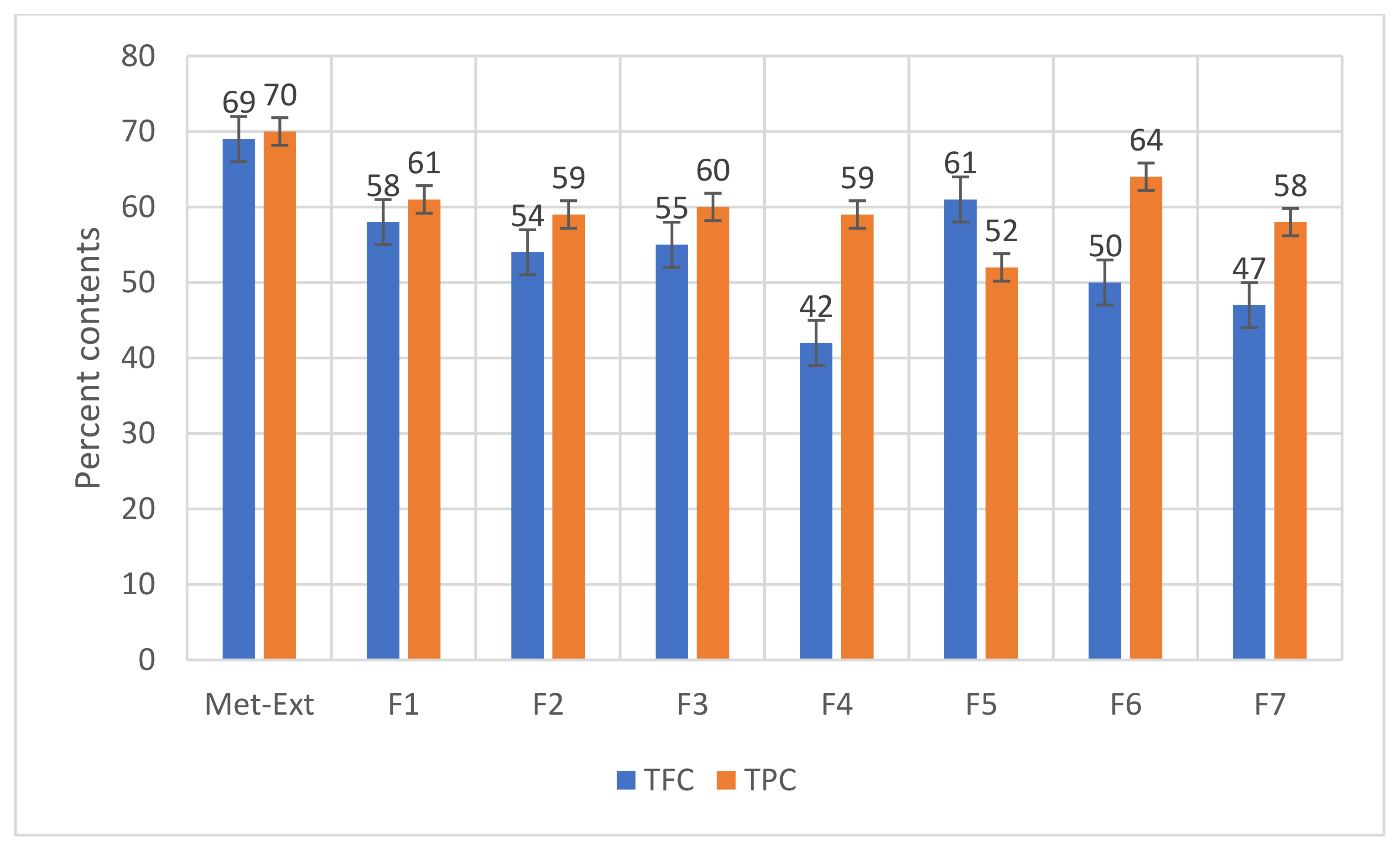
2.2. TFC and TPC in the Met-Ext and Purified Fractions of Cydonia oblonga
2.3. HPLC Analysis of Methanolic Extract of Cydonia oblonga
2.4. GC-MS Characterization of Methanolic Extract and Purified Fractions
2.5. Evaluation of Antibacterial Activity of Methanolic Extract and Fractions
2.6. Antioxidant Activity of Crude and Purified Fractions of Cydonia oblonga
2.7. In Vitro Antidiabetic Potential of Met + Ext and Purified Fractions
2.7.1. In Vitro α-Glucosidase Enzyme Inhibition Activity
2.7.2. In Vitro α Amylase Enzyme Inhibition Activity
3. Discussion
4. Material and Methods
4.1. Plant Sample Collection
4.2. Chemicals and Standards
4.3. Extraction and Fractionation
4.3.1. Phytochemical Analysis
4.3.2. Analysis of Total Phenolic Content and Total Flavonoids Content
4.3.3. HPLC Characterization
Sample Preparation for HPLC Analysis
HPLC-UV
GC-MS Analysis for Characterization
4.4. Antibacterial Activity
4.5. DPPH Radical Scavenging Activity
4.6. Antidiabetic Assays
4.6.1. Inhibition of α-Amylase
4.6.2. α-Glucosidase Inhibition Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, M.; Khan, K.-R.; Ahmad, S.; Aati, H.Y.; Ovatlarnporn, C.; Rehman, M.S.; Javed, T.; Khursheed, A.; Ghalloo, B.A.; Dilshad, R.; et al. Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development. Molecules 2022, 27, 4113. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D.; Singh, V.; Ali, M. Phytochemical and Pharmacological Profile of Pterocarpus Marsupium: A Review. Pharma Innov. J. 2016, 5, 31. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Luo, W.; Shoaib, R.M.; Li, G.l.; Hassan, S.S.; Yang, Z.; Xiao, X.; Tu, G.; Yan, S.K.; Ma, X.; et al. Guaiane-type sesquiterpenoids from Cinnamomum migao H.W. Li: And their anti-inflammatory activities. Phytochemistry 2021, 190, 112850. [Google Scholar] [CrossRef]
- Bent, S.; Ko, R. Commonly Used Herbal Medicines in the United States: A Review. Am. J. Med. 2004, 116, 478–485. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Kang, N.J.; Shin, S.H.; Lee, H.J.; Lee, K.W. Polyphenols as Small Molecular Inhibitors of Signaling Cascades in Carcinogenesis. Pharmacol. Ther. 2011, 130, 310–324. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Bomser, J.A.; Singletary, K.W.; Wallig, M.A.; Smith, M.A.L. Inhibition of TPA-Induced Tumor Promotion in CD-1 Mouse Epidermis by a Polyphenolic Fraction from Grape Seeds. Cancer Lett. 1999, 135, 151–157. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Farzan, M.; Choe, H.; Ohagen, A.; Gartner, S.; Busciglio, J.; Yang, X.; Hofmann, W.; Newman, W.; et al. CCR3 and CCR5 Are Co-Receptors for HIV-1 Infection of Microglia. Nature 1997, 385, 645–649. [Google Scholar] [CrossRef]
- Preuss, H.G.; Montamarry, S.; Echard, B.; Scheckenbach, R.; Bagchi, D. Long-Term Effects of Chromium, Grape Seed Extract, and Zinc on Various Metabolic Parameters of Rats. Mol. Cell. Biochem. 2001, 223, 95–102. [Google Scholar] [CrossRef]
- Asghar, A.; Tan, Y.C.; Zahoor, M.; Abidin, S.A.Z.; Yow, Y.Y.; Khan, E.; Lahiri, C. A Scaffolded Approach to Unearth Potential Antibacterial Components from Epicarp of Malaysian Nephelium lappaceum L. Sci. Rep. 2021, 11, 13859. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Seabra, R.M.; Silva, B.M. Phenolic Profile of Cydonia Oblonge Miller Leaves. J. Agric. Food Chem. 2007, 55, 7926–7930. [Google Scholar] [CrossRef]
- Li, X.; Bai, H.; Yang, Y.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular Antibacterial Materials for Combatting Antibiotic Resistance. Adv. Mater. 2019, 31, 1805092. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Barceló, A.; Rajpathak, S. Incidence and Prevalence of Diabetes Mellitus in the Americas. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2001, 10, 300–308. [Google Scholar] [CrossRef]
- International Diabetes Federation IDF. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation IDF: Brussels, Belgium, 2015. [Google Scholar]
- Muhtadi; Primarianti, A.U.; Sujono, T.A. Antidiabetic Activity of Durian (Durio Zibethinus murr.) and Rambutan (Nephelium lappaceum L.) Fruit Peels in Alloxan Diabetic Rats. Procedia Food Sci. 2015, 3, 255–261. [Google Scholar] [CrossRef]
- Sharma, B.; Balomajumder, C.; Roy, P. Hypoglycemic and Hypolipidemic Effects of Flavonoid Rich Extract from Eugenia Jambolana Seeds on Streptozotocin Induced Diabetic Rats. Food Chem. Toxicol. 2008, 46, 2376–2383. [Google Scholar] [CrossRef]
- Shemchuk, O.; Braga, D.; Grepioni, F.; Turner, R.J. Co-Crystallization of Antibacterials with Inorganic Salts: Paving the Way to Activity Enhancement. RSC Adv. 2020, 10, 2146–2149. [Google Scholar] [CrossRef]
- Marwa, S.K.; Khan, M.A.; Ur-Rehman, F.; Bhat, I.U. Aromatic Plant Species Mentioned in the Holy Qura’n and Ahadith and Their Ethnomedicinal Importance. Pak. J. Nutr. 2009, 8, 1472–1479. [Google Scholar] [CrossRef][Green Version]
- Ganopoulos, I.; Merkouropoulos, G.; Pantazis, S.; Tsipouridis, C.; Tsaftaris, A. Assessing Molecular and Morpho-Agronomical Diversity and Identification of ISSR Markers Associated with Fruit Traits in Quince (Cydonia Oblonga). Genet. Mol. Res. 2011, 10, 2729–2746. [Google Scholar] [CrossRef]
- Rop, O.; Balík, J.; Řezníček, V.; Juríková, T.; Škardová, P.; Salaš, P.; Sochor, J.; Mlček, J.; Kramářová, D. Chemical Characteristics of Fruits of Some Selected Quince (Cydonia oblonga Mill.) Cultivars. Czech J. Food Sci. 2011, 29, 65–73. [Google Scholar] [CrossRef]
- Erdoğan, T.; Gönenç, T.; Hortoğlu, Z.S.; Demirci, B.; Başer, K.H.; Kıvçak, B. Chemical composition of the essential oil of quince (Cydonia oblonga Miller) leaves. Med. Aromat. Plants 2012, 1, 134. [Google Scholar]
- Yamanaka, N.; Oda, O.; Nagao, S. Green Tea Catechins Such as (−)-Epicatechin and (−)-Epigallocatechin Accelerate Cu2+-Induced Low Density Lipoprotein Oxidation in Propagation Phase. FEBS Lett. 1997, 401, 230–234. [Google Scholar] [CrossRef]
- Nomoto, H.; Iigo, M.; Hamada, H.; Kojima, S.; Tsuda, H. Chemoprevention of Colorectal Cancer by Grape Seed Proanthocyanidin Is Accompanied by a Decrease in Proliferation and Increase in Apoptosis. Nutr. Cancer 2004, 49, 81–88. [Google Scholar] [CrossRef]
- Carvalho, M.; Branca, M.S.; Silva, R.; Valentão, P.; Paula, B.A. First Report on Cydonia oblonga Miller Anticancer Potential: Differential Antiproliferative Effect against Human Kidney and Colon Cancer Cells. J. Agric. Food Chem. 2010, 58, 3366–3370. [Google Scholar] [CrossRef]
- Adel, A.M.; El-Wahab, Z.H.A.; Ibrahim, A.A.; Al-Shemy, M.T. Characterization of Microcrystalline Cellulose Prepared from Lignocellulosic Materials. Part I. Acid Catalyzed Hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef]
- Djilali, A.B.; Mehraz, R.; Bouacem, K.; Benseddik, A.; Moualek, I.; Nabiev, M.; Benzara, A. Bioactive Substances of Cydonia oblonga Fruit: Insecticidal Effect of Tannins on Tribuliumm Confusum. Int. J. Fruit Sci. 2021, 21, 721–731. [Google Scholar] [CrossRef]
- Jain, M.; Kapadia, R.; Albert, S.; Mishra, S.H. Standardization of Feronia limonia L. Leaves by HPLC, HPTLC, Physico-Chemical and Histological Parameters. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 525–535. [Google Scholar]
- Tripathi, I.P.; Mishra, M.K.; Pardhi, Y.; Dwivedi, A.; Dwivedi, N.; Kamal, A.; Gupta, P. HPLC Analysis of Methanolic Extract of Some Medicinal Plant Leaves of Myrtaceae Family. Int. Pharm. Sci. 2012, 2, 49–53. [Google Scholar]
- Kabra, A.; Sharma, R.; Hano, C.; Kabra, R.; Martins, N.; Baghel, U.S. Phytochemical Composition, Antioxidant, and Antimicrobial Attributes of Different Solvent Extracts from Myrica Esculenta Buch.-Ham. Ex. d. Don Leaves. Biomolecules 2019, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.; Depuydt, P.; Vandewoude, K.; De Bacquer, D. Measuring the Impact of Multidrug Resistance in Nosocomial Infection. Curr. Opin. Infect. Dis. 2007, 20, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazraji, S. Phytochemical Screening and Antibacterial Activity of the Crude Extract of Cydonia oblonga Seeds. Glob. Adv. Res. J. Microbiol. 2013, 2, 137–140. [Google Scholar]
- Benzarti, S.; Hamdi, H.; Lahmayer, I.; Toumi, W.; Kerkeni, A.; Belkadhi, K.; Sebei, H. Total Phenolic Compounds and Antioxidant Potential of Quince (Cydonia oblonga Miller) Leaf Methanol Extract. Int. J. Innov. Appl. Stud. 2015, 13, 518. [Google Scholar]
- Karar, M.G.E.; Pletzer, D.; Jaiswal, R.; Weingart, H.; Kuhnert, N. Identification, Characterization, Isolation and Activity against Escherichia coli of Quince (Cydonia Oblonga) Fruit Polyphenols. Food Res. Int. 2014, 65, 121–129. [Google Scholar] [CrossRef]
- Urbanavičiūte, I.; Liaudanskas, M.; Bobinas, Č.; Šarkinas, A.; Rezgiene, A.; Viskelis, P. Japanese Quince (Chaenomeles japonica) as a Potential Source of Phenols: Optimization of the Extraction Parameters and Assessment of Antiradical and Antimicrobial Activities. Foods 2020, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, S.; Belkadhi, K.; Hamdi, H. Biological Activities of Phenolics from Leaves of Tunisian Cydonia oblonga Miller. Allelopath. J. 2018, 45, 229–242. [Google Scholar] [CrossRef]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective Effect of Quince (Cydonia oblonga Miller) Fruit against Oxidative Hemolysis of Human Erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef]
- Zeb, A. A Reversed Phase HPLC-DAD Method for the Determination of Phenolic Compounds in Plant Leaves. Anal. Methods 2015, 7, 7753–7757. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical Constituents of Some Nigerian Medicinal Plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Cruz, M.T.G.D.; Cordeiro, M.N.D.S.; Milhazes, N.; Borges, F.; Marques, M.P.M. Phenolic Acid Derivatives with Potential Anticancer Properties—A Structure-Activity Relationship Study. Part 1: Methyl, Propyl and Octyl Esters of Caffeic and Gallic Acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef]
- Kielhorn, S.; Thorngate, J.H. Oral Sensations Associated with the Flavan-3-Ols (+)-Catechin and (−)-Epicatechin. Food Qual. Prefer. 1999, 10, 109–116. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Marco, G.D.; Canini, A.; Bekkara, F.A. GC/MS Analysis, and Antioxidant and Antimicrobial Activities of Alkaloids Extracted by Polar and Apolar Solvents from the Stems of Anabasis Articulata. Med. Chem. Res. 2019, 28, 754–767. [Google Scholar] [CrossRef]
- Aslan, M.; Orhan, N.; Orhan, D.D.; Ergun, F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J. Ethnopharmacol. 2010, 128, 384–389. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The medical importance of Cydonia oblonga—A review. IOSR J. Pharm. 2016, 6, 87–99. [Google Scholar]

| Phytochemicals | Reagent | Analyses | Result |
|---|---|---|---|
| Flavonoids | Ferric chloride | Appearance of yellow color and colorless after HCL addition | + |
| Alkaloids | Dragendroff’s | Formation of orange–red color precipitate | + |
| Glycosides | Keller Killiani | Red to brown layer formation | + |
| Triterpenoids | Liebermann Burchard | Reddish–brown boundary | + |
| Tannins | Gelatine | Brownish to green precipitates | + |
| Retention Time (min) | Phenolic Compound Identity | Peak Area of Sample | Identification Reference |
|---|---|---|---|
| 2.616 | Malic acid | 7.64427 | Ref. Stand |
| 10.879 | Mandelic acid | 28.98360 | Ref. Stand |
| 12.373 | Caffeic acid | 21.41109 | Ref. Stand |
| 20.578 | Quercetin | 77.68702 | Ref. Stand |
| 29.537 | Catechin hydrate | 46.64664 | Ref. Stand |
| 30.408 | Morin | 134.41394 | Ref. Stand |
| S. No | Fraction | Compounds |
|---|---|---|
| 1 | F1 | BIS-(2-ethylhexyl)phtjalate, diisooctyl phthlate |
| 2 | F2 | carbamide |
| 3 | F3 | octasiloxane, dimethylsiloxanecyclictrimer |
| 4 | F4 | silicic acid, cyclotrisiloxane |
| 5 | F5 | 6-AH-cAMP, 4H-cyclopropa[5′,6′]benz[1′,2′,7,8]azule, 4-(4-chlorophenyl)-3-morpholinepyrol-2-yl)-butenedioic acid |
| 6 | F6 | isopropyamine |
| 7 | F7 | 1-propylhydrazine |
| Sample | Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| Microbial Strains | ||||||
| Escherichia coli | Salmonella typhi | Klebsiella pneumonia | Bacillus subtilis | Staphylococcus aureus | Streptococcus pneumonia | |
| Crude | 18 ± 0.7 | 19 ± 2.0 | 18 ± 0.6 | 19 ± 0.2 | 25 ± 1.8 | 20 ± 2.2 |
| F1 | 10 ± 0.6 | 10 ± 0.9 | 10 ± 0.8 | 10 ± 0.8 | 15± 0.4 | 12 ± 1.1 |
| F2 | 11 ± 0.2 | 14 ± 0.4 | 10 ± 1.3 | 15 ± 1.5 | 15 ± 1.1 | 20 ± 2.0 |
| F3 | 11 ± 1.9 | 10 ± 1.3 | 15 ± 0.9 | 10 ± 1.2 | 10 ± 1.2 | 16 ± 1.6 |
| F4 | 15 ± 1.4 | 13 ± 0.8 | 11 ± 1.0 | 12 ± 1.5 | 11 ± 0.5 | 13 ± 1.4 |
| F5 | 9 ± 1.6 | 10 ± 1.6 | 12 ± 0.6 | 16 ± 1.0 | 14 ± 0.3 | 11 ± 0.8 |
| F6 | 9 ± 08 | 12 ± 1.2 | 12 ± 1.9 | 10 ± 1.1 | 9 ± 1.7 | 11 ± 1.3 |
| F7 | 10 ± 0.4 | 9 ± 0.5 | 12 ± 1.3 | 11 ± 0.8 | 10 ± 1.3 | 12 ± 1.4 |
| S. No | Sample | Concentration (µg/mL) | % DPPH Scavenging | IC50 |
|---|---|---|---|---|
| 1 | Met-Ext | 1000 | 87.41 ± 0.54 | 120 |
| 500 | 85.12 ± 0.76 | |||
| 250 | 68.27 ± 0.85 | |||
| 125 | 52.78 ± 0.25 | |||
| 62.5 | 36.98 ± 0.63 | |||
| 2 | F1 | 1000 | 85.45 ± 0.85 | 115 |
| 500 | 74.23 ± 0.75 | |||
| 250 | 67.12 ± 0.91 | |||
| 125 | 57.34 ± 0.77 | |||
| 62.5 | 38.65 ± 0.48 | |||
| 3 | F2 | 1000 | 65.78 ± 0.68 | 200 |
| 500 | 60.27 ± 0.63 | |||
| 250 | 57.92 ± 0.78 | |||
| 125 | 46.74 ± 0.89 | |||
| 62.5 | 36.04 ± 0.43 | |||
| 4 | F3 | 1000 | 58.61 ± 0.58 | 380 |
| 500 | 52.05 ± 0.49 | |||
| 250 | 48.93 ± 0.75 | |||
| 125 | 44.21 ± 0.48 | |||
| 62.5 | 34.96 ± 0.81 | |||
| 5 | F4 | 1000 | 80.76 ± 0.59 | 110 |
| 500 | 68.23 ± 0.67 | |||
| 250 | 64.04 ± 0.83 | |||
| 125 | 54.92 ± 0.79 | |||
| 62.5 | 42.71 ± 0.58 | |||
| 6 | F5 | 1000 | 71.29 ± 0.49 | 200 |
| 500 | 67.61 ± 0.59 | |||
| 250 | 57.93 ± 0.59 | |||
| 125 | 46.97 ± 0.59 | |||
| 62.5 | 37.12 ± 0.69 | |||
| 7 | F6 | 1000 | 85.28 ± 0.94 | 105 |
| 500 | 81.60 ± 0.96 | |||
| 250 | 70.26 ± 0.86 | |||
| 125 | 55.82 ± 0.89 | |||
| 62.5 | 36.05 ± 0.99 | |||
| 8 | F7 | 1000 | 48.45 ± 0.62 | 110 |
| 500 | 40.38 ± 0.38 | |||
| 250 | 34.18 ± 0.85 | |||
| 125 | 31.41 ± 0.45 | |||
| 62.5 | 29.34 ± 0.63 | |||
| 10 | Ascorbic acid | 1000 | 94.88 ± 0.56 | 30 |
| 500 | 86.59 ± 0.45 | |||
| 250 | 78.64 ± 0.76 | |||
| 125 | 69.14 ± 0.25 | |||
| 62.5 | 62.87 ± 0.53 |
| Sample | Concentration (µg/mL) | % α-Glucosidase Inhibition | IC50 (µg/mL) | % α-Amylase Inhibition | IC50 (µg/mL) |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||||
| Met-Ext | 1000 | 78.21 ± 0.67 | 57 | 77.98 ± 0.57 | 52 |
| 500 | 73.51 ± 0.56 | 76.29 ± 0.36 | |||
| 250 | 64.79 ± 0.14 | 74.32 ± 0.25 | |||
| 125 | 60.28 ± 0.38 | 68.38 ± 0.68 | |||
| 62.5 | 52.51 ± 1.06 | 57.28 ± 0.52 | |||
| F1 | 1000 | 55.01 ± 0.29 | 500 | 79.72 ± 0.02 | 57 |
| 500 | 50.97 ± 0.39 | 78.22 ± 0.72 | |||
| 250 | 43.13 ± 0.34 | 74.02 ± 0.76 | |||
| 125 | 47.24 ± 0.29 | 67.97 ± 0.80 | |||
| 62.5 | 32.41 ± 0.74 | 52.58 ± 0.10 | |||
| F 2 | 1000 | 56.10 ± 0.24 | 494 | 79.97 ± 0.30 | 45 |
| 500 | 51.88 ± 0.38 | 79.27 ± 0.28 | |||
| 250 | 47.62 ± 0.91 | 76.22 ± 0.62 | |||
| 125 | 47.09 ± 0.01 | 75.82 ± 0.56 | |||
| 62.5 | 30.89 ± 1.99 | 70.18 ± 0.48 | |||
| F3 | 1000 | 62.44 ± 1.02 | 200 | 82.16 ± 0.48 | 49 |
| 500 | 57.61 ± 0.28 | 80.41 ± 0.92 | |||
| 250 | 55.88 ± 0.18 | 78.06 ± 0.65 | |||
| 125 | 48.19 ± 0.15 | 76.72 ± 0.53 | |||
| 62.5 | 43.27 ± 1.02 | 74.67 ± 0.59 | |||
| F4 | 1000 | 70.52 ± 0.15 | 370 | 77.37 ± 0.28 | 54 |
| 500 | 63.86 ± 0.03 | 76.57 ± 0.04 | |||
| 250 | 55.04 ± 0.08 | 74.47 ± 0.92 | |||
| 125 | 49.04 ± 0.71 | 68.88 ± 0.38 | |||
| 62.5 | 43.25 ± 0.90 | 57.18 ± 0.05 | |||
| F5 | 1000 | 62.18 ± 0.92 | 180 | 72.14 ± 0.30 | 220 |
| 500 | 59.92 ± 0.49 | 61.50 ± 0.58 | |||
| 250 | 52.92 ± 0.32 | 53.44 ± 0.28 | |||
| 125 | 48.84 ± 0.73 | 46.16 ± 0.74 | |||
| 62.5 | 36.73 ± 0.07 | 38.58 ± 0.48 | |||
| F6 | 1000 | 72.68 ± 0.22 | 125 | 74.24 ± 0.29 | 59 |
| 500 | 68.05 ± 0.52 | 67.38 ± 1.62 | |||
| 250 | 57.39 ± 0.99 | 62.67 ± 0.25 | |||
| 125 | 50.92 ± 0.27 | 56.52 ± 0.49 | |||
| 62.5 | 46.35 ± 0.89 | 51.81 ± 0.76 | |||
| F7 | 1000 | 57.33 ± 0.05 | 495 | 56.58 ± 0.10 | 500 |
| 500 | 51.61 ± 0.12 | 50.42 ± 0.46 | |||
| 250 | 46.84 ± 0.83 | 44.39 ± 0.78 | |||
| 125 | 39.60 ± 0.39 | 38.28 ± 0.60 | |||
| 62.5 | 34.68 ± 0.06 | 31.09 ± 1.05 | |||
| Acarbose | 1000 | 87.65 ± 0.71 | 30 | 85.99 ± 0.44 | 32 |
| 500 | 83.05 ± 0.65 | 83.61 ± 0.58 | |||
| 250 | 78.90 ± 1.02 | 76.85 ± 0.96 | |||
| 125 | 71.83 ± 0.99 | 70.47 ± 0.78 | |||
| 62.5 | 65.15 ± 0.75 | 64.89 ± 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abed, S.N.; Bibi, S.; Jan, M.; Talha, M.; Islam, N.U.; Zahoor, M.; Al-Joufi, F.A. Phytochemical Composition, Antibacterial, Antioxidant and Antidiabetic Potentials of Cydonia oblonga Bark. Molecules 2022, 27, 6360. https://doi.org/10.3390/molecules27196360
Abed SN, Bibi S, Jan M, Talha M, Islam NU, Zahoor M, Al-Joufi FA. Phytochemical Composition, Antibacterial, Antioxidant and Antidiabetic Potentials of Cydonia oblonga Bark. Molecules. 2022; 27(19):6360. https://doi.org/10.3390/molecules27196360
Chicago/Turabian StyleAbed, Shaymaa Najm, Sania Bibi, Marwa Jan, Muhammad Talha, Noor Ul Islam, Muhammad Zahoor, and Fakhria A. Al-Joufi. 2022. "Phytochemical Composition, Antibacterial, Antioxidant and Antidiabetic Potentials of Cydonia oblonga Bark" Molecules 27, no. 19: 6360. https://doi.org/10.3390/molecules27196360
APA StyleAbed, S. N., Bibi, S., Jan, M., Talha, M., Islam, N. U., Zahoor, M., & Al-Joufi, F. A. (2022). Phytochemical Composition, Antibacterial, Antioxidant and Antidiabetic Potentials of Cydonia oblonga Bark. Molecules, 27(19), 6360. https://doi.org/10.3390/molecules27196360






