Production of Siamenoside I and Mogroside IV from Siraitia grosvenorii Using Immobilized β-Glucosidase
Abstract
:1. Introduction
2. Results and Discussion
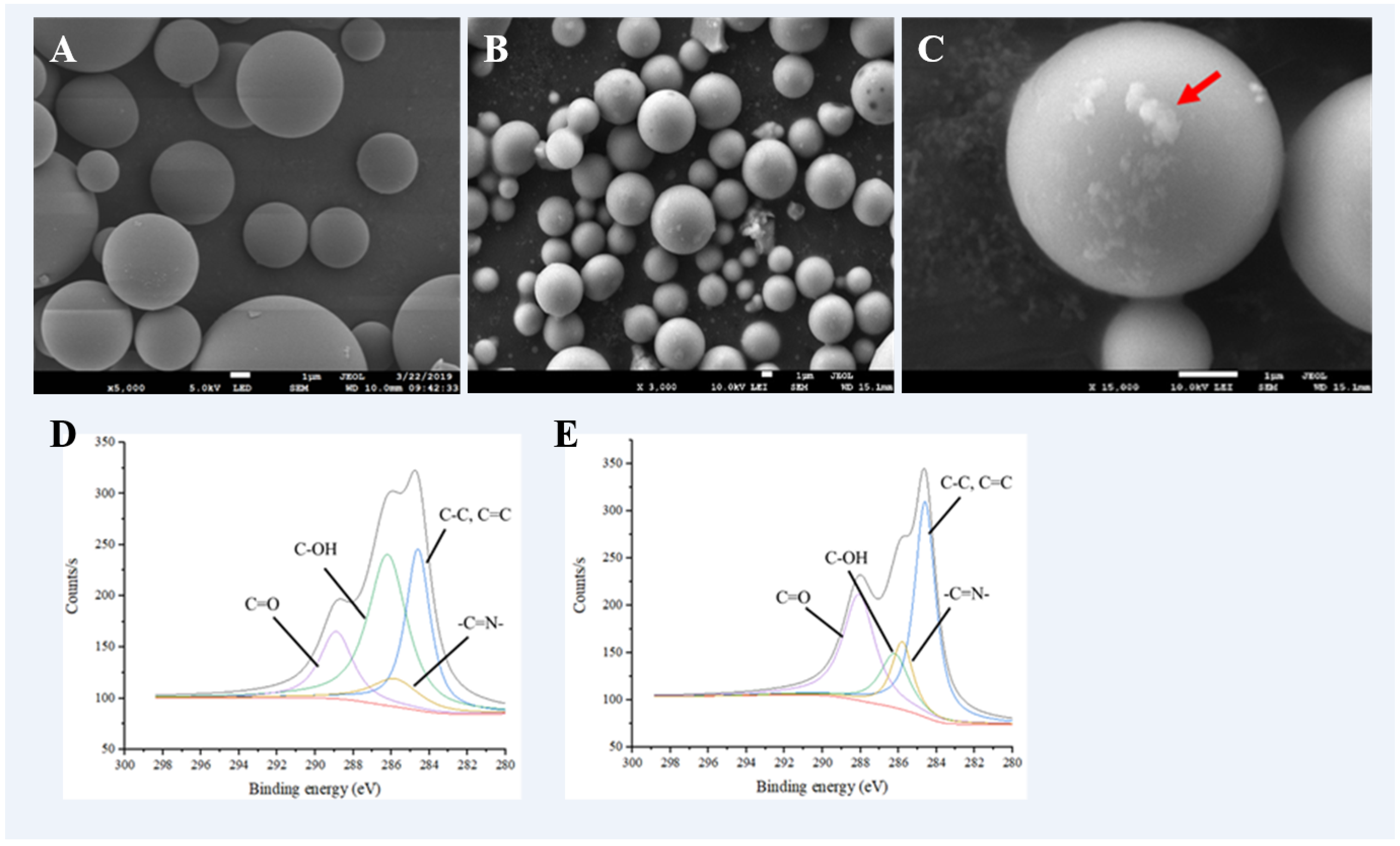
2.1. Morphology of Glass Microsphere
2.2. Verification of Enzyme Immobilisation
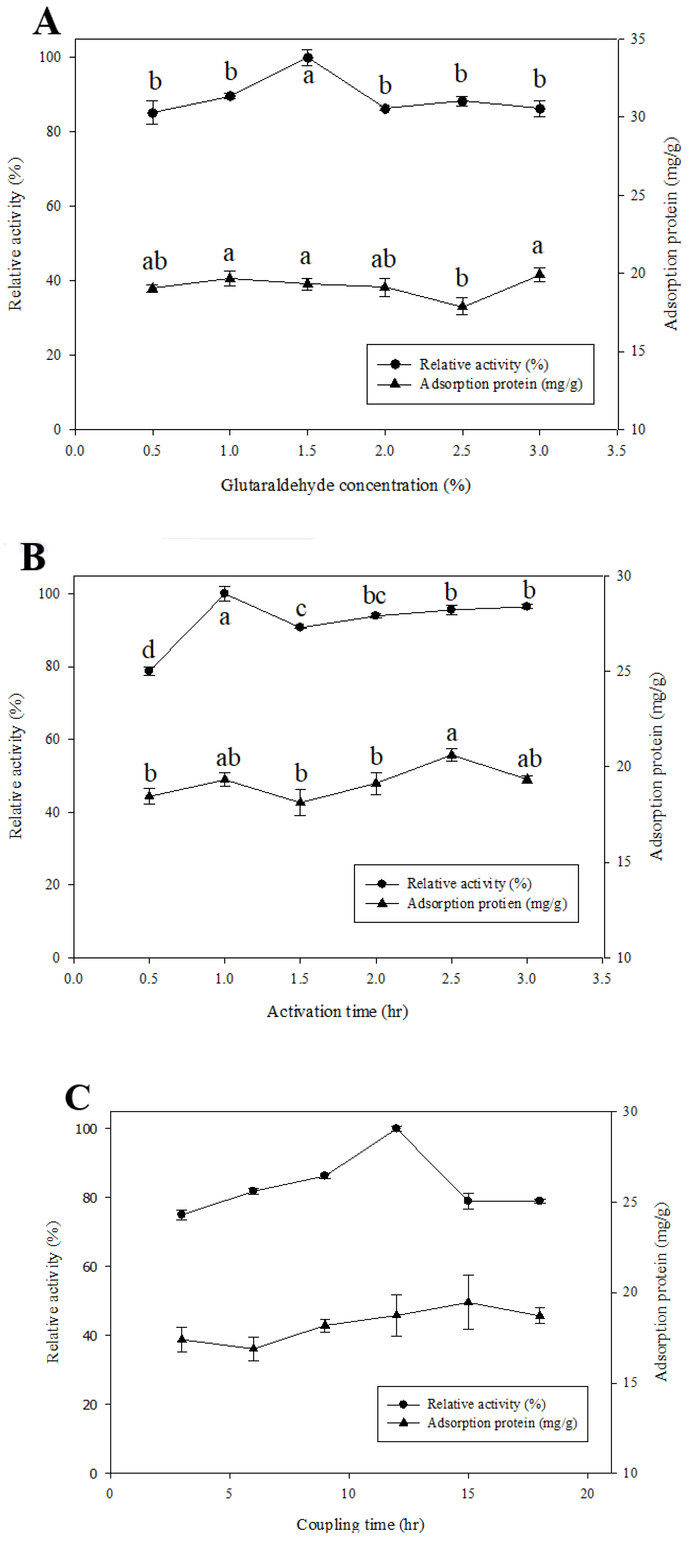
2.3. Determination of Optimal Conditions for the β-Glucosidase Immobilized System
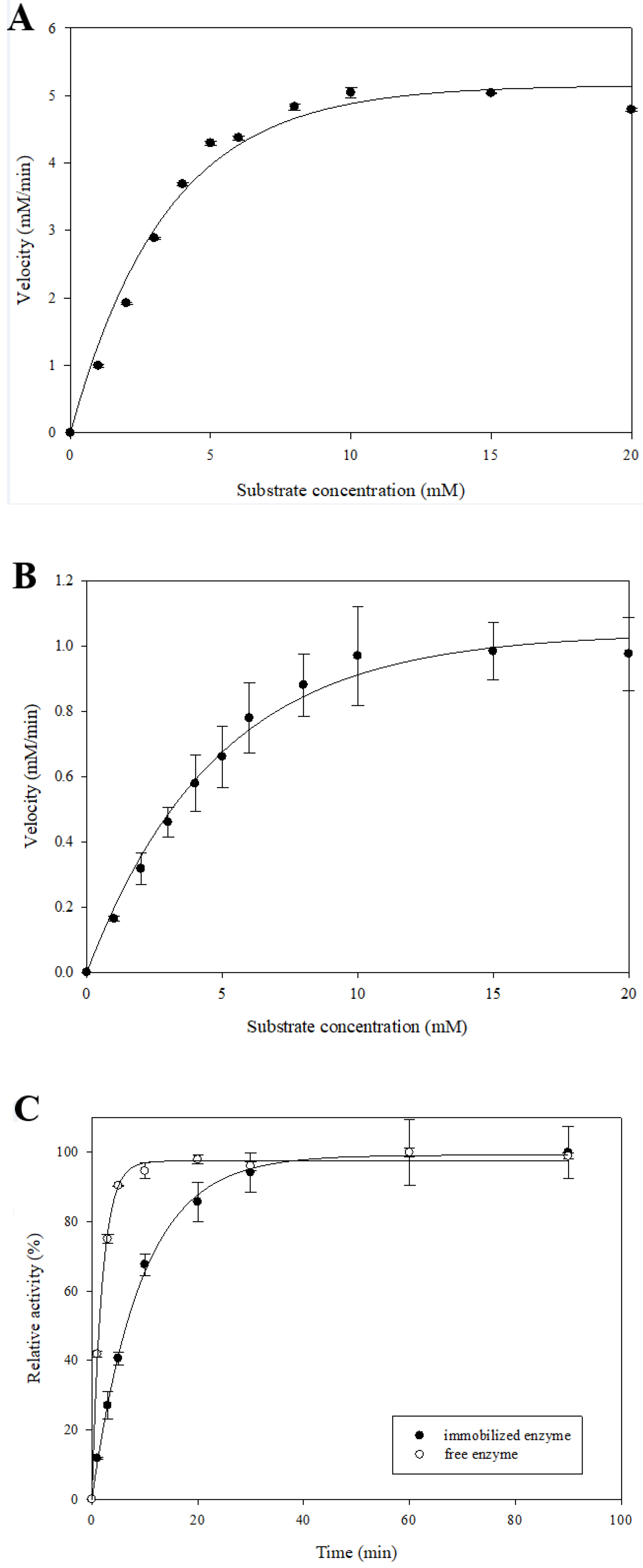
2.4. Determination of Kinetic Parameters for β-Glucosidase Immobilized System
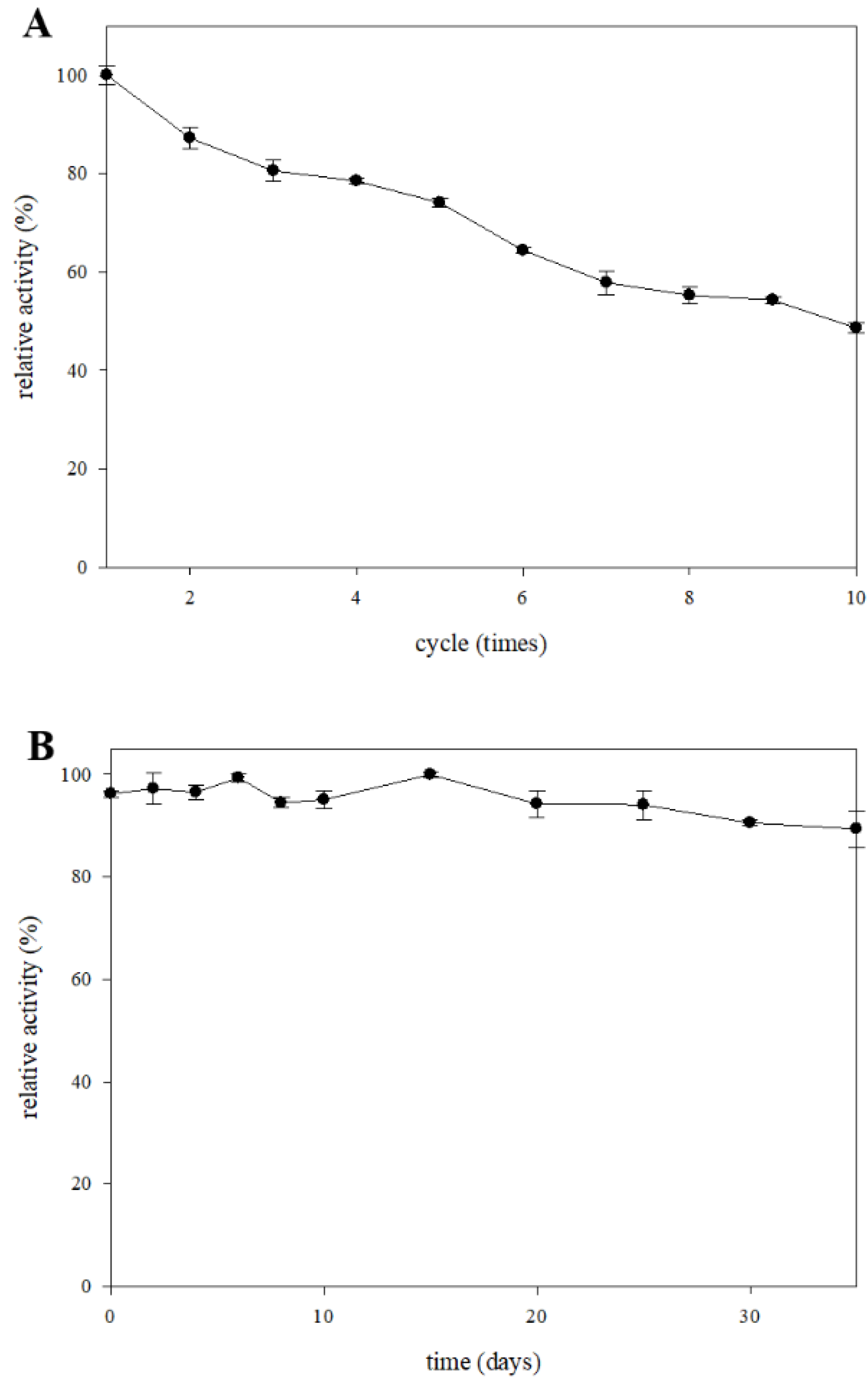
2.5. Storage Stability and Reusability of the β-Glucosidase Immobilized System
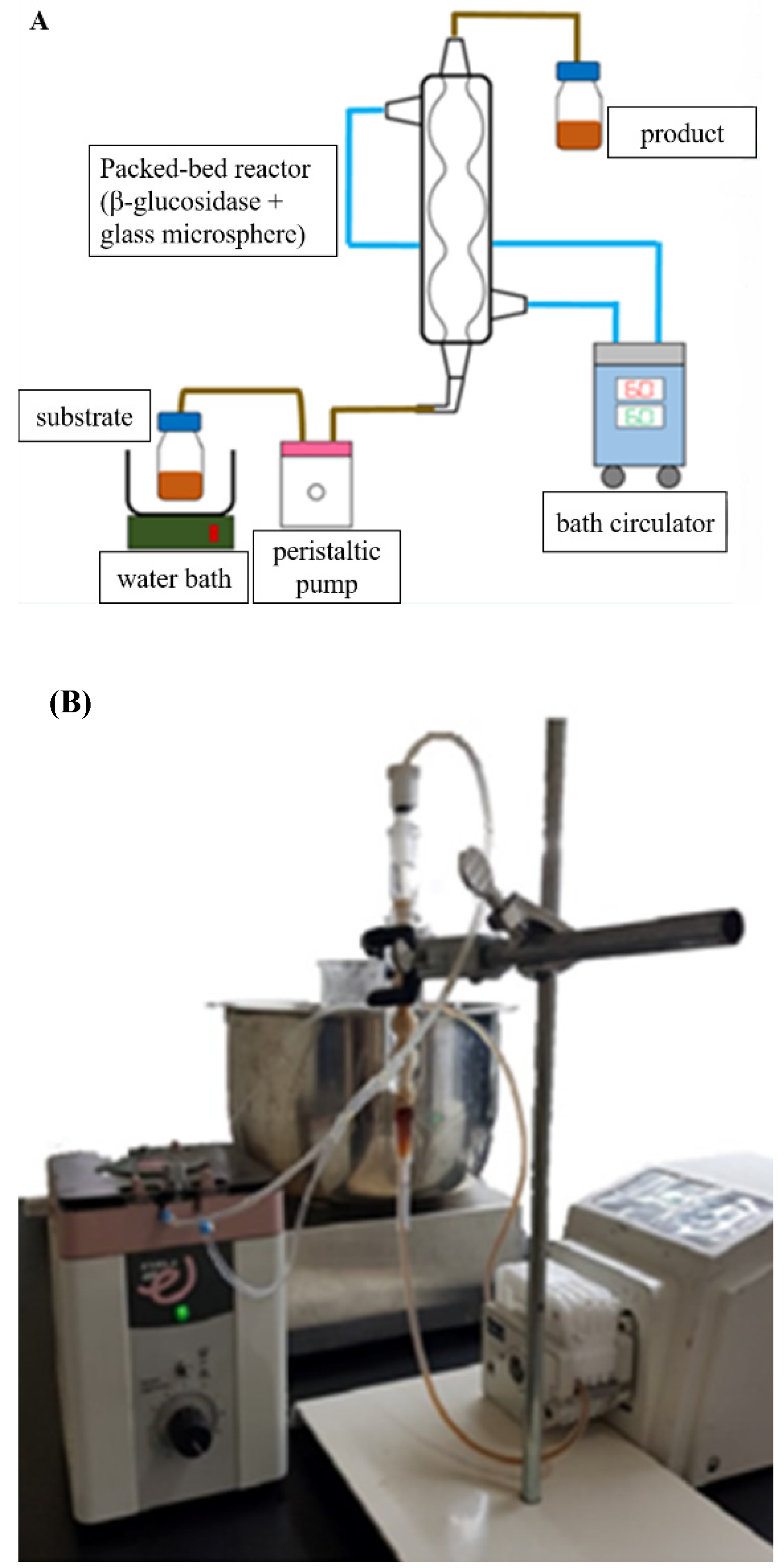
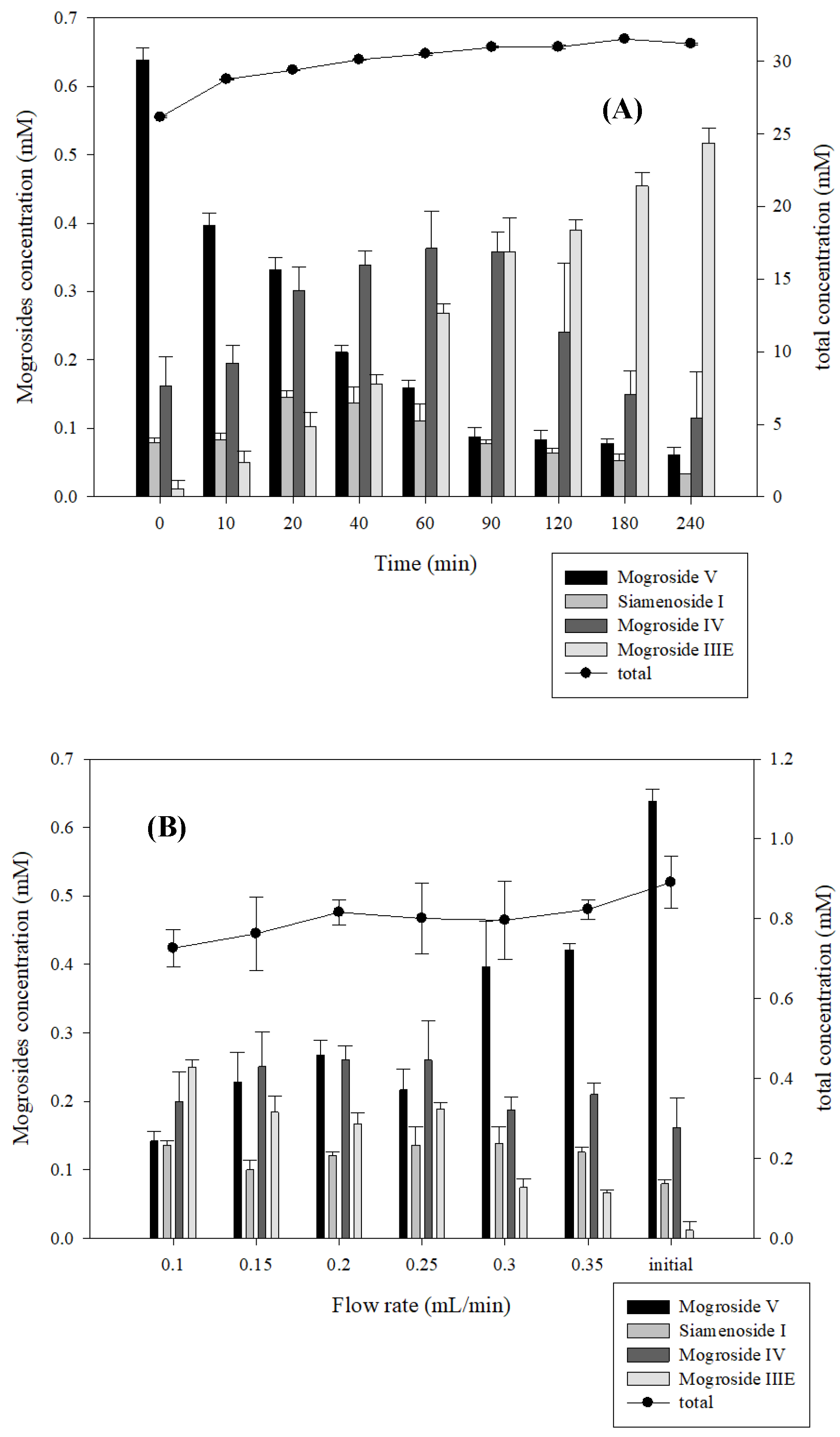
2.6. Production of Siamenoside I and Mogroside IV from Siraitia grosvenorii through the β-Glucosidase Immobilized Bioreactor
3. Materials and Methods
3.1. Materials
3.2. Enzyme Immobilization
3.3. Morphology Characterization
3.4. Determination of Reaction Conditions
3.5. Determination of Kinetic Parameters
3.6. Storage Stability and Reusability
3.7. Creation of the β-glucosidase Immobilized Bioreactor
3.8. Siraitia Grosvenorii Extraction
3.9. HPLC Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, C.; Dai, L.; Liu, Y.; Dou, D.; Sun, Y.; Ma, L. Pharmacological activities of mogrosides. Future Sci. 2018, 10, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ikeda, T.; Huang, Y.; Liu, J.; Nohara, T.; Sakamoto, T.; Nonaka, G.-I. Seasonal variation of mogrosides in Lo Han Kuo (Siraitia grosvenori) fruits. J. Nat. Med. 2007, 61, 307–312. [Google Scholar] [CrossRef]
- Pawar, R.S.; Krynitsky, A.J.; Rader, J.I. Sweeteners from plants—With emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvenorii (Swingle). Anal. Bioanal. Chem. 2013, 405, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Toriumi, M.; Mukainaka, T.; Banno, N.; Kimura, Y.; Hasegawa, J.-I.; Nishino, H. Inhibitory effects of cucurbitane glycosides and other triterpenoids from the fruit of Momordica grosvenori on epstein− barr virus early antigen induced by tumor promoter 12-O-tetradecanoylphorbol-13-acetate. J. Agric. Food Chem. 2002, 50, 6710–6715. [Google Scholar] [CrossRef]
- Yang, X.-R.; Xu, F.; Li, D.-P.; Lu, F.-L.; Liu, G.-X.; Wang, L.; Shang, M.-Y.; Huang, Y.-L.; Cai, S.-Q. Metabolites of Siamenoside I and their distributions in rats. Molecules 2016, 21, 176. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Wang, R.; Lee, C.-C.; Lo, Y.-C.; Lu, T.-J. Biotransformation of mogrosides from Siraitia grosvenorii Swingle by Saccharomyces cerevisiae. J. Agric. Food Chem. 2013, 61, 7127–7134. [Google Scholar] [CrossRef]
- Gong, G.; Zheng, Z.; Liu, H.; Wang, L.; Diao, J.; Wang, P.; Zhao, G. Purification and Characterization of a β-Glucosidase from Aspergillus niger and Its Application in the Hydrolysis of Geniposide to Genipin. J. Microbiol. Biotechnol. 2014, 24, 788–794. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Chen, K.-I.; Yao, Y.; Chen, H.-J.; Lo, Y.-C.; Yu, R.-C.; Cheng, K.-C. Hydrolysis of isoflavone in black soy milk using cellulose bead as enzyme immobilizer. J. Food Drug Anal. 2016, 24, 788–795. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Ting, Y.; Kuo, H.-C.; Hsieh, C.-W.; Hsu, H.-Y.; Wu, C.-N.; Cheng, K.-C. Enzymatic degradation of ginkgolic acids by laccase immobilized on core/shell Fe3O4/nylon composite nanoparticles using novel coaxial electrospraying process. Int. J. Biol. Macromol. 2021, 172, 270–280. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Liu, J.-M.; Chen, K.-I.; Hsieh, C.-W.; Chu, Y.-L.; Cheng, K.-C. Lactose-free milk preparation by immobilized lactase in glass microsphere bed reactor. Food Biophys. 2018, 13, 353–361. [Google Scholar] [CrossRef]
- Amaya-Delgado, L.; Hidalgo-Lara, M.; Montes-Horcasitas, M. Hydrolysis of sucrose by invertase immobilized on nylon-6 microbeads. Food Chem. 2006, 99, 299–304. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; Dias, J.L.; da Silva, O.S.; Porto, T.S. Immobilization of pectinase from Aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod. Process. 2018, 109, 9–18. [Google Scholar] [CrossRef]
- Homaei, A. Enzyme immobilization and its application in the food industry. Adv. Food Biotechnol. 2015, 9, 145–164. [Google Scholar]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sardar, M. Enzyme immobilization: An overview on nanoparticles as immobilization matrix. Biochem. Anal. Biochem. 2015, 4, 1. [Google Scholar]
- Mo, H.; Qiu, J.; Yang, C.; Zang, L.; Sakai, E.; Chen, J. Porous biochar/chitosan composites for high performance cellulase immobilization by glutaraldehyde. Enzym. Microb. Technol. 2020, 138, 109561. [Google Scholar] [CrossRef]
- Chang, J.; Lee, Y.-S.; Fang, S.-J.; Park, D.-J.; Choi, Y.-L. Hydrolysis of isoflavone glycoside by immobilization of β-glucosidase on a chitosan-carbon in two-phase system. Int. J. Biol. Macromol. 2013, 61, 465–470. [Google Scholar] [CrossRef]
- Wu, H.; Liang, Y.; Shi, J.; Wang, X.; Yang, D.; Jiang, Z. Enhanced stability of catalase covalently immobilized on functionalized titania submicrospheres. Mater. Sci. Eng. C 2013, 33, 1438–1445. [Google Scholar] [CrossRef]
- Kamin, R.A.; Wilson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Ye, L.; Liu, X.; Shen, G.-H.; Li, S.-S.; Luo, Q.-Y.; Wu, H.-J.; Chen, A.-J.; Liu, X.-Y.; Li, M.-L.; Pu, B. Properties comparison between free and immobilized wheat esterase using glass fiber film. Int. J. Biol. Macromol. 2019, 125, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kessi, E.; Arias, J. Using natural waste material as a matrix for the immobilization of enzymes: Chicken eggshell membrane powder for β-galactosidase immobilization. Appl. Biochem. Biotechnol. 2019, 187, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Yang, J.T.; Chen, K.I.; Wang, T.Y.; Lu, T.J.; Cheng, K.C. Hydrolyzation of mogrosides: Immobilized β-glucosidase for mogrosides deglycosylation from Lo Han Kuo. Food Sci. Nutr. 2019, 7, 834–843. [Google Scholar] [CrossRef]
- Ghattas, N.; Abidi, F.; Galai, S.; Marzouki, M.N.; Salah, A.B. Monoolein production by triglycerides hydrolysis using immobilized Rhizopus oryzae lipase. Int. J. Biol. Macromol. 2014, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.J. Recent advances in bioreactor engineering. Korean J. Chem. Eng. 2010, 27, 1035–1041. [Google Scholar] [CrossRef]
- Halim, S.F.A.; Kamaruddin, A.H.; Fernando, W. Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: Optimization using response surface methodology (RSM) and mass transfer studies. Bioresour. Technol. 2009, 100, 710–716. [Google Scholar] [CrossRef]
- Chen, K.I.; Chiang, C.Y.; Ko, C.Y.; Huang, H.Y.; Cheng, K.C. Reduction of phytic acid in soymilk by immobilized phytase system. J. Food Sci. 2018, 83, 2963–2969. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, N.; Wu, R.; Fu, Q.; Wang, H.; Zhang, Z.; Haji, Z.; Li, X.; Lian, X.; An, Y. Immobilization of β-Glucosidase BglC on Decanedioic Acid-Modified Magnetic Nanoparticles. Chem. Eng. Technol. 2018, 41, 1949–1955. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Zhang, M.; Wang, Y.; Wang, J.; Yau, L.; Jiang, Z.; Hu, P. Identification of flavonol and triterpene glycosides in Luo-Han-Guo extract using ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Food Compos. Anal. 2012, 25, 142–148. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, S.; Han, C.; Hou, X.; Long, Z.; Xu, K. Rapid identification and quantification of five major mogrosides in Siraitia grosvenorii (Luo-Han-Guo) by high performance liquid chromatography–triple quadrupole linear ion trap tandem mass spectrometry combined with microwave-assisted extraction. Microchem. J. 2014, 116, 142–150. [Google Scholar] [CrossRef]

| K (mM/min) | τ50 (min) | τcomplete (min) | |
|---|---|---|---|
| Free β-glucosidase | 0.52 | 1.33 | 4.43 |
| Immobilized β-glucosidase | 0.11 | 6.43 | 21.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Lin, C.-H.; Hou, C.-Y.; Lin, H.-W.; Hsieh, C.-W.; Cheng, K.-C. Production of Siamenoside I and Mogroside IV from Siraitia grosvenorii Using Immobilized β-Glucosidase. Molecules 2022, 27, 6352. https://doi.org/10.3390/molecules27196352
Chen H-Y, Lin C-H, Hou C-Y, Lin H-W, Hsieh C-W, Cheng K-C. Production of Siamenoside I and Mogroside IV from Siraitia grosvenorii Using Immobilized β-Glucosidase. Molecules. 2022; 27(19):6352. https://doi.org/10.3390/molecules27196352
Chicago/Turabian StyleChen, Hung-Yueh, Ching-Hsiang Lin, Chih-Yao Hou, Hui-Wen Lin, Chang-Wei Hsieh, and Kuan-Chen Cheng. 2022. "Production of Siamenoside I and Mogroside IV from Siraitia grosvenorii Using Immobilized β-Glucosidase" Molecules 27, no. 19: 6352. https://doi.org/10.3390/molecules27196352
APA StyleChen, H.-Y., Lin, C.-H., Hou, C.-Y., Lin, H.-W., Hsieh, C.-W., & Cheng, K.-C. (2022). Production of Siamenoside I and Mogroside IV from Siraitia grosvenorii Using Immobilized β-Glucosidase. Molecules, 27(19), 6352. https://doi.org/10.3390/molecules27196352








