Seawater Splitting for Hydrogen Generation Using Zirconium and Its Niobium Alloy under Gamma Radiation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
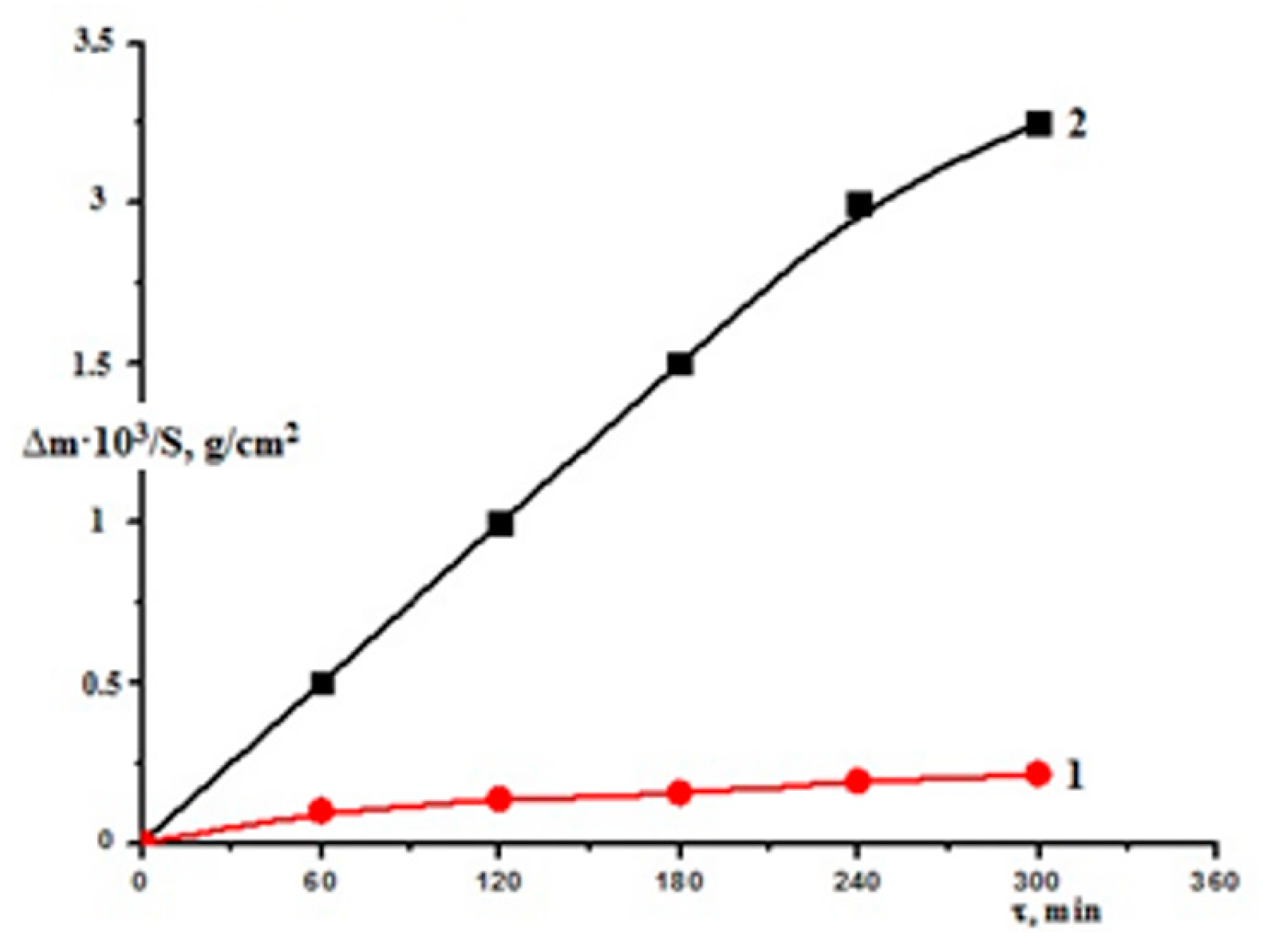
3.1. Characterization of the Catalysts
3.2. Hydrogen Generation
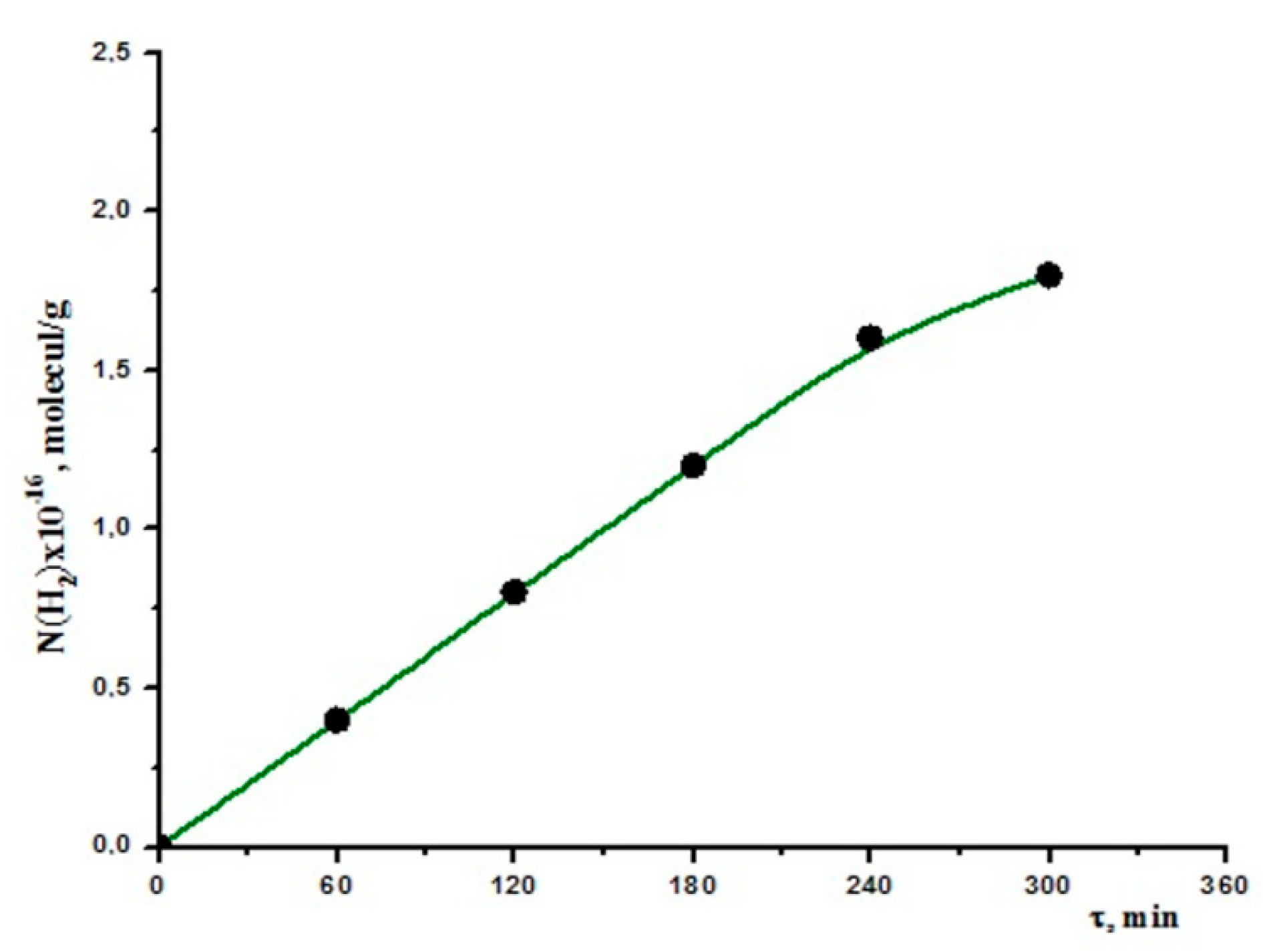
3.2.1. Seawater Decomposition without Any Catalyst
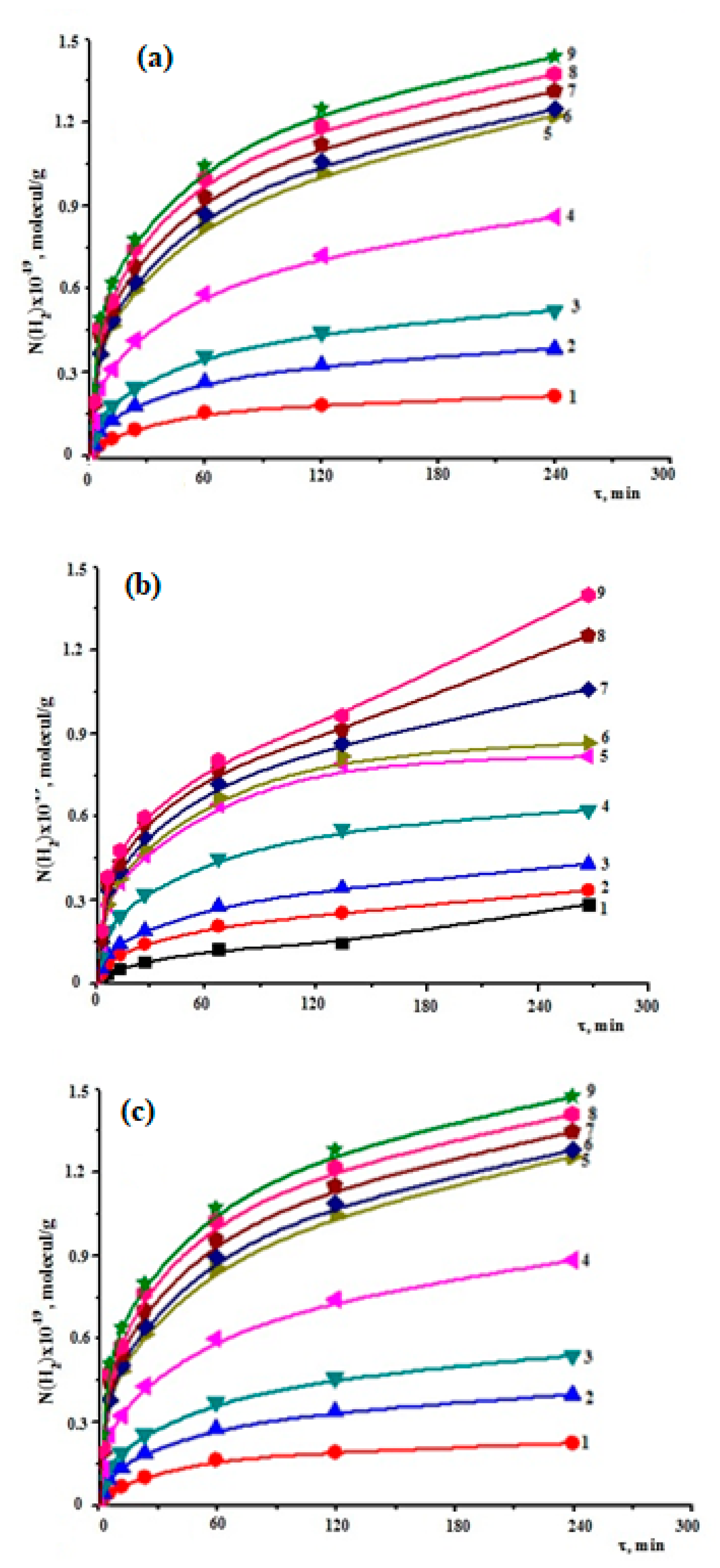
3.2.2. Seawater Decomposition in Presence of Zr
3.2.3. Seawater Decomposition in the Presence of Zr1%Nb Alloy
4. Activation Energy of Water Decomposition
5. Kinetics of Water Decomposition
6. Mechanism
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ali, I.; Alharbi, O.M.L.; Tkachev, A.; Galunin, E.; Burakov, A.; Grachev, V.A. Water Treatment by New Generation Graphene Materials: Hope for Bright Future. Environ. Sci. Pollut. Res. 2018, 25, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. Nano anti-cancer drugs: Pros and cons and future perspectives. Curr. Cancer Drug Targets 2011, 11, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Basheer, A.A.; Kucherova, A.; Memetov, N.; Pasko, T.; Ovchinnikov, K.; Pershin, V.; Kuznetsov, D.; Galunin, D.; Grachev, V.; et al. Advances in carbon nanomaterials as lubricants modifiers. J. Mol. Liq. 2019, 279, 251–266. [Google Scholar] [CrossRef]
- Ali, I.; Imanova, G.T.; Garibov, A.A.; Agayev, T.N.; Jabarov, S.H.; Almalki, A.S.; Alsubaie, A. Gamma rays mediated water splitting on nano-ZrO2 surface: Kinetics of molecular hydrogen formation. Radiat. Phys. Chem. 2021, 183, 109431. [Google Scholar] [CrossRef]
- Ali, I.; Imanova, G.T.; Mbianda, X.Y.; Alharbi, O.M. Role of the radiations in water splitting for hydrogen generation, Sustain. Energy Technol. Assess. 2022, 51, 101926. [Google Scholar]
- Basheer, A.A.; Ali, I. Water photo splitting for green hydrogen energy by green nanoparticles. Int. J. Hydrog. Energy 2019, 44, 11564–11572. [Google Scholar] [CrossRef]
- Imanova, G.T.; Agayev, T.N.; Jabarov, S.H. Investigation of structural and optical properties of zirconia dioxide nanoparticles by radiation and thermal methods. Mod. Phys. Lett. B 2021, 35, 2150050. [Google Scholar] [CrossRef]
- Sokolov, N.B. Computational Modeling of the Thermomechanical and Corrosive Condition of Fuel Rods in Case of Accidents with Contour Sealing; Mir: Obnisk, Russia, 1995; pp. 1–144. [Google Scholar]
- Smirnov, V.P.; Smirnov, A.V.; Tsykanov, V.A.; Asmolov, V.G. Results of Experimental Studies to Substantiate the Behavior of High-burned Reactors with Pressurized Water in Accidents with Loss of Coolant; Electrostal, Russia, 2000; pp. 1–120. Available online: https://www.osti.gov/etdeweb/biblio/20145945 (accessed on 30 July 2022).
- Petelguzov, I.A.; Rodak, A.G.; Roenko, N.M. Study of the Kinetics of Corrosion and the Structure of Fuel Pipes of the KTC-110 and E110 Alloys; Publishing House of Belgorod State University: Belgorod, Russia, 2001; Volume 1, pp. 145–149. [Google Scholar]
- Petelguzov, I.A.; Rodak, A.G.; Slabospitskaya, E.A.; Ishchenko, N.I. The kinetics of the corrosion process and the change in the structure of the calcium-thermal zirconium alloy Zr1Nb when heated in water vapor in the temperature range 660 ÷ 1200 °C. Phys. Chem. Mater. 2004, 3, 301–308. [Google Scholar]
- Voytovich, R.F. Oxidation of Zirconium and Its Alloys; NaukovaDumka: Kiev, Ukraine, 1989; pp. 1–240. [Google Scholar]
- Douglas, D.L. The metallurgy of zirconium. In Atomic Energy Review; pp. 1–464. Available online: https://www.iaea.org/publications/1990/atomic-energy-review-supplement-1971-the-metallurgy-of-zirconium-dl-douglass (accessed on 30 July 2022).
- Parfenov, B.G.; Gerasimov, V.V.; Venediktova, G.I. Corrosion of Zirconium and Its Alloys; Atomizdat: Mocsow, Russia, 1967; pp. 1–258. [Google Scholar]
- Krasnoruyky, I.S.; Petelguzov, I.A. Investigation of fuel rod models for the VVER-1000 reactor made of the calcium-thermal zirconium alloy Zr1Nb after long-term corrosion tests, Problems of Atomic Science and Technology. Ser. Phys. Radiat. Damage Radiat. Mater. 2003, 3, 101–107. [Google Scholar]
- Fomishkin, M.A.; Tonkov, V.Y.; Dolgov, Y.I. Investigation of Oxygen Diffusion in Zr1% Nb Alloy during High-Temperature Oxidation in Water Vapor. At. Energy 1988, 65, 321–326. [Google Scholar] [CrossRef]
- Al-Azmi, A.; Sajjad, K. New bidental sulfur-doped graphene quantum dots modified with gold as a catalyst for hydrogen generation. J. Colloid Interface Sci. 2022, 612, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Keshipour, S.; Asghari, A. A review on hydrogen generation by phthalocyanines. Int. J. Hydrog. Energy 2022, 47, 12865–12881. [Google Scholar] [CrossRef]
- Sajjad, K.; Shima, M.A. Nickel phthalocyanine@graphene oxide/TiO2 as an efficient degradation catalyst of formic acid toward hydrogen production. Sci. Rep. 2021, 11, 16148. [Google Scholar]
- Agaev, T.N.; Musayeva, S.Z.; Imanova, G.T. Studying the Kinetics of Formation of Molecular Hydrogen during the Radiolysis of Hexane and a Mixture of C6H14–H2O on a Surface of n-ZrO2. Russ. J. Phys. Chem. A 2021, 95, 270–272. [Google Scholar] [CrossRef]
- Imanova, G.T.; Agaev, T.N.; Garibov, A.A.; Melikova, S.Z.; Jabarov, S.H.; Akhundzada, H.V. Radiation-thermocatalytic and thermocatalytic properties of n-ZrO2-n-SiO2 systems in the process of obtaining hydrogen from water at different temperatures. J. Mol. Struct. 2021, 1241, 130651. [Google Scholar] [CrossRef]
- Imanova, G.T. Kinetics of radiation-heterogeneous and catalytic processes of water in the presence of zirconia nanoparticles. Adv. Phys. Res. 2020, 2, 94–101. [Google Scholar]
- Imanova, G.T. Gamma rays mediated hydrogen generation by water decomposition On nano-ZrO2 surface. Mod Approach Mater. Sci. 2021, 4, 508–514. [Google Scholar]
- Imanova, G.T. Molecular hydrogen production by radiolysis of water on the surface of nano-ZrO2 under the influence of gamma rays. Synth. Sint. 2022, 2, 9–13. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-Temperature Oxidation of Metals, Cambridge, 2nd ed.; University Press: Cambridge, UK, 2006. [Google Scholar]
- He, J.; Bai, X.; Ma, C.; Chen, C. The effect of ion implantation of Ar on the aqueous corrosion resistance of Zr-4 alloy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1995, 100, 59–64. [Google Scholar] [CrossRef]
- Roy, S.P.; Gupta, N.K.; Mukerjee, S.K.; Parida, S.C. Oxidation Kinetics of Zr-Nb Alloys in Dry Air in the Temperature Range 723–790 K. Ind. J. Adv. Chem. Sci. 2017, 5, 255–262. [Google Scholar]
- Christensen, H. Effects of water radiolysis on corrosion in nuclear reactors. Radiat. Phys. Chem. 1981, 18, 1–2. [Google Scholar] [CrossRef]
- Kholoud, E.S.; Abdelrahman, M.M.; Ali, A.; Nageh, K.A. Niobium–Zirconium Oxynitride Nanotube Arrays for Photoelectrochemical Water Splitting. Appl. Nano Mater. 2020, 3, 6078–6088. [Google Scholar]


| T (K) | WT(H2) | WR(H2) | WRT(H2) | G(H2), molec./100 eV |
|---|---|---|---|---|
| 473 | 4.25 × 1014 | 5.19 × 1014 | 9.44 × 1014 | 7.90 |
| 573 | 1.1 × 1015 | 1.08 × 1015 | 2.18 × 1015 | 16.1 |
| 673 | 2.3 × 1015 | 2.7 × 1015 | 5.0 × 1015 | 39.5 |
| 773 | 5.8 × 1016 | 4.2 × 1016 | 1.02 × 1017 | 60.8 |
| 873 | 1.2 × 1017 | 7.0 × 1016 | 1.9 × 1017 | 101.4 |
| 973 | 1.8 × 1017 | 6.0 × 1016 | 3.0 × 1017 | 106.7 |
| 1073 | 2.0 × 1017 | 0.9 × 1017 | 4.1 × 1017 | 304.3 |
| 1173 | 3.0 × 1017 | 1.1 × 1017 | 4.4 × 1017 | 306.6 |
| 1273 | 5.5 × 1017 | 2.1 × 1017 | 5.45 × 1017 | 307.1 |
| T (K) | WT(H2) | WR(H2) | WRT(H2) | G(H2), molec./100 eV |
|---|---|---|---|---|
| 473 | 5.2 × 1014 | 5.90 × 1014 | 11.1 × 1014 | 9.00 |
| 573 | 1.6 × 1015 | 1.3 × 1015 | 2.91 × 1015 | 19.7 |
| 673 | 2.8 × 1015 | 3.3 × 1015 | 6.1 × 1015 | 48.1 |
| 773 | 6.5 × 1016 | 6.3 × 1016 | 1.28 × 1017 | 91.3 |
| 873 | 1.35 × 1017 | 1.25 × 1017 | 2.6 × 1017 | 181.1 |
| 973 | 1.49 × 1017 | 1.3 × 1017 | 3.3 × 1017 | 185.1 |
| 1073 | 3.5 × 1017 | 3.0 × 1017 | 6.5 × 1017 | 434.7 |
| 1173 | 4.5 × 1017 | 3.1 × 1017 | 6.7 × 1017 | 434.9 |
| 1273 | 6.83 × 1017 | 3.2 × 1017 | 6.9 × 1017 | 437.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Imanova, G.; Agayev, T.; Aliyev, A.; Jabarov, S.; Albishri, H.M.; Alshitari, W.H.; Hameed, A.M.; Alharbi, A. Seawater Splitting for Hydrogen Generation Using Zirconium and Its Niobium Alloy under Gamma Radiation. Molecules 2022, 27, 6325. https://doi.org/10.3390/molecules27196325
Ali I, Imanova G, Agayev T, Aliyev A, Jabarov S, Albishri HM, Alshitari WH, Hameed AM, Alharbi A. Seawater Splitting for Hydrogen Generation Using Zirconium and Its Niobium Alloy under Gamma Radiation. Molecules. 2022; 27(19):6325. https://doi.org/10.3390/molecules27196325
Chicago/Turabian StyleAli, Imran, Gunel Imanova, Teymur Agayev, Anar Aliyev, Sakin Jabarov, Hassan M. Albishri, Wael Hamad Alshitari, Ahmed M. Hameed, and Ahmed Alharbi. 2022. "Seawater Splitting for Hydrogen Generation Using Zirconium and Its Niobium Alloy under Gamma Radiation" Molecules 27, no. 19: 6325. https://doi.org/10.3390/molecules27196325
APA StyleAli, I., Imanova, G., Agayev, T., Aliyev, A., Jabarov, S., Albishri, H. M., Alshitari, W. H., Hameed, A. M., & Alharbi, A. (2022). Seawater Splitting for Hydrogen Generation Using Zirconium and Its Niobium Alloy under Gamma Radiation. Molecules, 27(19), 6325. https://doi.org/10.3390/molecules27196325











