Designing a 0D/1D S-Scheme Heterojunction of Cadmium Selenide and Polymeric Carbon Nitride for Photocatalytic Water Splitting and Carbon Dioxide Reduction
Abstract
1. Introduction
2. Results and Discussion
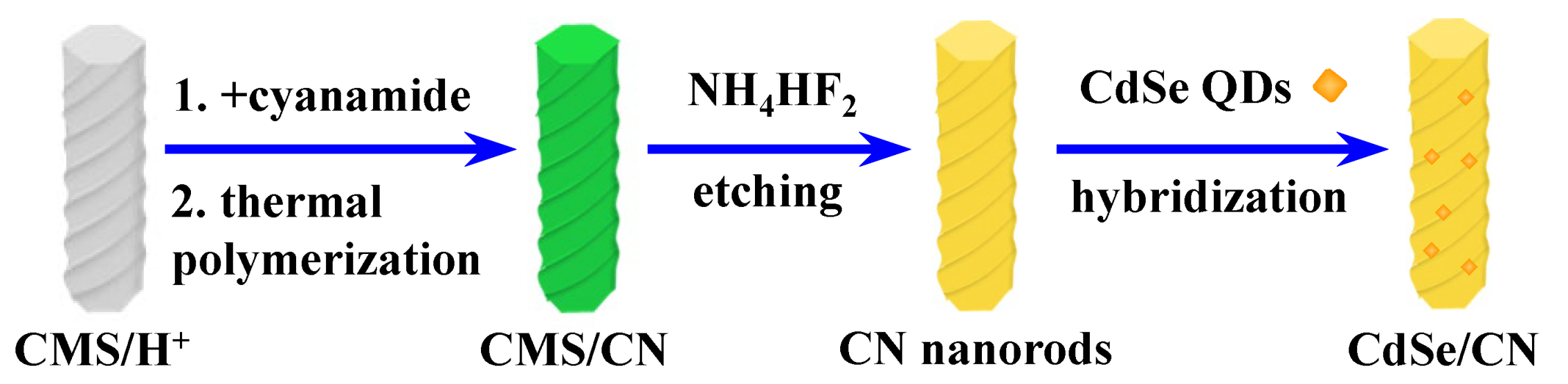
2.1. Preparation of Photocatalysts
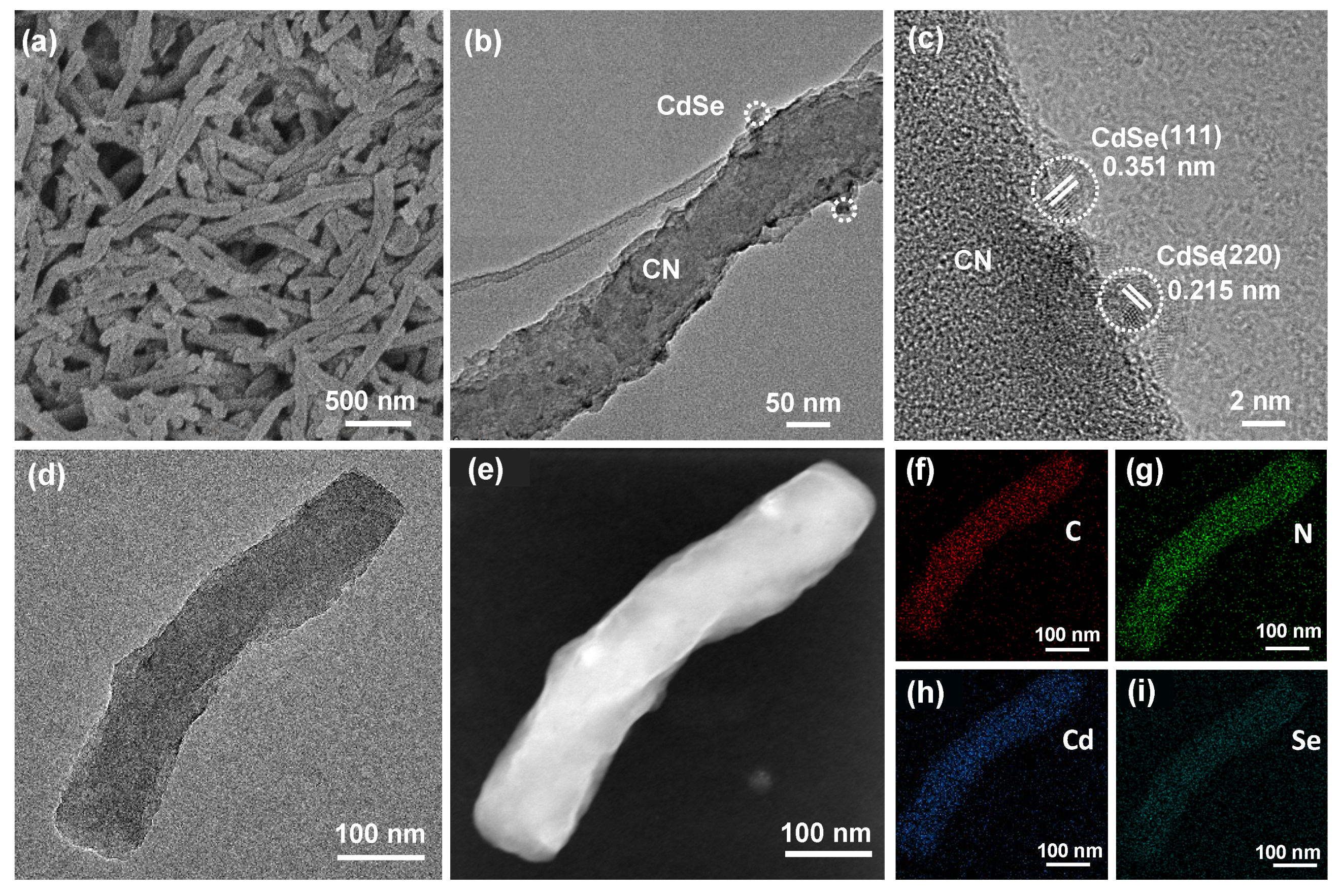
2.2. Morphological Characterization
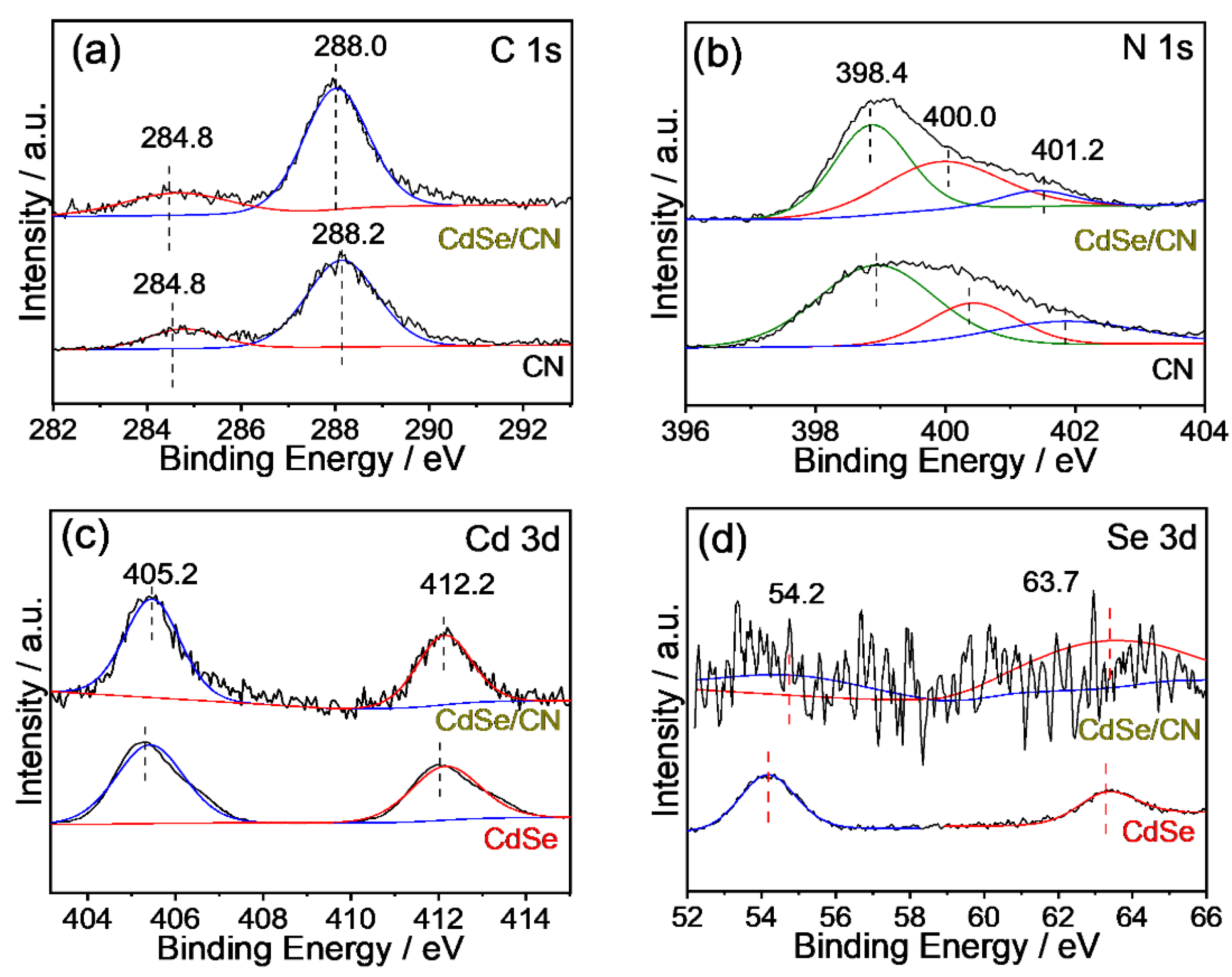
2.3. Structural Characterization
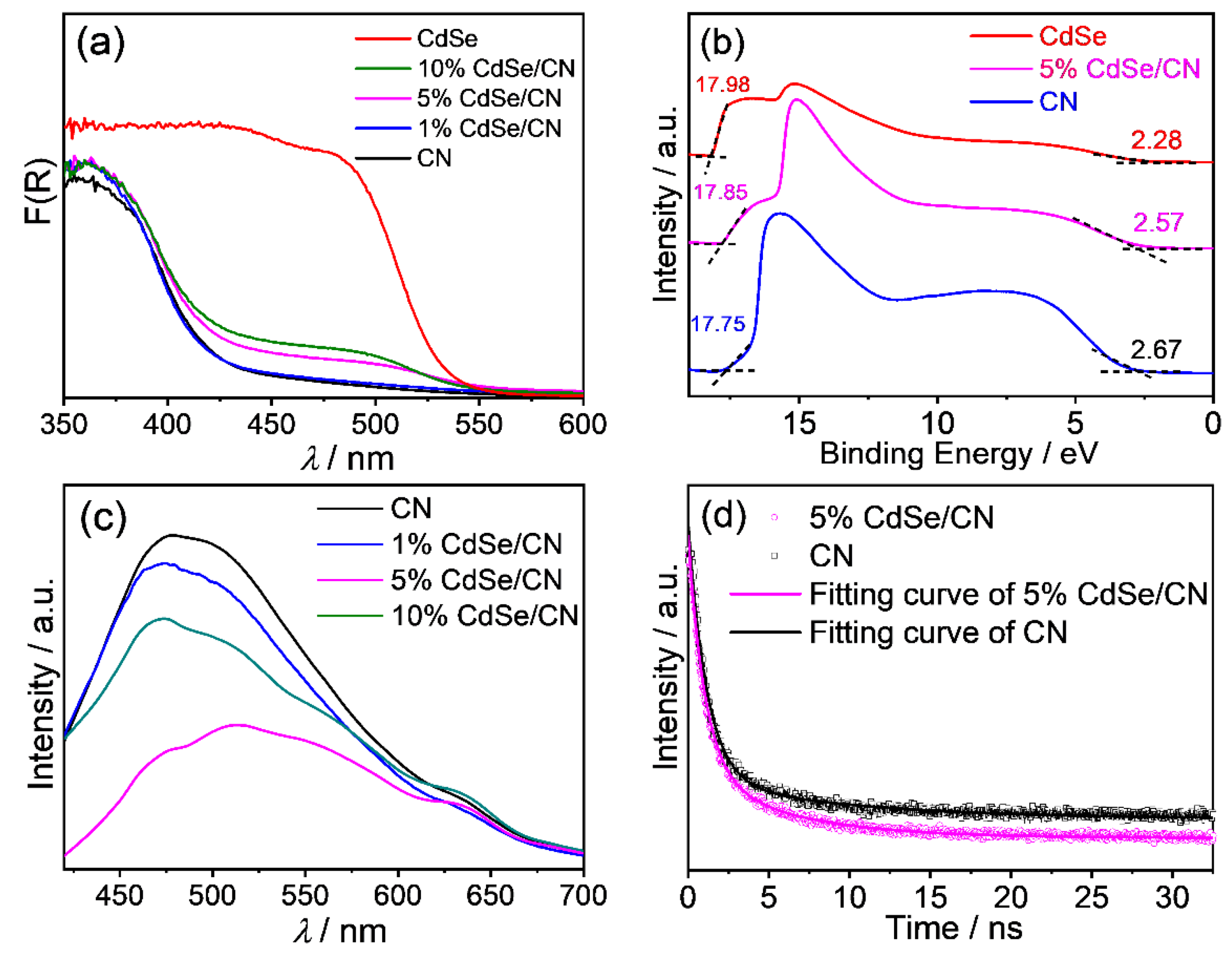
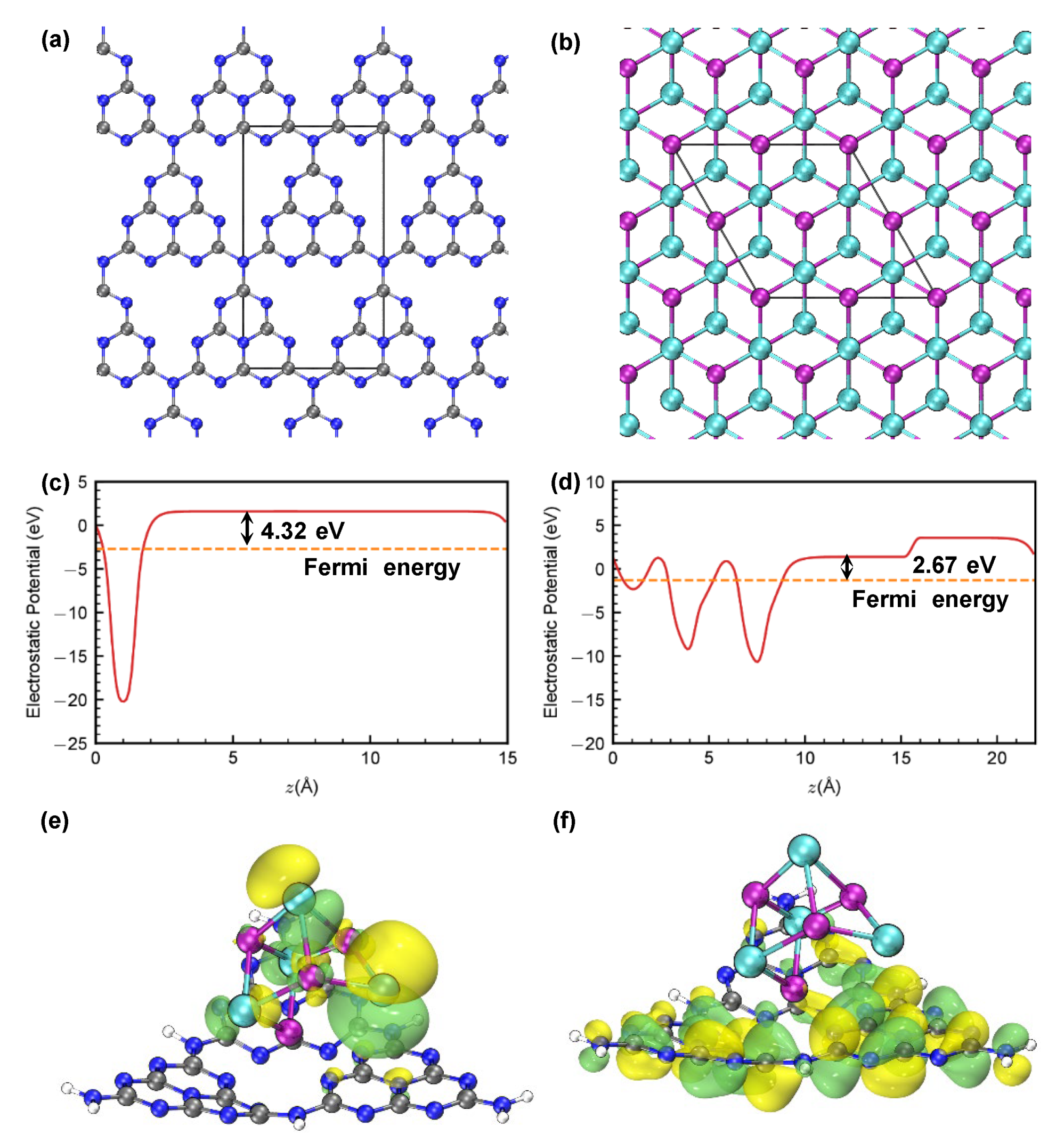
2.4. Photochemical Properties and Band Structure
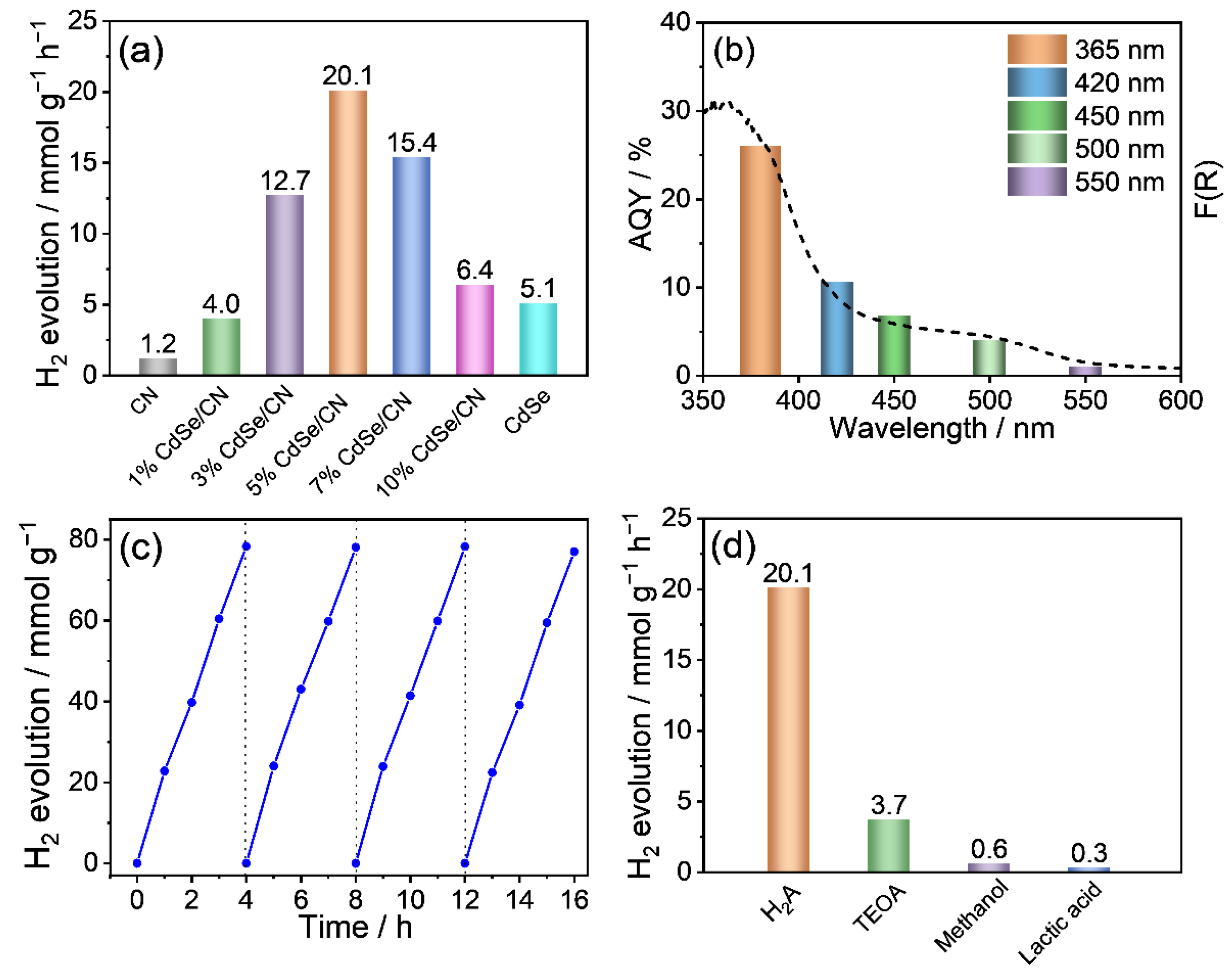
2.5. Photocatalytic Water-Splitting Activities
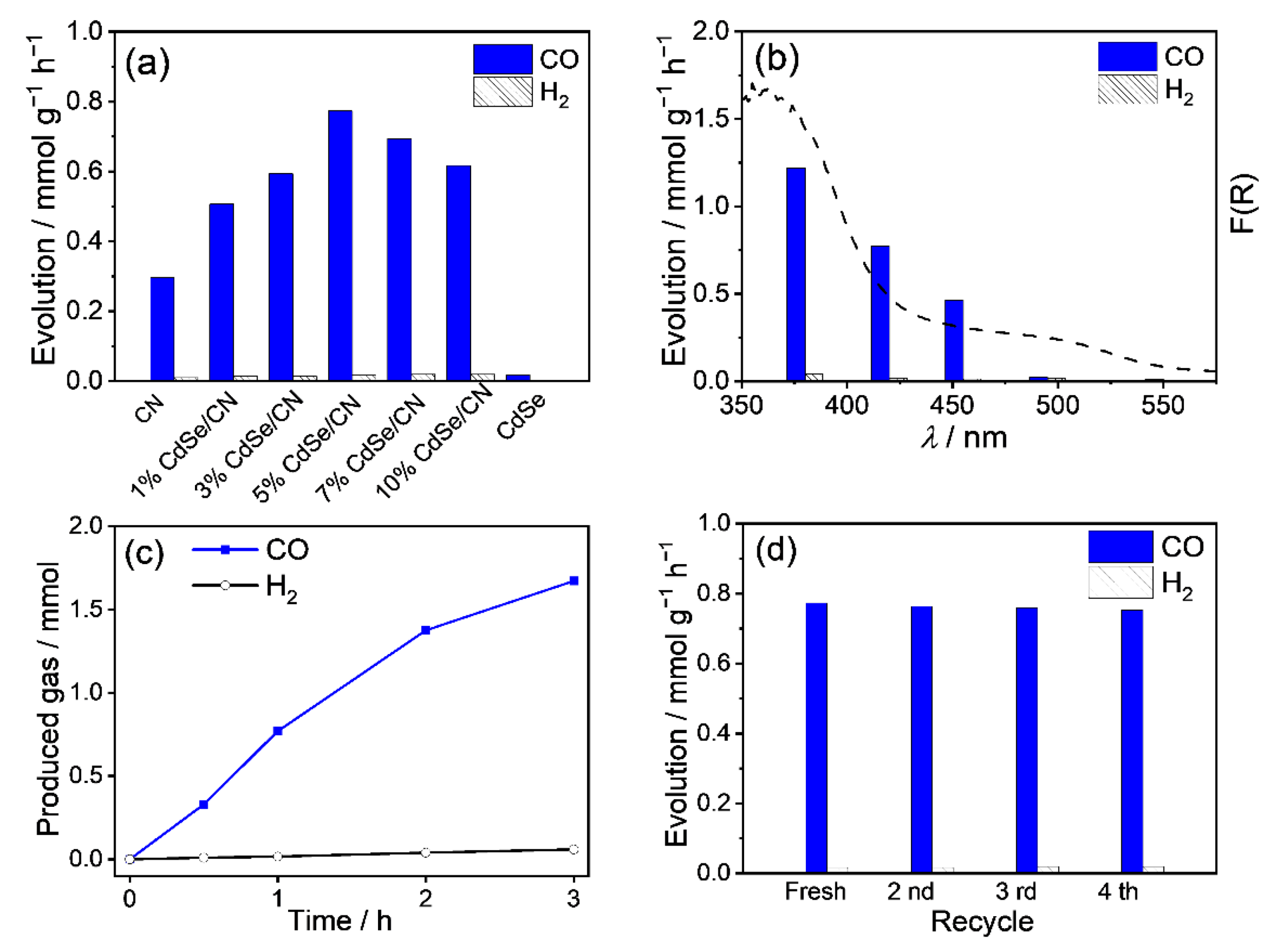
2.6. Photocatalytic CO2 Reduction Activities
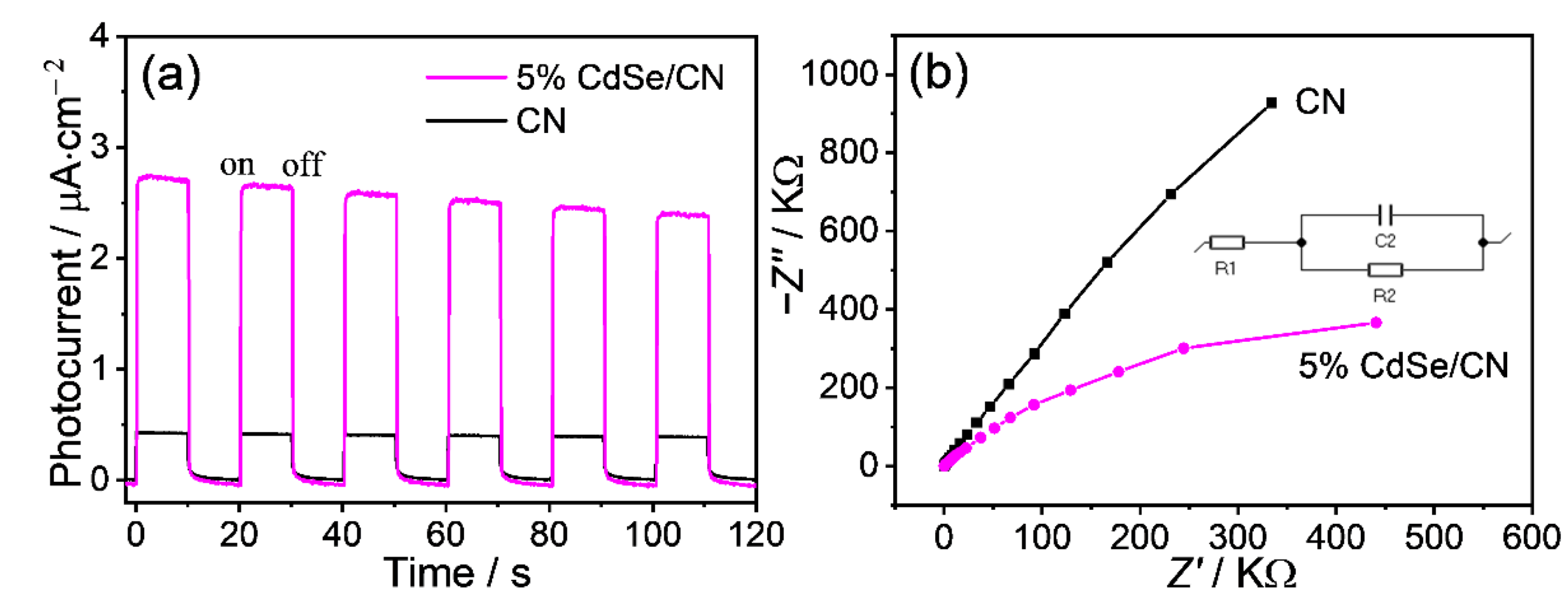
2.7. Charge Transfer Process
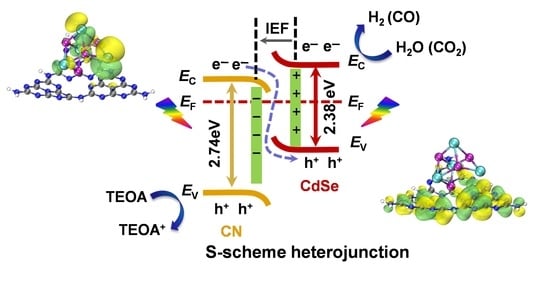
2.8. Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of N-Myristoylalanine (C14-d-Ala)
3.3. Synthesis of Chiral Mesoporous Silica Hard-Template
3.4. Preparation of CN Nanorods
3.5. Preparation of Water-Soluble CdSe QDs
3.6. Synthesis of CdSe/CN Composites
3.7. Characterizations
3.8. Photoelectrochemical Measurement
3.9. Photocatalytic Hydrogen Evolution
3.10. Apparent Quantum Efficiency for H2 Evolution Measurement
3.11. Photocatalytic CO2 Reduction
3.12. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.-H.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Diarmand-Khalilabad, H.; Ghosh, S. Nitrogen photofixation ability of g-C3N4 nanosheets/Bi2MoO6 heterojunction photocatalyst under visible-light illumination. J. Colloid Interf. Sci. 2020, 563, 81–91. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, J.; Li, X.; Guan, S.; Dai, M.; Wang, S. 1D/2D heterostructured photocatalysts: From design and unique properties to their environmental applications. Small 2020, 16, 2005051. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Ghosh, S.; Maiyalagan, T.; Basu, R.N. Band edge engineering of BiOX/CuFe2O4 heterostructures for efficient water splitting. ACS Appl. Energy Mater. 2022, 5, 3821–3833. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Pan, B.; Feng, M.; McDonald, T.J.; Manoli, K.; Wang, C.; Huang, C.H.; Sharma, V.K. Enhanced ferrate(VI) oxidation of micropollutants in water by carbonaceous materials: Elucidating surface functionality. Chem. Eng. J. 2020, 398, 125607. [Google Scholar] [CrossRef]
- Pan, B.; Wu, Y.; Qin, J.; Wang, C. Ultrathin Co0.85Se nanosheet cocatalyst for visible-light CO2 photoreduction. Catal. Today 2019, 335, 208–213. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, K.A.I.; Lin, W.; Landfester, K.; Wang, X. Heterogeneous photoredox flow chemistry for the scalable organosynthesis of fine chemicals. Nat. Commun. 2020, 11, 1239. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, L.; Shi, R.; Zhang, S.; Bian, X.; Wu, F.; Cao, X.; Waterhouse, G.I.N.; Zhang, T. Alkali etching of layered double hydroxide nanosheets for enhanced photocatalytic N2 reduction to NH3. Adv. Energy Mater. 2020, 10, 2002199. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Z.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.; Wang, X. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting polymers for oxygen evolution reaction under light illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef] [PubMed]
- Volokh, M.; Peng, G.; Barrio, J.; Shalom, M. Carbon nitride materials for water splitting photoelectrochemical cells. Angew. Chem. Int. Ed. 2019, 58, 6138–6151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fang, Y.; Wang, L.; Chen, X.; Lin, W.; Wang, X. Remarkable oxygen evolution by Co-doped ZnO nanorods and visible light. Appl. Catal. B Environ. 2021, 296, 120369. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Cui, J.; Li, X.; Zhang, Y.; Wang, C.; Yu, X.; Ye, J. Defective g-C3N4/covalent organic framework van der Waals heterojunction toward highly efficient S-scheme CO2 photoreduction. Appl. Catal. B Environ. 2022, 301, 120814. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Ye, X.; Guo, F.; Wang, X. Helical graphitic carbon nitrides with photocatalytic and optical activities. Angew. Chem. Int. Ed. 2014, 53, 11926–11930. [Google Scholar] [CrossRef]
- Chen, Y.; Su, F.; Xie, H.; Wang, R.; Ding, C.; Huang, J.; Xu, Y.; Ye, L. One-step construction of S-scheme heterojunctions of N-doped MoS2 and S-doped g-C3N4 for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 404, 126498. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for congo red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, D.; Yang, S.; Tian, Z.; Cheng, B.; Fan, J. Novel g-C3N4/g-C3N4 S-scheme isotype heterojunction for improved photocatalytic hydrogen generation. Appl. Surf. Sci. 2019, 495, 143555. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.; Ou, H.; Asiri, A.M.; Chen, Y.; Wang, X. Black phosphorus and polymeric carbon nitride heterostructure for photoinduced molecular oxygen activation. Adv. Funct. Mater. 2018, 28, 1705407. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Huo, Y.; Dai, K.; Li, S.; Chen, S. Two-dimensional sulfur- and chlorine-codoped g-C3N4/CdSe-amine heterostructures nanocomposite with effective interfacial charge transfer and mechanism insight. Appl. Catal. B Environ. 2021, 280, 119452. [Google Scholar] [CrossRef]
- Yao, G.; Liu, Y.; Liu, J.; Xu, Y. Facile synthesis of porous g-C3N4 with enhanced visible-light photoactivity. Molecules 2022, 27, 1754. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Y.; Gao, B.; Lin, B.; Wang, X. Black phosphorus and carbon nitride hybrid photocatalysts for photoredox reactions. Adv. Funct. Mater. 2020, 30, 2002021. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Xia, J.; Fang, Y.; Hou, Y.; Fu, X.; Shalom, M.; Wang, X. One-pot synthesis of CoS2 merged polymeric carbon nitride films for photoelectrochemical water splitting. ChemSusChem 2022, 15, e202200330. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, Y.; Yin, L.-C.; Niu, P.; Zhen, C.; Chen, R.; Kang, X.; Wu, F.; Liu, G. Constructing CdSe QDs modified porous g-C3N4 heterostructures for visible light photocatalytic hydrogen production. J. Mater. Sci. Technol. 2021, 95, 167–171. [Google Scholar] [CrossRef]
- Pan, J.; Liang, J.; Xu, Z.; Yao, X.; Qiu, J.; Chen, H.; Qin, L.; Chen, D.; Huang, Y. Rationally designed ternary CdSe/WS2/g-C3N4 hybrid photocatalysts with significantly enhanced hydrogen evolution activity and mechanism insight. Int. J. Hydrogen Energy 2021, 46, 30344–30354. [Google Scholar] [CrossRef]
- Li, C.; Zou, X.; Lin, W.; Mourad, H.; Meng, J.; Liu, Y.; Abdellah, M.; Guo, M.; Zheng, K.; Nordlander, E. Graphitic carbon nitride/CdSe quantum dot/iron carbonyl cluster composite for enhanced photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 6280–6289. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Dai, K.; Liang, C. Amine-modified S-scheme porous g-C3N4/CdSe-diethylenetriamine composite with enhanced photocatalytic CO2 reduction activity. ACS Appl. Energy Mater. 2021, 4, 956–968. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Dai, K. Organic amine surface modified one-dimensional CdSe0.8S0.2-diethylenetriamine/two-dimensional SnNb2O6 S-scheme heterojunction with promoted visible-light-driven photocatalytic CO2 reduction. Chin. J. Catal. 2022, 43, 255–264. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Ismail, A.A.; Bahnemann, D.W. Construction of visible light responsive CdSe/g-C3N4 nanocomposites for H2 production. Nanosci. Nanotechnol. Lett. 2019, 11, 1281–1291. [Google Scholar] [CrossRef]
- Hu, T.; Li, Z.; Lu, L.; Dai, K.; Zhang, J.; Li, R.; Liang, C. Inorganic-organic CdSe-diethylenetriamine nanobelts for enhanced visible photocatalytic hydrogen evolution. J. Colloid Interf. Sci. 2019, 555, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Rogach, A.L.; Kornowski, A.; Gao, M.; Eychmüller, A.; Weller, H. Synthesis and characterization of a size series of extremely small thiol-stabilized CdSe nanocrystals. J. Phys. Chem. B 1999, 103, 3065–3069. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-scale synthesis of nearly monodisperse CdSe/CdS core/shell nanocrystals using air-stable reagents via successive ion layer adsorption and reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, W.; Yu, S.; Xie, Z.; Wei, S.; Zhou, Y. CdSe quantum dots/g-C3N4 heterostructure for efficient H2 production under visible light irradiation. ACS Omega 2018, 3, 17762–17769. [Google Scholar] [CrossRef]
- Raziq, F.; Hayat, A.; Humayun, M.; Mane, S.K.B.; Faheem, M.B.; Ali, A.; Zhao, Y.; Han, S.; Cai, C.; Li, W.; et al. Photocatalytic solar fuel production and environmental remediation through experimental and DFT based research on CdSe-QDs-coupled P-doped-g-C3N4 composites. Appl. Catal. B Environ. 2020, 270, 118867. [Google Scholar] [CrossRef]
- Raheman, S.A.R.; Wilson, H.M.; Momin, B.M.; Annapure, U.S.; Jha, N. CdSe quantum dots modified thiol functionalized g-C3N4: Intimate interfacial charge transfer between 0D/2D nanostructure for visible light H2 evolution. Renew. Energy 2020, 158, 431–443. [Google Scholar] [CrossRef]
- Putri, L.K.; Ng, B.-J.; Ong, W.-J.; Lee, H.W.; Chang, W.S.; Mohamed, A.R.; Chai, S.-P. Energy level tuning of CdSe colloidal quantum dots in ternary 0D-2D-2D CdSe QD/B-rGO/O-gC3N4 as photocatalysts for enhanced hydrogen generation. Appl. Catal. B Environ 2020, 265, 118592. [Google Scholar] [CrossRef]
- Bao, Y.; Song, S.; Yao, G.; Jiang, S. S-scheme photocatalytic systems. Solar RRL 2021, 5, 2100118. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An inorganic/organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In situ irradiated xps investigation on S-scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Song, T.; Long, B.; Yin, S.; Ali, A.; Deng, G.-J. Designed synthesis of a porous ultrathin 2D CN@graphene@CN sandwich structure for superior photocatalytic hydrogen evolution under visible light. Chem. Eng. J. 2021, 404, 126455. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Ren, H.; Hou, Y.; Wang, X. Photocatalytic reduction of CO2 by graphitic carbon nitride polymers derived from urea and barbituric acid. Appl. Catal. B Environ. 2015, 179, 1–8. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 nanowire heterostructure photoanodes based on type II band alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Li, H.; Gong, H.; Jin, Z. Phosphorus modified Ni-MOF–74/BiVO4 S-scheme heterojunction for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2022, 307, 121166. [Google Scholar] [CrossRef]
- Takehara, M.; Yoshimura, I.; Takizawa, K.; Yoshida, R. Surface active N-acylglutamate: I. Preparation of long chain N-acylglutamic acid. J. Am. Oil Chem. Soc. 1972, 49, 157. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Z.; Ohsuna, T.; Terasaki, O.; Inoue, Y.; Sakamoto, K.; Nakanishi, T.; Ariga, K.; Che, S. Control of morphology and helicity of chiral mesoporous silica. Adv. Mater. 2006, 18, 593–596. [Google Scholar] [CrossRef]
- Jin, H.; Qiu, H.; Sakamoto, Y.; Shu, P.; Terasaki, O.; Che, S. Mesoporous silicas by self-assembly of lipid molecules: Ribbon, hollow sphere, and chiral materials. Chem. Eur. J. 2008, 14, 6413–6420. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Neugebauer, J.; Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al(111). Phys. Rev. B Condens. Matter 1992, 46, 16067–16080. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, Y.; Zhao, Z. Porous defect-modified graphitic carbon nitride via a facile one-step approach with significantly enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B Environ. 2018, 226, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z.; et al. Carbon quantum dot implanted graphite carbon nitride nanotubes: Excellent charge separation and enhanced photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 5765–5771. [Google Scholar] [CrossRef]
- Fang, H.B.; Zhang, X.H.; Wu, J.; Li, N.; Zheng, Y.Z.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Iqbal, W.; Qiu, B.; Zhu, Q.; Xing, M.; Zhang, J. Self-modified breaking hydrogen bonds to highly crystalline graphitic carbon nitrides nanosheets for drastically enhanced hydrogen production. Appl. Catal. B Environ. 2018, 232, 306–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Zong, S.; Cheng, C.; Shi, J.; Guo, P.; Guan, X.; Luo, B.; Shen, S.; Guo, L. Rapid high-temperature treatment on graphitic carbon nitride for excellent photocatalytic H2-evolution performance. Appl. Catal. B Environ. 2018, 233, 80–87. [Google Scholar] [CrossRef]
- Han, Q.; Cheng, Z.; Wang, B.; Zhang, H.; Qu, L. Significant enhancement of visible-light-driven hydrogen evolution by structure regulation of carbon nitrides. ACS Nano 2018, 12, 5221–5227. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Shi, J.-W.; Ma, D.; Fan, Z.; Cheng, L.; Sun, D.; Wang, Z.; Niu, C. WS2/graphitic carbon nitride heterojunction nanosheets decorated with CdS quantum dots for photocatalytic hydrogen production. ChemSusChem 2018, 11, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ou, H.; Fang, Y.; Wang, X. A facile steam reforming strategy to delaminate layered carbon nitride semiconductors for photoredox catalysis. Angew. Chem. Int. Ed. 2017, 56, 3992–3996. [Google Scholar] [CrossRef]
- Liu, J.; Liu, N.Y.; Li, H.; Wang, L.P.; Wu, X.Q.; Huang, H.; Liu, Y.; Bao, F.; Lifshitz, Y.; Lee, S.T.; et al. A critical study of the generality of the two step two electron pathway for water splitting by application of a C3N4/MnO2 photocatalyst. Nanoscale 2016, 8, 11956–11961. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, H.; Li, Y.; Zhang, M.; Zheng, Y. Designing a 0D/1D S-Scheme Heterojunction of Cadmium Selenide and Polymeric Carbon Nitride for Photocatalytic Water Splitting and Carbon Dioxide Reduction. Molecules 2022, 27, 6286. https://doi.org/10.3390/molecules27196286
Wang Y, Wang H, Li Y, Zhang M, Zheng Y. Designing a 0D/1D S-Scheme Heterojunction of Cadmium Selenide and Polymeric Carbon Nitride for Photocatalytic Water Splitting and Carbon Dioxide Reduction. Molecules. 2022; 27(19):6286. https://doi.org/10.3390/molecules27196286
Chicago/Turabian StyleWang, Yayun, Haotian Wang, Yuke Li, Mingwen Zhang, and Yun Zheng. 2022. "Designing a 0D/1D S-Scheme Heterojunction of Cadmium Selenide and Polymeric Carbon Nitride for Photocatalytic Water Splitting and Carbon Dioxide Reduction" Molecules 27, no. 19: 6286. https://doi.org/10.3390/molecules27196286
APA StyleWang, Y., Wang, H., Li, Y., Zhang, M., & Zheng, Y. (2022). Designing a 0D/1D S-Scheme Heterojunction of Cadmium Selenide and Polymeric Carbon Nitride for Photocatalytic Water Splitting and Carbon Dioxide Reduction. Molecules, 27(19), 6286. https://doi.org/10.3390/molecules27196286