Abstract
The extensive use of pesticides in agriculture has significantly impacted the environment and human health, as these pollutants are inadequately disposed of into water bodies. In addition, pesticides can cause adverse effects on humans and aquatic animals due to their incomplete removal from the aqueous medium by conventional wastewater treatments. Therefore, processes such as heterogeneous photocatalysis and adsorption by nanocomposites have received special attention in the scientific community due to their unique properties and ability to degrade and remove several organic pollutants, including pesticides. This report reviews the use of nanocomposites in pesticide adsorption and photocatalytic degradation from aqueous solutions. A bibliographic search was performed using the ScienceDirect, American Chemical Society (ACS), and Royal Society of Chemistry (RSC) indexes, using Boolean logic and the following descriptors: “pesticide degradation” AND “photocatalysis” AND “nanocomposites”; “nanocomposites” AND “pesticides” AND “adsorption”. The search was limited to research article documents in the last ten years (from January 2012 to June 2022). The results made it possible to verify that the most dangerous pesticides are not the most commonly degraded/removed from wastewater. At the same time, the potential of the supported nanocatalysts and nanoadsorbents in the decontamination of wastewater-containing pesticides is confirmed once they present reduced bandgap energy, which occurs over a wide range of wavelengths. Moreover, due to the great affinity of the supported nanocatalysts with pesticides, better charge separation, high removal, and degradation values are reported for these organic compounds. Thus, the class of the nanocomposites investigated in this work, magnetic or not, can be characterized as suitable nanomaterials with potential and unique properties useful in heterogeneous photocatalysts and the adsorption of pesticides.
1. Introduction
The intense populational growth and industrial expansion in the most diverse segments of society have led to a substantial increase in the demand for drinking water supply and large-scale food production [1]. Thus, to increase productivity at an economically profitable level, the employment of agrochemicals has been widely used to combat pests and weeds [2]. However, pesticides are characterized by low biodegradability, high bioaccumulative capacity arising from their physicochemical properties, and a long half-life, of 5–15 years, increasing their toxicity to the environment and humans [3,4]. Thus, pesticide persistence in soil, wastewater, ground, and surface water has proved to be a considerable environmental problem, and may be compounded along the food chain, reaching concentrations toxic to human health [5]. Due to their high stability, these compounds can contaminate areas distant from pulverization through water volatilization and soil absorption. Studies have associated exposure to compounds with hormonal changes in the immune, neurological, and cardiac systems, as well as with the development of neoplasms [6,7].
For this purpose, diverse techniques, such as adsorption and advanced oxidative processes (AOPs), which include Fenton, photo-Fenton, heterogeneous photocatalysis, and ozonation systems, have been explored for removing and degrading biopersistent organic compounds [8,9,10]. AOPs are based on the generation of free radicals, e.g., hydroxyl (HO•) and superoxide (O2−•) radicals, which have high oxidizing power in an aqueous solution and are able to degrade pollutants into lower molecular weight intermediates and inorganic precursors [11,12]. Heterogeneous photocatalysis is an advanced oxidative process that occurs through the photoactivation (by sunlight or artificial light) of a semiconductor, which uses water molecules and dissolved oxygen as reagents of oxi-reduction reactions [13]. This technique is very efficient and promising for the degradation of organic pollutants, including dyes, drugs, and pesticides [10,14,15]. Among the materials used, metallic nanooxides (zinc oxide and titanium dioxide) have been largely employed due to their excellent properties, such as low toxicity, good availability, chemical stability, large surface area/porosity, and photocorrosion [16,17]. However, these conventional nanocatalysts are characterized by their high bandgap energy, which is the energy required to start photocatalytic reactions. Additionally, due to their high surface energy, they tend to agglomerate during the photocatalytic process. Therefore, the association of these nanocatalysts with a second, less active material (called catalytic support or matrix) can solve these drawbacks, even when the active material is dispersed in low concentrations (ca. 0.5–5 wt%) on the support [18]. Thus, combining the two materials results in a new material called nanocomposite, in which the active substance is in the above-mentioned concentration range and this is named the reinforcement phase [19].
Nanocomposites are multiphase materials formed by a continuous and dispersed phase and have at least one dimension in the nanoscale [20]. The continuous phase (matrix) consists of a compound of polymeric, ceramic, or metallic origin, while the dispersed phase (reinforcement) is commonly derived from fibrous materials [21,22,23]. Nanocomposite materials are synthesized to combine individual properties and reduce limitations, such as physicochemical and thermal instability, expanding the scope of applications [22]. In parallel, at the nanoscale, the materials exhibit distinct behaviors to those found at the micrometer scale, such as volume/area relationship and increased reactivity [24].
Another technique widely used for pesticide removal from wastewater consists of adsorption, especially when using nanomaterials (adsorbents), due to its simplicity of operation, relatively low cost, and low energy requirements [25]. In addition, nanoadsorbents are characterized by their high specific surface area, chemical/thermal stability, and affinity for organic pollutants [26]. Although the efficiency of nanoadsorbents in the removal of organic compounds is remarkable, there are still limitations to conventional materials’ use, such as separation from the aqueous medium and the reuse of nanoadsorbents and nanocatalysts [27]. Recently, the development of nanocomposites as nanoadsorbents has been the subject of diverse research due to their increased surface area and physicochemical stability. Moreover, magnetic nanocomposites have been used as a good alternative to improve the stability, textural properties, and reuse of nanoadsorbents [28]. The facilities separate material from the aqueous medium and considerably increase their reuse, resulting in high adsorptive capacity [29]. Additionally, the same behavior is observed for magnetic nanocomposites as nanocatalysts. Using magnetic nanocatalysts allows the reuse of the material, increasing the cost-effectiveness and avoiding subsequent steps such as filtration and centrifugation [30].
When combined with other photoactive compounds, magnetic nanoparticles (MNPs) displayed prominent photocatalyst activity [31]. Moreover, the incorporation of MNPs on the surface of nanomaterials can increase surface area and affinity for the pollutant, resulting in enhanced adsorptive capacity [32]. Besides, due to their magnetic properties, the adsorbents/catalysts are easily removed from the liquid phase after reaction, without the need for centrifugation, and the use of other chemical compounds can decrease process costs [33,34,35].
Therefore, this work reports the use of nanocomposites as nanoadsorbents and nanocatalysts in the removal and degradation of pesticides, beyond the influence of diverse experimental conditions on the efficiency of these processes. Furthermore, this approach also extends to magnetic nanocomposites due to their potential and versatility in these processes. Further, the harmful effects of pesticides on the environment and public health are discussed.
The bibliographic search was performed using the Science Direct, American Chemical Society (ACS), and Royal Society of Chemistry (RSC) indexes, using Boolean logic and the following descriptors: “pesticide degradation” AND “photocatalysis” AND “nanocomposites”; “nanocomposites” AND “pesticides” AND “adsorption”. The search was limited to research article documents in the last ten years (from January 2012 to June 2022).
2. Contamination of Wastewater by Pesticides
Due to the considerable expansion of agriculture and extensive use of pesticides, an imbalance between water quality and quantity is observed worldwide. Furthermore, it is known that some pesticides are not completely removed from wastewater by biological treatments, mainly due to the damage these contaminants cause to the microorganisms used in the processes [36]. In addition, pesticide removal from aqueous solution by physicochemical processes, such as coagulation, flocculation, and decantation, is often inefficient [37].
Consequently, the demand for more efficient treatments has increased considerably among the processes used to remove pesticides from wastewater, especially heterogeneous photocatalysis and adsorption [38]. Advanced oxidative processes have several advantages, such as completely mineralizing pesticides and transforming them into non-toxic forms such as carbon dioxide and water [39]. Additionally, the possibility of using solar radiation as an energy source enables the photoactivation of the catalytic surface of the nanocatalyst, with a lower energy demand than the concentrations of chemical reagents applied in conventional effluent treatments [40].
Regarding adsorption, multiphasic nanoadsorbents (nanocomposites) have been widely explored due to their increased adsorptive capacity, dispersibility, and thermodynamic and physicochemical stability in aqueous media. Furthermore, nanoadsorbents are characterized as high specific surface area materials and efficient in removing organic compounds [28,41].
Pesticides Toxicity
Table 1 shows the pesticide classification according to degree of danger, chemical structure, and toxic effects on the environment and public health.
As shown in Table 1, most pesticides are classified as moderately toxic according to the World Health Organization [42]. The listed data inform the adverse effects caused in aquatic animals, especially zebrafish. However, these effects are also observed in investigations of non-aquatic animals, microorganisms, and human cell in vitro models. Among pesticides, organophosphate compounds present greater carcinogenic, cytotoxic, and endocrine-disrupting potential. This scenario shows that the removal of these compounds from waters and wastewater is fundamental.

Table 1.
Classification of pesticides in terms of hazardousness and chemical structure.
Table 1.
Classification of pesticides in terms of hazardousness and chemical structure.
| Pesticide | Class | Toxic Effects | Classification * | References |
|---|---|---|---|---|
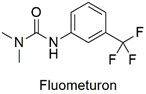 | Trifluoromethyl urea | Degeneration of renal tubule epithelial cells and hemorrhage in sheep | Unlikely to have acute problems with normal use | [43] |
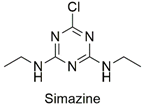 | Triazine | Endocrine disruptor, carcinogen, and degeneration of renal tubule epithelial cells of Cyprinus carpio species | Unlikely to present a hazard in normal use | [44,45] |
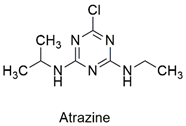 | Triazine | Hematological abnormalities, degenerative and hormonal changes, cardiotoxicity, and acute microalgae toxicity | Slightly hazardous | [46,47] |
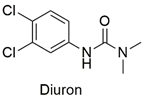 | Arylurea | Acute toxicity in zebrafish embryos and abnormalities in embryonic development, reduced cell viability, and production of reactive species in the HepG2 lineage | Slightly hazardous | [48,49] |
 | Anilinopyrimidine | Amphibian endocrine disruptor, cardiotoxicity, induction of reactive species, and apoptotic gene induction in zebrafish | Slightly hazardous | [50,51] |
 | Organophosphate | Cytotoxic, carcinogenic, and genotoxic to human lymphocytes | Slightly hazardous | [52,53] |
 | Triazine | Change in enzymatic activity and decrease in chlorophyll production in wheat crops, delay in larvae growth and carp development | Slightly hazardous | [54,55] |
 | Chlorotriazine | Effect on the swimming behavior of Danio rerio larvae, toxic effects on human liver and kidney cells, and primary DNA damage | Slightly hazardous | [56,57] |
 | Organophosphorus | Endocrine disruptor induces human breast cancer cell growth, exposure of mice to glyphosate causes anxiety and depression-like behaviors and effects on energy metabolism of peripheral blood mononuclear cells | Slightly hazardous | [58,59,60] |
 | Organophosphate | Neurotoxicity, alteration in trophoblastic layer integrity, induction of ꞵ-hCG expression | Moderately hazardous | [61,62] |
 | Neonicotinoid | Respiratory failure, induction of lymphocyte apoptosis, and alterations in spermatogenesis in rats | Moderately hazardous | [63,64,65] |
 | Pyrethroid | Reduced heart rate and altered thoracic limbic activity in Daphia magna | Moderately hazardous | [66] |
 | Organophosphate | Erythrocyte abnormalities in Danio rerio species, mutagenic and endocrine-disrupting properties | Moderately hazardous | [67,68,69] |
 | Phenylurea | Changes in the growth pattern of Lemna minor species reduced photosynthetic pigment production | Moderately hazardous | [70,71] |
 | Chloroacetanilide | Production of reactive oxygen species, induction of apoptosis and damage to sperm DNA and alteration in the gene expression of the species, and decrease in cell viability of Prorocentrum minimum | Moderately hazardous | [72,73] |
 | Organophosphate | Chromosomal alterations, DNA damage, carcinogenic effects, and neurotoxicity to mouse embryos | Moderately hazardous | [74,75,76] |
 | Triazine | Malformation in zebrafish larvae and oxidative stress and genotoxicity in Wistar rats | Moderately hazardous | [77,78] |
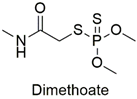 | Organophosphate | Enzymatic alterations in Cyprinus carpio and Galba truncatula species | Moderately hazardous | [79,80] |
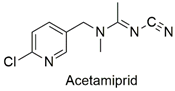 | Neonicotinoid | Reduced hatchability of Danio rerio eggs, heart rate, and growth changes. Physiological changes in Apis cerana cerana | Moderately hazardous | [81,82] |
 | Neonicotinoid | Invertebrate toxicity, increased body mass, and liver hypertrophy in Wistar rats | Moderately hazardous | [83,84] |
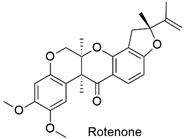 | Terpene | Neurotoxic and neurodegenerative agents for humans | Moderately hazardous | [85] |
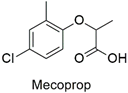 | Chlorophenoxy | Toxicity to the bacterial strain of Pseudomonas putida | Moderately hazardous | [86] |
 | Oxi-alkanoic acid | Changes in the organization of the cell membrane of Pseudomonas putida and uncoupling of oxidative phosphorylation in a cell lineage of the Metynnis roosevelti species | Moderately hazardous | [87,88] |
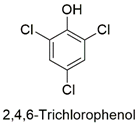 | p-Chlorophenol | Reproductive toxicity, developmental disturbance in Danio rerio embryos, mutations in p53 gene from zebrafish liver | Non-cancerous | [89,90] |
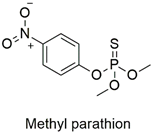 | Organophosphate | Endocrine disruptor, genotoxic, cardiotoxic and neurotoxic | Highly hazardous | [91,92,93,94] |
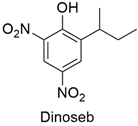 | Dinitrophenol | Depletion of ATP levels, disruption of liver metabolism | Highly toxic. Obsolete as a pesticide in the European Union and the United States | [95,96] |
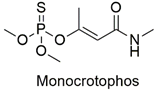 | Organophosphate | Cytological anomalies in Catla catla fish and neurotoxicity in humans | Highly hazardous | [97,98] |
 | Neonicotinoid | Production of reactive species, bioaccumulation, alteration in growth and reproduction of the species Eisenia fetida | Acute oral toxicity | [99,100] |
 | Neonicotinoid | Endocrine disruptor for the species Eremias argus and responsible for the loss of body mass in birds, induction of lipid peroxidation, oxidative stress, and DNA damage | Extremely toxic to aquatic life | [101,102,103] |
 | Neonicotinoid | Reduction of antioxidant activity and oxidative damage in Chironomus ripar and endocrine disruption in flies | Uninformed | [101,104] |
 | Neonicotinoid | Genotoxicity, effects on antioxidant enzymes in zebrafish and earthworm | Uninformed | [105,106] |
* Adapted from Who [42].
3. Decontamination Methods
3.1. Nanocomposites
Nanocomposites are solid materials composed of two phases, the matrix and the reinforcement phase, with one of the constituents having dimensions in nanometers [22,107]. The matrix phase can be ceramic, polymeric, or metallic in origin and the reinforcement phase can be any class of nanomaterial.
From this perspective, metallic nanoparticles, graphene oxide, chitosan, and reduced graphene oxide can be used as reinforcement, which are dispersed on the matrix to generate materials with high mechanical resistance, good optical properties, and high surface area [28,108,109]. Thus, nanocomposites can be easily applied to water and wastewater treatment, catalysis, and structural applications. Furthermore, the synthesis of nanocomposites can reduce surface energy and the tendency for magnetic nanoparticles to agglomerate, increasing the physicochemical stability [110,111].
Due to cost-effectiveness and ease of application, polymeric and ceramic nanocomposites are commonly used in various applications. Polymeric-based matrix nanocomposites can be prepared by either in situ intercalation polymerization, melt intercalation of the pre-polymer solution, or sol–gel synthesis [112], while ceramic-based matrix nanocomposites can be synthesized by either sol–gel synthesis, powder process or polymer precursor process, for example [113].
3.1.1. Heterogeneous Supported Nanocatalysts
Supported nanocatalysts consist of an active nanomaterial dispersed on a less active material called a catalytic support. They are widely used in heterogeneous photocatalysis due to their high surface area, considerable photocatalytic activity, and chemical/thermal stability [114].
The application of supported nanocatalysts (nanocomposites) helps overcome some drawbacks commonly encountered when applying isolated nanocatalysts, including nanoparticle agglomeration due to their high surface energy and poor dispersion in the aqueous solution [115]. Additionally, these catalytic supports increase the contact between organic pollutants and the catalytic surface [116].
Among the supported nanocatalysts used for organic pollutant degradation, TiO2, CuO, Fe2O3, and ZnO, supported on zeolites or silica, can be highlighted due to their significant photocatalytic activity, high surface area, and thermo-chemical stability [117]. Additionally, supported catalysts show reduced bandgap energy due to better charge separation by the presence of the support. Thus, the possibility of using this supported nanocatalyst even in the visible light range of spectrum compliments their potential applicability in light-driven processes, in particular, heterogenous photocatalysis [118]. Moreover, these nanocatalysts can be easily prepared by alternative synthetic methods, such as those based on leaves or plant extracts, microorganism strains, and industrial waste [119]. Plant-based biosynthesis of nanocatalysts is based on the reduction and stabilization of the metallic precursor. In contrast, biogenic synthesis using microorganism strains is based on a biocatalytic reduction reaction that transforms the precursor into nanoparticle suspensions, followed by nucleation and stabilization [120]. Figure 1 shows a schematic representation of the heterogeneous photocatalysis process for application in the degradation of organic pollutants.
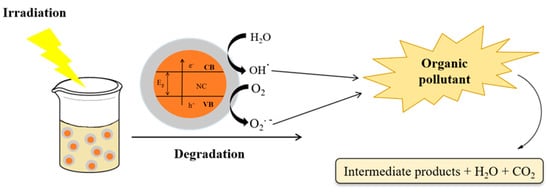
Figure 1.
Scheme showing degradation of organic pollutants by heterogeneous photocatalysis.
Photocatalytic Degradation of Pesticides
All scientific works discussed were found in indexed platforms, and research articles were selected as the basis for investigation. Table 2 shows the main results observed in this work.

Table 2.
Photocatalytic degradation of pesticides using supported nanocatalysts.
From Table 2, it was possible to observe a tendency for specific pesticide degradation, such as chlorpyrifos, atrazine, and imidacloprid. This is due to the wide use of these pesticides in agriculture, generating residues that can be released into wastewater [138,139,140].
It is worth mentioning that the utilization of magnetic nanocatalysts has been increasing once they have been characterized by improved photocatalytic activity compared to non-magnetic ones. In addition, magnetic nanocatalysts can solve operational drawbacks faced in heterogeneous photocatalysis by applying isolated nanocatalysts such as nanoparticle agglomeration and difficult recovery of the solid material after treatment [141]. Thus, in the following sections, the mechanisms involved in the pesticide degradation on support nanocatalysts will be described, as well as the enhancement of these nanocatalysts by adding a dopant material, which can be magnetic or non-magnetic.
Degradation of Pesticides by Heterogeneous Photocatalysis
Table 2 shows that pesticide degradation percentages are reported in the range of 80 to 90% after 30 to 180 min, with initial pesticide and nanocatalyst concentrations ranging from 15 to 50 mol L−1 and 0.1 to 0.5 g L−1, respectively. In addition, the selected scientific works report the good dispersibility of the nanocatalysts on the catalytic support, resulting in a nanocomposite with high chemical stability and preserved photocatalytic activity after 4 to 6 cycles of heterogeneous photocatalysis.
Effect of Initial Pesticide Concentration
Regarding the initial pesticide concentration, it is important to notice that an increase in this parameter leads to a decrease in the degradation percentage; at a high pollutant concentration, the number of pesticides molecules and active sites of the nanocomposite are altered, which results in an insufficient number of sites available to receive pesticide molecules and degrade them [142]. Therefore, an ideal initial concentration that favors photocatalytic pesticide degradation can be assumed, yielding higher oxidation percentages. In most scientific works, the concentration of 15 mol L−1 seems to be favorable for pesticide degradation (considering 0.1–0.3 g L−1) due the abundance of active sites available for pesticide molecules on the catalytic surfaces at this concentration [124,129]. In contrast, at higher concentrations (about 20 mg L−1 or more), pesticide molecules hindered light absorption by the nanocatalyst, which reduces hydroxyl radical generation and consequently degradation of the target pollutant [143].
Effect of Nanocatalyst Concentration and Dopant Incorporation
Based on the investigated scientific papers presented in Table 2, it is noticeable that an increase in the supported nanocatalyst concentration (metallic nanoparticles dispersed on catalytic support) favors the photocatalytic degradation of pesticides. This is due to the imbalance between the number of molecules of the pollutant in an aqueous solution and the number of active sites on the nanocatalyst [109]. Therefore, the constant rate values (k1, considering the Langmuir–Hinshelwood equation for the pseudo-first-order kinetic model) increase considerably, which results in greater pesticide degradation [132]. However, at extremely high nanocatalyst concentrations, pesticide degradation is reduced due to the tendency for nanocatalyst particles to agglomerate and increase the opacity of the aqueous solution, which reduces the photocatalytic activity of the supported nanocatalyst [144].
It is worth pointing out that there is a limiting value responsible for considerable pesticide degradation percentages in aqueous solution regarding dopant material. The reason for this is the same as for the increase in nanocatalyst concentration.
At the same time, it can be noticed that the incorporation of two materials (nanocomposite) can yield greater nanocatalyst surface area and greater light absorption in the electromagnetic spectrum, allowing the application of this nanocatalyst under UV and visible light radiation for heterogeneous photocatalysis.
Effect of pH
The solution pH has a considerable effect on pesticide degradation in heterogeneous photocatalysis. According to the scientific articles investigated (Table 2), it was possible to verify that the solution pH affects the surface charge of the nanocatalyst. The net charge of the nanocatalyst surface is measured by the point of zero charge (pHPZC), at which the nanocatalyst has a net charge of zero [145]. At a pH lower than pHPZC, the surface of the nanocatalyst is protonated and, therefore, positive. In contrast, at a pH higher than pHPZC, the surface of the nanocatalyst is deprotonated and, therefore, negative.
The pH interferes with pesticide degradation as it changes the charges associated with the surface of the nanocomposites used in heterogeneous photocatalysis. Thus, the surface charge is strongly related to the adsorption of pesticide molecules before their photocatalytic oxidation [29]. When the pesticide molecule has anionic character (imidacloprid), the adsorption on the surface of the nanocatalyst is favored at acidic pH. On the other hand, when the pesticide molecule has more acidic character, i.e., chlorpyrifos, the adsorption onto the catalyst surface is favored at alkaline pH, in which pHPZC is greater than the solution pH.
Additionally, the solution pH can affect the reaction between hydroxyl ions and positive holes of the supported nanocatalyst and, therefore, determine a greater or lesser extent of pesticide degradation [128]. The above-mentioned reaction seems to be favored at higher solution pH (alkaline pH), resulting in more hydroxyl radicals, which are responsible for pesticide photocatalytic degradation.
Effect of Temperature
In some scientific research involving heterogeneous photocatalysis and pesticide degradation (Table 2), the effect of temperature on pesticide photocatalytic degradation was investigated. The effects of temperature on heterogeneous photocatalysis using supported nanocatalysts, magnetic or non-magnetic, are associated with the energy required for the adsorption and desorption of pesticide molecules. Some studies found that the adsorption of imidacloprid was facilitated by increasing the temperature from 20 to 80 ° C. Due to the endothermic nature of the pesticide adsorption–degradation process, which is the majority of cases studied, pesticide absorption on the catalytic surface and adsorption rate increase with temperature [131]. In addition, the degradation rate seems to be increased at temperatures above the atmospheric temperature (i.e., 25 ± 5 °C).
Effect of Nanocatalyst Reuse
In most scientific research involving heterogeneous photocatalysis and pesticide degradation (Table 2), the effect of nanocomposite reuse is evaluated. According to the results presented in the selected studies, the combination of two or more nanomaterials in the nanocatalyst composition yields greater photocatalytic activity, even after five cycles of reuse. However, it is worth pointing out that when one of the nanocomposite phases has magnetic properties, its efficiency in pesticide degradation improves considerably after various photocatalytic cycles. Therefore, magnetic nanomaterials have been highlighted due to their versatility, ease of operation (magnetic separation), and reuse capacity. In this view, about 72 to 80% of pesticide degradation is reported even after six to seven cycles of heterogeneous photocatalysis [128,129].
3.1.2. Adsorption
Adsorption is a physicochemical process and surface phenomenon wherein a fluid (liquid or gas) interacts with a solid surface (adsorbent), resulting in the mass transfer of a solute from a fluid phase to the solid surface [146]. In this system, the degree of interaction of ions and molecules depends on the concentration, pH, temperature, and available specific surface are [147]. The attractive forces between the adsorbent and the adsorbate can be divided into physical and chemical adsorption [148,149].
Chemical adsorption (chemisorption) involves the transfer of electrons and chemical bond formation. On the other hand, physical adsorption results from weak intermolecular interactions called van der Waals forces, electrostatic interactions, H-bonds, and π–π bonds [150].
Regarding the nature of adsorption, some parameters must be observed, such as adsorbent selectivity, homogeneity, and heterogeneity of the solid nanomaterial and the adsorption rate, which can be fast or slow. Furthermore, depending on the process, the adsorbate can accumulate at the adsorbent interface in a monolayer or multilayer [151]. For a further explanation, Figure 2 illustrates the adsorption mechanisms in mono and multilayer models.
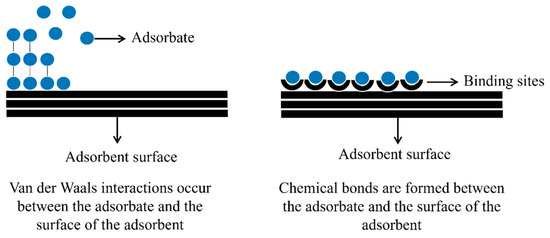
Figure 2.
Illustration of the adsorption process in mono and multilayer.
Adsorption is a relatively simple technology with low cost and energy demand and can effectively remove a range of chemical compounds from aqueous medium. However, the effectiveness of the process depends on the intrinsic properties of adsorbents, such as particle diameter, pore volume, and chemical/thermal stability [152]. In this context, diverse strategies have been explored, such as the use of thermal treatments, modification, and surface doping that aim not only to increase the adsorptive capacity but also to reduce costs associated with adsorbent synthesis [153,154]. For instance, 70% of the cost of an adsorption operation is relative to the adsorbent [155].
Adsorption of Pesticides into Nanocomposites
The research was performed (Table 3) to verify the efficiency of nanocomposites used as nanoadsorbents.

Table 3.
Pesticide adsorption by magnetic and non-magnetic nanocomposites.
Based on the results presented in Table 3, it was possible to verify that the pesticide adsorption studies using nanocomposites were efficient for the removal of organic compounds from aqueous solution, especially in the class of organophosphates neonicotinoids and triazines.
Moreover, the studies were heterogeneous enough and contemplated different compounds belonging to the same chemical class. However, in most articles, the nanoadsorbent showed selectivity and higher adsorption capacity when compared to isolated materials, i.e., applied individually. Hybrid materials were usually synthesized to combine individual properties and make them multifunctional [112].
In this study, it was found that nanocomposites have good performance in the adsorption process. In addition, the efficiency and stability of the adsorbent were maintained after several adsorption/desorption cycles. Furthermore, because some materials exhibit magnetic behavior, the adsorbent can be easily separated from the solution by the application of an external magnetic field. Figure 3 shows a simple approach to magnetic nanocomposites used for pesticide adsorption, magnetic retention, removal, and reuse.
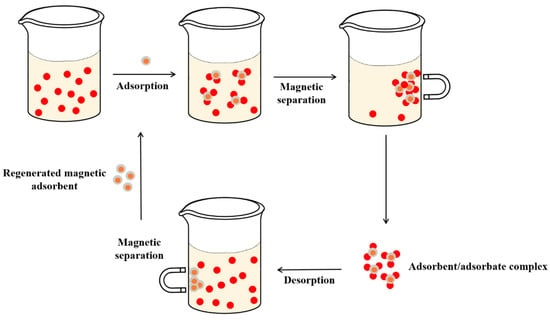
Figure 3.
Use of magnetic nanoadsorbents in liquid phase adsorption.
Effect of Initial Adsorbate Concentration and Adsorbent Dosage
Studying the effect of experimental parameters such as initial adsorbate concentration, adsorbent dosage, and adsorption contact time is essential to determine the most favorable conditions for contaminant removal and to investigate the phenomenon of adsorption.
Muda et al. [162] demonstrated that the maximum adsorption capacity was directly proportional to the increase in the initial concentration of adsorbate and adsorbent mass, mainly due to the high probability of adsorbate molecules binding to the adsorption sites [160]. Likewise, the gradual increase in the initial concentration of adsorbate and the amount of adsorbent can reduce the contaminant removal percentage due to the saturation of higher energy sites and the interaction between the adsorbent molecules [165].
Another important factor for the adsorption efficiency is the contact time between adsorbent and adsorbate. In most of the studies, adsorption showed distinct behavior over time. In the initial stages, a fast adsorption rate is observed (mass transfer of liquid phase to the solid phase) and a tendency to plateau in the subsequent steps because of saturation of the adsorption sites (equilibrium between vacancy sites and adsorbed molecules) [145]. At the same time, the nanocomposite content (ratio mass: mass) of the of constituent materials can, in some cases, affect the efficiency of the process, either positively or negatively. For example, Gupta et al. [166] reported that the combination of cellulose nanofibers and cadmium sulfide resulted in more efficient nanomaterials for removing chlorpyrifos, mainly due to the increase in the specific area and, consequently, the greater number of adsorption sites available. However, the increase in biopolymer charge (>10%) significantly reduced the adsorbent performance due to the aggregation of particles and the steric hindrance promoted by the functional groups present in the polymeric chains.
Effect of pH
The pH of the solution is an important parameter that influences the adsorption capacity since it is related to the surface properties of the adsorbent, degree of speciation of chemical compounds, and competition of ions in solution for adsorbent sites [28,161]. Nevertheless, the ideal pH for each process is quite specific, as it depends on the adsorbent’s surface charge and the adsorbate’s intrinsic characteristics, such as the acid dissociation constant (pKa) and chemical stability to pH changes. In addition, the study of pH effects can also help to understand the possible chemical or physical mechanisms involved in the removal of the adsorbate [167,168].
Recently, Abukhadra et al. [160] developed a polymeric nanocomposite containing bentonite for methyl-parathion removal. The study of the effect of pH verified a considerable increase in the adsorption capacity in alkaline medium (pH = 9.0). This adsorption behavior is related to the point of zero charge (pHPZC) of the adsorbent and deprotonation at basic pH, which results in strong electrostatic attraction with the cationic species of methyl-parathion.
However, van der Waals forces are not the only interactions existing between the adsorbent and the adsorbate since the pH of the solution may not influence the process [145]. According to the chemical structure of compounds and functional groups, the interaction between adsorbent/adsorbate systems can occur through stacking bonds π–π, hydrogen bonds, and ionic exchange [156,161].
Effect of Temperature on Adsorption
The efficiency of the adsorption process is highly dependent on experimental conditions, such as temperature. Evaluating the effect of temperature on adsorption behavior is an important step considering a future application in wastewater treatment systems [111].
In an adsorption system, the temperature can affect the adsorption velocity and kinetic energy, resulting in an increase or decrease in the removal percentage and adsorption capacity [156,169]. The effects of temperature on adsorption occur due to viscosity, rate diffusion, and equilibrium state [170].
Adsorption is a spontaneous process that can be endothermic or exothermic [171,172]. Observations concerning the temperature dependence of adsorption of pesticides were reported. All studies that evaluated the effect of temperature on adsorption assumed an endothermic reaction, i.e., the reaction became more favorable at higher temperatures [156,157,159,160].
Equilibrium and Adsorption Kinetics
Adsorption isotherms are helpful mathematical equations to describe the equilibrium of adsorption; that is, the amount of solute adsorbed on the surface of the adsorbent as a function of concentration in the liquid phase at a constant temperature [173]. Furthermore, they are relevant tools for understanding the interaction between the adsorbent and the adsorbate, which can help process design [174]. Several isothermal models can describe the adsorption phenomenon. However, the most commonly reported in the literature are the Langmuir, Freundlich, and Temkin isotherms.
The Langmuir isotherm is a theoretical model used to describe processes on a uniform, non-porous surface. The model is based on the hypothesis that adsorption occurs in monolayers with uniform energy distribution, in which the molecules of the adsorbed solutes do not interact with each other [175]. The expression of the Langmuir isotherm is represented by Equation (1):
where qe is the amount of adsorbate adsorbed per unit of mass of adsorbent in equilibrium (mg g−1), qm is the maximum adsorbed amount (mg g−¹), KL is the adsorption equilibrium constant or Langmuir constant (L mg−1), and Ce is the concentration of adsorbate in the solution at equilibrium (mg L−1).
The Freundlich isotherm is an empirical mathematical model that represents the adsorption equilibrium of a fluid (liquid or gas) on the surface of a solid material. This equation assumes that the adsorption occurs on heterogeneous surfaces and adsorption sites of different energy levels [176]. The Freundlich isotherm is expressed by Equation (2):
where KF is the adsorption equilibrium constant or Freundlich constant ((mg g−1) (L mg−1)−1/n), and 1/n is the heterogeneity factor.
The Temkin isotherm is an empirical model used to describe the indirect interactions between the adsorbent and the adsorbate and assumes that the heat of adsorption decreases as the surface of the adsorbent is covered. Unlike the Freundlich isothermal model, the system energy decreases in a non-logarithmic form (uniform energy distribution) [177]. Temkin’s model is given by Equation (3) and is applied in studies to measure the energetic contributions of the adsorption process. Thus, the process temperature must be considered.
where B is the constant associated with adsorption heat (kJ mol−1), T is the temperature (K), R is the universal constant of ideal gases (8.314 J mol−1 K−1), b is the constant associated with the interaction between adsorbate and adsorbent, and KT is the Temkin constant (L mg−1).
Furthermore, the Dubinin–Radushkevich (DR) model is often reported as a good fit for the experimental data to the adsorption isotherms [178]. However, this empirical model assumes active sites with Gaussian energy distribution and is widely applied only to intermediate ranges of adsorbate concentration, as it presents an unrealistic behavior [179].
Kinetic models have been widely used to assess adsorbent performance, construct the kinetic profile, and investigate the possible mechanisms involved in mass transfer between liquid and solid phases [180]. Among the proposed models, the most common are pseudo-first-order (PFO), pseudo-second-order (PSO), and Elovich kinetics [181,182].
The PFO model was initially proposed by Lagergren and describes that the adsorption process is independent of the initial concentration of adsorbate and which adsorbent has some active sites, with the external/internal diffusion being the step determining the adsorption rate. Moreover, the model suggests that the interactions between the molecules of the adsorbent and the adsorbed substance are relatively weak attractive forces, indicating a physisorption process. The PFO kinetics is based on the non-competition of molecules for an active site (1:1 ratio) and is expressed by Equation (4):
where qt is the amount of solute adsorbed on the solid surface at time t (mg g−1), and k1 is the pseudo-first-order kinetic constant (min−1).
The PSO model assumes that adsorption on active sites is the control step of the adsorption process, where the adsorption rate increases proportionally with the available adsorption sites. Thus, adsorption occurs in specific sites, and adsorbate molecules do not interact with each other. In terms of adsorption mechanisms, if this model presents the best adjustment of the experimental data (higher values of R2), the electrons transfer is the limiting rate of the process, indicating a phenomenon of a chemical nature. The pseudo-second-order kinetics, however, consider the competition between the adsorbate molecules for an active site (2:1 ratio) and are expressed by Equation (5):
where k2 is the pseudo-second-order kinetic constant (g mg−1 min−1), and t is the contact time (min)
Elovich model is largely used to describe the chemisorption of solutes onto solid sorbents and can be expressed by Equation (6):
where a is the constant that describes the initial sorption rate (mg g−1 min−1) and b is the constant related to surface coverage and activated energy for chemisorption (g mg−1).
From the scientific articles shown in Table 3, it was possible to verify that the kinetic models of PPO and PSO are the most used to measure the adsorption rates of pesticides on nanoadsorbents (magnetic and non-magnetic). Regarding the adsorption equilibrium models, the Langmuir and Freundlich isotherms are applied in adsorption studies in the aqueous phase to represent a good adjustment of the experimental data.
The Langmuir isotherm was reported as the better model for adjusting the equilibrium of the pesticide’s adsorption, suggesting adsorption in monolayers with uniform distribution of active sites of nanocomposites. Furthermore, it is assumed that there is no competition between the adsorbent molecules for a single active site of the adsorbent.
Concerning the kinetic models, the PSO model demonstrated the best adjustment of experimental data, indicating that mass transfer as a function of a concentration gradient is the limiting step of adsorption. Furthermore, the number adsorbate and adsorbent sites had a 2:1 relationship [183]. Furthermore, this model suggests that the transfer of pesticides to the solid surface occurs predominantly by chemical mechanisms, i.e., chemisorption.
4. Conclusions and Future Perspectives
The present work made it possible to verify the potential and extensive application of magnetic and conventional nanomaterials in either degradation or removal of pesticides from the aqueous solution. Thus, it is observed that the degradation of these pollutants occurs mainly by heterogeneous photocatalysis, whereas the removal of wastewater is mainly by adsorption processes.
The pesticides methyl-parathion, dinoseb, and monocrotophos are the most dangerous. However, photodegradation and adsorption are effective for moderately toxic pollutants, such as chlorpyrifos and atrazine. Values of up to 90 and 99% are reported for degradation and the removal of pesticides from wastewater. This result demonstrates a certain lack of research involving the adsorption or photodegradation of highly hazardous pesticides.
At the same time, the wide-ranging use of magnetic nanocomposites is justified by their operational ease, reuse, and chemical or thermal stability. In addition, the percentage of materials used in the synthesis of nanoadsorbents can affect the interaction between the adsorbent and the adsorbate. The pH of the solution, initial concentration of adsorbate, contact time, and surface area are factors determining the efficiency and optimization of the process.
Photocatalytic degradation is reported under visible light and UV radiation due to the addition of different nanomaterials that make up the reinforcement layer, and the nanocomposite matrix is combined. Further, the catalytic efficiency is considerably enhanced by the incorporation two materials in the nanocatalyst structure. Therefore, it was possible to show the potential of nanocomposites in the degradation/removal of pesticides in aqueous solutions.
Studies involving the scale-up of adsorption and photocatalytic processes should be encouraged, considering the higher reuse capabilities and efficiency of nanocomposites, especially the magnetic ones. In this view, heterogeneous photocatalysis for the degradation of pesticides at higher concentrations and adsorption of these pollutants in fixed-bed adsorbers should be conducted to expand the breakthrough performed by the research discussed in this review. In addition, the search for alternative sources to produce nanocomposite matrixes, such as residual or abundant materials (biomass), can intensify the use of these nanocomposites in heterogeneous photocatalysis and the adsorption of pesticides. At the same time, ecotoxicity and cytotoxicity studies should be performed to assess the safety of the use of these materials and their implementation at a large scale.
Author Contributions
F.S.B., C.S., L.R.O. and S.K. writing—original draft preparation; L.F.O.S., W.L.S., G.L.D. and C.R.B.R. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Nor applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Xu, P.; Chen, M.; Tang, W.; Zeng, G.; Gong, J.; Zhang, P.; Ye, S. Using nanomaterials to facilitate the phytoremediation of contaminated soil. Crit. Rev. Environ. Sci. Tecnol. 2019, 49, 791–824. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- García-García, C.R.; Parrón, T.; Requena, M.; Alarcón, R.; Tsatsakis, A.M.; Hernández, A.F. Occupational pesticide exposure and adverse health effects at the clinical, hematological and biochemical level. Life Sci. 2016, 145, 274–283. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu GP, S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Pesticide chemical research in toxicology: Lessons from nature. Chem. Res. Toxicol. 2017, 30, 94–104. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S.V. Ozone as a novel emerging technology for the dissipation of pesticide residues in foods—A review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, X.; Liu, Z.; Zhang, Y.; Wang, J.; Xu, D. Adsorption and removal of organophosphorus pesticides from environmental water and soil samples by using magnetic multi-walled carbon nanotubes@ organic framework ZIF-8. J. Mater. Sci. 2018, 53, 10772–10783. [Google Scholar] [CrossRef]
- Abdennouri, M.; Baâlala, M.; Galadi, A.; El Makhfouk, M.; Bensitel, M.; Nohair, K.; Sadiq, M.; Boussaoud, A.; Barka, N. Photocatalytic degradation of pesticides by titanium dioxide and titanium pillared purified clays. Arab. J. Chem. 2016, 9, S313–S318. [Google Scholar] [CrossRef]
- Barka, N.; Qourzal, S.; Assabbane, A.; Nounah, A.; Ait-Ichou, Y. Photocatalytic degradation of an azo reactive dye, Reactive Yellow 84, in water using an industrial titanium dioxide coated media. Arab. J. Chem. 2010, 3, 279–283. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karamitabar, Y.; Changani, F.; Heidarinejad, Z. High performance degradation of phenol from aqueous media using ozonation process and zinc oxide nanoparticles as a semiconductor photo catalyst in the presence of ultraviolet radiation. Desalin. Water Treat. 2019, 166, 105–114. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Silva, W.L.; Lansarin, M.A.; Livotto, P.R.; Santos JH, Z. Photocatalytic degradation of drugs by supported titania-based catalysts produced from petrochemical plant residue. Powder Technol. 2015, 279, 166–172. [Google Scholar] [CrossRef]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Kamat, P.V. TiO2 Nanostructures: Recent Physical Chemistry Advances. J. Phys. Chem. C 2012, 116, 11849–11851. [Google Scholar] [CrossRef]
- Hernández, S.; Hidalgo, D.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Cauda, V.; Tresso, E.; Saracco, G. Comparison of photocatalytic and transport properties of TiO2 and ZnO nanostructures for solar-driven water splitting. Phys. Chem. Chem. Phys. 2015, 17, 7775–7786. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, V.C. Synthesis and characterization of ZnO/CuO nanocomposite by electrochemical method. Mater. Sci. Semicond. Process. 2017, 57, 173–177. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Da Silva Bruckmann, F.; Ledur, C.M.; da Silva, I.Z.; Dotto, G.L.; Rhoden CR, B. A DFT theoretical and experimental study about tetracycline adsorption onto magnetic graphene oxide. J. Mol. Liq. 2022, 353, 118837. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Woodhead Publishing: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Nunes, F.B.; Da Silva Bruckmann, F.; Da Rosa Salles, T.; Rhoden, C.B.R. Study of phenobarbital removal from the aqueous solutions employing magnetite-functionalized chitosan. Environ. Sci. Pollut. Res. 2022, 12, 908–931. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yoldi, M.; Fuentes-Ordoñez, E.; Korili, S.; Gil, A. Zeolite synthesis from industrial wastes. Microporous Mesoporous Mater. 2019, 287, 183–191. [Google Scholar] [CrossRef]
- Mintova, S.; Jaber, M.; Valtchev, V. Nanosized microporous crystals: Emerging applications. Chem. Soc. Rev. 2015, 44, 7207–7233. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Tran, H.N.; Fu, C.C.; Lu, Y.T.; Juang, R.S. Roles of adsorption and photocatalysis in removing organic pollutants from water by activated carbon–supported titania composites: Kinetic aspects. J. Taiwan Inst. Chem. Eng. 2020, 109, 51–61. [Google Scholar] [CrossRef]
- Bruckmann, F.S.; Rossato Viana, A.; Tonel, M.Z.; Fagan, S.B.; Garcia WJ, D.S.; Oliveira AH, D.; Dorneles, L.S.; Mortari, S.R.; Da Silva, W.L.; Da Silva, I.Z.; et al. Influence of magnetite incorporation into chitosan on the adsorption of the methotrexate and in vitro cytotoxicity. Environ. Sci. Pollut. Res. 2022, 29, 1–22. [Google Scholar] [CrossRef]
- Rahimi, B.; Jafari, N.; Abdolahnejad, A.; Farrokhzadeh, H.; Ebrahimi, A. Application of efficient photocatalytic process using a novel BiVO/TiO2-NaY zeolite composite for removal of acid orange 10 dye in aqueous solutions: Modeling by response surface methodology (RSM). J. Environ. Chem. Eng. 2019, 7, 103253. [Google Scholar] [CrossRef]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef]
- Feijoo, S.; González-Rodríguez, J.; Fernández, L.; Vázquez-Vázquez, C.; Feijoo, G.; Moreira, M.T. Fenton and photo-fenton nanocatalysts revisited from the perspective of life cycle assessment. Catalysts 2020, 10, 23. [Google Scholar] [CrossRef]
- Rhoden CR, B.; Bruckmann, F.S.; Salles, T.R.; Kaufmann Jr, C.G.; Mortari, S.R. Study from the influence of magnetite onto removal of hydrochlorothiazide from aqueous solutions applying magnetic graphene oxide. J. Water Process. Eng. 2021, 43, 102262. [Google Scholar] [CrossRef]
- Park, C.M.; Kim, Y.M.; Kim, K.H.; Wang, D.; Su, C.; Yoon, Y. Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: A mini review. Chemosphere 2019, 221, 392–402. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Bruckmann, F.D.S.; Pimentel, A.C.; Viana, A.R.; Salles, T.d.R.; Krause, L.M.F.; Mortari, S.R.; Da Silva, I.Z.; Rhoden, C.R.B. Synthesis, characterization and cytotoxicity evaluation of magnetic nanosilica in L929 cell line. Discip. Sci. Ser. Cienc. Nat. Tecnol. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Abbas, G.; Hassan, N.; Farhan, M.; Haq, I.; Karar, H. Effect of selected insecticides on Helicoverpa armigera Hubner (Lepidoptera: Noctuidae) on tomato (Lycopersicon esculentum Miller) and their successful management. Adv. Entomol. 2015, 3, 16. [Google Scholar] [CrossRef]
- Khan, S.B.; Hou, M.; Shuang, S.; Zhang, Z. Morphological influence of TiO2 nanostructures (nanozigzag, nanohelics and nanorod) on photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2017, 400, 184–193. [Google Scholar] [CrossRef]
- Kanchi, S. Nanotechnology for water treatment. J. Environ. Anal. Chem. 2014, 1, 1–3. [Google Scholar] [CrossRef]
- Abbasi, M.; Rafique, U.; Murtaza, G.; Ashraf, M.A. Synthesis, characterization and photocatalytic performance of ZnS coupled Ag2S nanoparticles: A remediation model for environmental pollutants. Arab. J. Chem. 2018, 11, 827–837. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C: Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and water treatment: Applications and emerging opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. 2020. Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 26 June 2021).
- Mohamed, O.S.; Ahmed, K.E.; Adam, S.E.; Idris, O.F. Toxicity of cotoran (fluometuron) in Desert sheep. Vet. Hum. Toxicol. 1995, 37, 214–216. [Google Scholar] [PubMed]
- Silva, M.; Iyer, P. Toxicity endpoint selections for a simazine risk assessment. Birth Defects Res. B Dev. Reprod. Toxicol. 2014, 101, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Velisek, J.; Stara, A.; Machova, J.; Svobodova, Z. Effects of long-term exposure to simazine in real concentrations on common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2012, 76, 79–86. [Google Scholar] [CrossRef]
- Gammon, D.W.; Aldous, C.N.; Carr Jr, W.C.; Sanborn, J.R.; Pfeifer, K.F. A risk assessment of atrazine use in California: Human health and ecological aspects. Pest Manag. Sci. 2005, 61, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, Y.; Hu, N.; Ma, J.; Sun, S.; Cao, W.; Klobučar, G.; Hu, C.; Zhao, Y. To evaluate the toxicity of atrazine on the freshwater microalgae Chlorella sp. using sensitive indices indicated by photosynthetic parameters. Chemosphere 2020, 244, 125514. [Google Scholar] [CrossRef]
- Velki, M.; Di Paolo, C.; Nelles, J.; Seiler, T.B.; Hollert, H. Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity. Chemosphere 2017, 180, 65–76. [Google Scholar] [CrossRef]
- Kao, C.M.; Ou, W.J.; Lin, H.D.; Eva, A.W.; Wang, T.L.; Chen, S.C. Toxicity of diuron in HepG2 cells and zebrafish embryos. Ecotoxicol. Environ. Saf. 2019, 172, 432–438. [Google Scholar] [CrossRef]
- Bernabò, I.; Guardia, A.; Macirella, R.; Tripepi, S.; Brunelli, E. Chronic exposures to fungicide pyrimethanil: Multi-organ effects on Italian tree frog (Hyla intermedia). Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Meng, Y.; Zhong, K.; Xiao, J.; Huang, Y.; Wei, Y.; Tang, L.; Chen, L.; Wu, J.; Ma, J.; Cao, Z.; et al. Exposure to pyrimethanil induces developmental toxicity and cardiotoxicity in zebrafish. Chemosphere 2020, 255, 126889. [Google Scholar] [CrossRef]
- Olakkaran, S.; Purayil, A.K.; Antony, A.; Mallikarjunaiah, S.; Puttaswamygowda, G.H. Oxidative stress-mediated genotoxicity of malathion in human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 849, 503138. [Google Scholar] [CrossRef]
- Anjitha, R.; Antony, A.; Shilpa, O.; Anupama, K.P.; Mallikarjunaiah, S.; Gurushankara, H.P. Malathion induced cancer-linked gene expression in human lymphocytes. Environ. Res. 2020, 182, 109131. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H. Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol. Environ. Saf. 2009, 72, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Velisek, J.; Stara, A.; Koutnik, D.; Machova, J. Effects of prometryne on early life stages of common carp (Cyprinus carpio L.). Pestic. Biochem. Phys. 2015, 118, 58–63. [Google Scholar] [CrossRef]
- Pérez, J.; Domingues, I.; Monteiro, M.; Soares, A.M.; Loureiro, S. Synergistic effects caused by atrazine and terbuthylazine on chlorpyrifos toxicity to early-life stages of the zebrafish Danio rerio. Environ. Sci. Pollut. Res. 2013, 20, 4671–4680. [Google Scholar] [CrossRef]
- Želježić, D.; Žunec, S.; Bjeliš, M.; Benković, V.; Mladinić, M.; Tariba, B.L.; Pavičić, I.; Čermak, A.M.M.; Kašuba, V.; Milić, M.; et al. Effects of the chloro-s-triazine herbicide terbuthylazine on DNA integrity in human and mouse cells. Environ. Sci. Pollut. Res. 2018, 25, 19065–19081. [Google Scholar] [CrossRef] [PubMed]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Aitbali, Y.; Ba-M’hamed, S.; Elhidar, N.; Nafis, A.; Soraa, N.; Bennis, M. Glyphosate based-herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol. Teratol. 2018, 67, 44–49. [Google Scholar] [CrossRef]
- Peillex, C.; Pelletier, M. The impact and toxicity of glyphosate and glyphosate-based herbicides on health and immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef]
- Ridano, M.E.; Racca, A.C.; Flores-Martín, J.B.; Reyna, L.; Genti-Raimondi, S.; Panzetta-Dutari, G.M. Effect of Chlorpyrifos on human extravillous-like trophoblast cells. Reprod. Toxicol. 2019, 90, 118–125. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Dawood, M.A.; Elbadawy, M.; Aleya, L.; Alkahtani, S. Spirulina platensis reduced oxidative damage induced by chlorpyrifos toxicity in Nile tilapia (Oreochromis niloticus). Animals 2020, 10, 473. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; Freire AF, P.A.; Zanuncio, J.C.; Bozdoğan HSerrão, J.E. Toxicity and cytotoxicity of the insecticide imidacloprid in the midgut of the predatory bug, Podisus nigrispinus. Ecotoxicol. Environ. Saf. 2019, 167, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.P.; Li, J.W.; Yang, F.W.; Yin, X.F.; Ren, F.Z.; Fang, B.; Pang, G.F. Spermiogenesis Toxicity of Imidacloprid in Rats, Possible Role of CYP3A4. Chemosphere 2021, 282, 131120. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Gawarammana, I.; Robertson, T.A.; Roberts, M.S.; Palangasinghe, C.; Zawahir, S.; Jayamanne, S.; Kandasamy, J.; Eddleston, M.; Buckley, N.A.; et al. Acute human self-poisoning with imidacloprid compound: A neonicotinoid insecticide. PLoS ONE 2009, 4, e5127. [Google Scholar] [CrossRef] [PubMed]
- Bownik, A.; Kowalczyk, M.; Banczerowskim, J. Lambda-cyhalothrin affects swimming activity and physiological responses of Daphnia magna. Chemosphere 2019, 216, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.M.; Mostakim, G.M.; Moniruzzaman, M.; Rahman, U.O.; Islam, M.S. Distortion of micronuclei and other peripheral erythrocytes caused by fenitrothion and their recovery assemblage in zebrafish. Toxicol. Rep. 2021, 8, 415–421. [Google Scholar] [CrossRef]
- Oiwa, M.; Yamaguchi, K.; Hayashi, H.; Saitoh, T. Rapid sorption of fenitrothion on didodecyldimethylammonium bromide-montmorillonite organoclay followed by the degradation into less toxic 3-methyl-4-nitrophenolate. J. Environ. Chem. Eng. 2020, 8, 104000. [Google Scholar] [CrossRef]
- Wang, D.; Naito, H.; Nakajima, T. The Toxicity of Fenitrothion and Permethrin; IntechOpen: London, UK, 2012; pp. 85–98. [Google Scholar]
- Varga, M.; Žurga, P.; Brusić, I.; Horvatić, J.; Moslavac, M. Growth inhibition and recovery patterns of common duckweed Lemna minor L. after repeated exposure to isoproturon. Ecotoxicology 2020, 29, 1538–1551. [Google Scholar] [CrossRef]
- Varga, M.; Horvatić, J.; Žurga, P.; Brusić, I.; Moslavac, M. Phytotoxicity assessment of isoproturon on growth and physiology of non-targeted aquatic plant Lemna minor L.—A comparison of continuous and pulsed exposure with equivalent time-averaged concentrations. Aquat. Toxicol. 2019, 213, 105225. [Google Scholar] [CrossRef]
- Grizard, G.; Ouchchane, L.; Roddier, H.; Artonne, C.; Sion, B.; Vasson, M.P.; Janny, L. In vitro alachlor effects on reactive oxygen species generation, motility patterns and apoptosis markers in human spermatozoa. Reprod. Toxicol. 2007, 23, 55–62. [Google Scholar] [CrossRef]
- Kim, H.; Wang, H.; Abassi, S.; Ki, J.S. The herbicide alachlor severely affects photosystem function and photosynthetic gene expression in the marine dinoflagellate Prorocentrum minimum. J. Environ. Sci. Health–B 2020, 55, 620–629. [Google Scholar] [CrossRef]
- Sh, G.; Shabestani Monfared, A.; Zabihi, E.; Khoshbin Khoshnazar, A.; Asadi, J.; Abedian, Z.; Borzoueisileh, S. Changes in the radiation toxicity of human lymphoblastic t-cell line (Jurkat) by a common pesticide: Diazinon. J. Biomed. Phys. Eng. 2020, 10, 147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Luo, S.M.; Ma, J.Y.; Shen, W.; Yin, S. Cytotoxicity and DNA damage caused from diazinon exposure by inhibiting the PI3K-AKT pathway in porcine ovarian granulosa cells. J. Agric. Food Chem. 2018, 67, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Saraei, F.; Sadraie, S.H.; Kaka, G.R.; Sadoughi, M.; Afzal Nejad, N.; Sarahian, N. Effects of maternal diazinon exposure on frontal cerebral cortical development in mouse embryo. Int. J. Neurosci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.D.; Hsu, L.S.; Chien, C.C.; Chen, S.C. Proteomic analysis of ametryn toxicity in zebrafish embryos. Environ. Toxicol. 2018, 33, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Cancian, G.; Neodini, D.N.; Mano, D.R.; Capucho, C.; Predes, F.S.; Barbieri, R.; Oliveira, C.A.; Pigoso, A.A.; Dolder, H.; et al. Toxicological evaluation of ametryn effects in Wistar rats. Exp. Toxicol. Pathol. 2015, 67, 525–532. [Google Scholar] [CrossRef]
- Shadegan, M.R.; Banaee, M. Effects of dimethoate alone and in combination with Bacilar fertilizer on oxidative stress in common carp, Cyprinus carpio. Chemosphere 2018, 208, 101–107. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Taheri, S.; Hedayatzadeh, F. Sub-lethal effects of dimethoate alone and in combination with cadmium on biochemical parameters in freshwater snail, Galba truncatula. Comp. Biochem. Physiol. Part-C: Toxicol. Pharmacol. 2019, 220, 62–70. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Xiong, J.; Mehler, W.T.; You, J. Developmental toxicity of a neonicotinoid insecticide, acetamiprid to zebrafish embryos. J. Agric. Food Chem. 2019, 67, 2429–2436. [Google Scholar] [CrossRef]
- Han, W.; Yang, Y.; Gao, J.; Zhao, D.; Ren, C.; Wang, S.; Zhao, S.; Zhong, Y. Chronic toxicity and biochemical response of Apis cerana cerana (Hymenoptera: Apidae) exposed to acetamiprid and propiconazole alone or combined. Ecotoxicology 2019, 28, 399–411. [Google Scholar] [CrossRef]
- Silva CD, L.; Brennan, N.; Brouwer, J.M.; Commandeur, D.; Verweij, R.A.; van Gestel, C.A. Comparative toxicity of imidacloprid and thiacloprid to different species of soil invertebrates. Ecotoxicology 2017, 26, 555–564. [Google Scholar] [CrossRef]
- Alarcan, J.; Waizenegger, J.; Solano MD, L.M.; Lichtenstein, D.; Luckert, C.; Peijnenburg, A.; Stoopen, G.; Sharma, R.P.; Kumar, V.; Marx-Stoeltingb, P.; et al. Hepatotoxicity of the pesticides imazalil, thiacloprid and clothianidin–Individual and mixture effects in a 28-day study in female Wistar rats. Food Chem. Toxicol. 2020, 140, 111306. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Block, K.; Lau, P.; Gribaldo, L.; Pardo, C.A.; Barreras, P.; Smirnova, L.; Wiersma, D.; Zhao, L.; Harris, G.; et al. Rotenone exerts developmental neurotoxicity in a human brain spheroid model. Toxicol. Appl. Pharmacol. 2018, 354, 101–114. [Google Scholar] [CrossRef]
- Piotrowska, A.; Syguda, A.; Wyrwas, B.; Chrzanowski, Ł.; Heipieper, H.J. Toxicity evaluation of selected ammonium-based ionic liquid forms with MCPP and dicamba moieties on Pseudomonas putida. Chemosphere 2017, 167, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Salvo, L.M.; Malucelli MI, C.; da Silva JR, M.; Alberton, G.C.; Silva De Assis, H.C. Toxicity assessment of 2, 4-D and MCPA herbicides in primary culture of fish hepatic cells. J. Environ. Sci. Health-B 2015, 50, 449–455. [Google Scholar] [CrossRef]
- Piotrowska, A.; Syguda, A.; Chrzanowski, Ł.; Heipieper, H.J. Toxicity of synthetic herbicides containing 2, 4-D and MCPA moieties towards Pseudomonas putida mt-2 and its response at the level of membrane fatty acid composition. Chemosphere 2016, 144, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, M.; Liu, J.; Wang, X.; Wang, C.; Ai, W.; Chen, S.; Wang, H. Combined toxicity of triclosan, 2, 4-dichlorophenol and 2, 4, 6-trichlorophenol to zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2018, 57, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Zhu, H.; Hu, P.; Zhao, Q. Genotoxic effect of 2,4,6-trichlorophenol on P53 gene in zebrafish liver. Environ. Toxicol. Chem. 2009, 28, 603–608. [Google Scholar] [CrossRef]
- Hegde, S.; Poojary, K.K.; Rasquinha, R.; Crasta, D.N.; Gopalan, D.; Mutalik, S.; Siddiqui, S.; Adiga, S.K.; Kalthur, G. Epigallocatechin-3-gallate (EGCG) protects the oocytes from methyl parathion-induced cytoplasmic deformities by suppressing oxidative and endoplasmic reticulum stress. Pestic. Biochem. Phys. 2020, 167, 104588. [Google Scholar] [CrossRef]
- Edwards, F.L.; Yedjou, C.G.; Tchounwou, P.B. Involvement of oxidative stress in methyl parathion and parathion-induced toxicity and genotoxicity to human liver carcinoma (HepG2) cells. Environ. Toxicol. 2013, 28, 342–348. [Google Scholar] [CrossRef]
- Urióstegui-Acosta, M.; Tello-Mora, P.; de Jesús Solís-Heredia, M.; Ortega-Olvera, J.M.; Piña-Guzmán, B.; Martín-Tapia, D.; González-Mariscal, L.; Quintanilla-Vega, B. Methyl parathion causes genetic damage in sperm and disrupts the permeability of the blood-testis barrier by an oxidant mechanism in mice. Toxicology 2020, 438, 152463. [Google Scholar] [CrossRef]
- Wilson-Frank, C. Proteomics in Biomarkers of Chemical Toxicity. In Biomarkers in Toxicology; Kishor, K., Sahu, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1153–1163. [Google Scholar] [CrossRef]
- Salla GB, F.; Bracht, L.; de Sá-Nakanishi, A.B.; Parizotto, A.V.; Bracht, F.; Peralta, R.M.; Bracht, A. Distribution, lipid-bilayer affinity and kinetics of the metabolic effects of dinoseb in the liver. Toxicol. Appl. Pharmacol. 2017, 329, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Pires, I.; Rodrigues, P. Teratological effects of pesticides in vertebrates: A review. J. Environ. Sci. Health-B 2020, 55, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Anbumani, S.; Mohankumar, M.N. Cytogenotoxicity assessment of monocrotophos and butachlor at single and combined chronic exposures in the fish Catla catla (Hamilton). Environ. Sci. Pollut. Res. 2015, 22, 4964–4976. [Google Scholar] [CrossRef]
- Tripathi, V.K.; Kumar, V.; Pandey, A.; Vatsa, P.; Dhasmana, A.; Singh, R.P.; Appikonda SH, C.; Hwang, I.; Lohani, M. Monocrotophos induces the expression of xenobiotic metabolizing cytochrome P450s (CYP2C8 and CYP3A4) and neurotoxicity in human brain cells. Mol. Neurobiol. 2017, 54, 3633–3651. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Xu, J.; You, X.; Chen, D.; Wang, F.; Li, Y. Biochemical and genetic toxicity of dinotefuran on earthworms (Eisenia fetida). Chemosphere 2017, 176, 156–164. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; Li, Y.; Wang, X.; Wang, F. Enantioselective bioaccumulation and toxicity of the neonicotinoid insecticide dinotefuran in earthworms (Eisenia fetida). J. Agric. Food Chem. 2018, 66, 4531–4540. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zeng, T.; Li, W.; Yang, L.; Guo, B. Accumulation and toxicity of thiamethoxam and its metabolite clothianidin to the gonads of Eremias argus. Sci. Total Environ. 2019, 667, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Addy-Orduna, L.M.; Brodeur, J.C.; Mateo, R. Oral acute toxicity of imidacloprid, thiamethoxam and clothianidin in eared doves: A contribution for the risk assessment of neonicotinoids in birds. Sci. Total Environ. 2019, 650, 1216–1223. [Google Scholar] [CrossRef]
- Şekeroğlu, Z.A.; Şekeroğlu, V.; Aydın, B.; Yedier, S.K.; Ilkun, E. Clothianidin induces DNA damage and oxidative stress in bronchial epithelial cells. Environ. Mol. Mutagen. 2020, 61, 647–655. [Google Scholar] [CrossRef]
- Saraiva, A.S.; Sarmento, R.A.; Rodrigues, A.C.; Campos, D.; Fedorova, G.; Žlábek, V.; Gravato, C.; Pestana JL, T.; Soares, A.M. Assessment of thiamethoxam toxicity to Chironomus riparius. Ecotoxicol. Environ. Saf. 2017, 137, 240–246. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, X.; Wang, J.; Zhu, L.; Wang, J.; Wang, G.; Chen, Y.; Kim, Y.M. Oxidative stress and genotoxicity of nitenpyram to earthworms (Eisenia foetida). Chemosphere 2021, 264, 128493. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, J.; Zhu, L.; Chen, A.; Wang, J. Toxic effects of nitenpyram on antioxidant enzyme system and DNA in zebrafish (Danio rerio) livers. Ecotoxicol. Environ. Saf. 2015, 122, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zheng, S.; Zheng, Y.G. Polymer Matrix Composites and Technology, 1st ed.; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- da Rosa Salles, T.; de Bitencourt Rodrigues, H.; da Silva Bruckmann, F.; Alves LC, S.; Mortari, S.R.; Rhoden CR, B. Graphene oxide optimization synthesis for application on laboratory of Universidade Franciscana. Discip. Sci. Sér. Ciên. Nat. Tecnol. 2020, 21, 15–26. [Google Scholar] [CrossRef]
- Oviedo, L.R.; Muraro PC, L.; Pavoski, G.; Espinosa DC, R.; Ruiz YP, M.; Galembeck, A.; Rhoden CR, B.; Silva, W.L. Synthesis and characterization of nanozeolite from (agro)industrial waste for application in heterogeneous photocatalysis. Environ. Sci. Pollut. Res. 2021, 28. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Geilich, B.M.; Webster, T.J. Synthesis, characterization, and antimicrobial activity of an ampicillin-conjugated magnetic nanoantibiotic for medical applications. Int. J. Nanomedicine. 2014, 9, 3801–3814. [Google Scholar] [CrossRef]
- da Rosa Salles, T.; da Silva Bruckamann, F.; Viana, A.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. J. Polym. Environ. 2022, 30, 2695–2713. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Camargo PH, C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Ayekoe, C.Y.P.; Robert, D.; Lanciné, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbo River (Ivory-Coast). Catal Today 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Cross, A.; Miller, J.T.; Danghyan, V.; Mukasyan, A.S.; Wolf, E.E. Highly active and stable Ni-Cu supported catalysts prepared by combustion synthesis for hydrogen production from ethanol. Appl. Cat. A Gen. 2019, 572, 124–133. [Google Scholar] [CrossRef]
- Ziyu, L.; Zhigang, J.; Wenwen, L.; Jianhong, L.; Shan, J.; Shengbiao, L.; Rongsun, Z. Synthesis of Ag/AgCl nanoparticles immobilized on CoFe2O4 fibers and their photocatalytic degradation for methyl orange. Rare Met. Mater. Eng. 2017, 46, 3669–3674. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Nazarkovsky, M.; Farah, S.; Pawar, R.P.; Basu, A.; Domb, A.J. Nanotechnology for water purification: Applications of nanotechnology methods in wastewater treatment. Water Purif. 2017, 1, 33–74. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Hushmandrad, S. Solar photodecolorization of methylene blue by CuO/X zeolite as a heterogeneous catalyst. Appl. Catal. A-Gen. 2010, 388, 149–159. [Google Scholar] [CrossRef]
- Sadiqab, H.; Sher, F.; Sehar, S.; Lima, E.C.; Zhang, S.; Iqbal HM, N.; Zafar, F.; Nuhanovic’, M. Green synthesis of ZnO nanoparticles from Syzygium Cumini leaves extract with robust photocatalysis applications. J. Mol. Liq. 2021, 335, 1–48. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, V.; Jolly, S.S.; Kim, K.H.; Rawat, M.; Kukkar, D.; Tsang, Y.F. Biogenic synthesis of silver nanoparticles and its photocatalytic applications for removal of organic pollutants in water. J. Ind. Eng. Chem. 2019, 80, 247–257. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Tolosana-Moranchel, Á.; Duran-Valle, C.; Faraldos, M.; Bahamonde, A. Optimizing P25-rGO composites for pesticides degradation: Elucidation of photo-mechanism. Catal. Today 2019, 328, 172–177. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Singh, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Nguyen, V.H. Highly effective degradation of imidacloprid by H2O2/fullerene decorated P-doped g-C3N4 photocatalyst. J. Environ. Chem. Eng. 2020, 8, 104483. [Google Scholar] [CrossRef]
- Zangiabadi, M.; Saljooqi, A.; Shamspur, T.; Mostafavi, A. Evaluation of GO nanosheets decorated by CuFe2O4 and CdS nanoparticles as photocatalyst for the degradation of dinoseb and imidacloprid pesticides. Ceram. Int. 2020, 46, 6124–6128. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Taher, M.A.; Tamaddon, A. Facile synthesis and characterization of magnetic nanocomposite ZnO/CoFe2O4 hetero-structure for rapid photocatalytic degradation of imidacloprid. Heliyon 2019, 5, e02870. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Nikitha, M.; Ramkumar, K.; Meenakshi, S. Immobilization of MIL-88 (Fe) anchored TiO2-chitosan (2D/2D) hybrid nanocomposite for the degradation of organophosphate pesticide: Characterization, mechanism and degradation intermediates. J. Hazard. Mater. 2021, 406, 124728. [Google Scholar] [CrossRef]
- Boruah, P.K.; Das, M.R. Dual responsive magnetic Fe3O4-TiO2/graphene nanocomposite as an artificial nanozyme for the colorimetric detection and photodegradation of pesticide in an aqueous medium. J. Hazard. Mater. 2020, 385, 121516. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, M.A.; Butt, K.M.; Chong, K.K.; Chang, W.S. Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2-SiO2 nanocomposite and photophysical properties. J. Saudi Chem. Soc. 2019, 23, 561–575. [Google Scholar] [CrossRef]
- Rashidimoghaddam, M.; Saljooqi, A.; Shamspur, T.; Mostafavi, A. Constructing S-doped Ni–Co LDH intercalated with Fe3O4 heterostructure photocatalysts for enhanced pesticide degradation. New J. Chem. 2020, 44, 15584–15592. [Google Scholar] [CrossRef]
- Soltani-Nezhad, F.; Saljooqi, A.; Mostafavi, A.; Shamspur, T. Synthesis of Fe3O4/CdS–ZnS nanostructure and its application for photocatalytic degradation of chlorpyrifos pesticide and brilliant green dye from aqueous solutions. Ecotoxicol. Environ. Saf. 2020, 189, 109886. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Yadav, J.; Shanker, U. Green synthesis of sunlight responsive zinc oxide coupled cadmium sulfide nanostructures for efficient photodegradation of pesticides. J. Colloid Interface Sci. 2021, 601, 689–703. [Google Scholar] [CrossRef]
- Soltani-Nezhad, F.; Saljooqi, A.; Shamspur, T.; Mostafavi, A. Photocatalytic degradation of imidacloprid using GO/Fe3O4/TiO2-NiO under visible radiation: Optimization by response level method. Polyhedron 2019, 165, 188–196. [Google Scholar] [CrossRef]
- Premalatha, N.; Miranda, L.R. Surfactant modified ZnO–Bi2O3 nanocomposite for degradation of lambda-cyhalothrin pesticide in visible light: A study of reaction kinetics and intermediates. J. Environ. Manag. 2019, 246, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, M.K.; Kaur, S.; Pal, B.; Singh, S. Morphological influence of ZnO nanostructures and their Cu loaded composites for effective photodegradation of methyl parathion. Solid State Sci. 2020, 99, 106045. [Google Scholar] [CrossRef]
- Liu, X.; Zong, H.; Tan, X.; Wang, X.; Qiu, J.; Kong, F.; Fang, S. Facile synthesis of modified carbon nitride with enhanced activity for photocatalytic degradation of atrazine. J. Environ. Chem. Eng. 2021, 9, 105807. [Google Scholar] [CrossRef]
- Nyankson, E.; Efavi, J.K.; Agyei-Tuffour, B.; Manu, G. Synthesis of TiO2–Ag3PO4 photocatalyst material with high adsorption capacity and photocatalytic activity: Application in the removal of dyes and pesticides. RSC Adv. 2021, 11, 17032–17045. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. Ternary semiconductor Znx Ag1−x S nanocomposites for efficient photocatalytic degradation of organophosphorus pesticides. Photochem. Photobiol. Sci. 2018, 17, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.K.; Kataria, J.; Bhardwaj, V.K.; Sharma, S. Green biomimetic preparation of efficient Ag–ZnO heterojunctions with excellent photocatalytic performance under solar light irradiation: A novel biogenic-deposition-precipitation approach. Nanoscale Adv. 2019, 1, 1035–1044. [Google Scholar] [CrossRef]
- John, E.M.; Shaike, J.M. Chlorpyrifos: Pollution and remediation. Environ. Chem. Lett. 2015, 13, 269–291. [Google Scholar] [CrossRef]
- Wu, B.; Arnold, W.A.; Ma, L. Photolysis of atrazine: Role of triplet dissolved organic matter and limitations of sensitizers and quenchers. Water Res. 2020, 190, 1–44. [Google Scholar] [CrossRef]
- Sumon, K.A.; Ritika, A.K.; Peeters, E.T.; Rashid, H.; Bosma, R.H.; Rahman, M.S.; Fatema, M.K.; Van den Brink, P.J. Effects of imidacloprid on the ecology of sub-tropical freshwater microcosms. Environ. Pollut. 2018, 236, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Zabihi-Mobarakeh, H.; Nezamzadeh-Ejhieh, A. Application of supported TiO2 onto iranian clinoptilolite nanoparticles in the photodegradation of mixture of aniline and 2,4-dinitroaniline aqueous solution. J. Ind. Eng. Chem. 2015, 26, 315–321. [Google Scholar] [CrossRef]
- Verma, A.; Sheoran, M.; Toor, A.P. Titanium dioxide mediated photocatalytic degradation of malathion in aqueous phase. Indian J. Chem. Technol. 2013, 20, 46–51. [Google Scholar] [CrossRef]
- Jonidi-Jafari, A.; Shirzad-Siboni, M.; Yang, J.K.; Naimi-Joubani, M.; Farrokhi, M. Photocatalytic degradation of Diazinon with illuminated ZnO-TiO2 composite. J.Taiwan Inst. Chem. Eng. 2015, 50, 100–107. [Google Scholar] [CrossRef]
- Daneshvar, A.; Khataee, R. Removal of Azo Dye C.I. Acid Red 14 from Contaminated Water using Fenton, UV/H2O2, UV/H2O2/Fe(II), UV/H2O2/Fe(III) and UV/H2O2/Fe(III)/Oxalate Processes: A Comparative Study. J. Environ. Sci. Health Part A 2006, 41, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Wanjeri VW, O.; Sheppard, C.J.; Prinsloo AR, E.; Ngila, J.C.; Ndungu, P.G. Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J. Environ. Chem. Eng. 2018, 6, 1333–1346. [Google Scholar] [CrossRef]
- Geankoplis, C.J.; Hersel, A.A.; Lepek, D.H. Transport Processes and Separation Process Principles, 5th ed.; Earson Education: New York, NY, USA, 2018. [Google Scholar]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Tien, C. Introduction of Adsorption–Basics, Analysis and Applications, 1st ed.; Book Aid International: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Da Silva Bruckmann, F.; Zuchetto, T.; Ledur, C.M.; dos Santos, C.L.; da Silva, W.L.; Fagan, S.B.; da Silva, I.Z.; Rhoden CR, B. Methylphenidate adsorption onto graphene derivatives: Theory and experiment. New J. Chem. 2022, 46, 4283–4291. [Google Scholar] [CrossRef]
- Hu, H.; Xu, K. Physicochemical technologies for HRPs and risk control. In High-Risk Pollutants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–207. [Google Scholar] [CrossRef]
- Scheufele, F.B.; Módenes, A.N.; Borba, C.E.; Ribeiro, C.; Espinoza-Quiñones, F.R.; Bergamasco, R.; Pereira, N.C. Monolayer–multilayer adsorption phenomenological model: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 2016, 284, 1328–1341. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, G.; Guo, X.; Xu, Y. Designing a novel N-doped adsorbent with ultrahigh selectivity for CO2: Waste biomass pyrolysis and two-step activation. Biomass Convers. Biorefin. 2020, 11, 2843–2854. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Zhang, Y.; Lin, T.; Zeng, G.; Zhang, S.; Wang, Y.; He, W.; Zhang, M.; Long, H. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour. Technol. 2019, 288, 121511. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Lv, X.; Li, S. Graphene Oxide–Crospolyvinylpyrrolidone Hybrid Microspheres for the Efficient Adsorption of 2,4,6-Trichlorophenol. ACS Omega 2020, 5, 18862–18871. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Nikou, M. Modeling of removal of an organophosphorus pesticide from aqueous solution by amagnetic metal-organic framework composite. Chin. J. Chem. Eng. 2021, 40, 323–335. [Google Scholar] [CrossRef]
- Nodeh, H.R.; Ibrahim WA, W.; Kamboh, M.A.; Sanagi, M.M. New magnetic graphene-based inorganic–organic sol-gel hybrid nanocomposite for simultaneous analysis of polar and non-polar organophosphorus pesticides from water samples using solid-phase extraction. Chemosphere 2017, 166, 21–30. [Google Scholar] [CrossRef]
- Boruah, P.K.; Sharma, B.; Hussain, N.; Das, M.R. Magnetically recoverable Fe3O4/graphene nanocomposite towards efficient removal of triazine pesticides from aqueous solution: Investigation of the adsorption phenomenon and specific ion effect. Chemosphere 2017, 168, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Luqman, M. Insight into carbohydrate polymers (chitosan and 2-hydroxyethyl methacrylate/methyl methacrylate) intercalated bentonite-based nanocomposites as multifunctional and environmental adsorbents for methyl parathion pesticide. Int. J. Biol. Macromol. 2021, 167, 335–344. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Xu, D.; Huang, X.; Xu, X.; Zheng, S.; Zhang, Y.; Lin, H. Metal–organic framework preparation using magnetic graphene oxide–β-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr. Polym. 2017, 175, 584–591. [Google Scholar] [CrossRef]
- Muda, M.S.; Kamari, A.; Bakar, S.A.; Yusoff SN, M.; Fatimah, I.; Phillip, E.; Din, S.M. Chitosan-graphene oxide nanocomposites as water-solubilising agents for rotenone pesticide. J. Mol. Liq. 2020, 318, 114066. [Google Scholar] [CrossRef]
- Gámiz, B.; Hermosín, M.C.; Cornejo, J.; Celis, R. Hexadimethrine-montmorillonite nanocomposite: Characterization and application as a pesticide adsorbent. Appl. Surf. Sci. 2015, 332, 606–613. [Google Scholar] [CrossRef]
- Soulé, M.Z.; Fernández, M.A.; Montes, M.L.; Suárez-García, F.; Sánchez, R.T.; Tascón, J.M.D. Montmorillonite-hydrothermal carbon nanocomposites: Synthesis, characterization and evaluation of pesticides retention for potential treatment of agricultural wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124192. [Google Scholar] [CrossRef]
- Dehaghi, S.M.; Rahmanifar, B.; Moradi, A.M.; Azar, P.A. Removal of permethrin pesticide from water by chitosan–zinc oxide nanoparticles composite as an adsorbent. J. Saudi Chem. Soc. 2014, 18, 348–355. [Google Scholar] [CrossRef]
- Gupta, K.; Kumar, V.; Tikoo, K.B.; Kaushik, A.; Singhal, S. Encrustation of cadmium sulfide nanoparticles into the matrix of biomass derived silanized cellulose nanofibers for adsorptive detoxification of pesticide and textile waste. Chem. Eng. J. 2020, 385, 123700. [Google Scholar] [CrossRef]
- Qiu, Y.; Xiao, X.; Cheng, H.; Zhou, Z.; Sheng, G.D. Influence of environmental factors on pesticide adsorption by black carbon: pH and model dissolved organic matter. Environ. Sci. Technol. 2009, 43, 4973–4978. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigm. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Seczkowska, M.; Deryło-Marczewska, A.; Blachnio, M. Adsorption equilibrium and kinetics of selected phenoxyacid pesticides on activated carbon: Effect of temperature. Adsorption 2016, 22, 777–790. [Google Scholar] [CrossRef]
- Doğan, M.; Alkan, M.; Demirbaş, Ö.; Özdemir, Y.; Özmetin, C. Adsorption kinetics of maxilon blue GRL onto sepiolite from aqueous solutions. Chem. Eng. J. 2006, 124, 89–101. [Google Scholar] [CrossRef]
- Calisto, J.S.; Pacheco, I.S.; Freitas, L.L.; Santana, L.K.; Fagundes, W.S.; Amaral, F.A.; Canobre, S.C. Adsorption kinetic and thermodynamic studies of the 2, 4–dichlorophenoxyacetate (2, 4-D) by the [Co–Al–Cl] layered double hydroxide. Heliyon 2019, 5, e02553. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Shu, X.; Fang, H.; Sun, X.; Pan, Y.; Ma, L. Equilibrium, kinetic and thermodynamic studies on the adsorption of atrazine in soils of the water fluctuation zone in the Three-Gorges Reservoir. Environ. Sci. Eur. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment–Fundamentals, Processes, and Modelling, 1st ed.; Walter De Gruyter GmbH & Co. KG: Berlin, Germany, 2012. [Google Scholar]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.; McKay, G. Adsorption of acid dyes on chitosan—Equilibrium isotherm analyses. Process. Biochem. 2004, 39, 695–704. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.d. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef]
- Kamga, F.T. Modeling adsorption mechanism of paraquat onto Ayous (Triplochiton scleroxylon) wood sawdust. Appl. Water Sci. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Travis, C.L.; Etnier, E.L. A Survey of Sorption Relationships for Reactive Solutes in Soil. J. Env. Qual 1981, 10, 8–17. [Google Scholar] [CrossRef]
- Çeleb, O.; Üzüm, Ç.; Shahwan, T.; Erten, H.N. A radiotracer study of the adsorption behavior of aqueous Ba2+ ions on nanoparticles of zero-valent iron. J. Hazard. Mat. 2007, 148, 761–767. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Özacar, M.; Sengil, I.A. A kinetic study of metal complex dye sorption onto pine sawdust. Process. Biochem. 2005, 40, 565–572. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).