Valorisation of Lignocellulosic Wastes, the Case Study of Eucalypt Stumps Lignin as Bioadsorbent for the Removal of Cr(VI)
Abstract
1. Introduction
2. Results and Discussion
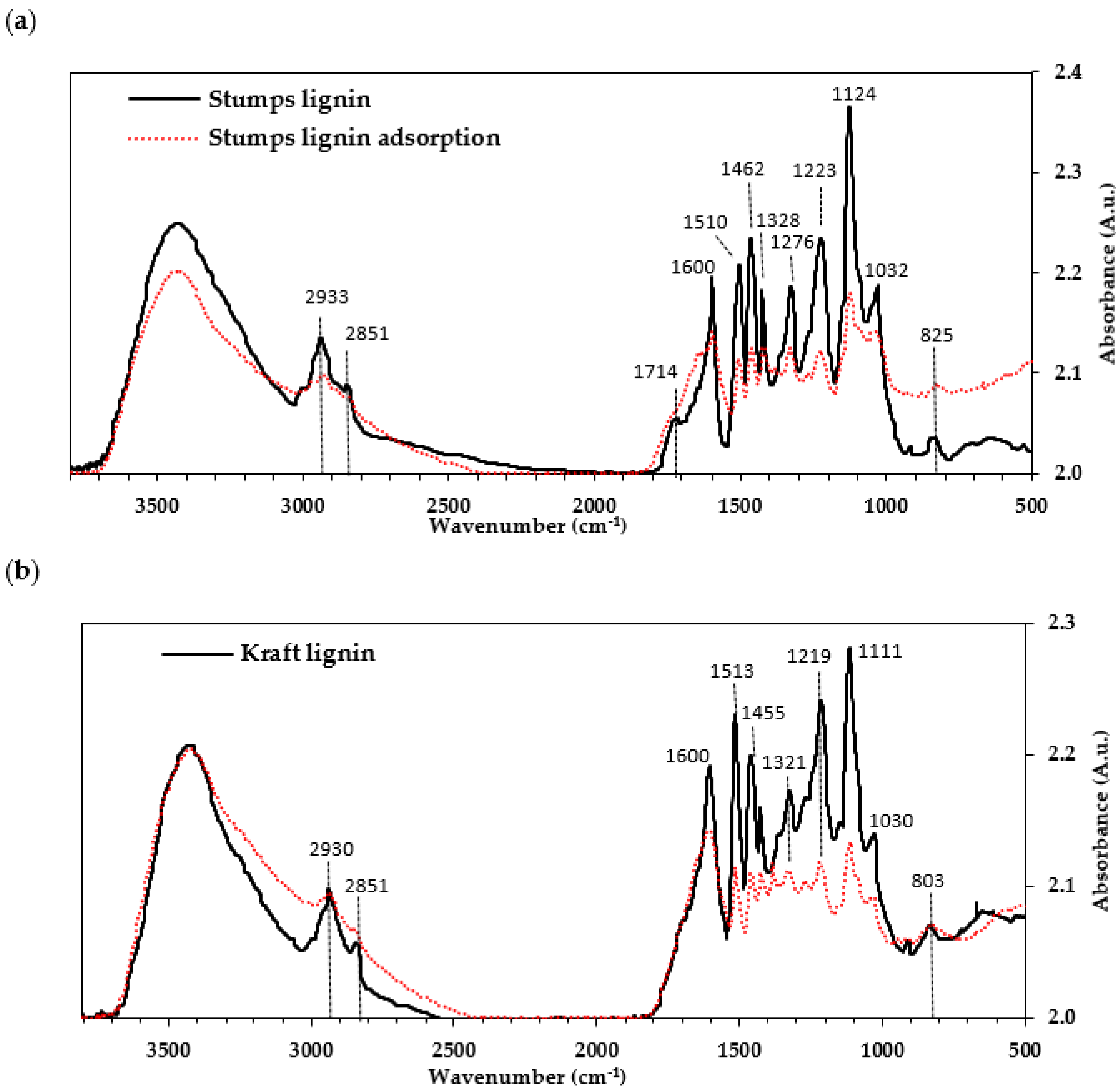
2.1. Lignin Characterisation
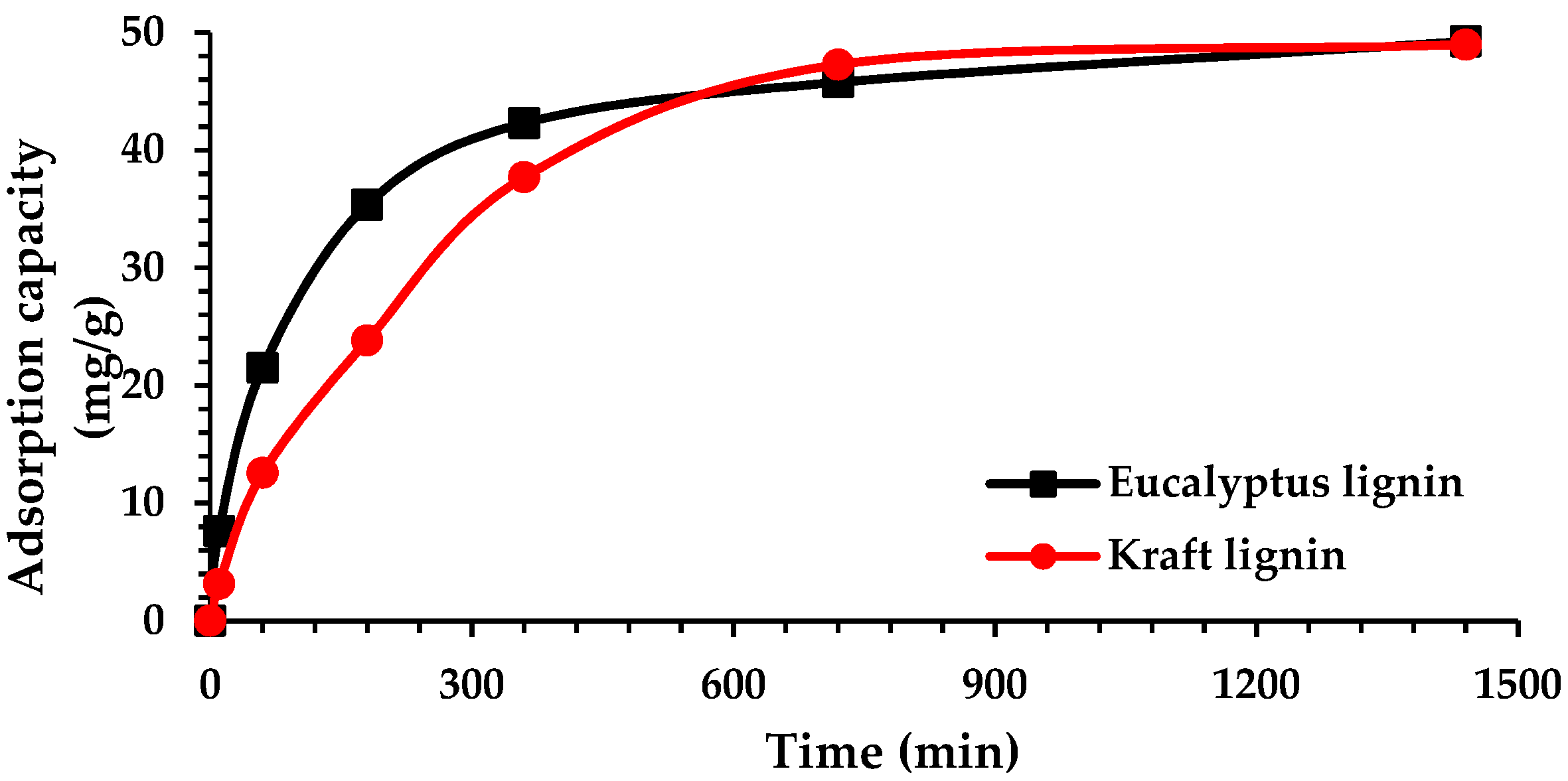
2.2. Adsorption Kinetics
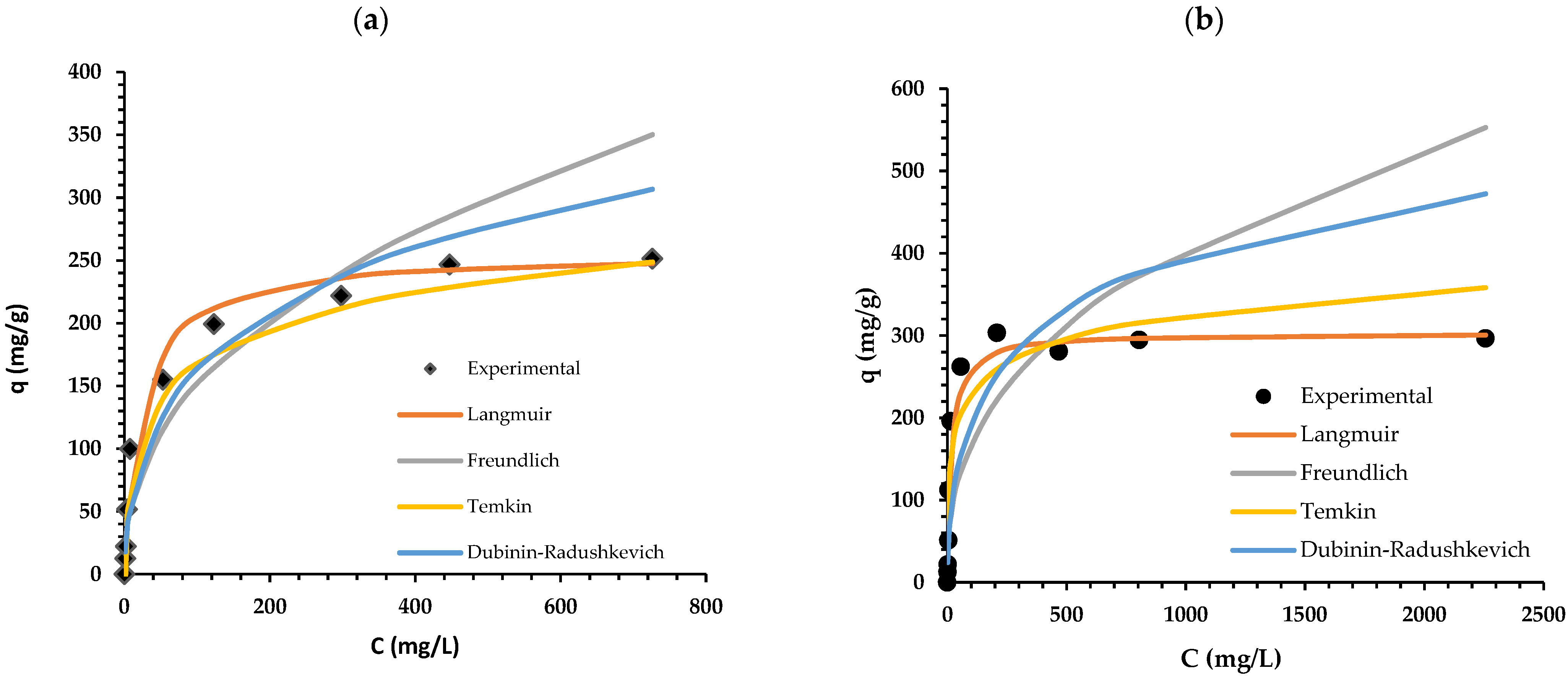
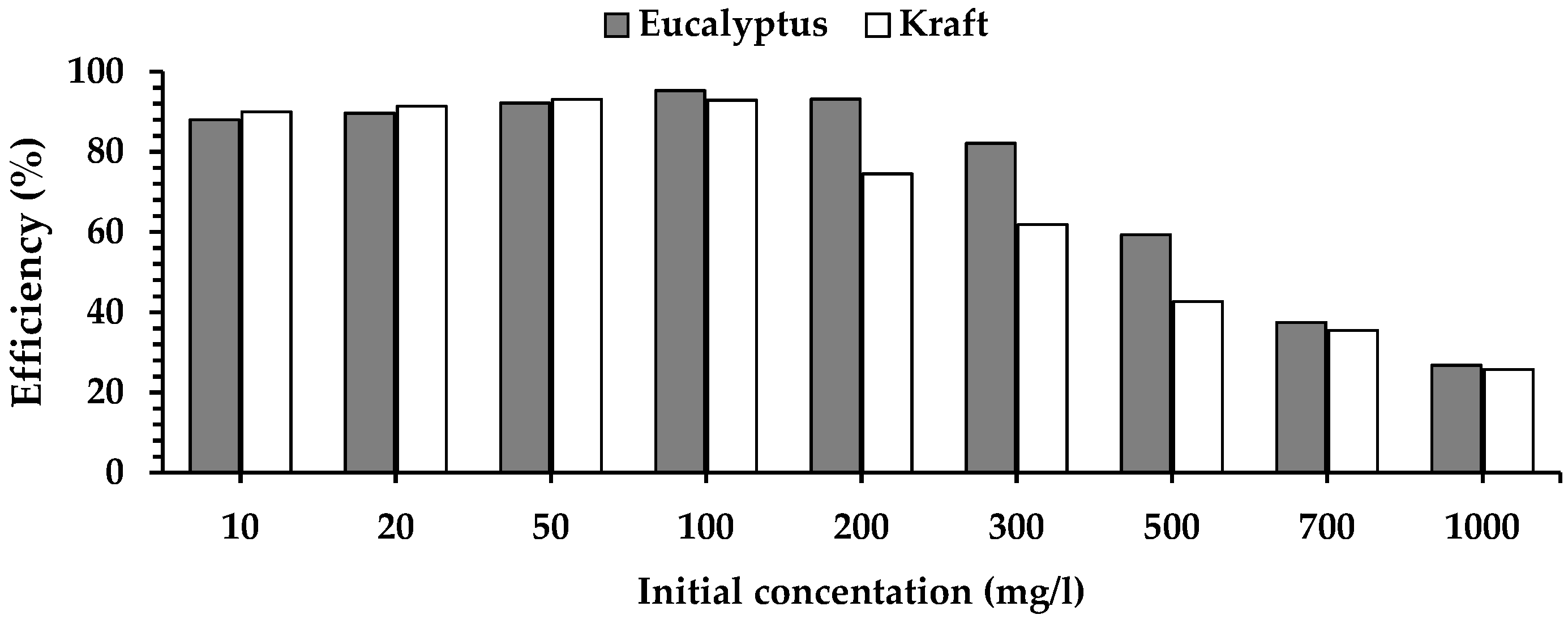
2.3. Isotherm Study
3. Materials and Methods
3.1. Eucalypt Stump Lignin
3.2. Kraft Lignin
3.3. Characterisation of the Lignins
3.3.1. Analytical Pyrolysis (Py-GC/MS)
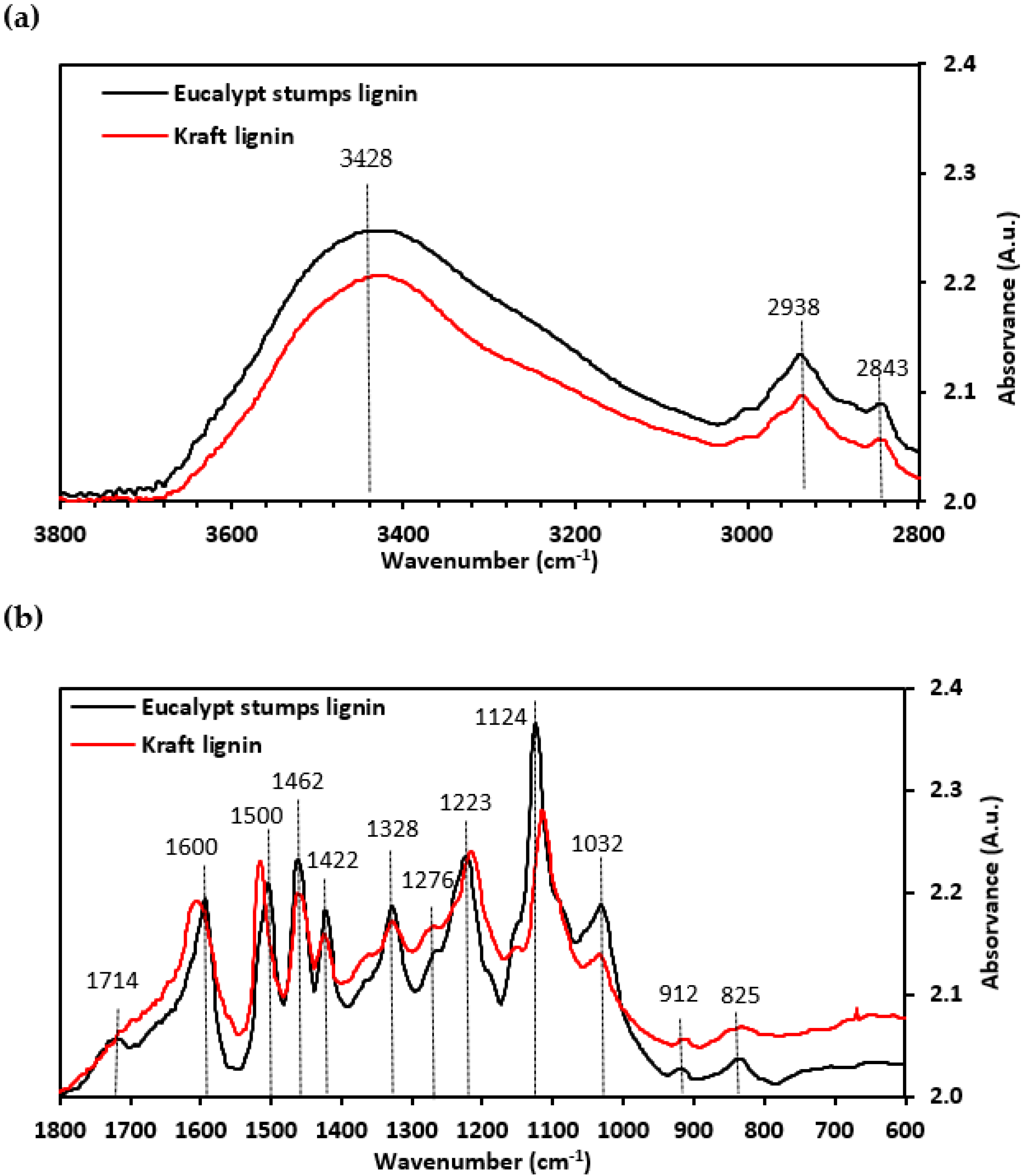
3.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
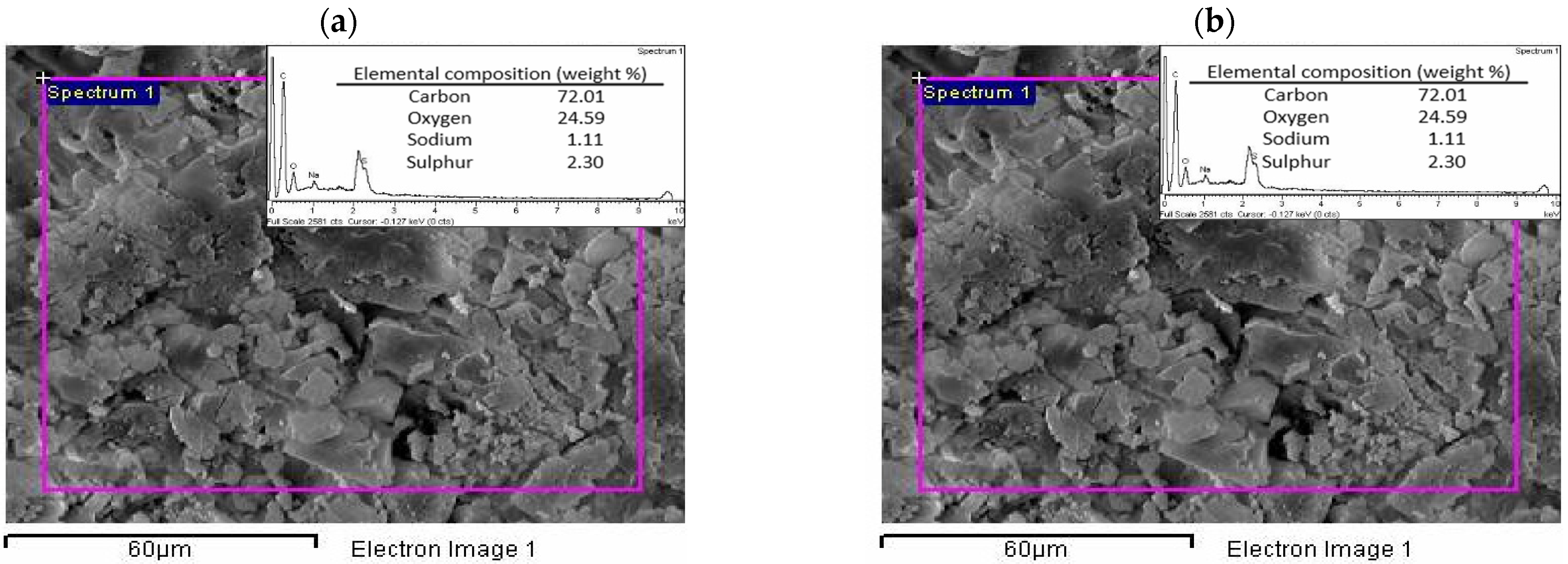
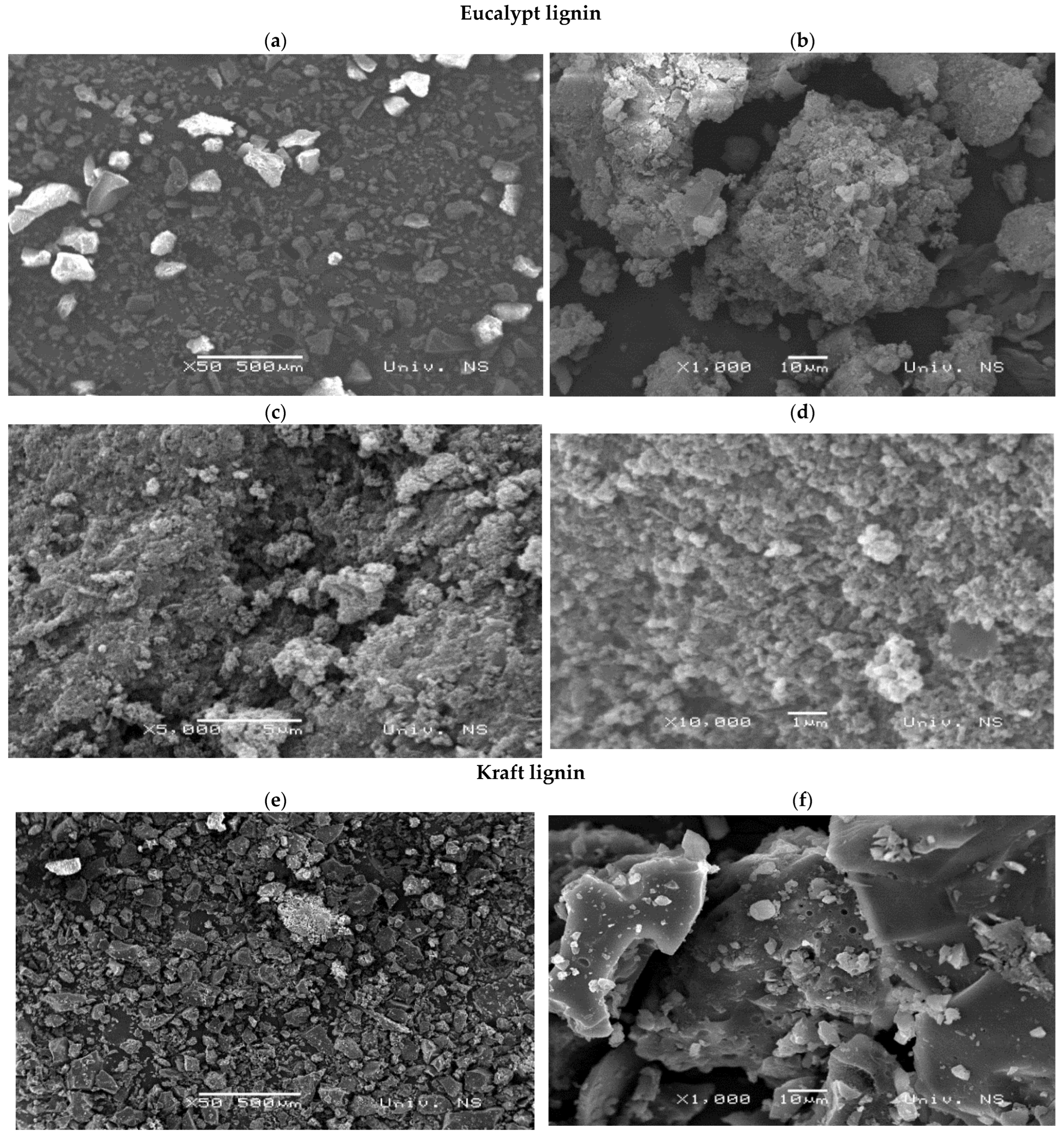
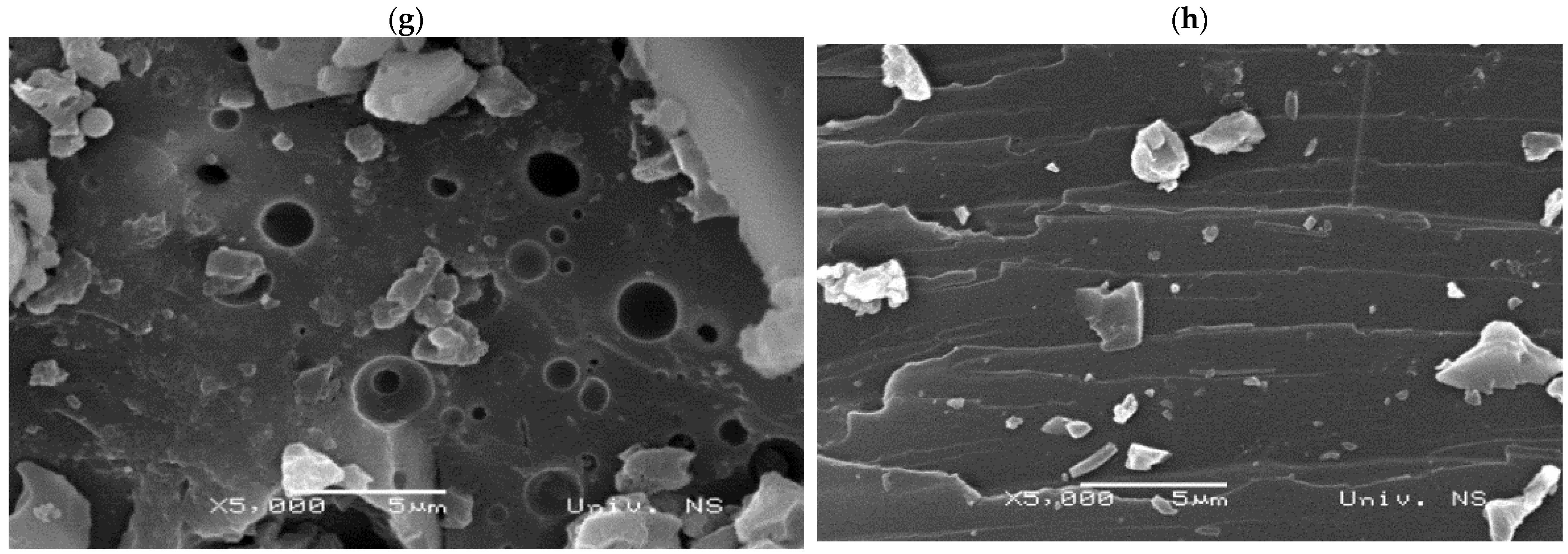
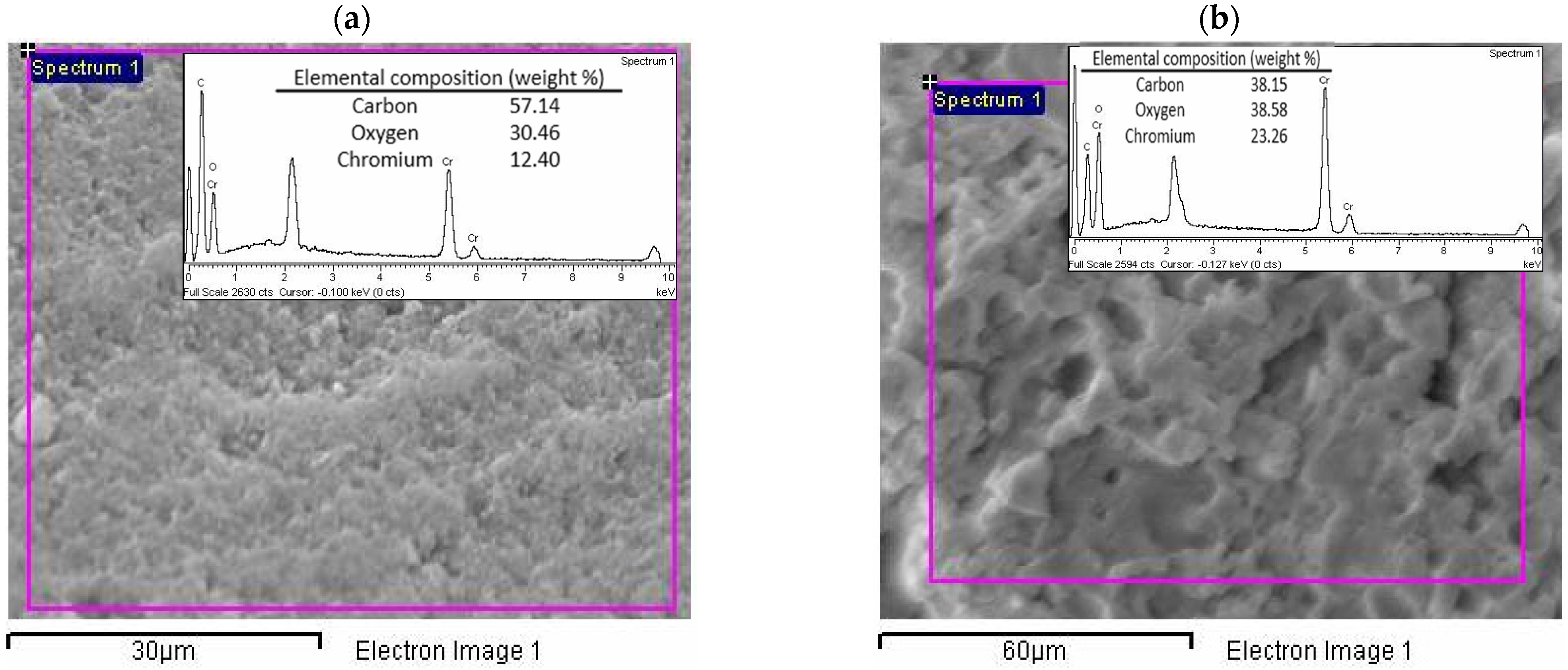
3.3.3. Elemental Analysis, Scanning Electron Microscopy (SEM), and Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
3.3.4. Determination of the Point of Zero Charge (pHpzc)
3.4. Batch Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bortoluz, J.; Cemin, A.; Bonetto, L.R.; Ferrarini, F.; Esteves, V.I.; Giovanela, M. Isolation, characterization and valorization of lignin from Pinus elliottii sawdust as a low-cost biosorbent for zinc removal. Cellulose 2019, 26, 4895–4908. [Google Scholar] [CrossRef]
- Lourenço, A.; Pereira, H. Compositional Variability of Lignin in Biomass. In Lignin—Trends and Applications; Poletto, M., Ed.; InTechOpen: London, UK, 2018; Chapter 3; pp. 65–98. ISBN 978-953-51-3901-0. Available online: https://www.intechopen.com/books/lignin-trends-and-applications (accessed on 23 August 2022).
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods and applications. New J. Chem. 2020, 45, 6971–7392. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; Xu, F.; Sun, R.-C. Role of lignin in a biorefinery: Separation characterization and valorization. J. Chem. Technol. Biotechnol. 2012, 88, 346–352. [Google Scholar] [CrossRef]
- Harmita, H.; Karthikeyan, K.; Pan, X. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Hübner, A.; Wiltowski, T.S. Chromium Adsorption by Lignin. Energy Sources 2000, 22, 45–56. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Wiltowski, T.S.; Murphy, D.; Lalvani, L.S. Metal Removal from Process Water by Lignin. Environ. Technol. 1997, 18, 1163–1168. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin—A biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef]
- Šćiban, M.B.; Klašnja, M.T.; Antov, M.G. Study of the biosorption of different heavy metal ions onto Kraft lignin. Ecol. Eng. 2011, 37, 2092–2095. [Google Scholar] [CrossRef]
- Cemin, A.; Ferrarini, F.; Poletto, M.; Bonetto, L.R.; Bortoluz, J.; Lemée, L.; Guégan, R.; Esteves, V.I.; Giovanela, M. Characterization and use of a lignin sample extracted from Eucalyptus grandis sawdust for the removal of methylene blue dye. Int. J. Biol. Macromol. 2021, 170, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, H. Industrial lignins: The potential for efficient removal of Cr(VI) from wastewater. Environ. Sci. Pollut. Res. 2022, 29, 10467–10481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, Y.; Niu, Y.; Dong, X.; Lou, H.; Zhou, H. Lignosulfonate/N-butylaniline hollow microspheres for the removal of Cr(VI): Fabrication, adsorption isotherm and kinetics. J. Water Process Eng. 2022, 46, 102588. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, H. A novel nitrogen-doped porous car bon derived from black liquor for efficient removal of Cr(VI) and tetracycline: Comparison with lignin porous carbon. J. Clean. Prod. 2022, 333, 130106. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Netzahuatl-Muñoz, A.R.; Cristiani-Urbina, M.D.C.; Cristiani-Urbina, E. Chromium Biosorption from Cr(VI) Aqueous Solutions by Cupressus lusitanica Bark: Kinetics, Equilibrium and Thermodynamic Studies. PLoS ONE 2015, 10, e0137086. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; Ortega-Toro, R.; Mancilla-Bonilla, H.; Espinoza-León, F. Potential Use of Residual Sawdust of Eucalyptus globulus Labill in Pb (II) Adsorption: Modelling of the Kinetics and Equilibrium. Appl. Sci. 2021, 11, 3125. [Google Scholar] [CrossRef]
- Gominho, J.; Lourenço, A.; Miranda, I.; Pereira, H. Chemical and fuel properties of stumps biomass from Eucalyptus globulus plantations. Ind. Crop. Prod. 2012, 39, 12–16. [Google Scholar] [CrossRef]
- Gominho, J.; Lopes, C.; Lourenço, A.; Simões, R.; Pereira, H. Eucalyptus globulus Stumpwood as a Raw Material for Pulping. BioResources 2014, 9, 4038–4049. [Google Scholar] [CrossRef][Green Version]
- Gominho, J.; Costa, R.; Lourenco, A.; Neiva, D.; Pereira, H. The effect of different pre-treatments to improve delignification of eucalypt stumps in a biorefinery context. Bioresour. Technol. Rep. 2019, 6, 89–95. [Google Scholar] [CrossRef]
- Pinto, F.; Gominho, J.; André, R.N.; Gonçalves, D.; Miranda, M.; Varela, F.; Neves, D.; Santos, J.; Lourenço, A.; Pereira, H. Improvement of gasification performance of Eucalyptus globulus stumps with torrefaction and densification pre-treatments. Fuel 2017, 206, 289–299. [Google Scholar] [CrossRef]
- Luís, A.; Neiva, D.M.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Bioassay-guided fractionation, GC–MS identification and in vitro evaluation of antioxidant and antimicrobial activities of bioactive compounds from Eucalyptus globulus stump wood methanolic extract. Ind. Crop. Prod. 2016, 91, 97–103. [Google Scholar] [CrossRef]
- Evtuguin, D.V.; Neto, C.P.; Silva, A.M.S.; Domingues, P.M.; Amado, F.M.L.; Robert, D.; Faix, O. Comprehensive Study on the Chemical Structure of Dioxane Lignin from Plantation Eucalyptus globulus Wood. J. Agric. Food Chem. 2001, 49, 4252–4261. [Google Scholar] [CrossRef]
- Lourenço, A.; Gominho, J.; Pereira, H. Chemical Characterisation of Lignocellulosic Materials by Analytical Pyrolysis. In Analytical Pyrolysis; Kusch, P., Ed.; InTechOpen: London, UK, 2019; Chapter 2; pp. 9–30. ISBN 978-1-78984-958-5. Available online: https://www.intechopen.com/books/analytical-pyrolysis/chemical-characterization-of-lignocellulosic-materials-by-analytical-pyrolysis (accessed on 23 August 2022).
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Sun, R.-C. Structural elucidation of whole lignin from Eucalyptus based on preswelling and enzymatic hydrolysis. Green Chem. 2014, 17, 1589–1596. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.; van Dam, J.E. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crop. Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Hydrogen Bonding in Lignin: A Fourier Transform Infrared Model Compound Study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Gabov, K.; Gosselink, R.J.A.; Smeds, A.I.; Fardim, P. Characterization of Lignin Extracted from Birch Wood by a Modified Hydrotropic Process. J. Agric. Food Chem. 2014, 62, 10759–10767. [Google Scholar] [CrossRef]
- De Meester, B.; Calderón, B.M.; De Vries, L.; Pollier, J.; Goeminne, G.; Van Doorsselaere, J.; Chen, M.; Ralph, J.; Vanholme, R.; Boerjan, W. Tailoring poplar lignin without yield penalty by combining a null and haploinsufficient CINNAMOYL-CoA REDUCTASE2 allele. Nat. Commun. 2020, 11, 5020. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.; Kusch, P.; Schulze, M.; Kamm, B. Qualitative and Quantitative Analysis of Lignin Produced from Beech Wood by Different Conditions of the Organosolv Process. J. Polym. Environ. 2016, 24, 85–97. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; Taddei, A.R.; D’Annibale, A.; Romagnoli, M.; Mugnozza, G.S. Isolation and characterization of lignin from beech wood and chestnut sawdust for the preparation of lignin nanoparticles (LNPs) from wood industry side-streams. Holzforschung 2018, 72, 961–972. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2008, 7, 79–84. [Google Scholar] [CrossRef]
- Ofomaja, A.E. Intraparticle diffusion process for lead(II) biosorption onto mansonia wood sawdust. Bioresour. Technol. 2010, 101, 5868–5876. [Google Scholar] [CrossRef]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Batch nickel removal from aqueous solution by sphagnum moss peat. Water Res. 1995, 29, 1327–1332. [Google Scholar] [CrossRef]
- Zeldowitsch, J. Uber den mechanismus der katalytischen oxydationvon CO and MnO2. Acta Physicochim. 1934, 1, 364–449. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Mechanism of Cr(VI) uptake onto sagwan sawdust derived biochar and statistical optimization via response surface methodology. Biomass Convers. Biorefinery 2020, 1–17. [Google Scholar] [CrossRef]
- Vinodhini V, Das N Mechanism of Cr(VI) biosorption by neem sawdust. Am.-Eurasian J. Sci. Res. 2009, 4, 324–329.
- Suksabye, P.; Thiravetyan, P. Cr(VI) adsorption from electroplating plating wastewater by chemically modified coir pith. J. Environ. Manag. 2012, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Faix, O.; Fortman, I.; Bremer, J.; Meier, D. Thermal degradation products of wood. Gas chromatographic separation and mass spectrometric characterisation of lignin derived products. Holz als Roh-und Werkstoff 1991, 48, 281–285. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D. Pyrolysis-GC-MS characterization of forage materials. J. Agric. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- Smičiklas, I.; Milonjić, S.; Pfendt, P.; Raičević, S. The point of zero charge and sorption of cadmium (II) and strontium (II) ions on synthetic hydroxyapatite. Sep. Purif. Technol. 2000, 18, 185–194. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al-Muhtaseb, A.H.; Al-Laqtah, N.A.; Walker, G.M.; Allen, S.J.; Ahmad, M.N. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]



| Peak Number | RT | Compound | Origin | Eucalypt Lignin | Kraft Lignin |
|---|---|---|---|---|---|
| 1 | 23.36 | phenol | H | 0.2 | 1.6 |
| 2 | 24.09 | guaiacol | G | 2.2 | 14.2 |
| 3 | 26.76 | p-cresol | H | 0.1 | 0.3 |
| 4 | 28.26 | 4-methylguaiacol | G | 3.5 | 2.9 |
| 5 | 30.27 | 4-ethylphenol | H | 0.1 | 0.1 |
| 6 | 31.74 | 4-ethylguaiacol | G | 0.7 | 1.2 |
| 7 | 32.87 | methylsyringol | S | 0.1 | 0.4 |
| 8 | 34.13 | 4-vinylguaiacol | G | 3.6 | 6.9 |
| 9 | 35.09 | eugenol | G | 0.1 | 0.3 |
| 10 | 35.20 | 4-propylguaiacol | G | 0.2 | 0.2 |
| 11 | 36.32 | syringol | S | 6.5 | 30.7 |
| 12 | 36.78 | isoeugenol | G | 0.3 | 0.2 |
| 13 | 37.37 | cis isoeugenol | G | 0.4 | 0.3 |
| 14 | 39.43 | trans isoeugenol | G | 1.8 | 1.3 |
| 15 | 39.98 | 4-methylsyringol | S | 8.5 | 5.4 |
| 16 | 40.46 | vanillin | G | 1.4 | 0.5 |
| 17 | 40.88 | 1-(4-hydroxy-3-methoxyphenyl)propyne | G | 0.2 | 0.1 |
| 18 | 40.88 | 1-(4-hydroxy-3-methoxyphenyl)propyne | G | 0.2 | 0.1 |
| 19 | 42.70 | homovanillin | G | 1.4 | 0.1 |
| 20 | 42.82 | 4-ethylsyringol | S | 1.7 | 1.7 |
| 21 | 43.57 | acetoguaiacone | G | 1.1 | 0.9 |
| 22 | 45.06 | 4-vinylsyringol | S | 6.1 | 5.2 |
| 23 | 45.48 | guaiacylacetone | G | 0.8 | 0.7 |
| #24 | 45.71 | 4-allylsyringol | S | 1.1 | 0.3 |
| #25 | 45.71 | 4-propylsyringol | S | 1.1 | 0.3 |
| 26 | 46.65 | propioguaiacone | G | 0.5 | 0.2 |
| #27 | 47.17 | coniferyl alcohol | G | 0.8 | 0.1 |
| #28 | 47.17 | guaiacyl vinyl ketone | G | 0.8 | 0.1 |
| 29 | 47.62 | cis 4-propenylsyringol | S | 1.2 | 0.4 |
| 30 | 48.94 | 4-propinylsyringol | S | 1.0 | 0.0 |
| 31 | 49.25 | 4-propinylsyringol | S | 0.6 | 0.3 |
| 32 | 49.14 | 1,6-anhydro-β-D-glucopyranose | Sugar | 0.6 | 0.3 |
| 33 | 49.76 | trans 4-propenylsyringol | S | 5.9 | 1.7 |
| 34 | 50.01 | dihydroconiferyl alcohol | G | 0.1 | 0.0 |
| 35 | 50.97 | syringaldehyde | S | 6.2 | 0.8 |
| 36 | 52.45 | homosyringaldehyde | S | 3.6 | 0.1 |
| 37 | 53.05 | syringic acid methyl ester | S | 0.2 | 0.1 |
| 38 | 53.30 | acetosyringone | S | 4.2 | 1.5 |
| 39 | 54.58 | trans coniferaldehyde | G | 1.1 | 0.8 |
| 40 | 54.71 | syringylacetone | S | 2.6 | 0.2 |
| 41 | 55.76 | propiosyringone | S | 1.3 | 0.4 |
| #42 | 56.30 | syringyl vinyl ketone | S | 1.4 | 0.1 |
| #43 | 56.33 | sinapyl alcohol isomer | S | 1.9 | 0.1 |
| 44 | 62.87 | trans sinapaldehyde | S | 6.8 | 0.2 |
| Total lignin (% of total area) | 83.5 | 83.1 | |||
| Syringyl units (S) | 61.9 | 50.0 | |||
| Guaiacyl units (G) | 21.1 | 31.1 | |||
| p-Hydroxyphenyl units (H) | 0.4 | 2.0 | |||
| S/G ratio | 2.9 | 1.6 | |||
| H:G:S | 1:50:146 | 1:16:26 | |||
| Element | Eucalypt Stump Lignin | Kraft Lignin |
|---|---|---|
| C | 58.2 | 60.5 |
| H | 5.8 | 5.1 |
| N | 0.0 | 0.0 |
| S | 0.0 | 2.0 |
| O | 36.0 | 32.5 |
| Model | Equation | Parameter | Eucalypt Lignin | Kraft Lignin | Ref. |
|---|---|---|---|---|---|
| Pseudo-first-order | qe (mg/g) | 50.00 | 50.00 | [37] | |
| k1 (1/min) | 0.00253 | 0.00276 | |||
| R2 | 0.9626 | 0.944 | |||
| Pseudo-second-order | qe (mg/g) | 51.55 | 56.49 | [38] | |
| k2 (g/mg min) | 0.00835 | 0.0000915 | |||
| R2 | 0.9995 | 0.9951 | |||
| Elovich | α | 5.45·106 | 5.64⋅1012 | [39] | |
| β | 0.1112 | 0.0934 | |||
| R2 | 0.9823 | 0.9507 |
| Model | Equation | Parameter | Adsorbent | Ref. | |
|---|---|---|---|---|---|
| Eucalyptus | Kraft | ||||
| Langmuir | qm (mg/g) | 256.4 | 303.0 | [40] | |
| KL | 0.0385 | 0.0565 | |||
| R2 | 0.9979 | 0.9997 | |||
| Freundlich | KF | 21.2 | 28.7 | ||
| 1/n | 0.42 | 0.38 | |||
| R2 | 0.8642 | 0.7065 | |||
| Temkin | b | 3,098,523.9 | 3,098,523.8 | ||
| AT | 28282.5 | 126753.5 | |||
| R2 | 0.9916 | 0.8634 | |||
| Dubinin–Radushkevich | qm (mg/g) | 538.9 | 644.3 | ||
| Ad | −5 × 10−9 | −5 × 10−9 | |||
| R2 | 0.9141 | 0.7989 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, A.; Kukić, D.; Vasić, V.; Costa, R.A.; Antov, M.; Šćiban, M.; Gominho, J. Valorisation of Lignocellulosic Wastes, the Case Study of Eucalypt Stumps Lignin as Bioadsorbent for the Removal of Cr(VI). Molecules 2022, 27, 6246. https://doi.org/10.3390/molecules27196246
Lourenço A, Kukić D, Vasić V, Costa RA, Antov M, Šćiban M, Gominho J. Valorisation of Lignocellulosic Wastes, the Case Study of Eucalypt Stumps Lignin as Bioadsorbent for the Removal of Cr(VI). Molecules. 2022; 27(19):6246. https://doi.org/10.3390/molecules27196246
Chicago/Turabian StyleLourenço, Ana, Dragana Kukić, Vesna Vasić, Ricardo A. Costa, Mirjana Antov, Marina Šćiban, and Jorge Gominho. 2022. "Valorisation of Lignocellulosic Wastes, the Case Study of Eucalypt Stumps Lignin as Bioadsorbent for the Removal of Cr(VI)" Molecules 27, no. 19: 6246. https://doi.org/10.3390/molecules27196246
APA StyleLourenço, A., Kukić, D., Vasić, V., Costa, R. A., Antov, M., Šćiban, M., & Gominho, J. (2022). Valorisation of Lignocellulosic Wastes, the Case Study of Eucalypt Stumps Lignin as Bioadsorbent for the Removal of Cr(VI). Molecules, 27(19), 6246. https://doi.org/10.3390/molecules27196246









