Thermodynamic Properties of Crystalline Cellulose Allomorphs Studied with Dispersion-Corrected Density Functional Methods
Abstract
1. Introduction
2. Computational Methods
3. Results
3.1. Geometry Optimization and Thermodynamics
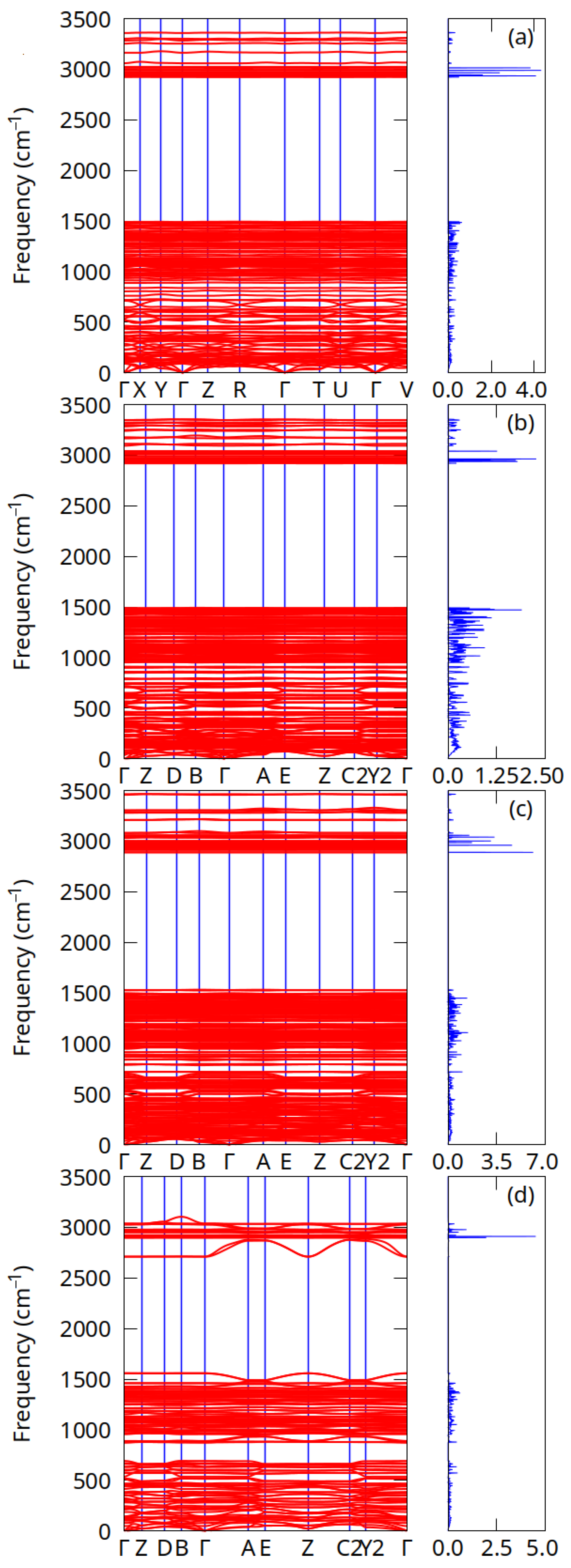
3.2. Phonon Dispersion Relations
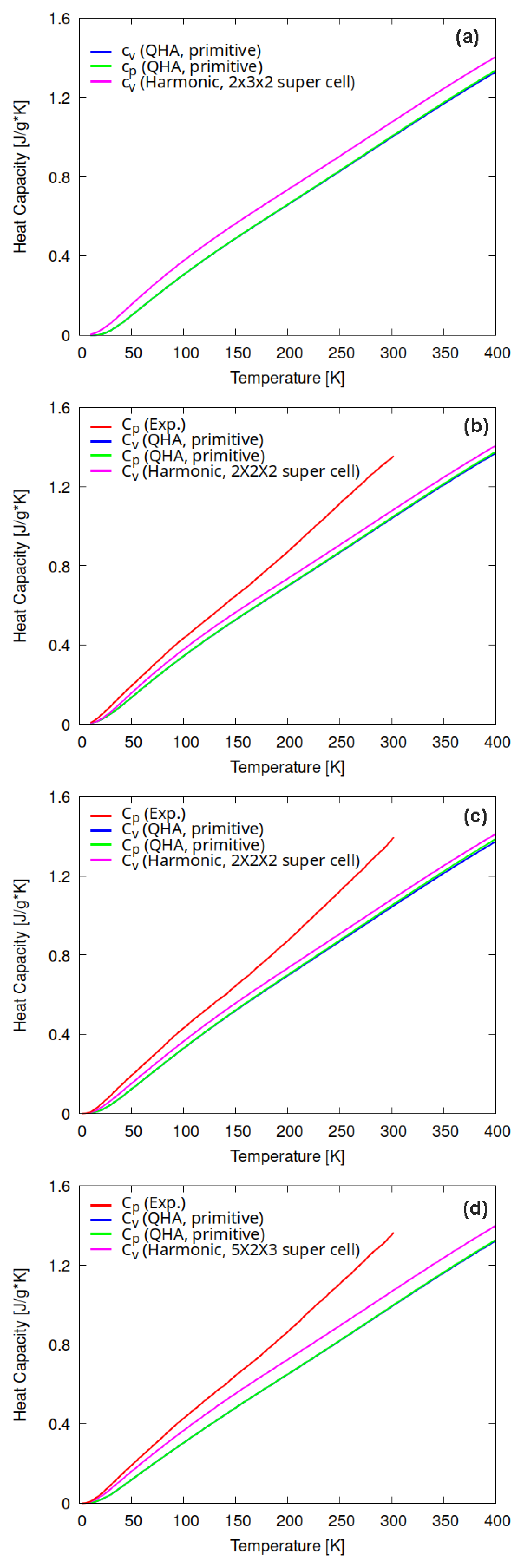
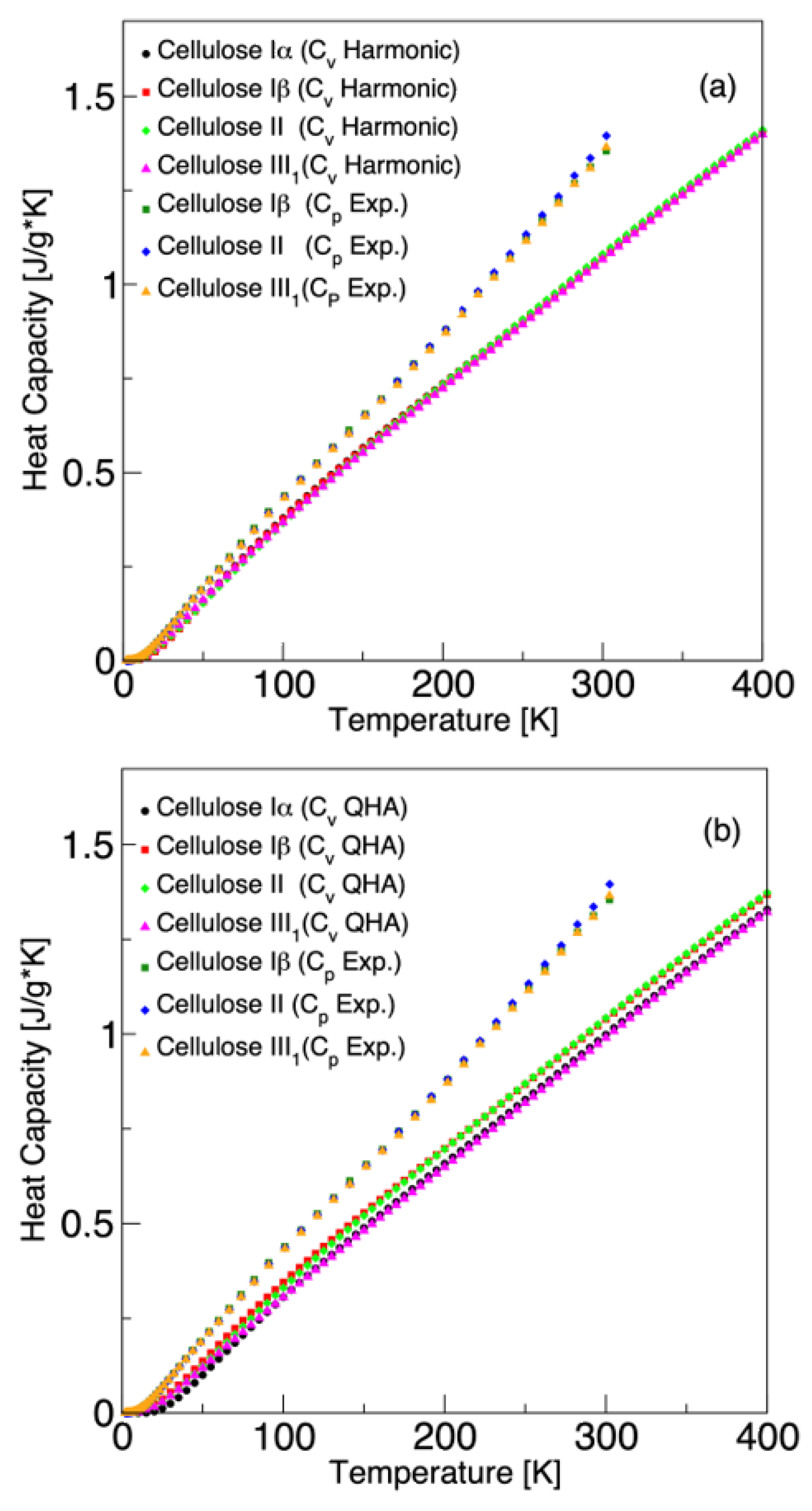
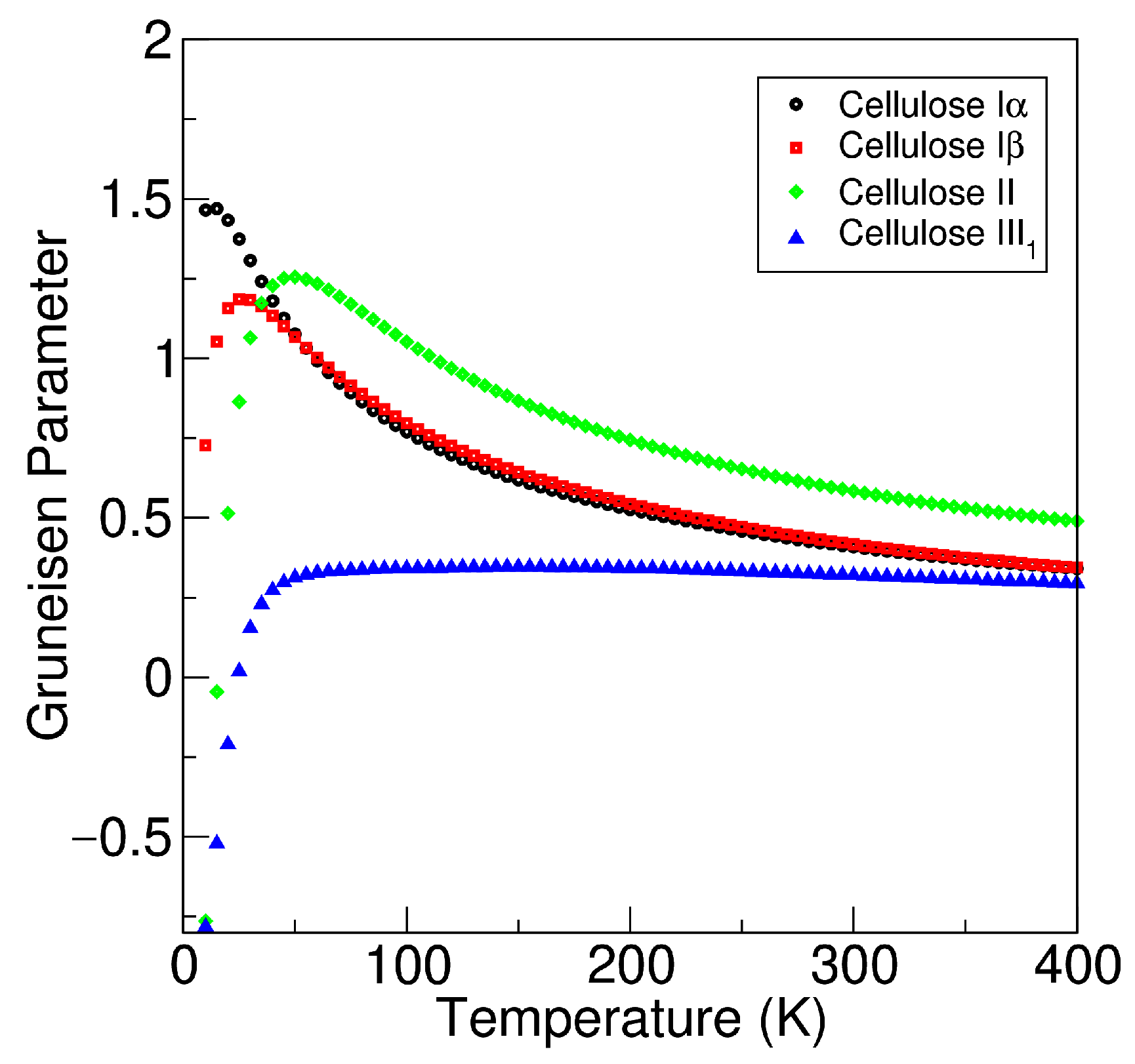
3.3. Specific Heat Capacity and Gruneisen Parameter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrier, W.G. The crystal and molecular structure of β-D-glucose. Acta Cryst. 1963, 16, 1023–1031. [Google Scholar] [CrossRef]
- Atalla, R.H.; VanderHart, D.L. Native Cellulose: A Composite of Two Distinct Crystalline Forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iα from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Hayashi, J.; Sufoka, A.; Ohkita, J.; Watanabe, S. The confirmation of existences of cellulose IIII, IIIII, IVI, and IVII by the X-ray method. J. Polym. Sci. Polym. Lett. Ed. 1975, 13, 23–27. [Google Scholar] [CrossRef]
- Gardiner, E.S.; Sarko, A. Packing analysis of carbohydrates and polysaccharides. 16. The crystal structures of celluloses IVI and IVII. Can. J. Chem 1985, 63, 173–180. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- O’sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray Structure of Mercerized Cellulose II at 1 Å Resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef]
- Wada, M.; Chanzy, H.; Nishiyama, Y.; Langan, P. Cellulose IIII Crystal Structure and Hydrogen Bonding by Synchrotron X-ray and Neutron Fiber Diffraction. Macromolecules 2004, 37, 8548–8555. [Google Scholar] [CrossRef]
- Wada, M.; Nishiyama, Y.; Chanzy, H.; Forsyth, T.; Langan, P. The structure of celluloses. Powder Diffr. 2008, 23, 92–95. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Schliesser, J.; Mittal, A.; Decker, S.R.; Santos, A.F.L.; Freitas, V.L.; Urbas, A.; Lang, B.E.; Heiss, C.; Ribeiro da Silva, M.D.; et al. A thermodynamic investigation of the cellulose allomorphs: Cellulose(am), cellulose Iβ(cr), cellulose II(cr), and cellulose III(cr). J. Chem. Thermodyn. 2015, 81, 184–226. [Google Scholar] [CrossRef]
- Dri, F.L.; Shang, S.; Hector, L.G.; Saxe, P.; Liu, Z.K.; Moon, R.J.; Zavattieri, P.D. Anisotropy and temperature dependence of structural, thermodynamic, and elastic properties of crystalline cellulose Iβ: A first-principles investigation. Model. Simul. Mater. Sci. Eng. 2014, 22, 085012. [Google Scholar] [CrossRef]
- Liu, Z.; Chung, P.W. Critical Evaluation of Reactive Force Fields for Vibrational Spectra: Case Study of Crystalline Cellulose Iβ. Propellants, Explos. Pyrotech. 2022, 47, e202100376. [Google Scholar] [CrossRef]
- Dong, R.Y.; Dong, Y.; Li, Q.; Wan, C. Ballistic-diffusive phonon transport in cellulose nanocrystals by ReaxFF molecular dynamics simulations. Int. J. Heat Mass Transf. 2020, 148, 119155. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. WIREs Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Srivastava, D.; Kuklin, M.S.; Ahopelto, J.; Karttunen, A.J. Electronic band structures of pristine and chemically modified cellulose allomorphs. Carbohydr. Polym. 2020, 243, 116440. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Erba, A. On combining temperature and pressure effects on structural properties of crystals with standard ab initio techniques. J. Chem. Phys. 2014, 141, 124115. [Google Scholar] [CrossRef]
- Erba, A.; Shahrokhi, M.; Moradian, R.; Dovesi, R. On how differently the quasi-harmonic approximation works for two isostructural crystals: Thermal properties of periclase and lime. J. Chem. Phys. 2015, 142, 044114. [Google Scholar] [CrossRef] [PubMed]
- Erba, A.; Maul, J.; De La Pierre, M.; Dovesi, R. Structural and elastic anisotropy of crystals at high pressures and temperatures from quantum mechanical methods: The case of Mg2SiO4 forsterite. J. Chem. Phys. 2015, 142, 204502. [Google Scholar] [CrossRef] [PubMed]
- Erba, A.; Maul, J.; Demichelis, R.; Dovesi, R. Assessing thermochemical properties of materials through ab initio quantum-mechanical methods: The case of α-Al2O3. Phys. Chem. Chem. Phys. 2015, 17, 11670–11677. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Togo, A. Phonopy Website. Available online: https://phonopy.github.io/phonopy/ (accessed on 18 September 2022).
- Hinuma, Y.; Pizzi, G.; Kumagai, Y.; Oba, F.; Tanaka, I. Band structure diagram paths based on crystallography. Comput. Mater. Sci. 2017, 128, 140–184. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
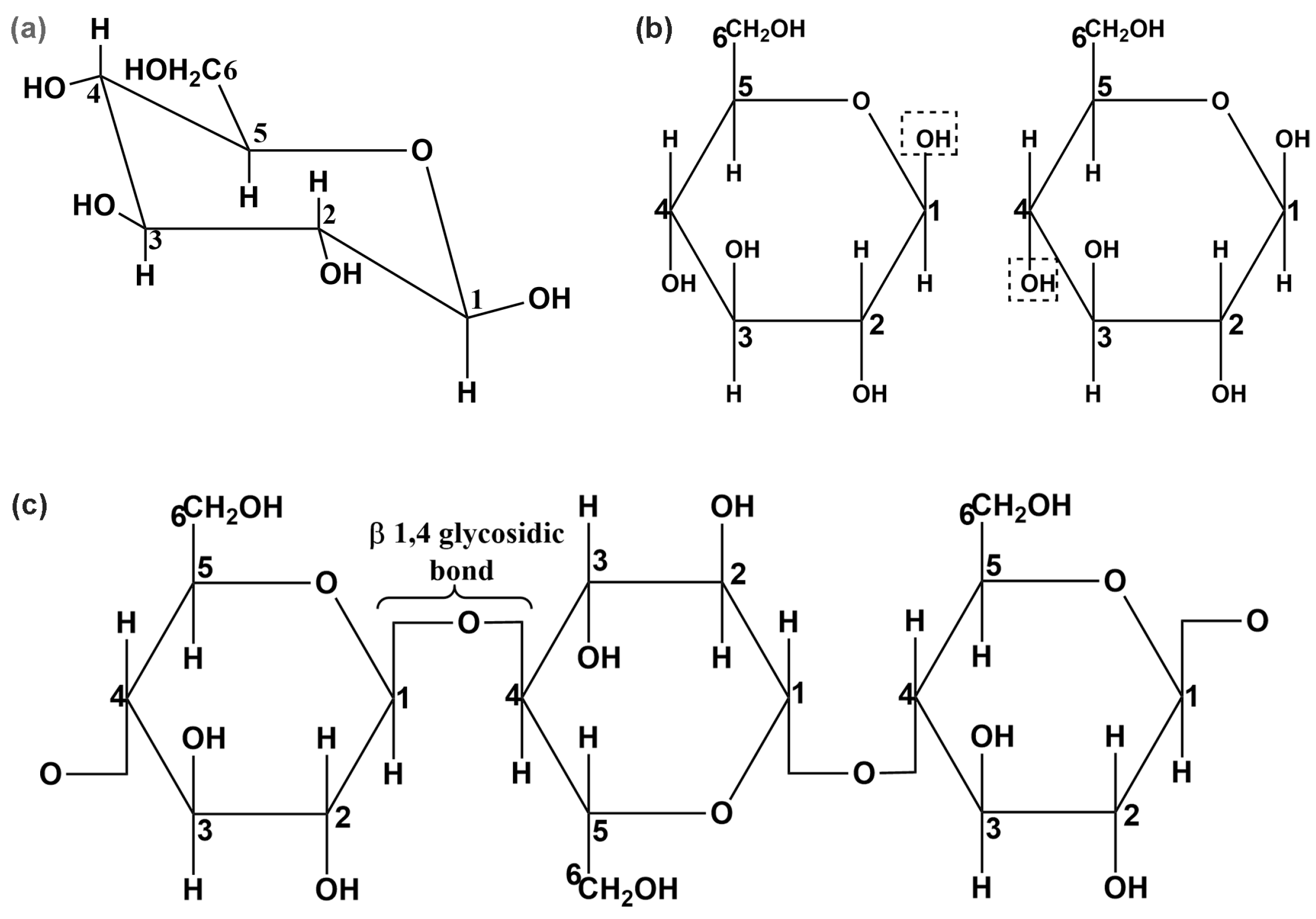
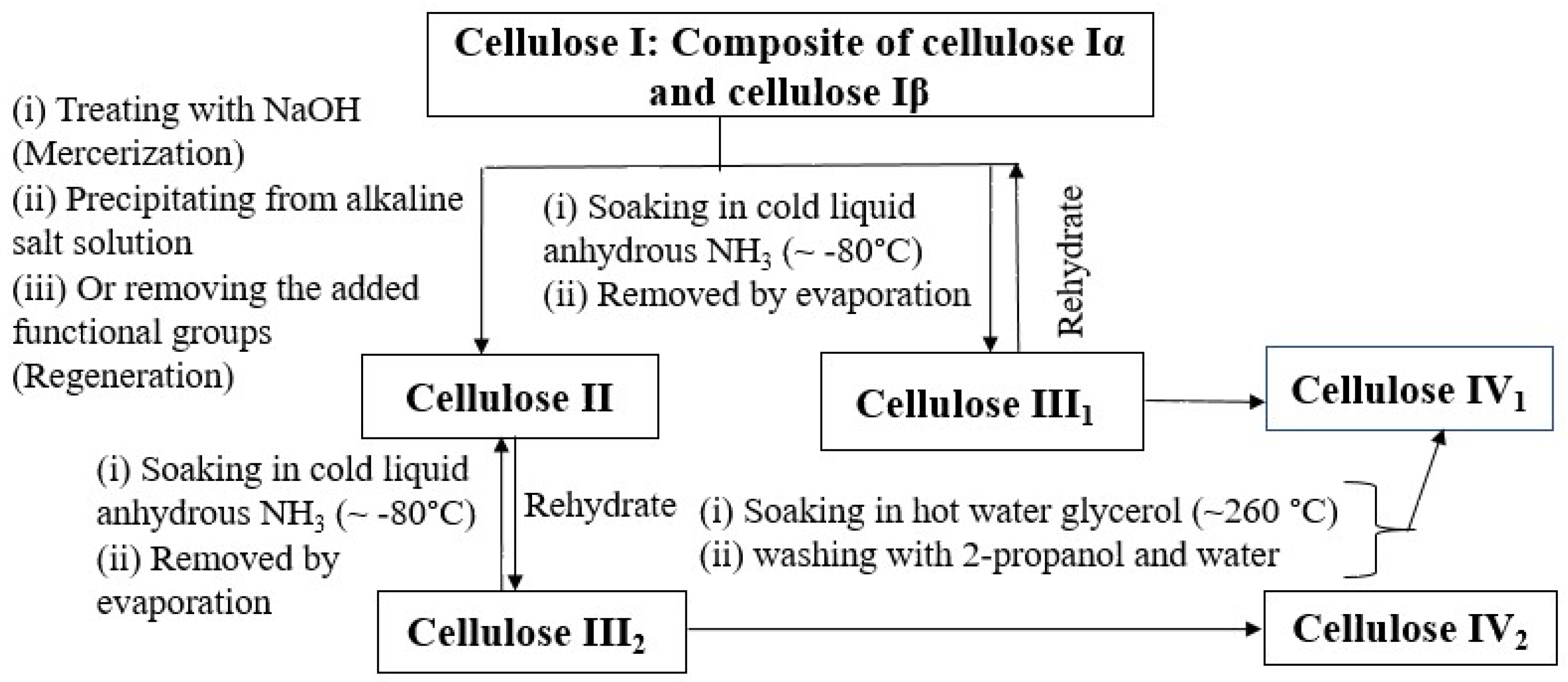
| I [3] | I [4] | II [9] | III [10] | |
|---|---|---|---|---|
| Crystal system | Triclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | ||||
| a (Å) | 6.54 (6.72) | 8.11 (8.20) | 7.88 (8.10) | 7.59 (7.85) |
| b (Å) | 10.39 (10.40) | 10.40 (10.38) | 10.45 (10.31) | 10.34 (10.31) |
| c (Å) | 5.84 (5.96) | 7.50 (7.78) | 8.45 (9.03) | 4.34 (4.45) |
| 117.7 (118.1) | 90 | 90 | 90 | |
| 114.9 (114.8) | 95.92 (96.5) | 114.11 (117.1) | 101.12 (105.1) | |
| 81.6 (80.4) | 90 | 90 | 90 | |
| Z | 1 | 2 | 2 | 1 |
| 6.6 | 0 | 27.5 | 14.0 | |
| 10.5 | 0 | 25.8 | 10.3 | |
| 10.7 | 0 | 25.5 | 11.3 | |
| 5.3 | 0 | 24.5 | 7.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, D.; Ahopelto, J.; Karttunen, A.J. Thermodynamic Properties of Crystalline Cellulose Allomorphs Studied with Dispersion-Corrected Density Functional Methods. Molecules 2022, 27, 6240. https://doi.org/10.3390/molecules27196240
Srivastava D, Ahopelto J, Karttunen AJ. Thermodynamic Properties of Crystalline Cellulose Allomorphs Studied with Dispersion-Corrected Density Functional Methods. Molecules. 2022; 27(19):6240. https://doi.org/10.3390/molecules27196240
Chicago/Turabian StyleSrivastava, Divya, Jouni Ahopelto, and Antti J. Karttunen. 2022. "Thermodynamic Properties of Crystalline Cellulose Allomorphs Studied with Dispersion-Corrected Density Functional Methods" Molecules 27, no. 19: 6240. https://doi.org/10.3390/molecules27196240
APA StyleSrivastava, D., Ahopelto, J., & Karttunen, A. J. (2022). Thermodynamic Properties of Crystalline Cellulose Allomorphs Studied with Dispersion-Corrected Density Functional Methods. Molecules, 27(19), 6240. https://doi.org/10.3390/molecules27196240






