Abstract
Symmetrical diaryl sulfides and diaryl disulfides have been efficiently and selectively constructed via the homocoupling of sodium arenesulfinates. The selectivity of products relied on the different reaction systems: symmetrical diaryl sulfides were predominately obtained under the Pd(OAc)2 catalysis, whereas symmetrical diaryl sulfides were exclusively yielded in the presence of the reductive Fe/HCl system.
1. Introduction
Symmetrical sulfides [1,2,3] and disulfides [4,5,6,7] are ubiquitous structural motifs, and their corresponding derivatives have found prevalent existence in many biologically active molecules, pharmaceuticals, ligands, functional materials, and natural products. Owing to their importance, various synthetic methodologies have been developed for the preparation of these two classes of sulfur compounds [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Although the cross-coupling reactions of various sulfur surrogates with other aromatic reagents, such as aromatic halides, arenediazonium salts, and others, are generally efficient for the construction of these two symmetric structures [24,25,26,27,28,29,30,31,32,33,34,35,36], the homocoupling of arylsulfonyl derivatives [37,38] and thiols [39,40,41,42,43,44,45,46,47,48] has proven to be the most straightforward and convenient strategy in terms of simplicity. Despite these major advances, the utility of environmentally friendly sulfur sources for symmetric sulfides and disulfides is still highly desirable.
Due to their stable, greener, and inexpensive features, sodium sulfinates have been utilized as ideal sulfur donors and widely applied as the coupling partners in C-S cross-coupling reactions, such as sulfonylation [49,50], thiosulfonylation [51], sulfinylation [52,53], and sulfenylation [54,55,56,57,58,59]. Especially by reductive coupling, the sodium sulfinates could serve as sulfenylation reagents for the synthesis of unsymmetric sulfides under Pd, Cu, or I2 catalysts (Scheme 1a) [54,55,56,57,58,59]. However, the application of sodium sulfinates for the construction of symmetric disulfides by homocoupling reactions was still less explored. On the other hand, although several reaction systems, such as EtOP(O)H2, TiCl4/Sm, WCl6/NaI, WCl6/Zn, MoCl6/Zn, Cp2TiCl2/Sm, and Silphos, have been reported to mediate the conversion of sodium arenesulfinates into symmetric diaryl disulfides by reductive coupling (Scheme 1b) [60,61,62,63], they generally suffered from limited substrate scope, expensive reagents, or complicated procedures. Herein, we would like to report an efficient strategy for the selective synthesis of symmetrical diaryl sulfides and diaryl disulfides using sodium sulfinates as sulfenylation reagents through homocoupling.
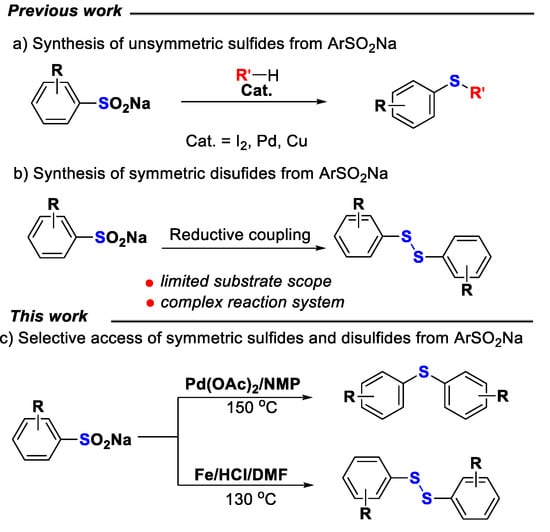
Scheme 1.
Sulfides and disulfides construction from sodium sulfinates.
2. Results and Discussion
Our initial study started with sodium phenylsulfinate 1a as the model substrate to explore the formation of diphenylsulfide 2a. First, by screening different solvents, NMP was proven to be the most effective out of the others, such as DMF and DMSO (Table 1, entry 3 vs. 1−2). The catalyst played a decisive role in this reaction: among the common metal catalysts, Pd(OAc)2 was the best one to afford diphenylsulfide 2a in 47% yield (entry 3 vs. 4–6). With CuI and Ni(OAc)2 as catalysts, the product 2a was not detected at all (entries 4 and 5). Only a trace amount of 2a was observed when FeCl3 was employed (entry 6). The yield could be improved to 60% by increasing reaction temperature to 150 °C (entry 7 vs. 3 and 8). Most importantly, a decrease of catalyst loading to 2 mol% could further increase the yield to 89%, and no better result was observed by continuously reducing the catalyst loading (entry 10 vs. 7, 9–11). The desired 2a was not detected when sodium benzenesulfinate was replaced with benzenesulfinic acid in the presence of NaOH under the same conditions (entry 12).

Table 1.
Optimization of reaction conditions for sodium benzenesulfinate to diphenylsulfide [a].
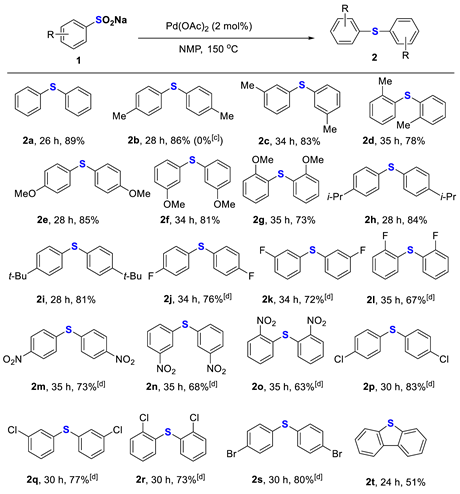
Having built the optimal conditions for the construction of diphenylsulfide 2a, we turned our attention to explore the generality of sodium sulfinates. As shown in Table 2, a variety of substrates could undergo the homocoupling to afford symmetrical diaryl sulfides with high chemoselectivity. It was found that sodium benzenesulfinates with electron-donating groups such as 4-methyl, 3-methyl, 2-methyl, 4-methoxyl, 3-methoxyl, 2-methoxyl, 4-isopropyl, and 4-tert-butyl on the phenyl ring gave the corresponding products 2b–2i in good yields. Electron-withdrawing groups, such as F, Cl, Br, and NO2, were also well-tolerated to provide the desired products 2j–2s in moderate to good yields that were somewhat lower than the electron-donating groups offered. To our delight, the intramolecular formation of sulfides was also tried, and the desired dibenzothiophene 2t was produced in a 51% yield.

Table 2.
Scope of sodium arylsulfinates to diaryl sulfides [a,b].
During our studies on the synthesis of diphenylsulfide 2a, 1,2-diphenyldisulfide 3a was accidentally detected when using CuI as a reductant. This discovery encouraged us to search for optimal conditions for the reductive coupling of sodium benzenesulfinate for 1,2-diphenyldisulfide 3a. Fortunately, using Fe/HCl as the reductive system, diphenyldisulfide 3a was isolated as the major product. Subsequently, the investigation of the concentration of hydrochloric acid revealed that increasing the concentration led to the higher yield, and 12 mol/L of hydrochloric acid gave up to 96% yield (Table 3, entry 4 vs. 1−3). The highest yield was obtained when 4.0 equiv. of HCl was used (Table 2, entry 4). Increasing or decreasing the amount resulted in lower yields (entries 5 and 6 vs. 4). It was found that 2.0 equiv. of Fe was suitable for this transformation, and other amounts did not improve the yield further (entry 4 vs. 7 and 8). More notably, the similar high yield was provided when the time was shortened to 9 h (entry 9). However, sodium benzenesulfinate generated in situ by the reaction of NaOH and the equivalent of 4-methylbenzenesulfinic acid only afforded the target product (3b) in a 59% yield (entry 10).

Table 3.
Optimization of reaction conditions for sodium benzenesulfinate to diphenyldisulfide [a].
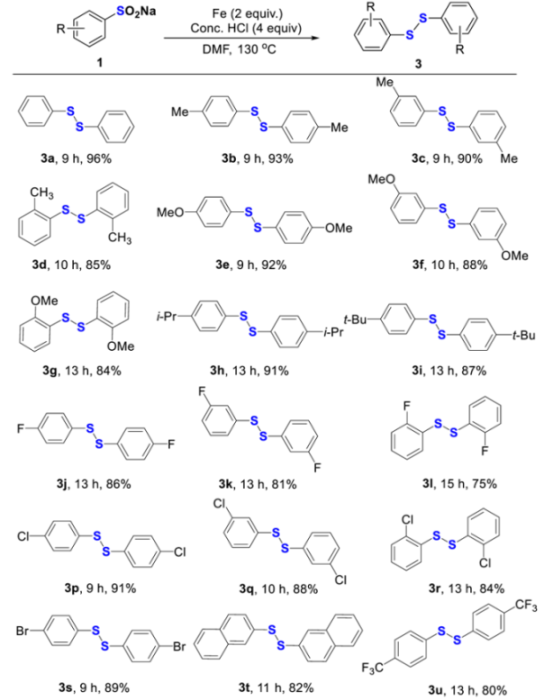
With the optimized reaction conditions in hand, we next focused on the evaluation of the scope of the coupling partner to symmetric disulfides, and the results are summarized in Table 4. To our delight, it was found that the reaction could be compatible with a broad range of functional groups, furnishing the corresponding products in good to excellent yields. Although various functional groups, including electronically diverse (3a–3u) and sterically hindered (3d, 3g, 3h, 3i, 3l, and 3r) ones are readily tolerated, some substantial influence of electronic properties and steric hindrance of the substituents was observed. The substrates possessing an electron-rich group (Me, MeO, i-Pr, and t-Bu) showed higher yields than those bearing an electron-poor group (F, Cl, Br, and CF3) (3b–3i vs. 3j–3l, 3p–3s, and 3u). Among substrates, sodium ortho-substituted arylsulfinates, which are sterically hindered, gave relatively lower yields (3d vs. 3b, 3c, and 3g vs. 3e, 3f, and 3l vs. 3j, 3k, and 3r vs. 3p and 3q). In addition, an 82% yield was obtained when sodium 2-naphthylsulfinate was employed as a substrate (3t). Notably, sodium 3-carboxybenzenesulfinate and sodium thiophene-2-sulfinate could be not transformed into the corresponding disulfides.

Table 4.
Scope of sodium arylsulfinates to diaryl disulfides [a,b].
To further evaluate the utility of these two protocols, two gram-scale reactions were subsequently carried out (Scheme 2). The corresponding products 2a and 3a could be afforded in 88% and 94% yields in a 10 mmol scale, respectively, demonstrating the practicability of the present methodology.
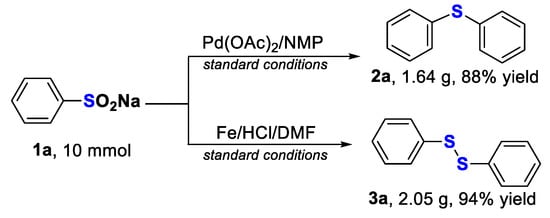
Scheme 2.
Gram-scale reactions of sodium benzenesulfinate.
To elucidate the reaction mechanism for the homocoupling of sodium arylsulfinates, several control experiments were conducted (Scheme 3). The formation of both symmetric diphenyl disulfide 3a and diphenyl sulfide 2a was not detected after the addition of the radical scavenger 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO, 2 equiv.) to the standard reaction systems (Scheme 3a,b), indicating that both of these two transformations underwent a free-radical process. For the synthesis of 2a, the disulfide 3a was detected by mass spectrometry. The preparation of disulfides from sodium arylsulfinates under Pd(OAc)2 catalysis was also demonstrated by Xiang and co-workers [55]. In addition, the transformation from 3a to 2a could be successfully realized in the presence of a catalytic amount of Pd(OAc)2 and sodium sulfinate 1a (Scheme 3c,d).
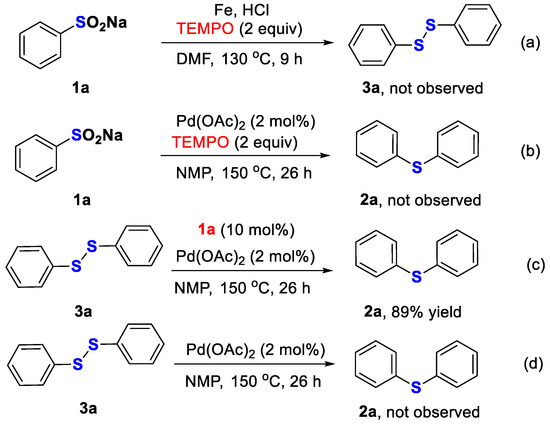
Scheme 3.
Control experiments for the homocoupling of sodium phenylsulfinate.
Based on the results of the control experiments and literature reports [64,65], a plausible mechanism for the homocoupling of sodium arylsulfinate 1 to the selective access to symmetric sulfide 2 and disulfide 3 is shown in Scheme 4. First, in the reductive Fe/HCl system, disulfide 3a could be generated via the homocoupling of the thiyl radical A, which comes from the radical reduction of sodium phenylsulfinate 2a (Scheme 4a). Alternatively, disulfide 3a could be also formed in the presence of catalytic Pd(OAc)2. After disulfide 3a was formed, the Pd(II)-insertion to the S-S bond produced the metal-intermediate B, which underwent ligand exchange to form intermediate C. The thermal extrusion of SO2 of intermediate C resulted in the formation of intermediate D [66], which underwent the reductive elimination to give the target sulfide 2a and regenerate Pd(0) into the next catalytic cycle (Scheme 4b).
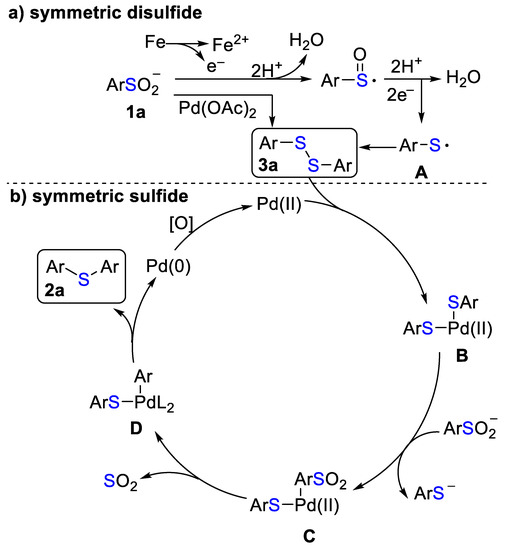
Scheme 4.
Proposed mechanism for the homocoupling of sodium arylsulfinates.
3. Materials and Methods
Unless otherwise indicated, all reagents and solvents were purchased from commercial sources and used without further purification. Deuterated solvents were purchased from Sigma–Aldrich(Shanghai, China.). Refinement of the mixed system was achieved through column chromatography, which was performed on silica gel (200–300 mesh) with petroleum ether (solvent A)/ethyl acetate (solvent B) gradients as elution. In addition, all yields were referred to the isolated yields (average of two runs) of the compounds, unless otherwise specified. The known compounds were partly characterized by melting points (for solid samples), 1H NMR, and compared to authentic samples or the literature data. Melting points were measured with an RD-II digital melting point apparatus (Henan, China) and were uncorrected. 1H NMR data were acquired on a Bruker Advance 600 MHz spectrometer (Bruker, Germany). using CDCl3 as solvent. Chemical shifts are reported in ppm from tetramethylsilane, with the solvent CDCl3 resonance as the internal standard (CDCl3 = 7.26). Spectra are reported as follows: chemical shift (δ = ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constants (Hz), integration, and assignment. 13C NMR data were collected at 100 MHz, with complete proton decoupling. The chemical shifts are reported in ppm downfield to the central CDCl3 resonance (δ = 77.0). High-resolution mass spectra were performed on a micrOTOF-Q II instrument (Bruker, Germany), with an ESI source.
3.1. Typical Procedure for Symmetric Diaryl Sulfides 2
The mixture of sodium arylsulfinate 1 (0.4 mmol) and Pd(OAc)2 (2 mg, 2 mol%) in NMP (1.0 mL) was stirred at 150 °C (oil bath) until the substrate was completely consumed, which was determined by TLC. Finally, the reaction mixture was purified by silica gel column chromatography (PE: EA = 40: 1) to afford the desired coupling product diarylsulfides 2.
3.2. Typical Procedure for Symmetric Diaryl Sisulfides 3
The mixture of sodium arylsulfinate 1 (0.2 mmol), Fe powder (23 mg, 2.0 equiv), and HCl (12 M, 67.0 μL) in DMF (1.0 mL) was stirred at 130 °C (oil bath), until the substrate was completely consumed, which was determined by TLC. Finally, the reaction mixture was purified by silica gel column chromatography (PE: EA = 40: 1) to afford the desired coupling product diaryldisulfides 3.
3.3. Gram-Scale Reaction of Sodium Benzenesulfinate to Diphenylsulfide
The mixture of sodium benzenesulfinate 1a (1.64 g, 10 mmol) and the catalyst, Pd(OAc)2 (45 mg, 2 mol%) in NMP (10 mL), was stirred at 150 °C (oil bath) until the substrate was completely consumed, which was determined by TLC. Finally, the reaction mixture was purified by silica gel column chromatography to afford the coupling product diphenylsulfide 2a (1.640 g, 88% yield).
3.4. Gram-Scale Reaction of Sodium Benzenesulfinate to 1,2-Diphenyldisulfane
The mixture of sodium benzenesulfinate 1a (1.640 g, 10 mmol), Fe powder (1.150 g, 2.0 equiv.), and 12 mol/L HCl (3.35 mL) in DMF (15 mL) was stirred at 130 °C (oil bath) until the substrate was completely consumed, which was determined by TLC. Finally, the reaction mixture was purified by silica gel column chromatography to afford the coupling product 1,2-diphenyldisulfane 3a (2.050 g, 94% yield).
3.5. Characterization Data for Homo-Coupling Products of Sodium Arylsulfinates
3.5.1. Characterization Data for the Products of Diaryl sulfides
Diphenyl sulfide (2a) [8]. Colorless liquid (33.1 mg, 89% yield); Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.35−7.33 (m, 4H), 7.32−7.27 (m, 4H), 7.26−7.22 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 135.9, 131.2, 129.3, and 127.1; HRMS (ESI) m/z [M + H]+ calculated for C12H11S 187.0576, found 187.0579.
4,4’-Dimethyldiphenyl sulfide (2b) [67]. White solid (36.8 mg, 86% yield); mp 57−58 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.23 (dt, J = 4.8, 2.4 Hz, 4H), 7.10 (d, J = 7.8 Hz, 4H), 2.33 (s, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 136.9, 132.7, 131.1, 129.9, and 21.0; HRMS (ESI) m/z [M + H]+ calculated for C14H15S 215.0889, found 215.0885.
3,3’-Dimethyldiphenyl sulfide (2c) [68]. Colorless liquid (35.5 mg, 83% yield); Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.20−7.16 (m, 4H), 7.13 (d, J = 7.8 Hz, 2H), 7.05 (d, J = 7.8 Hz, 2H), and 2.31 (s, 6H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C14H15S 215.0889, found 215.0885.
Di-o-tolylsulfide (2d) [8]. White solid (33.4 mg, 78% yield); mp 64−65 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.35−7.22 (m, 2H), 7.16 (td, J = 6.0, 1.2 Hz, 2H), 7.11−7.08 (m, 2H), 7.05 (dd, J = 6.6, 1.2 Hz, 2H), and 2.38 (s, 6H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C14H15S 215.0889, found 215.0885.
Bis(4-methoxyphenyl)sulfide (2e) [8]. White solid (41.8 mg, 85% yield); mp 44−46 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.29−7.26 (m, 4H), 6.83 (dt, J = 9.0, 2.4 Hz, 4H), and 3.79 (s, 6H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C14H15O2S 247.0787, found 247.0785.
Bis(3-methoxyphenyl)sulfide (2f) [8]. White solid (39.9 mg, 81% yield); mp 45−47 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.24−7.18 (m, 2H), 6.96−6.92 (m, 2H), 6.90−6.88 (m, 2H), 6.81−6.77 (m, 2H), and 3.76 (s, 6H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C14H15O2S 247.0787, found 247.0785.
Bis(2-methoxyphenyl)sulfide (2g) [8]. White solid (35.9 mg, 73% yield); mp 73−74 °C; Rf = 0.6 (petroleum ether); 1H NMR (400 MHz, CDCl3) δ 7.28−7.21 (m, 2H), 7.06 (dd, J = 5.6, 2.0 Hz, 2H), 6.93−6.90 (dd, J = 7.6, 0.8 Hz, 2H), 6.87−6.85 (dd, J = 6.4, 1.2 Hz, 2H), and 3.87 (s, 6H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C14H15O2S 247.0787, found 247.0785.
4,4’-Diisopropyldiphenyl sulfide (2h). White solid (45.4 mg, 84% yield); mp 73−75 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHZ, CDCl3) δ 7.28−7.25 (m, 4H), 7.17−7.14 (m, 4H), 2.92−2.82 (m, 2H), and 1.24 (d, J = 6.6 Hz, 12H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 147.8, 132.8, 131.0, 127.3, 33.7, and 23.9; HRMS (ESI) m/z [M + K]+ calculated for C18H22SK 309.1074, found 309.1073.
4,4’-Di-tert-butyldiphenyl sulfide (2i) [69]. White solid (48.3 mg, 81% yield); mp 83−84 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.34−7.30 (m, 4H), 7.28 (t, J = 1.8 Hz, 2H), 7.26 (t, J = 2.4 Hz, 2H), and 1.30 (s, 18H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C20H27S 299.1828, found 299.1821.
4,4’-Difluorodiphenyl sulfide (2j) [8]. Colorless liquid (33.7 mg, 76% yield); Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.33−7.28 (m, 4H) and 7.03−6.98 (m, 4H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9F2S 223.0388, found 223.0393; 19F NMR (376 MHz, CDCl3) δ -114.3 ppm.
3,3’-Difluorodiphenyl sulfide(2k) [68]. Colorless liquid (32.0 mg, 72% yield); Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.33−7.26 (m, 2H), 7.16−7.11 (m, 2H), 7.06−7.01 (m, 2H), and 6.99−6.94 (m, 2H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9F2S 223.0388, found 223.0393; 19F NMR (376 MHz, CDCl3) δ -111.5 ppm.
Bis(2-fluorophenyl)sulfide (2l) [70]. Colorless liquid (29.8 mg, 67% yield); Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.31−7.22 (m, 4H) and 7.14−7.06 (m, 4H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9F2S 223.0388, found 223.0393; 19F NMR (376 MHz, CDCl3) δ -108.7 ppm.
Bis(4-nitrophenyl)sulfide (2m) [8]. White solid (40.3 mg, 73% yield); mp 156−158 °C; Rf = 0.5 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 8.06 (dt, J = 9.0, 2.4 Hz, 2H), 7.58−7.52 (m, 2H), 7.48−7.44 (m, 2H), and 7.17 (dt, J = 9.0, 2.4 Hz, 2H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9N2O4S 277.0278, found 277.0281.
Bis(3-nitrophenyl)sulfide (2n) [71]. White solid (37.5 mg, 68% yield); mp 42−44 °C; Rf = 0.5 (petroleum ether); 1H NMR (400 MHz, CDCl3) δ 8.36 (t, J = 4.0 Hz, 2H), 8.15−8.06 (m, 2H), 7.86−7.77 (m, 2H), and 7.53 (t, J = 12.0 Hz, 2H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9N2O4S 277.0278, found 277.0282.
Bis(2-nitrophenyl)sulfide (2o) [8]. Yellow solid (34.8 mg, 63% yield); mp 123−124 °C; Rf = 0.5 (petroleum ether); 1H NMR (400 MHz, CDCl3) δ 8.41 (dd, J = 8.4, 1.2 Hz, 2H), 8.07 (dd, J = 8.4, 1.2 Hz, 2H), 7.84−7.77 (m, 2H), and 7.61−7.54 (m, 2H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9N2O4S 277.0278, found 277.0281.
4,4’-Dichlorodiphenyl sulfide (2p) [8]. White solid (42.2 mg, 83% yield); mp 88−89 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.30−7.27 (m, 4H) and 7.26−7.23 (m, 4H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9Cl2S 254.9797, found 254.9792.
Bis(3-clorophenyl)sulfide (2q) [71]. Colorless liquid (39.1 mg, 77% yield); Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.32−7.31 (m, 2H), 7.26−7.23 (m, 4H), and 7.22−7.19 (m, 2H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9Cl2S 254.9797, found 254.9792.
Bis(2-clorophenyl)sulfide (2r) [68]. White solid (37.1 mg, 73% yield); mp 68−70 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.47 (dd, J = 7.8, 1.2 Hz, 2H), 7.24 (td, J = 7.8, 1.8 Hz, 2H), 7.19 (td, J = 7.8, 1.2 Hz, 2H), and 7.14 (dd, J = 7.8, 1.8 Hz, 2H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9Cl2S 254.9797, found 254.9792.
Bis(4-bromophenyl)sulfide (2s) [8]. White solid (54.7 mg, 80% yield); mp 110−111 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.45−7.40 (m, 4H) and 7.21−7.16 (m, 4H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C12H9Br2S 342.8786, found 342.8784.
Dibenzo[b,d]thiophene (2t) [72]. white solid (38 mg, 51% yield); m.p. 95–96 °C. Rf = 0.8 (PE/EA = 20:1); 1H NMR (400 MHz, CDCl3) δ 8.17 (m, 2H), 7.87 (dd, 2 H, J = 8.0, 4.0 Hz), and 7.47 (m, 4 H). The NMR data were consistent with the previous report (see spectra at Supplementary Materials).
3.5.2. Characterization Data for the Products of Diaryl Disulfides
Diphenyl disulfide (3a) [73]. White solid (21.0 mg, 96% yield); mp 61−62 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.54−7.45 (m, 4H), 7.33−7.26 (m, 4H), and 7.25−7.19 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 137.0, 129.0, 127.5, and 127.1; HRMS (ESI) m/z [M]+ calculated for C12H10S2 218.0224, found 218.0217.
4-Methylphenyl disulfide (3b) [73]. White solid (22.9 mg, 93% yield); mp 47−48 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.39 (d, J = 8.4 Hz, 4H), 7.11 (d, J = 7.8 Hz, 4H), and 2.32 (s, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 137.4, 133.9, 129.8, 128.6, and 21.0; HRMS (ESI) m/z [M]+ calculated for C14H14S2 246.0537, found 246.0517.
3-Methylphenyl disulfide (3c) [73]. White solid (22.1 mg, 90% yield); mp 112−114 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.31 (d, J = 7.2 Hz, 4H), 7.19 (t, J = 7.4 Hz, 2H), 7.04 (d, J = 7.2 Hz, 2H), and 2.32 (s, 6H) ppm; HRMS (ESI) m/z [M]+ calculated for C14H14S2 246.0537, found 246.0519.
Di(o-methylphenyl)disulfide (3d) [73]. White solid (20.9 mg, 85% yield); mp 40−42 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.24−7.21 (m, 2H), 7.19−7.15 (m, 2H), 7.11−7.07 (m, 2H), 7.06 (dd, J = 7.8, 1.8 Hz, 2H), and 2.37 (s, 6H) ppm; HRMS (ESI) m/z [M]+ calculated for C14H14S2 246.0537, found 246.0517.
Di(4-methoxyphenyl)disulfide (3e) [34]. White solid (25.6 mg, 92% yield); mp 45−47 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.31−7.27 (m, 4 H), 6.87−6.81 (m, 4 H), and 3.79 (s, 6H) ppm; HRMS (ESI) m/z [M]+ calculated for C14H14O2S2 278.0435, found 278.0423.
Di(3-methoxyphenyl)disulfide (3f) [34]. White solid (24.5 mg, 88% yield); mp 106−108 °C; Rf = 0.6 (petroleum ether); 1H NMR (400 MHz, CDCl3) δ 7.18−7.11 (m, 2H), 7.03−6.99 (m, 2H), 6.88−6.81 (m, 2H), 6.73−6.67 (m, 2H), and 3.69 (s, 6H) ppm; HRMS (ESI) m/z [M]+ calculated for C14H14O2S2 278.0435, found 278.0423.
Di(2-methoxyphenyl)disulfide (3g) [34]. White solid (23.4 mg, 84% yield); mp 120−121 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.53 (dd, J = 6.6, 1.2 Hz, 2 H), 7.21−7.16 (m, 2 H), 6.93−6.88 (m, 2 H), 6.86 (d, J = 7.8 Hz, 2 H), and 3.90 (s, 6 H) ppm; HRMS (ESI) m/z [M]+ calculated for C14H14O2S2 278.0435, found 278.0427.
1,2-bis(4-isopropylphenyl)disulfane (3h) [73]. White solid (27.5 mg, 91% yield); mp 79−81 °C; Rf = 0.6 (Petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.44 (dt, J = 8.4, 4.8 Hz, 4H), 7.17 (dt, J = 7.8, 4.2 Hz, 4H), 2.93−2.86 (m, 2H), and 1.24 (d, J = 7.2 Hz, 12H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C18H23S2 303.1236, found 303.1226.
Bis(4-tert-butylphenyl) disulfide (3i) [74]. White solid (28.7 mg, 87% yield); mp 88−89 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.46 (d, J = 9.0 Hz, 4H), 7.34 (d, J = 8.4 Hz, 4H), and 1.31 (s, 18H) ppm; HRMS (ESI) m/z [M]+ calculated for C20H26S2 330.1476, found 330.1462.
Bis(4-fluorophenyl) disulfide (3j) [34]. White solid (21.8 mg, 86% yield); mp 112−114 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.48−7.41 (m, 4H) and 7.05−6.98 (m, 4H) ppm; HRMS (ESI) m/z [M]+ calculated for C12H8F2S2 254.0035, found 254.0029; 19F NMR (376 MHz, CDCl3) δ -113.4 ppm.
Bis(3-fluorophenyl) disulfide (3k) [75]. White solid (20.6 mg, 81% yield); mp 93−94 °C; Rf = 0.6 (Petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.31−7.27 (m, 2H), 7.26 (s, 1H), 7.25−7.23 (m, 2 H), 7.22 (t, J = 1.8 Hz, 1H), and 6.96−6.91 (m, 2H) ppm; HRMS (ESI) m/z [M]+ calculated for C12H8F2S2 254.0035, found 254.0030; 19F NMR (376 MHz, CDCl3) δ -111.1 ppm.
Bis(2-fluorophenyl)disulfide (3l) [75]. Slight yellow oil (19.9 mg, 75% yield); Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.59 (td, J = 7.8, 1.8 Hz, 2 H), 7.28−7.25 (m, 2H), 7.12 (td, J = 7.8, 1.2 Hz, 2 H), and 7.08−7.04 (m, 2H) ppm; HRMS (ESI) m/z [M]+ calculated for C12H8F2S2 254.0035, found 254.0029; 19F NMR (376 MHz, CDCl3) δ -109.9 ppm.
4,4’-Dichlorodiphenyl disulfide (3p) [73]. White solid (26.0 mg, 91% yield); mp 68−70 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.40 (dt, J = 8.4, 4.8 Hz, 4H) and 7.28 (dt, J = 8.4, 4.8 Hz, 4H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 135.1, 133.7, 129.4, and 129.3; HRMS (ESI) m/z [M]+ calculated for C12H8Cl2S2 285.9444, found 285.9421.
Bis(3-clorophenyl)disulfide (3q) [73]. White solid (25.2 mg, 88% yield); mp 80−82 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.48 (d, J = 2.4 Hz, 1 H), 7.37−7.32 (m, 2 H), 7.26 (d, J = 2.4 Hz, 1H), and 7.24−7.20 (m, 4H) ppm; HRMS (ESI) m/z [M]+ calculated for C12H8Cl2S2 285.9444, found 285.9425.
Bis(2-clorophenyl)disulfide (3r) [76]. White solid (24.0 mg, 84% yield); mp 90−91 °C; Rf = 0.6 (petroleum ether); 1H NMR (400 MHz, CDCl3) δ 7.48 (dd, J = 8.0, 1.6 Hz, 2H), 7.30 (dd, J = 7.2, 1.6 Hz, 2H), 7.14 (td, J = 7.6, 1.2 Hz, 2H), and 7.09 (td, J = 7.6, 1.6 Hz, 2H) ppm; HRMS (ESI) m/z [M]+ calculated for C12H8Cl2S2 285.9444, found 285.9421.
Bis(4-bromophenyl)disulfide (3s) [75]. White solid (33.3 mg, 89% yield); mp 110−112 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.44−7.41 (m, 4 H) and 7.35−7.32 (m, 4 H) ppm; HRMS (ESI) m/z [M]+ calculated for C12H8Br2S2 373.8434, found 373.8414.
2,2’-Dinaphthyl disulfide (3t) [77]. White solid (26.1 mg, 82% yield); mp 139−141 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.99 (d, J = 1.8 Hz, 2H), 7.82−7.76 (m, 4H), 7.75−7.71 (m, 2H), 7.62 (dd, J = 9.0, 6.6 Hz, 2H), and 7.49−7.44 (m, 4H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C20H15S2 319.0610, found 319.0614.
Bis(4-trifluoromethylphenyl)disulfide (3u) [42]. White solid (28.3 mg, 80% yield); mp 119−120 °C; Rf = 0.6 (petroleum ether); 1H NMR (600 MHz, CDCl3) δ 7.60−7.55 (m, 8H) ppm; HRMS (ESI) m/z [M + H]+ calculated for C14H9F6S2 355.0044, found 355.0039; 19 F NMR (376 MHz, CDCl3) δ -62.5 ppm.
4. Conclusions
In summary, we have developed an efficient protocol for the selective access to symmetrical diaryl sulfides and disulfides using sodium sulfinates as sulfenylation reagents via homocoupling reaction. The utilization of readily available sodium sulfinates as coupling partners and good functional group tolerance with modest to excellent yields for most substrates enable these two types of novel transformations to become attractive alternatives for the preparation of the corresponding sulfur compounds. More importantly, sodium sulfinates were used for the first time to access symmetrical diaryl sulfides. The convinced mechanism, selectivity, and synthetic application of this transformation are still under investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27196232/s1, 1H, 13C, 19F NMR, IR and HPLC spectra.
Author Contributions
Conceptualization, X.L. and W.-C.G.; methodology, X.-Z.Y.; formal analysis, Y.-L.N.; data curation, X.-Z.Y.; writing—original draft preparation, X.L.; writing—review and editing, M.W. and W.-C.G.; superversion, W.-L.W.; funding acquisition, W.-C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21901179, and the Natural Science Foundation of Shanxi Province, grant numbers 20210302123141 and 202103021224067.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Pasquini, S.; Mugnaini, C.; Tintori, C.; Botta, M.; Trejos, A.; Arvela, R.K.; Larhed, M.; Witvrouw, M.; Michiels, M.; Christ, F.; et al. Investigations on the 4-Quinolone-3-carboxylic Acid Motif. 1. Synthesis and Structure−Activity Relationship of a Class of Human Immunodeficiency Virus type 1 Integrase Inhibitors. J. Med. Chem. 2008, 51, 5125. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C−S, C−Se, and C−Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596. [Google Scholar] [CrossRef] [PubMed]
- Brigg, S.; Pribut, N.; Basson, A.E.; Avgenikos, M.; Venter, R.; Blackie, M.A.; van Otterlo, W.A.L.; Pelly, S.C. Novel indole sulfides as potent HIV-1 NNRTIs. Bioorg. Med. Chem. Lett. 2016, 26, 1580. [Google Scholar] [CrossRef] [PubMed]
- Srogl, J.; Hývl, J.; Révészb, Á.; Schröder, D. Mechanistic insights into a copper–disulfide interaction in oxidation of imines by disulfides. Chem. Commun. 2009, 3463. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Wei, Y. Iodine-catalyzed oxidative system for 3-sulfenylation of indoles with disulfides using DMSO as oxidant under ambient conditions in dimethyl carbonate. Green Chem. 2012, 14, 2066. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-Cleavage-Triggered Chemosensors and Their Biological Applications. Chem. Rev. 2013, 113, 5071. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Tang, B.; Liang, S.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200. [Google Scholar] [CrossRef]
- Ke, F.; Qu, Y.; Jiang, Z.; Li, Z.; Wu, D.; Zhou, X. An Efficient Copper-Catalyzed Carbon−Sulfur Bond Formation Protocol in Water. Org. Lett. 2011, 13, 454. [Google Scholar] [CrossRef]
- García, N.; García-García, P.; Fernández-Rodríguez, M.A.; Rubio, R.; Pedrosa, M.R.; Arnáiz, F.J.; Sanz, R. Pinacol as a New Green Reducing Agent: Molybdenum- Catalyzed Chemoselective Reduction of Sulfoxides and Nitroaromatics. Adv. Synth. Catal. 2012, 354, 321. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, H.; Gao, H.; Xi, C. Cu-Catalyzed Synthesis of Diaryl Thioethers and S-Cycles by Reaction of Aryl Iodides with Carbon Disulfide in the Presence of DBU. J. Org. Chem. 2013, 78, 5001. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, K.T.; Jeon, H.B. Deoxygenation of Sulfoxides to Sulfides with Thionyl Chloride and Triphenylphosphine: Competition with the Pummerer Reaction. J. Org. Chem. 2013, 78, 6328. [Google Scholar] [CrossRef]
- García, N.; García-García, P.; Fernández-Rodríguez, M.A.; García, D.; Pedrosa, M.R.; Arnáiz, F.J.; Sanz, R. An unprecedented use for glycerol: Chemoselective reducing agent for sulfoxides. Green Chem. 2013, 15, 999. [Google Scholar] [CrossRef]
- Mitsudome, T.; Takahashi, Y.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Hydrogenation of Sulfoxides to Sulfides under Mild Conditions Using Ruthenium Nanoparticle Catalysts. Angew. Chem. Int. Ed. 2014, 53, 8348. [Google Scholar] [CrossRef]
- Touchy, A.S.; Siddiki, S.M.A.H.; Onodera, W.; Kon, K.; Shimizu, K. Hydrodeoxygenation of sulfoxides to sulfides by a Pt and MoOx co-loaded TiO2 catalyst. Green Chem. 2016, 18, 2554. [Google Scholar] [CrossRef]
- Wu, R.; Huang, K.; Qiu, G.; Liu, J.-B. Synthesis of Thioethers from Sulfonyl Chlorides, Sodium Sulfinates, and Sulfonyl Hydrazides. Synthesis 2019, 51, 3567. [Google Scholar] [CrossRef]
- Barba, F.; Ranz, F.; Batanero, B. Electrochemical transformation of diazonium salts into diaryl disulfides. Tetrahedron Lett. 2009, 50, 6798. [Google Scholar] [CrossRef]
- Taniguchi, N. Copper-catalyzed chalcogenation of aryl iodides via reduction of chalcogen elements by aluminum or magnesium. Tetrahedron 2012, 68, 10510. [Google Scholar] [CrossRef]
- Xiao, X.; Feng, M.; Jiang, X. New Design of a Disulfurating Reagent: Facile and Straightforward Pathway to Unsymmetrical Disulfanes by Copper-Catalyzed Oxidative Cross-Coupling. Angew. Chem. Int. Ed. 2016, 55, 14121. [Google Scholar] [CrossRef]
- Abbasi, M.; Nowrouzi, N.; Borazjani, S.G. Conversion of organic halides to disulfanes using KCN and CS2. Tetrahedron Lett. 2017, 58, 4251. [Google Scholar] [CrossRef]
- Xiao, X.; Xue, J.; Jiang, X. Polysulfurating reagent design for unsymmetrical polysulfide construction. Nat. Commun. 2018, 9, 2191. [Google Scholar] [CrossRef]
- Xue, J.; Jiang, X. Unsymmetrical polysulfidation via designed bilateral disulfurating reagents. Nat. Commun. 2020, 11, 4170. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pratt, D.A. A Divergent Strategy for Site-Selective Radical Disulfuration of Carboxylic Acids with Trisulfide-1,1-Dioxides. Angew. Chem. Int. Ed. 2021, 60, 15598. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; Rao, W.; Ackermann, L.; Wang, S.-Y. Efficient preparation of unsymmetrical disulfides by nickel-catalyzed reductive coupling strategy. Nat. Commun. 2022, 13, 2588. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Peng, W.-T.; Lee, Y.-H.; Chang, Y.-L.; Chen, Y.-J.; Lai, Y.-C.; Jheng, N.-Y.; Chen, H.-Y. Use of Base Control to Provide High Selectivity between Diaryl Thioether and Diaryl Disulfide for C–S Coupling Reactions of Aryl Halides and Sulfur and a Mechanistic Study. Organometallics 2013, 32, 5514. [Google Scholar] [CrossRef]
- Kamal, A.; Srinivasulu, V.; Murty, J.N.S.R.C.; Shankaraiah, N.; Nagesh, N.; Reddy, T.S.; Rao, A.V.S. Copper Oxide Nanoparticles Supported on Graphene Oxide- Catalyzed S-Arylation: An Efficient and Ligand-Free Synthesis of Aryl Sulfides. Adv. Synth. Catal. 2013, 355, 2297. [Google Scholar] [CrossRef]
- Cai, M.; Yao, R.; Chen, L.; Zhao, H. A simple, efficient and recyclable catalytic system for carbon–sulfur coupling of aryl halides with thioacetamide. J. Mol. Catal. A Chem. 2014, 395, 349. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Gorginpour, F.; Samadi, A. Dithiooxamide as an Effective Sulfur Surrogate for Odorless High-Yielding Carbon–Sulfur Bond Formation in Wet PEG200 as an Eco-Friendly, Safe, and Recoverable Solvent. Eur. J. Org. Chem. 2015, 2914. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Taherinia, Z. The first report on the preparation of peptide nanofibers decorated with zirconium oxide nanoparticles applied as versatile catalyst for the amination of aryl halides and synthesis of biaryl and symmetrical sulfides. New. J. Chem. 2017, 41, 9414. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Q.; Xu, W.; Zeng, M.-T.; Dong, Z.-B. Nickel-Catalyzed C–S Coupling: Synthesis of Diaryl Sulfides Starting from Phenyldithiocarbamates and Iodobenzenes. Eur. J. Org. Chem. 2017, 5795. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Dong, Z.-B. Phenyldithiocarbamates: Efficient Sulfuration Reagents in the Chan–Lam Coupling Reaction. Eur. J. Org. Chem. 2018, 815. [Google Scholar] [CrossRef]
- Dong, Z.-B.; Balkenhohl, M.; Tan, E.; Knochel, P. Synthesis of Functionalized Diaryl Sulfides by Cobalt-Catalyzed Coupling between Arylzinc Pivalates and Diaryl Disulfides. Org. Lett. 2018, 20, 7581. [Google Scholar] [CrossRef] [PubMed]
- Arguello, J.E.; Schmidt, L.C.; Penenory, A.B. “One-Pot” Two-Step Synthesis of Aryl Sulfur Compounds by Photoinduced Reactions of Thiourea Anion with Aryl Halides. Org. Lett. 2003, 5, 4133. [Google Scholar] [CrossRef] [PubMed]
- Soleiman-Beigi, M.; Mohammadi, F. A novel copper-catalyzed, one-pot synthesis of symmetric organic disulfides from alkyl and aryl halides: Potassium 5-methyl-1,3,4-oxadiazole-2-thiolate as a novel sulfur transfer reagent. Tetrahedron Lett. 2012, 53, 7028. [Google Scholar] [CrossRef]
- Li, Z.K.; Ke, F.; Deng, H.; Xu, H.L.; Xiang, H.F.; Zhou, X.G. Synthesis of disulfides and diselenides by copper-catalyzed coupling reactions in water. Org. Biomol. Chem. 2013, 11, 2943. [Google Scholar] [CrossRef]
- Soleiman-Beigi, M.; Hemmati, M. An efficient, one-pot and CuCl-catalyzed route to the synthesis of symmetric organic disulfides via domino reactions of thioacetamide and aryl (alkyl) halides. Appl. Organometal. Chem. 2013, 27, 734. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Zhang, Y.; Chang, H.; Gao, W.; Wei, W. Synthesis and nano-Pd catalyzed chemoselective oxidation of symmetrical and unsymmetrical sulfides. Org. Biomol. Chem. 2019, 17, 3048. [Google Scholar] [CrossRef]
- Olah, G.A.; Narang, S.C.; Field, L.D.; Karpeles, R. Synthetic methods and reactions. 101. Reduction of sulfonic acids and sulfonyl derivatives to disulfides with iodide in the presence of boron halides. J. Org. Chem. 1981, 46, 2408. [Google Scholar] [CrossRef]
- Zheng, Y.; Qing, F.-L.; Huang, Y.; Xu, X.-H. Tunable and Practical Synthesis of Thiosulfonates and Disulfides from Sulfonyl Chlorides in the Presence of Tetrabutylammonium Iodide. Adv. Synth. Catal. 2016, 358, 3477. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Mallakpour, S.E.; Adibi, H. Selective and Efficient Oxidation of Sulfides and Thiols with Benzyltriphenylphosphonium Peroxymonosulfate in Aprotic Solvent. J. Org. Chem. 2002, 67, 8666. [Google Scholar] [CrossRef]
- Banfield, S.C.; Omori, A.T.; Leisch, H.; Hudlicky, T. Unexpected Reactivity of the Burgess Reagent with Thiols: Synthesis of Symmetrical Disulfides. J. Org. Chem. 2007, 72, 4989. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Aerobic oxidation of thiols to disulfides using iron metal–organic frameworks as solid redoxcatalysts. Chem. Commun. 2010, 46, 6476. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Tanaka, K.; Nishiyama, K.; Ando, W. Aerobic Oxidation of Thiols to Disulfides Catalyzed by Diaryl Tellurides under Photosensitized Conditions. J. Org. Chem. 2011, 76, 4173. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Rodenas, T.; Sabater, M.J. Aerobic oxidation of thiols to disulfides by heterogeneous gold catalysts. Chem. Sci. 2012, 3, 398. [Google Scholar] [CrossRef]
- Li, X.-B.; Li, Z.-J.; Gao, Y.-J.; Meng, Q.-Y.; Yu, S.; Weiss, R.G.; Tung, C.-H.; Wu, L.-Z. Mechanistic Insights into the Interface-Directed Transformation of Thiols into Disulfides and Molecular Hydrogen by Visible-Light Irradiation of Quantum Dots. Angew. Chem. Int. Ed. 2014, 53, 2085. [Google Scholar] [CrossRef]
- Tabrizian, E.; Amoozadeh, A.; Rahmani, S. Sulfamic acid-functionalized nano-titanium dioxide as an efficient, mild and highly recyclable solid acid nanocatalyst for chemoselective oxidation of sulfides and thiols. RSC Adv. 2016, 6, 21854. [Google Scholar] [CrossRef]
- Samanta, S.; Ray, S.; Ghosh, A.B.; Biswas, P. 3,6-Di(pyridin-2-yl)-1,2,4,5-tetrazine (pytz) mediated metal-free mild oxidation of thiols to disulfides in aqueous medium. RSC Adv. 2016, 6, 39356. [Google Scholar] [CrossRef]
- Paul, S.; Islam, S.M. Oxidative dehydrogenation of thiols to disulfides at room temperature using silica supported iron oxide as an efficient solid catalyst. RSC Adv. 2016, 6, 95753. [Google Scholar] [CrossRef]
- Laudadio, G.; Straathof, N.J.W.; Lanting, M.D.; Knoops, B.; Hessel, V.; Noël, T. An environmentally benign and selective electrochemical oxidation of sulfides and thiols in a continuous-flow microreactor. Green Chem. 2017, 19, 4061. [Google Scholar] [CrossRef]
- Ning, Y.; Ji, Q.; Liao, P.; Anderson, E.A.; Bi, X. Silver-Catalyzed Stereoselective Aminosulfonylation of Alkynes. Angew. Chem. Int. Ed. 2017, 56, 13805. [Google Scholar] [CrossRef]
- Yue, H.; Zhu, C.; Rueping, M. Cross-Coupling of Sodium Sulfinates with Aryl, Heteroaryl, and Vinyl Halides by Nickel/Photoredox Dual Catalysis. Angew. Chem. Int. Ed. 2018, 57, 1371. [Google Scholar] [CrossRef]
- Cao, L.; Luo, S.-H.; Jiang, K.; Hao, Z.-F.; Wang, B.-W.; Pang, C.-M.; Wang, Z.-Y. Disproportionate Coupling Reaction of Sodium Sulfinates Mediated by BF3·OEt2: An Approach to Symmetrical/Unsymmetrical Thiosulfonate. Org. Lett. 2018, 20, 4754. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Li, P.; Zhang, Y.; Wang, L. A Sulfenylation Reaction: Direct Synthesis of 3-Arylsulfinylindoles from Arylsulfinic Acids and Indoles in Water. Org. Lett. 2015, 17, 832. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-Z.; Li, H.-J.; Yang, H.-R.; Zhang, Z.-Y.; Xie, L.-J.; Wu, Y.-C. TMSOTf-Promoted Sulfinylation of Electron-Rich Aromatics with Sodium Arylsulfinates. Synlett 2020, 31, 349. [Google Scholar]
- Xiao, F.; Xie, H.; Liu, S.; Deng, G.-J. Iodine-Catalyzed Regioselective Sulfenylation of Indoles with Sodium Sulfinates. Adv. Synth. Catal. 2014, 356, 364. [Google Scholar] [CrossRef]
- Guo, S.; He, W.; Xiang, J.; Yuan, Y. Palladium-catalyzed direct thiolation of ethers with sodium sulfinates. Tetrahedron Lett. 2014, 55, 6407. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Lu, G.-P.; Cai, C.; Yi, W.-B. Odorless, One-Pot Regio- and Stereoselective Iodothiolation of Alkynes with Sodium Arenesulfinates under Metal-Free Conditions in Water. Org. Lett. 2015, 17, 3310. [Google Scholar] [CrossRef]
- Wang, B.W.; Jiang, K.; Li, J.-X.; Luo, S.-H.; Wang, Z.-Y.; Jiang, H.-F. 1,1-Diphenylvinylsulfide as a Functional AIEgen Derived from the Aggregation-Caused-Quenching Molecule 1,1-Diphenylethene through Simple Thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338. [Google Scholar] [CrossRef]
- Liu, Y.; Lam, L.Y.; Ye, J.; Blanchard, N.; Ma, C. DABCO-promoted Diaryl Thioether Formation by Metal-catalyzed Coupling of Sodium Sulfinates and Aryl Iodides. Adv. Synth. Catal. 2020, 362, 2326. [Google Scholar] [CrossRef]
- Lam, L.Y.; Ma, C. Chan–Lam-Type C–S Coupling Reaction by Sodium Aryl Sulfinates and Organoboron Compounds. Org. Lett. 2021, 23, 6164. [Google Scholar] [CrossRef]
- Pinnick, H.W.; Reynolds, M.A.; McDonald, R.T., Jr.; Brewster, W.D. Reductive coupling of aromatic sulfinate salts to disulfides. J. Org. Chem. 1980, 45, 930. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhang, Y.M. The Reduction of Arylsulfonyl Chlorides and Sodium Arylsulfinates with TiCl4/Sm System. A Novel Method for the Preparation of Diaryldisulfides. Syn. Commun. 1996, 26, 135. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Karimi, B. Efficient Deoxygenation of Sulfoxides to Thioethers and Reductive Coupling of Sulfonyl Chlorides to Disulfides with Tungsten Hexachloride. Synthesis 1999, 1999, 500. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Jamalian, A. Deoxygenation of Sulfoxides and Reductive Coupling of Sulfonyl Chlorides, Sulfinates and Thiosulfonates Using Silphos [PCl3-n(SiO2)n] as a Heterogeneous Phosphine Reagent. Synlett 2005, 9, 1447. [Google Scholar] [CrossRef]
- Still, I.W.J.; Watson, I.D.G. An efficient synthetic route to aryl thiocyanates from arenesulfinates. Synth. Commun. 2001, 31, 1355. [Google Scholar] [CrossRef]
- Emmett, E.J.; Hayter, B.R.; Willis, M.C. Palladium-Catalyzed Synthesis of Ammonium Sulfinates from Aryl Halides and a Sulfur Dioxide Surrogate: A Gas- and Reductant-Free Process. Angew. Chem. Int. Ed. 2014, 53, 10204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, J.; Chen, J.; Cao, F.; Hou, Y.; Yang, Y.; Deng, X.; Yang, J.; Wu, L.; Shao, X.; et al. Dechalcogenization of Aryl Dichalcogenides to Synthesize Aryl Chalcogenides via Copper Catalysis. ACS Catal. 2020, 10, 2707. [Google Scholar] [CrossRef]
- Li, Y.-M.; Nie, C.-P.; Wang, H.-P.; Verpoort, F.; Duan, C.-Y. A Highly Efficient Method for the Copper-Catalyzed Selective Synthesis of Diaryl Chalcogenides from Easily Available Chalcogen Sources. Eur. J. Org. Chem. 2011, 2011, 7331–7338. [Google Scholar] [CrossRef]
- Li, X.-K.; Yuan, T.-J.; Chen, J.-M. Efficient Copper(I)-Catalyzed S-Arylation of KSCN with Aryl Halides in PEG-400. Chin. J. Chem. 2012, 30, 651–655. [Google Scholar] [CrossRef]
- Carpino, L.A.; Gao, H.S.; Ti, G.S.; Segev, D. Thioxanthene Dioxide Based Amino-Protecting Groups Sensitive to Pyridine Bases and Dipolar Aprotic Solvents. J. Org. Chem. 1989, 54, 5887–5897. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Li, Y.; Zhang, B.-C.; Yang, Y.-N.; Wang, X.-S. Palladium-Catalyzed Enantioselective C-H Olefination of Diaryl Sulfoxides via Parallel Kinetic Resolution and Desymmetrization. Angew. Chem. Int. Ed. 2018, 57, 5129–5133. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.-R.; Karimzadeh, M.; Azizi, G. Highly Efficient and Magnetically Separable Nano-CuFe2O4 Catalyzed S-Arylation of Thiourea by Aryl/Heteroaryl Halides. Chin. Chem. Lett. 2014, 25, 1382–1386. [Google Scholar] [CrossRef]
- García-López, J.-A.; Çetin, M.; Greaney, M.F. Synthesis of Hindered Biaryls via Aryne Addition and in Situ Dimerization. Org. Lett. 2015, 17, 2649–2651. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, H.; Wang, C.-P.; Wan, J.-P.; Wen, C.-P. Bio-Based Green Solvent Mediated Disufide Synthesis via Thiol Couplings Free of Catalyst and Additive. RSC Adv. 2013, 3, 21369–21372. [Google Scholar] [CrossRef]
- Murahashi, S.I.; Zhang, D.Z.; Iida, H.; Miyawaki, T.; Uenaka, M.; Uenaka, K.; Meguro, K. Flavin-Catalyzed Aerobic Oxidation of Sulfides and Thiols with Formic Acid/Triethylamine. Chem. Commun. 2014, 50, 10295–10298. [Google Scholar] [CrossRef] [PubMed]
- Ruano, J.L.G.; Parra, A.; Alemán, J. Efficient Synthesis of Disulfides by Air Oxidation of Thiols under Sonication. Green Chem. 2008, 10, 706–711. [Google Scholar] [CrossRef]
- Chai, P.J.; Li, Y.S.; Tan, C.X. An Efficient and Convenient Method for Preparation of Disulfides from Thiols Using Air as Oxidant Catalyzed by Co-Salophen. Chin. Chem. Lett. 2011, 22, 1403–1406. [Google Scholar] [CrossRef]
- Wang, L.; Clive, D.L. [[(tert-Butyl)dimethylsilyl]oxy]methyl Group for Sulfur Protection. Org. Lett. 2011, 42, 1734–1737. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).