The Antioxidative Effects of Picein and Its Neuroprotective Potential: A Review of the Literature
Abstract
1. Background and Motivation
2. Pathogenesis of Neurodegenerative Diseases
3. Antioxidant and Neuroprotective Agents Suggested for the Treatment of Neurodegenerative Diseases
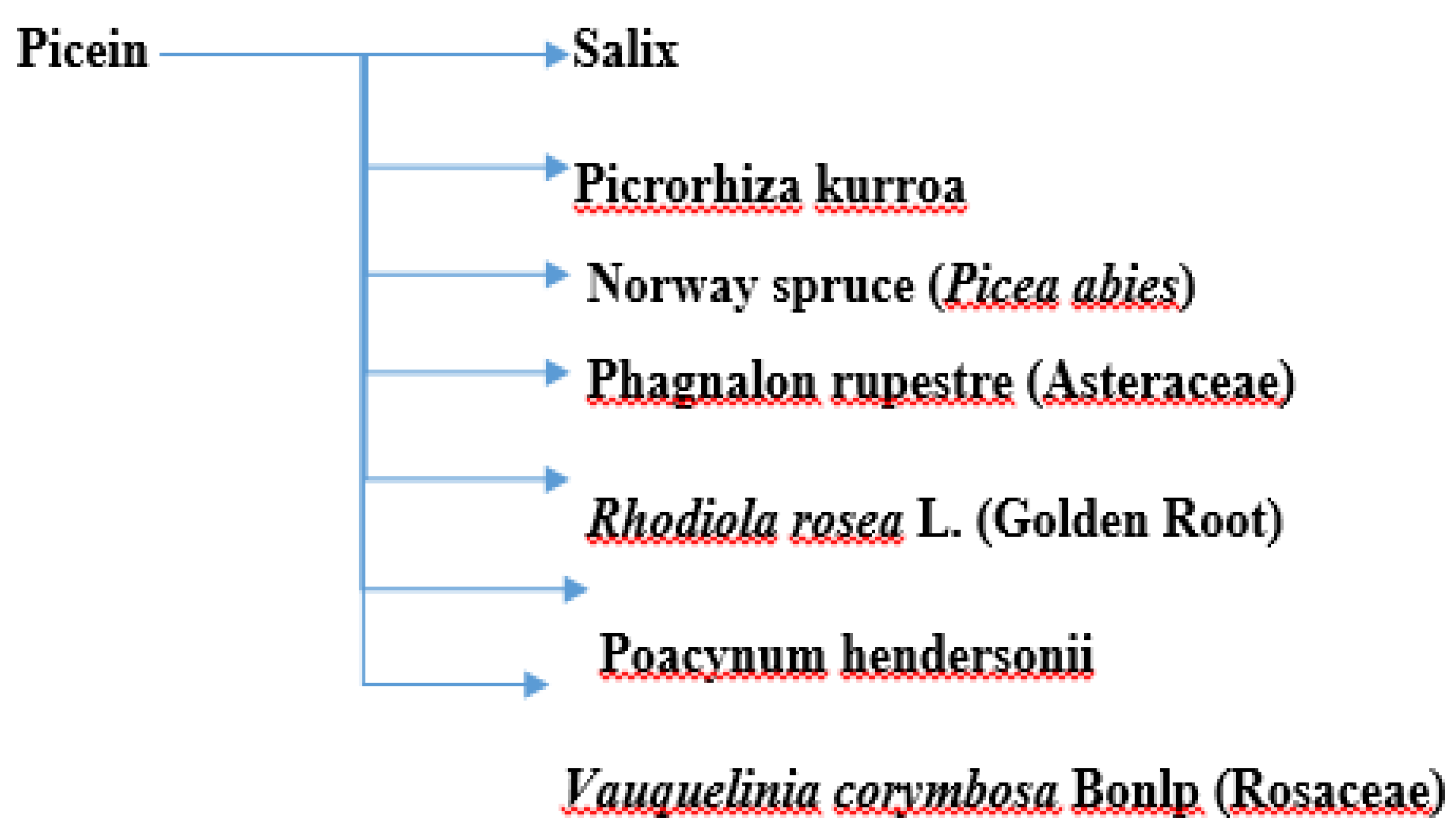
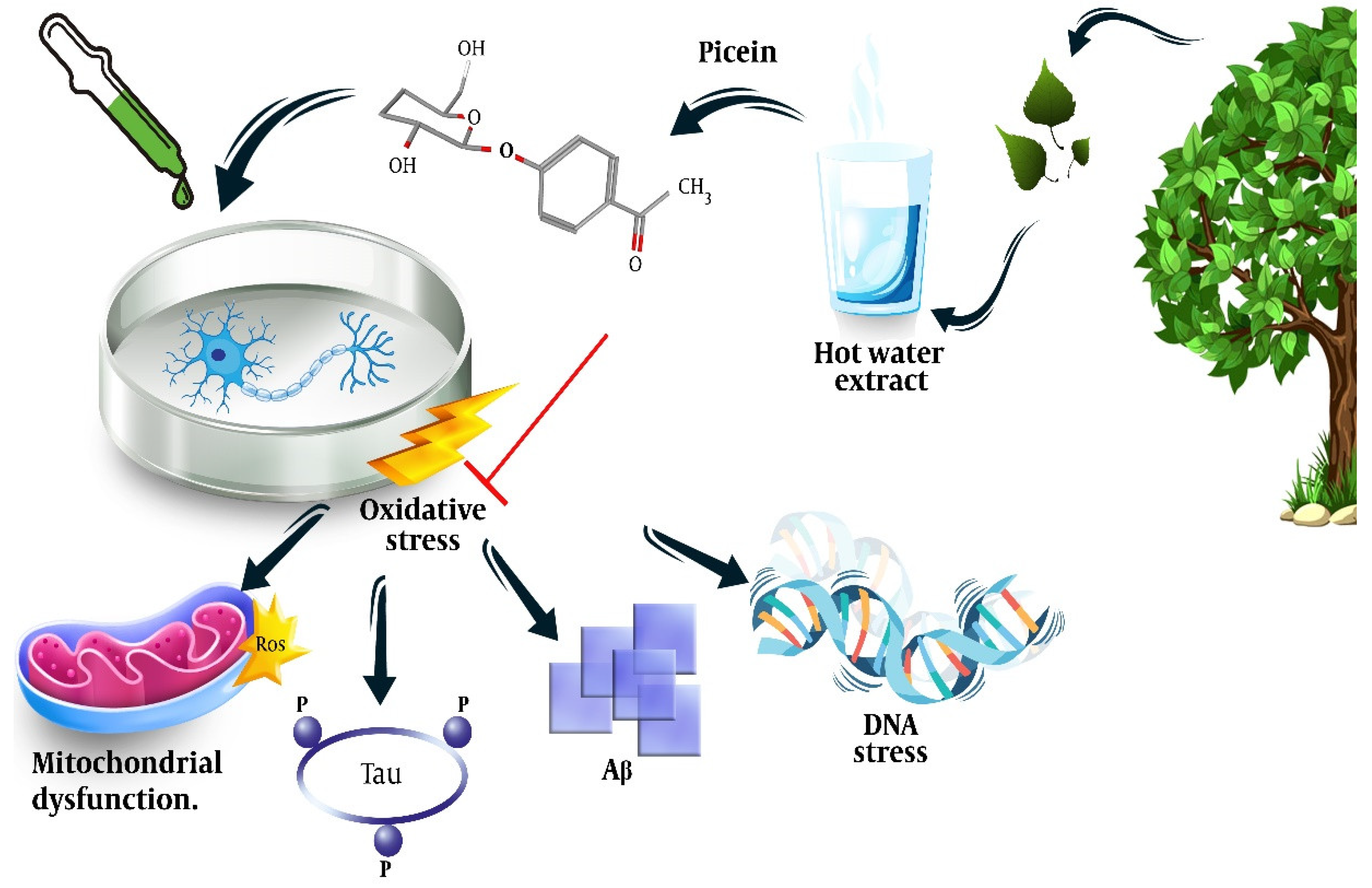
4. Antioxidative and Neuroprotective Properties of Picein
4.1. Willow (Salix)
4.2. Picrorhiza kurroa
4.3. Norway Spruce (Picea abies)
4.4. Other Plants
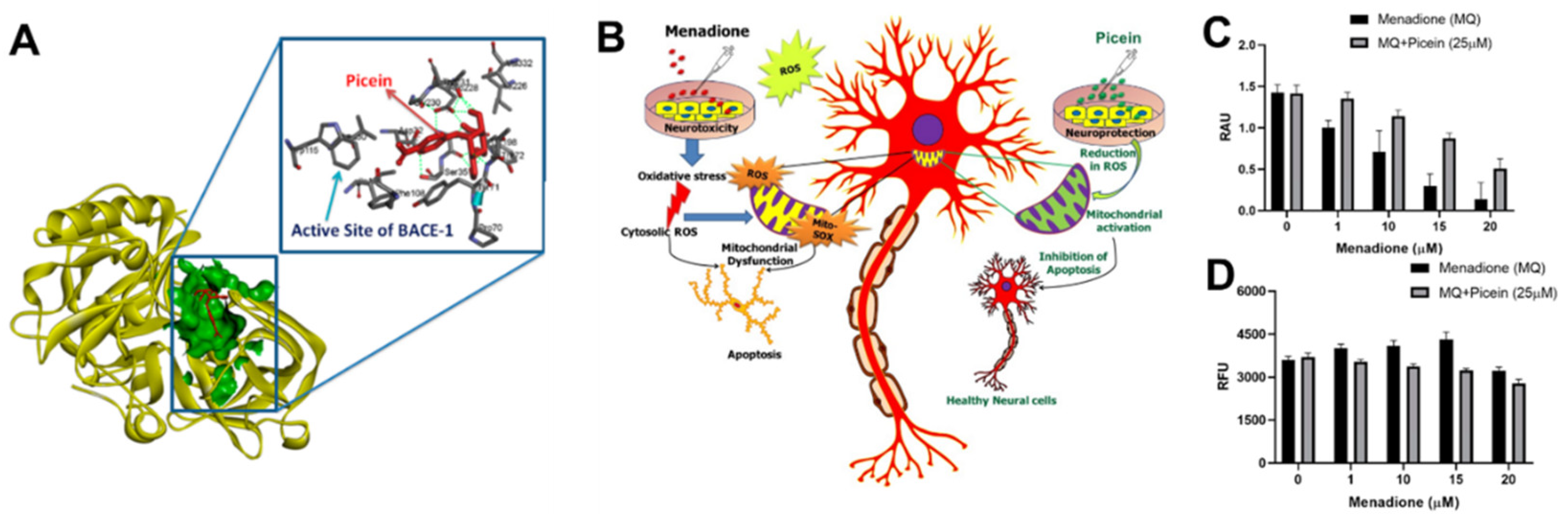
5. The Potential Role of Picein in the Treatment of Alzheimer’s Disease
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; George, B.P.; Leff, B.; Willis, A.W. The coming crisis: Obtaining care for the growing burden of neurodegenerative conditions. Neurology 2013, 80, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, P.; Tropepi, D. Drugs and clinical trials in neurodegenerative diseases. Ann. Dell’istituto Super. Sanità 2011, 47, 49–54. [Google Scholar]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; Lang, A.E. Disease-modifying strategies for Parkinson’s disease. Mov. Disord. 2015, 30, 1442–1450. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G. Pathogenesis and disease-modifying therapy in Alzheimer’s disease: The flat line of progress. Arch. Med. Res. 2012, 43, 694–698. [Google Scholar] [CrossRef]
- Dunkel, P.; Chai, C.L.; Sperlagh, B.; Huleatt, P.B.; Matyus, P. Clinical utility of neuroprotective agents in neurodegenerative diseases: Current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin. Investig. Drugs 2012, 21, 1267–1308. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxidative Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: Role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef]
- Lai, A.Y.; McLaurin, J. Clearance of amyloid-β peptides by microglia and macrophages: The issue of what, when and where. Future Neurol. 2012, 7, 165–176. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376. [Google Scholar]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 2015, 6, 206. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxidative Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Elyasi, L.; Ghazvini, H. The protective effects of citrus aurantium extract on a 6-hydroxydopamine-induced model of Parkinson’s disease in male rats. Anat. Sci. J. 2020, 17, 1–6. [Google Scholar]
- Elyasi, L.; Jahanshahi, M.; Ghazvini, H.; Nikmahzar, E. The protective effects of citrus aurantium flower extract against 6-hydroxydopamine-mediated cell damage in human neuroblastoma SH-SY5Y cells. Int. J. Morphol. 2018, 36, 435–440. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Hyun, S.; Shin, D. Chemical-mediated targeted protein degradation in neurodegenerative diseases. Life 2021, 11, 607. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative diseases: Regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef]
- Gao, Y.-L.; Wang, N.; Sun, F.-R.; Cao, X.-P.; Zhang, W.; Yu, J.-T. Tau in neurodegenerative disease. Ann. Transl. Med. 2018, 6, 175. [Google Scholar] [CrossRef]
- Tracy, T.E.; Gan, L. Tau-mediated synaptic and neuronal dysfunction in neurodegenerative disease. Curr. Opin. Neurobiol. 2018, 51, 134–138. [Google Scholar] [CrossRef]
- Yan, R.; Vassar, R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef]
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Martin, L.J. Biology of mitochondria in neurodegenerative diseases. Prog. Mol. Biol. Transl. Sci. 2012, 107, 355–415. [Google Scholar] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dehghanian, F.; Kalantaripour, T.P.; Esmaeilpour, K.; Elyasi, L.; Oloumi, H.; Pour, F.M.; Asadi-Shekaari, M. Date seed extract ameliorates β-amyloid-induced impairments in hippocampus of male rats. Biomed. Pharmacother. 2017, 89, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, L.; Eftekhar-Vaghefi, S.H.; Esmaeili-Mahani, S. Morphine protects SH-SY5Y human neuroblastoma cells against 6-hydroxydopamine–induced cell damage: Involvement of anti-oxidant, calcium blocking, and anti-apoptotic properties. Rejuvenat. Res. 2014, 17, 255–263. [Google Scholar] [CrossRef]
- Ratheesh, G.; Tian, L.; Venugopal, J.R.; Ezhilarasu, H.; Sadiq, A.; Fan, T.-P.; Ramakrishna, S. Role of medicinal plants in neurodegenerative diseases. Biomanuf. Rev. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Sharma, D.; Salvi, M. Neuroprotective effects of Withania somnifera dunal.: A possible mechanism. Neurochem. Res. 2009, 34, 1975–1983. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Effects of Ashwagandha (roots of Withania somnifera) on neurodegenerative diseases. Biol. Pharm. Bull. 2014, 37, 892–897. [Google Scholar] [CrossRef]
- Kurapati, K.R.V.; Atluri, V.S.R.; Samikkannu, T.; Nair, M.P. Ashwagandha (Withania somnifera) reverses β-amyloid1-42 induced toxicity in human neuronal cells: Implications in HIV-associated neurocognitive disorders (HAND). PLoS ONE 2013, 8, e77624. [Google Scholar] [CrossRef]
- Kumar, S.; Seal, C.J.; Howes, M.; Kite, G.C.; Okello, E.J. In vitro protective effects of Withania somnifera (L.) dunal root extract against hydrogen peroxide and β-amyloid (1–42)-induced cytotoxicity in differentiated PC12 cells. Phytother. Res. 2010, 24, 1567–1574. [Google Scholar] [CrossRef]
- Prakash, J.; Yadav, S.K.; Chouhan, S.; Singh, S.P. Neuroprotective role of Withania somnifera root extract in Maneb–Paraquat induced mouse model of parkinsonism. Neurochem. Res. 2013, 38, 972–980. [Google Scholar] [CrossRef]
- Prakash, J.; Chouhan, S.; Yadav, S.K.; Westfall, S.; Rai, S.N.; Singh, S.P. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem. Res. 2014, 39, 2527–2536. [Google Scholar] [CrossRef]
- Baitharu, I.; Jain, V.; Deep, S.N.; Hota, K.B.; Hota, S.K.; Prasad, D.; Ilavazhagan, G. Withania somnifera root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J. Ethnopharmacol. 2013, 145, 431–441. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Al Hilo, A.S.; Al-Balas, Q.A.; El-Salem, K.; El-Elimat, T.; Alali, F.Q. Withania somnifera root powder protects againist post-traumatic stress disorder-induced memory impairment. Mol. Biol. Rep. 2019, 46, 4709–4715. [Google Scholar] [CrossRef]
- Irfan, M.; Kwak, Y.-S.; Han, C.-K.; Hyun, S.H.; Rhee, M.H. Adaptogenic effects of Panax ginseng on modulation of cardiovascular functions. J. Ginseng Res. 2020, 44, 538–543. [Google Scholar] [CrossRef]
- Kim, C.-J.; Ryu, H.-Y.; Lee, S.; Lee, H.-J.; Chun, Y.-S.; Kim, J.-K.; Yu, C.-Y.; Ghimire, B.; Lee, J.-G. Neuroprotective effect and antioxidant potency of fermented cultured wild ginseng root extracts of Panax ginseng CA meyer in mice. Molecules 2021, 26, 3001. [Google Scholar] [CrossRef]
- Dou, J.; Heinonen, J.; Vuorinen, T.; Xu, C.; Sainio, T. Chromatographic recovery and purification of natural phytochemicals from underappreciated willow bark water extracts. Sep. Purif. Technol. 2021, 261, 118247. [Google Scholar] [CrossRef]
- Cho, I.-H. Effects of Panax ginseng in neurodegenerative diseases. J. Ginseng Res. 2012, 36, 342. [Google Scholar] [CrossRef]
- Lee, S.-T.; Chu, K.; Sim, J.-Y.; Heo, J.-H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Heo, J.-H.; Lee, S.-T.; Chu, K.; Oh, M.J.; Park, H.-J.; Shim, J.-Y.; Kim, M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur. J. Neurol. 2008, 15, 865–868. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 1256, 111–122. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, Y.; He, Y.; Shen, T.; Ji, L.; Li, F.; Hu, W. Vina-ginsenoside R4 from panax ginseng leaves alleviates 6-OHDA-induced neurotoxicity in PC12 cells via the PI3K/Akt/GSK-3β signaling pathway. J. Agric. Food Chem. 2020, 68, 15239–15248. [Google Scholar] [CrossRef]
- Tu, L.-H.; Ma, J.; Liu, H.-P.; Wang, R.-R.; Luo, J. The neuroprotective effects of ginsenosides on calcineurin activity and tau phosphorylation in SY5Y cells. Cell. Mol. Neurobiol. 2009, 29, 1257–1264. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease. Mol. Med. Rep. 2019, 19, 2975–2998. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, D.; Lee, H.L.; Kim, C.-E.; Jung, K.; Kang, K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Goozee, K.; Shah, T.; Sohrabi, H.R.; Rainey-Smith, S.R.; Brown, B.; Verdile, G.; Martins, R.N. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Substantiation for the use of curcumin during the development of neurodegeneration after brain ischemia. Int. J. Mol. Sci. 2020, 21, 517. [Google Scholar] [CrossRef]
- Hu, S.; Maiti, P.; Ma, Q.; Zuo, X.; Jones, M.R.; Cole, G.M.; Frautschy, S.A. Clinical development of curcumin in neurodegenerative disease. Expert Rev. Neurother. 2015, 15, 629–637. [Google Scholar] [CrossRef]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- El Nebrisi, E.; Javed, H.; Ojha, S.K.; Oz, M.; Shehab, S. Neuroprotective effect of Curcumin on the nigrostriatal pathway in a 6-hydroxydopmine-induced rat model of Parkinson’s disease is mediated by α7-nicotinic receptors. Int. J. Mol. Sci. 2020, 21, 7329. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Sahebkar, A.; Askari, G.; Johnston, T.P.; Alikiaii, B.; Bagherniya, M. The clinical use of curcumin on neurological disorders: An updated systematic review of clinical trials. Phytother. Res. 2021, 35, 6862–6882. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Sun, Z.-K.; Yang, H.-Q.; Chen, S.-D. Traditional Chinese medicine: A promising candidate for the treatment of Alzheimer’s disease. Transl. Neurodegener. 2013, 2, 6. [Google Scholar] [CrossRef]
- Fu, L.-M.; Li, J.-T. A systematic review of single chinese herbs for Alzheimer’s disease treatment. Evid.-Based Complementary Altern. Med. 2011, 2011, 640284. [Google Scholar] [CrossRef]
- Christen, Y. Ginkgo biloba and neurodegenerative disorders. Front. Biosci.-Landmark 2004, 9, 3091–3104. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, P.; Li, J.; Liu, T.; Zhang, Y.; Wang, Q.; Zhang, J.; Lu, X.; Fan, X. Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease. J. Pharm. Anal. 2021, 11, 220–231. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Guo, H. An updated review of randomized clinical trials testing the improvement of cognitive function of Ginkgo biloba extract in healthy people and Alzheimer’s patients. Front. Pharmacol. 2020, 10, 1688. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Albu, C.; Radu, G.L. Verbascum phlomoides and Solidago virgaureae herbs as natural source for preventing neurodegenerative diseases. J. Herb. Med. 2016, 6, 180–186. [Google Scholar] [CrossRef]
- Kesari, K.K.; Dhasmana, A.; Shandilya, S.; Prabhakar, N.; Shaukat, A.; Dou, J.; Rosenholm, J.M.; Vuorinen, T.; Ruokolainen, J. Plant-derived natural biomolecule picein attenuates menadione induced oxidative stress on neuroblastoma cell mitochondria. Antioxidants 2020, 9, 552. [Google Scholar] [CrossRef]
- Noleto-Dias, C.; Wu, Y.; Bellisai, A.; Macalpine, W.; Beale, M.H.; Ward, J.L. Phenylalkanoid glycosides (non-salicinoids) from wood chips of Salix triandra× dasyclados hybrid willow. Molecules 2019, 24, 1152. [Google Scholar] [CrossRef]
- Tawfeek, N.; Mahmoud, M.F.; I Hamdan, D.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus salix: An updated review. Front. Pharmacol. 2021, 12, 50. [Google Scholar] [CrossRef]
- Di Caprio, R.; Monfrecola, G.; Balato, A.; Balato, N.; Gasparri, F.; Micillo, R.; Lembo, S. The anti-inflammatory and antioxidant properties of 1, 2-decanediol and willow bark extract in lipopolysaccharide-stimulated keratinocytes. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2017, 154, 624–631. [Google Scholar] [CrossRef]
- di Giacomo, V.; Ferrante, C.; Ronci, M.; Cataldi, A.; Di Valerio, V.; Rapino, M.; Recinella, L.; Chiavaroli, A.; Leone, S.; Vladimir-Knežević, S.; et al. Multiple pharmacological and toxicological investigations on Tanacetum parthenium and Salix alba extracts: Focus on potential application as anti-migraine agents. Food Chem. Toxicol. 2019, 133, 110783. [Google Scholar] [CrossRef]
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; van Nederkassel, A.M.; Vander Heyden, Y.; Krauze-Baranowska, M.; Glód, D.; Baczek, T. Chromatographic analysis of salicylic compounds in different species of the genus Salix. J. Sep. Sci. 2007, 30, 2958–2966. [Google Scholar] [CrossRef]
- Maistro, E.L.; Terrazzas, P.M.; Perazzo, F.F.; Gaivão, I.O.N.D.M.; Sawaya, A.C.H.F.; Rosa, P.C.P. Salix alba (white willow) medicinal plant presents genotoxic effects in human cultured leukocytes. J. Toxicol. Environ. Health Part A 2019, 82, 1223–1234. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, S.J. Efficacy and safety of white willow bark (Salix alba) extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef]
- Durak, A.; Gawlik-Dziki, U.; Sugier, D. Coffee enriched with willow (Salix purpurea and Salix myrsinifolia) bark preparation–Interactions of antioxidative phytochemicals in a model system. J. Funct. Foods 2015, 18, 1106–1116. [Google Scholar] [CrossRef]
- Durak, A.; Gawlik-Dziki, U. The study of interactions between active compounds of coffee and willow (Salix sp.) bark water extract. BioMed Res. Int. 2014, 2014, 386953. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Łojkowska, E. Chromatographic analysis of simple phenols in some species from the genus Salix. Phytochem. Anal. 2010, 21, 463–469. [Google Scholar] [CrossRef]
- Sulima, P.; Krauze-Baranowska, M.; Przyborowski, J.A. Variations in the chemical composition and content of salicylic glycosides in the bark of Salix purpurea from natural locations and their significance for breeding. Fitoterapia 2017, 118, 118–125. [Google Scholar] [CrossRef]
- Heiska, S.; Tikkanen, O.-P.; Rousi, M.; Julkunen-Tiitto, R. Bark salicylates and condensed tannins reduce vole browsing amongst cultivated dark-leaved willows (Salix myrsinifolia). Chemoecology 2007, 17, 245–253. [Google Scholar] [CrossRef]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 470–478. [Google Scholar] [CrossRef]
- Lavola, A.; Maukonen, M.; Julkunen-Tiitto, R. Variability in the composition of phenolic compounds in winter-dormant Salix pyrolifolia in relation to plant part and age. Phytochemistry 2018, 153, 102–110. [Google Scholar] [CrossRef]
- Fischbach, R.J.; Kossmann, B.; Panten, H.; Steinbrecher, R.; Heller, W.; Seidlitz, H.K.; Sandermann, H.; Hertkorn, N.; Schnitzler, J.-P. Seasonal accumulation of ultraviolet-B screening pigments in needles of Norway spruce (Picea abies (L.) Karst.). Plant Cell Environ. 1999, 22, 27–37. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Kowalski, R.; Rój, E.; Warmiński, K.; Krzyżaniak, M.; Gil, L.; Stolarski, M.J. Characterization of bioactive compounds in the biomass of black locust, poplar and willow. Trees 2019, 33, 1235–1263. [Google Scholar] [CrossRef]
- Heller, W.; Rosemann, D.; Osswald, W.; Benz, B.; Schönwitz, R.; Lohwasser, K.; Kloosa, M.; Sandermann, H., Jr. Biochemical response of Norway spruce (Picea abies (L.) Karst.) towards 14-month exposure to ozone and acid mist: Part I—Effects on polyphenol and monoterpene metabolism. Environ. Pollut. 1990, 64, 353–366. [Google Scholar] [CrossRef]
- Dou, J.; Xu, W.; Koivisto, J.J.; Mobley, J.K.; Padmakshan, D.; Kögler, M.; Xu, C.; Willför, S.M.; Ralph, J.; Vuorinen, T. Characteristics of hot water extracts from the bark of cultivated willow (Salix sp.). ACS Sustain. Chem. Eng. 2018, 6, 5566–5573. [Google Scholar] [CrossRef]
- Jeon, S.H.; Chun, W.; Choi, Y.J.; Kwon, Y.S. Cytotoxic constituents from the bark of Salix hulteni. Arch. Pharmacal Res. 2008, 31, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, W.; Liu, F.; Zhang, P.; Tang, F.; Zeng, L.; Tang, K. Separation of active components tyrosol and salidroside from Rhodiola rosea crude extract by two-step multistage fractionation extraction. Chem. Eng. Process.-Process Intensif. 2022, 172, 108800. [Google Scholar] [CrossRef]
- Corradi, E.; Schmidt, N.; Räber, N.; De Mieri, M.; Hamburger, M.; Butterweck, V.; Potterat, O. Metabolite profile and antiproliferative effects in HaCaT cells of a Salix reticulata extract. Planta Med. 2017, 83, 1149–1158. [Google Scholar]
- Yang, H.; Lee, S.H.; Sung, S.H.; Kim, J.; Kim, Y.C. Neuroprotective compounds from Salix pseudo-lasiogyne twigs and their anti-amnesic effects on scopolamine-induced memory deficit in mice. Planta Med. 2013, 79, 78–82. [Google Scholar] [CrossRef]
- Masood, M.; Arshad, M.; Qureshi, R.; Sabir, S.; Amjad, M.S.; Qureshi, H.; Tahir, Z. Picrorhiza kurroa: An ethnopharmacologically important plant species of Himalayan region. Pure Appl. Biol. 2015, 4, 407. [Google Scholar] [CrossRef]
- Verma, P.C.; Basu, V.; Gupta, V.; Saxena, G.; Ur Rahman, L. Pharmacology and chemistry of a potent hepatoprotective compound Picroliv isolated from the roots and rhizomes of Picrorhiza kurroa royle ex benth.(kutki). Curr. Pharm. Biotechnol. 2009, 10, 641–649. [Google Scholar] [CrossRef]
- Morikawa, T.; Inoue, N.; Nakanishi, Y.; Manse, Y.; Matsuura, H.; Okino, K.; Hamasaki, S.; Yoshikawa, M.; Muraoka, O.; Ninomiya, K. Collagen synthesis-promoting and collagenase inhibitory activities of constituents isolated from the rhizomes of Picrorhiza kurroa royle ex benth. Fitoterapia 2020, 143, 104584. [Google Scholar] [CrossRef]
- Kant, K.; Walia, M.; Agnihotri, V.; Pathania, V.; Singh, B. Evaluation of antioxidant activity of Picrorhiza kurroa (leaves) extracts. Indian J. Pharm. Sci. 2013, 75, 324. [Google Scholar]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Plant polyketide synthases: A fascinating group of enzymes. Plant Physiol. Biochem. 2009, 47, 167–174. [Google Scholar] [CrossRef]
- Turtola, S.; Sallas, L.; Holopainen, J.K.; Julkunen-Tiitto, R.; Kainulainen, P. Long-term exposure to enhanced UV-B radiation has no significant effects on growth or secondary compounds of outdoor-grown Scots pine and Norway spruce seedlings. Environ. Pollut. 2006, 144, 166–171. [Google Scholar] [CrossRef]
- Stolter, C.; Niemelä, P.; Ball, J.P.; Julkunen-Tiitto, R.; Vanhatalo, A.; Danell, K.; Varvikko, T.; Ganzhorn, J.U. Comparison of plant secondary metabolites and digestibility of three different boreal coniferous trees. Basic Appl. Ecol. 2009, 10, 19–26. [Google Scholar] [CrossRef]
- Løkke, H. Picein and piceol concentrations in Norway spruce. Ecotoxicol. Environ. Saf. 1990, 19, 301–309. [Google Scholar] [CrossRef]
- Jensen, J.; Løkke, H. 4-hydroxyacetophenone and its glucoside picein as chemical indicators for stress in Picea abies/4-Hydroxyacetophenon und sein Glucosid Picein als chemische Indikatoren für Stress in Picea abies. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 1990, 97, 328–338. [Google Scholar]
- Ganthaler, A.; Stöggl, W.; Kranner, I.; Mayr, S. Foliar phenolic compounds in Norway spruce with varying susceptibility to Chrysomyxa rhododendri: Analyses of seasonal and infection-induced accumulation patterns. Front. Plant Sci. 2017, 8, 1173. [Google Scholar] [CrossRef]
- Parent, G.J.; Méndez-Espinoza, C.; Giguère, I.; Mageroy, M.H.; Charest, M.; Bauce, É.; Bohlmann, J.; MacKay, J.J. Hydroxyacetophenone defenses in white spruce against spruce budworm. Evol. Appl. 2020, 13, 62–75. [Google Scholar] [CrossRef]
- Bahnweg, G.; Schubert, R.; Kehr, R.D.; Müller-Starck, G.; Heller, W.; Langebartels, C.; Sandermann, H., Jr. Controlled inoculation of Norway spruce (Picea abies) with Sirococcus conigenus: PCR-based quantification of the pathogen in host tissue and infection-related increase of phenolic metabolites. Trees 2000, 14, 435–441. [Google Scholar] [CrossRef]
- Sarıkahya, N.B.; Pekmez, M.; Arda, N.; Kayce, P.; Yavaşoğlu, N.Ü.K.; Kırmızıgül, S. Isolation and characterization of biologically active glycosides from endemic Cephalaria species in Anatolia. Phytochem. Lett. 2011, 4, 415–420. [Google Scholar] [CrossRef]
- Méndez-Espinoza, C.; Parent, G.J.; Lenz, P.; Rainville, A.; Tremblay, L.; Adams, G.; McCartney, A.; Bauce, E.; Mackay, J. Genetic control and evolutionary potential of a constitutive resistance mechanism against the spruce budworm (Choristoneura fumiferana) in white spruce (Picea glauca). Heredity 2018, 121, 142–154. [Google Scholar] [CrossRef]
- Góngora, L.; Máñez, S.; Giner, R.M.; Recio, M.C.; Gray, A.I.; Ríos, J.-L. Phenolic glycosides from Phagnalon rupestre. Phytochemistry 2002, 59, 857–860. [Google Scholar] [CrossRef]
- Abreu, P.M.; Braham, H.; Jannet, H.B.; Mighri, Z.; Matthew, S. Antioxidant compounds from Ebenus pinnata. Fitoterapia 2007, 78, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2003, 51, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fan, J.; Wang, P.; Zhu, L.; Jin, Y.; Peng, Y.; Du, S. Isolation, identification and antioxidative capacity of water-soluble phenylpropanoid compounds from Rhodiola crenulata. Food Chem. 2012, 134, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Imura, K.; Miyake, S.; Ninomiya, K.; Matsuda, H.; Yamashita, C.; Muraoka, O.; Hayakawa, T.; Yoshikawa, M. Promoting the effect of chemical constituents from the flowers of Poacynum hendersonii on adipogenesis in 3T3-L1 cells. J. Nat. Med. 2012, 66, 39–48. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; Pérez-Vásquez, A.; Torres-Piedra, M.; Bye, R.; Linares, E.; Mata, R. α-Glucosidase inhibitors from Vauquelinia corymbosa. Molecules 2015, 20, 15330–15342. [Google Scholar] [CrossRef]
- Lai, L.B.; Gopalan, V.; Glew, R.H. Continuous spectrophotometric assays for β-glucosidases acting on the plant glucosides l-picein and prunasin. Anal. Biochem. 1992, 200, 365–369. [Google Scholar] [CrossRef]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef]
- Vassar, R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. MN 2004, 23, 105–114. [Google Scholar] [CrossRef]
- Vassar, R.; Kandalepas, P.C. The β-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimer’s Res. Ther. 2011, 3, 20. [Google Scholar] [CrossRef]
- Das, B.; Yan, R. A close look at BACE1 inhibitors for Alzheimer’s disease treatment. CNS Drugs 2019, 33, 251–263. [Google Scholar] [CrossRef]
- Zhu, K.; Peters, F.; Filser, S.; Herms, J. Consequences of pharmacological BACE inhibition on synaptic structure and function. Biol. Psychiatry 2018, 84, 478–487. [Google Scholar] [CrossRef]
- Mullard, A. BACE inhibitor bust in Alzheimer trial. Nat. Rev. Drug Discov. 2017, 16, 155–156. [Google Scholar] [CrossRef]
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Shen, W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology 2014, 34, 370–377. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elyasi, L.; Rosenholm, J.M.; Jesmi, F.; Jahanshahi, M. The Antioxidative Effects of Picein and Its Neuroprotective Potential: A Review of the Literature. Molecules 2022, 27, 6189. https://doi.org/10.3390/molecules27196189
Elyasi L, Rosenholm JM, Jesmi F, Jahanshahi M. The Antioxidative Effects of Picein and Its Neuroprotective Potential: A Review of the Literature. Molecules. 2022; 27(19):6189. https://doi.org/10.3390/molecules27196189
Chicago/Turabian StyleElyasi, Leila, Jessica M. Rosenholm, Fatemeh Jesmi, and Mehrdad Jahanshahi. 2022. "The Antioxidative Effects of Picein and Its Neuroprotective Potential: A Review of the Literature" Molecules 27, no. 19: 6189. https://doi.org/10.3390/molecules27196189
APA StyleElyasi, L., Rosenholm, J. M., Jesmi, F., & Jahanshahi, M. (2022). The Antioxidative Effects of Picein and Its Neuroprotective Potential: A Review of the Literature. Molecules, 27(19), 6189. https://doi.org/10.3390/molecules27196189






