Iodine-Mediated Alkoxyselenylation of Alkenes and Dienes with Elemental Selenium
Abstract
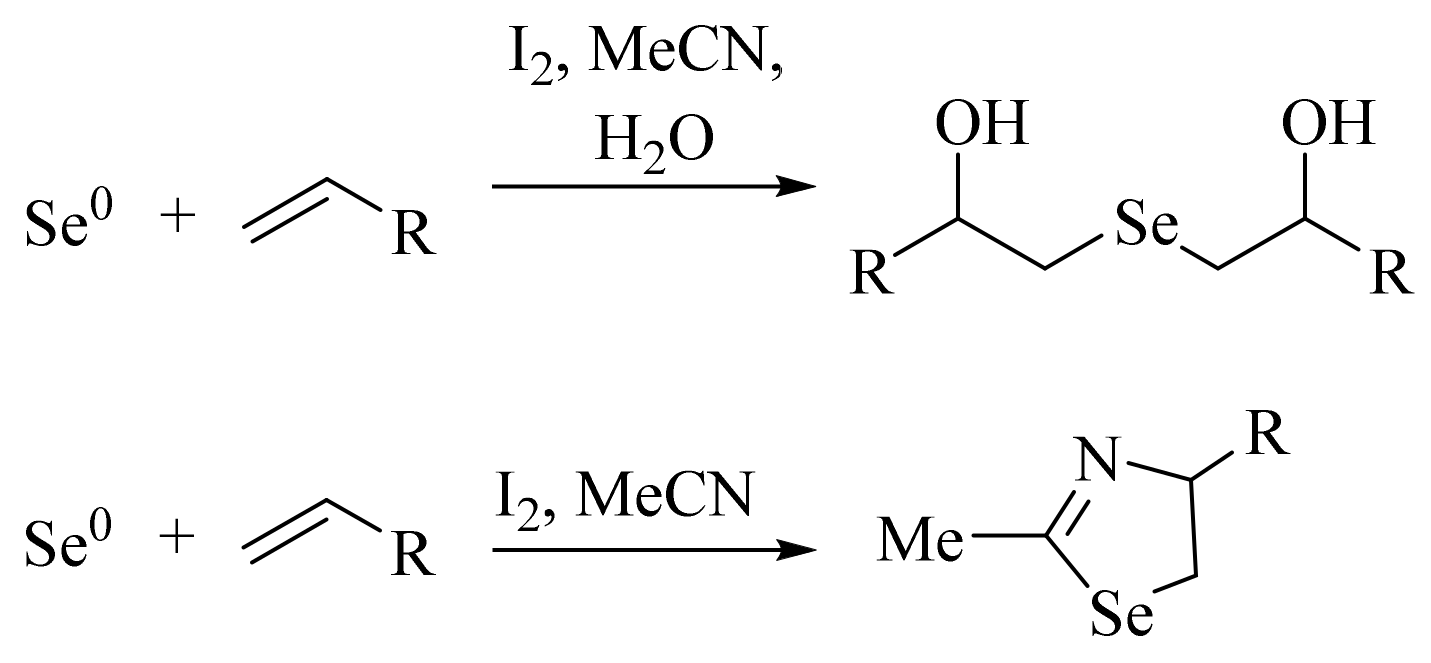
:1. Introduction
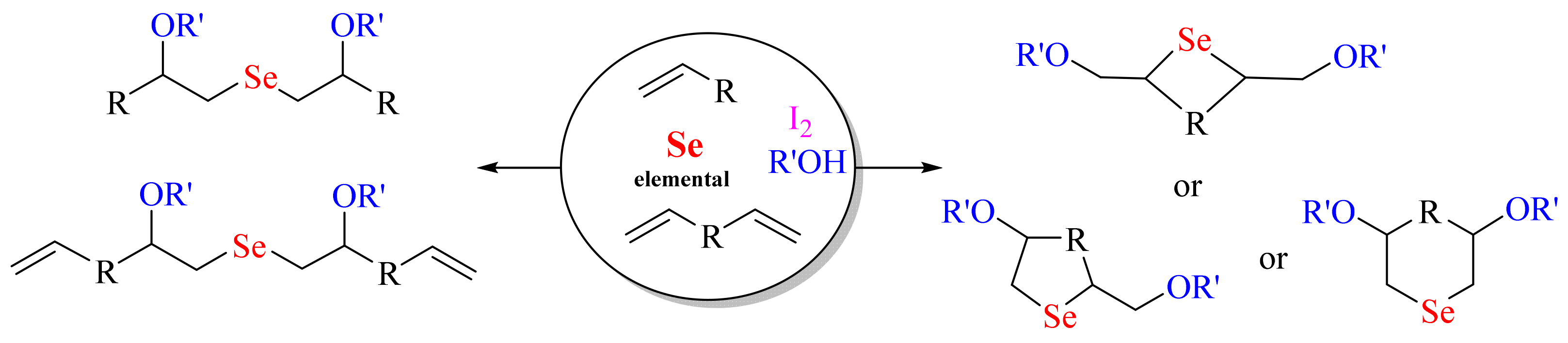
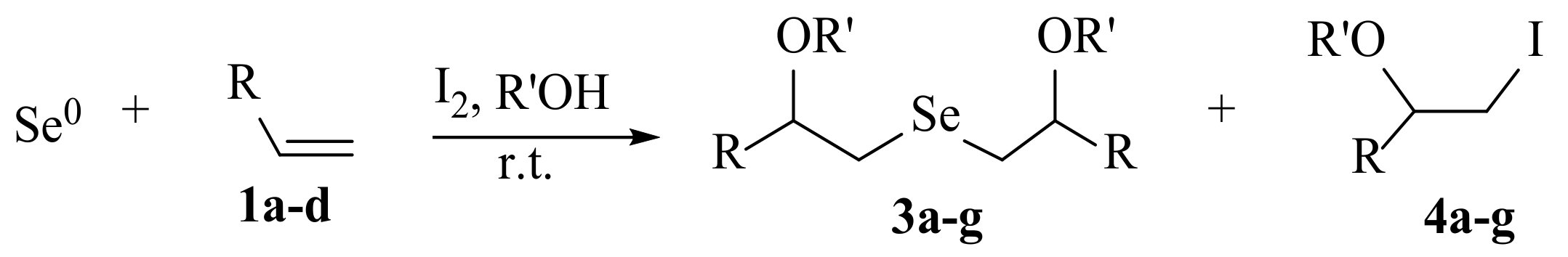
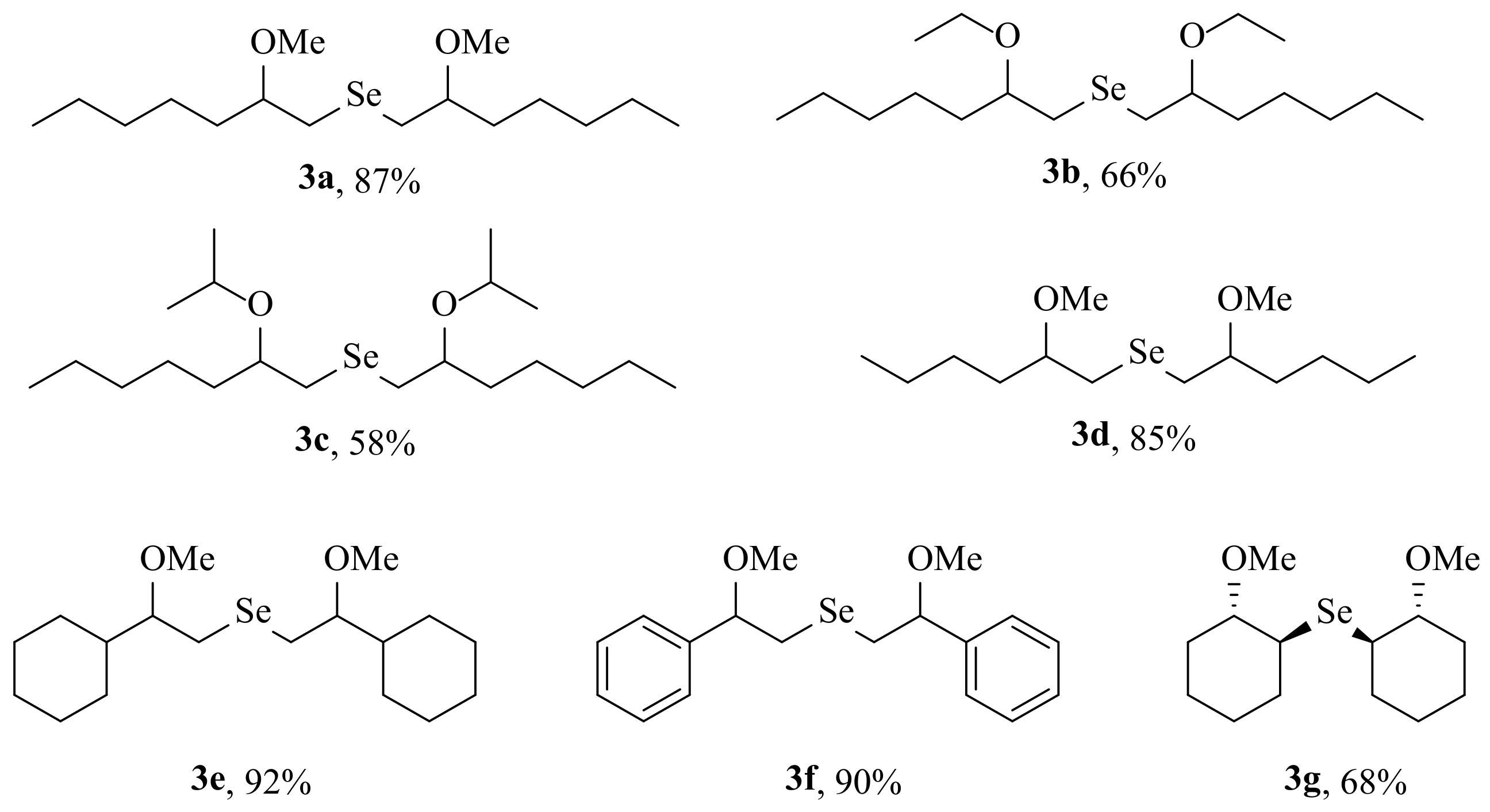
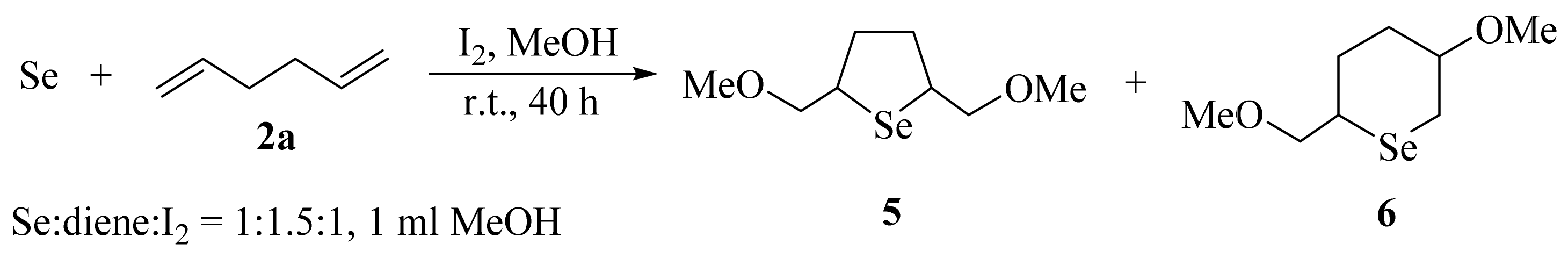
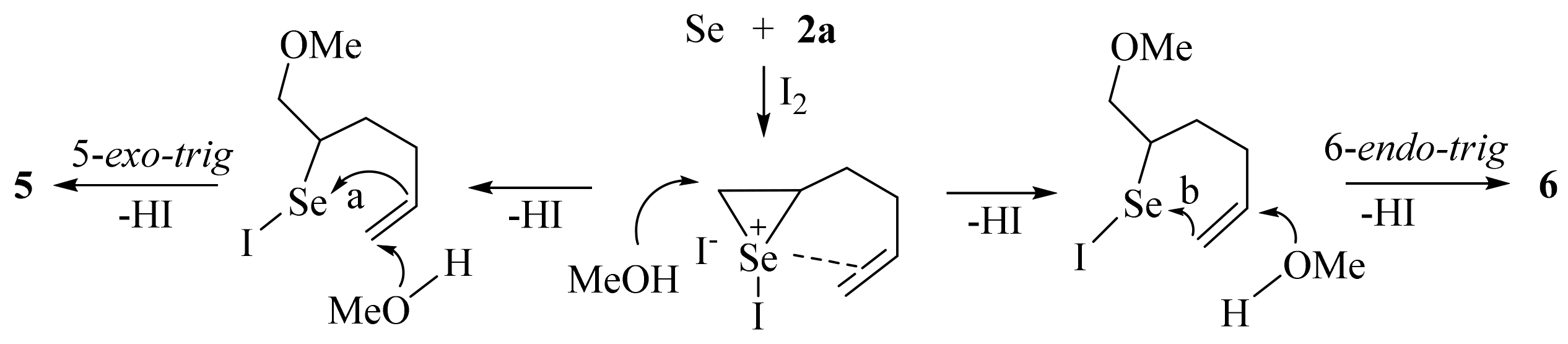
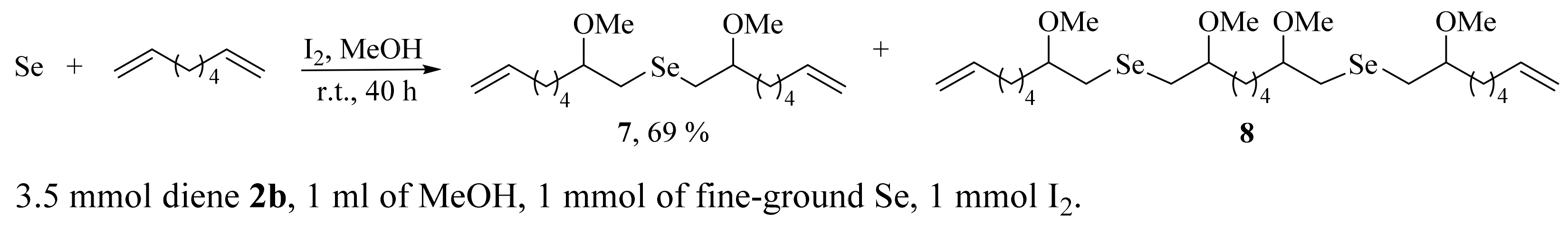
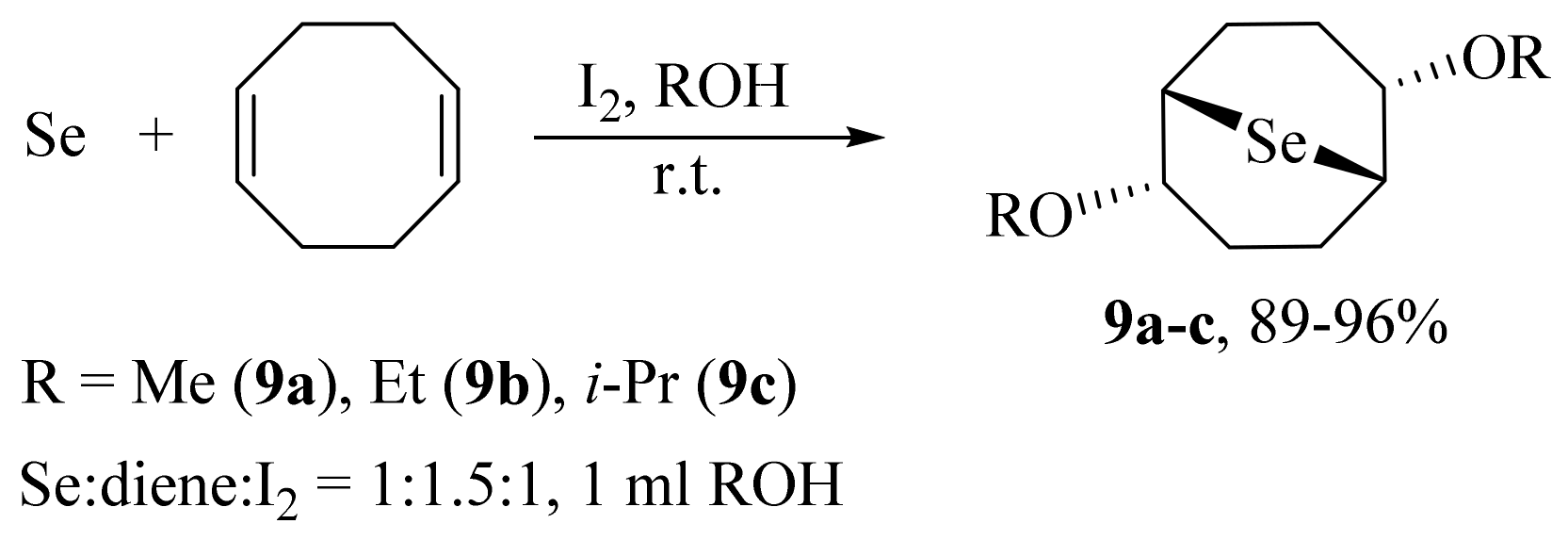
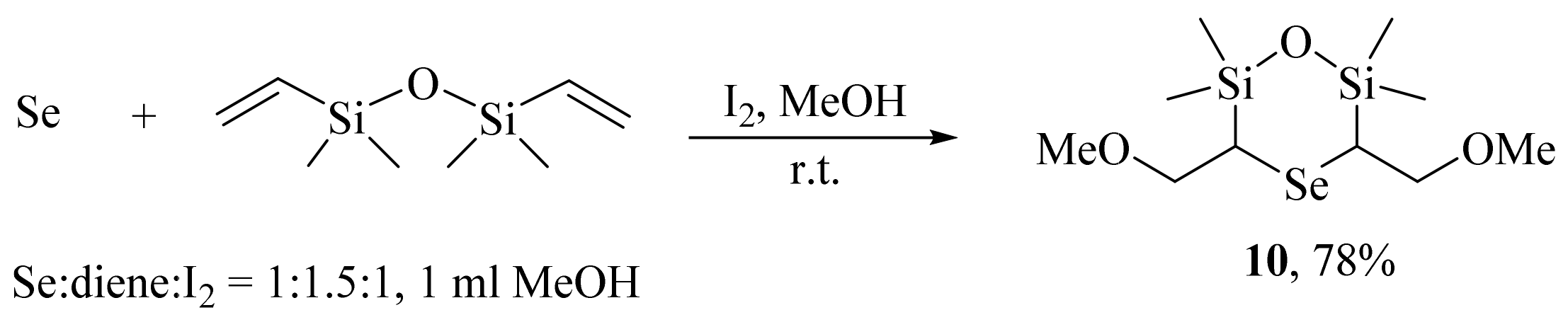
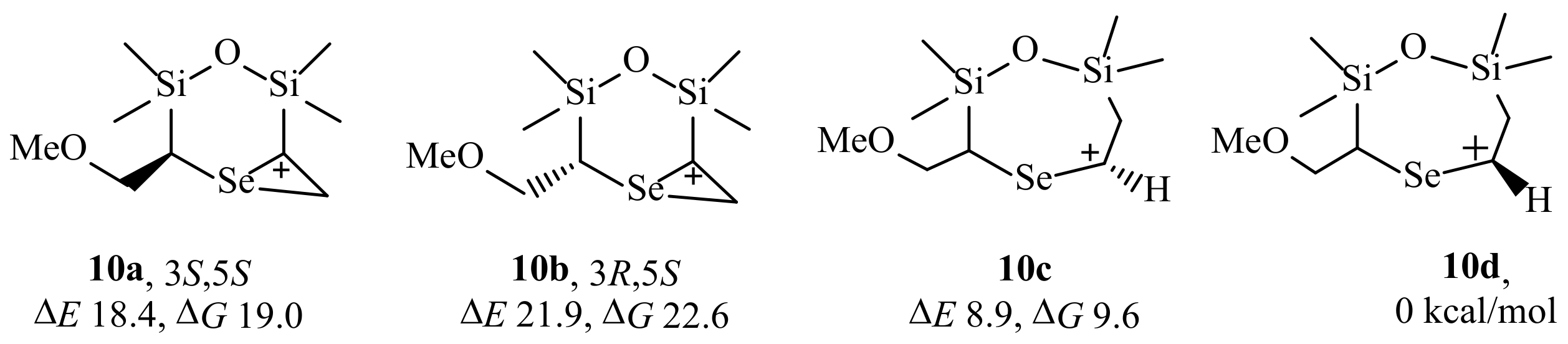
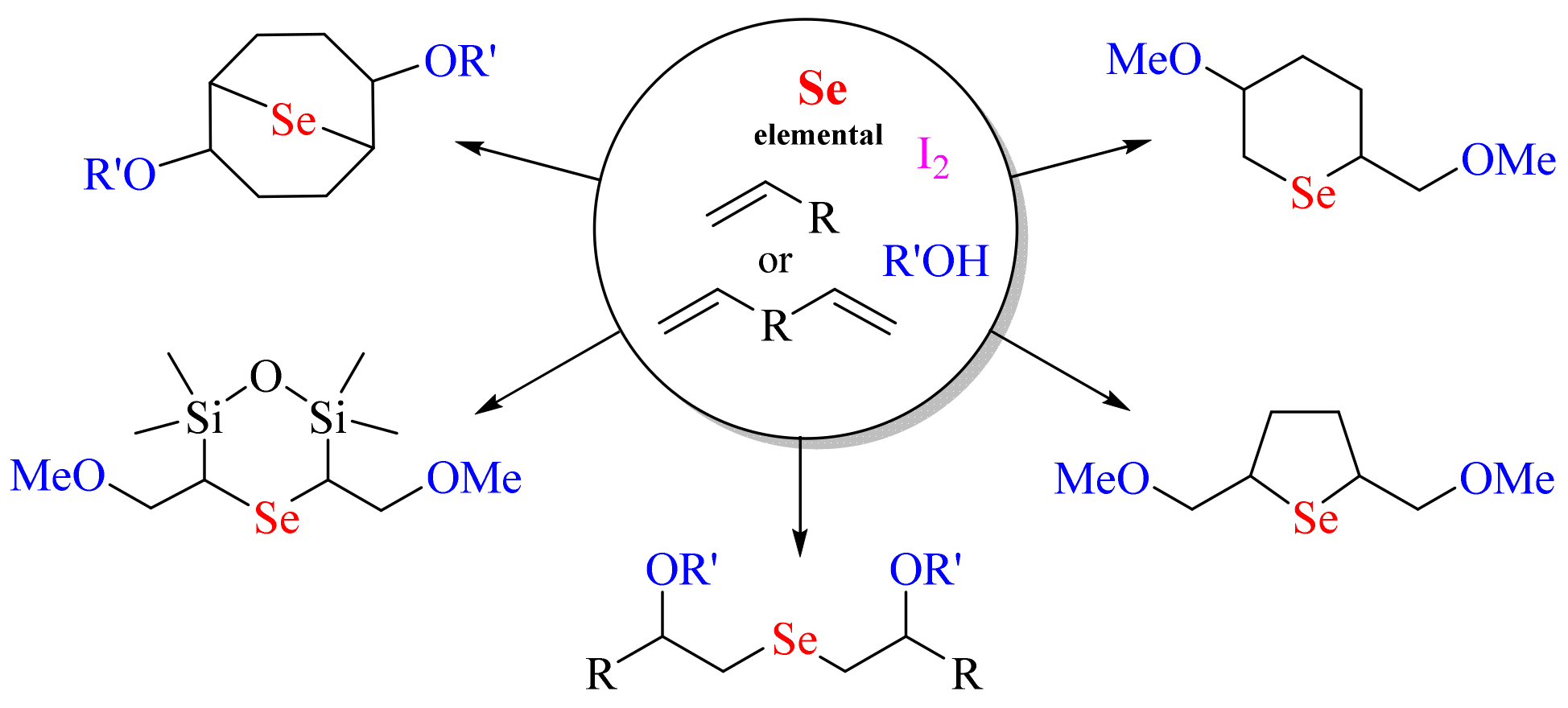
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Preparation of Selenides 3a–g and 7
3.3. General Procedure for the Preparation of Selenides 5, 6, 9a–c, and 10
3.4. Characterization Data of Products 3a–g, 5, 6, 9a–c and 10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wirth, T. (Ed.) Organoselenium Chemistry: Synthesis and Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wirth, T. (Ed.) Organoselenium Chemistry. Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Makhal, P.N.; Nandi, A.; Kaki, V.R. Insights into the recent synthetic advances of organoselenium compounds. Chem. Select 2021, 6, 663–679. [Google Scholar] [CrossRef]
- Azeredo, J.B.; Penteado, F.; Nascimento, V.; Sancineto, L.; Braga, A.L.; Lenardao, E.J.; Santi, C. Green Is the Color: An Update on Ecofriendly Aspects of Organoselenium Chemistry. Molecules 2022, 27, 1597. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compoundds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.K.; Priyadarsini, K.I. (Eds.) Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; RSC: London, UK, 2017. [Google Scholar]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and therapeutic potential of selenazo compounds. J. Med. Chem. 2019, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Pinz, M.; Reis, A.S.; Duarte, V.; da Rocha, M.J.; Goldani, B.S.; Alves, D.; Savegnago, L.; Luchese, C.; Wilhelm, E.A. 4-Phenylselenyl-7-chloroquinoline, a new quinoline derivative containing selenium, has potential antinociceptive and anti-inflammatory actions. Eur. J. Pharmacol. 2016, 780, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; de Lourenço, D.A.; Domingues, M.; Smaniotto, T.Â.; Birmann, P.T.; Vieira, B.; Sonego, M.S.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Anhedonic- and anxiogenic-like behaviors and neurochemical alterations are abolished by a single administration of a selenium-containing compound in chronically stressed mice. Compr. Psychoneuroendocrinol. 2021, 6, 100054. [Google Scholar] [CrossRef]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; de Lourenço, D.A.; Smaniotto, T.Â.; Segatto, N.; Vieira, B.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain Behav. Immun. 2020, 84, 229–241. [Google Scholar] [CrossRef]
- Sancineto, L.; Mariotti, A.; Bagnoli, L.; Marini, F.; Desantis, J.; Iraci, N.; Santi, C.; Pannecouque, C.; Tabarrini, O. Design and Synthesis of DiselenoBisBenzamides (DISeBAs) as Nucleocapsid Protein 7 (NCp7) Inhibitors with anti-HIV Activity. J. Med. Chem. 2015, 58, 9601–9614. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Yechun, X.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Boil. 2020, 37, 101715. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Botwina, P.; Menichetti, E.; Bagnoli, L.; Rosati, O.; Marini, F.; Fonseca, S.F.; Abenante, L.; Alves, D.; Dabrowska, A.; et al. Seleno-Functionalization of Quercetin Improves the Non-Covalent Inhibition of Mpro and Its Antiviral Activity in Cells against SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 7048. [Google Scholar] [CrossRef]
- Santi, C.; Marini, F.; Lenardão, E.J. Synthetic Advances on Bioactive Selenium Compounds. Looking Beyond the Traditional Idea of Glutathione Peroxidase Mimics as Antioxidants. In Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Chapter 2; Royal Society of Chemistry: London, UK, 2017; pp. 35–76. [Google Scholar]
- Sands, K.N.; Tuck, T.A.; Back, T.G. Cyclic seleninate esters, spirodioxyselenuranes and related compounds: New classes of biological antioxidants that emulate glutathione peroxidase. Chem. Eur. J. 2018, 24, 9714–9728. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, Z.; Zhang, H.; Gao, J.J.; Yang, K.R.; Fan, W.Y.; Wu, R.X.; Feng, M.L.; Zhu, W.; Zhu, Y.P. Iodine-Mediated Domino Cyclization for One-Pot Synthesis of Indolizine-Fused Chromones via Metal-Free sp3 C–H Functionalization. J. Org. Chem. 2022, 87, 835–845. [Google Scholar] [CrossRef]
- Kitamura, T.; Komoto, R.; Oyamada, J.; Higashi, M.; Kishikawa, Y. Iodine-Mediated Fluorination of Alkenes with an HF Reagent: Regioselective Synthesis of 2-Fluoroalkyl Iodides. J. Org. Chem. 2021, 86, 18300–18303. [Google Scholar] [CrossRef]
- Cui, H.; Liu, X.; Wei, W.; Yang, D.; He, C.; Zhang, T.; Wang, H. Molecular iodine-mediated difunctionalization of alkenes with nitriles and thiols leading to β-acetamido sulfides. J. Org. Chem. 2016, 81, 2252–2260. [Google Scholar] [CrossRef]
- Vieira, A.A.; Azeredo, J.B.; Godoi, M.; Santi, C.; da Silva Junior, E.N.; Braga, A.L. Catalytic chalcogenylation under greener conditions: A solvent-free sulfur- and seleno-functionalization of olefins via I2/DMSO oxidant system. J. Org. Chem. 2015, 80, 2120–2127. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Mu, S.; Li, Y.; Feng, R.; Sun, K. Eco-Friendly Selenoamidation of Alkenes with Sulfamides and Organoseleniums: Rapid access to β-Amidoselenides. Asian J. Org. Chem. 2018, 7, 720–723. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, H.J.; Yan, J. Iodine-mediated regioselective hydroxyselenenylation of alkenes: Facile access to β-hydroxy selenides. Chin. Chem. Lett. 2018, 29, 479–481. [Google Scholar] [CrossRef]
- Tanini, D.; Degl’Innocenti, A.; Capperucci, A. Bis (trimethylsilyl) selenide in the Selective Synthesis of β-Hydroxy, β-Mercapto, and β-Amino Diorganyl Diselenides and Selenides Through Ring Opening of Strained Heterocycles. Eur. J. Org. Chem. 2015, 2015, 357–369. [Google Scholar] [CrossRef]
- Leng, T.; Wu, G.; Zhou, Y.B.; Gao, W.; Ding, J.; Huang, X.; Liu, M.; Wu, H. Silver-Catalyzed One-Pot Three-Component Selective Synthesis of β-Hydroxy Selenides. Adv. Synth. Catal. 2018, 360, 4336–4340. [Google Scholar] [CrossRef]
- Arai, K.; Kumakura, F.; Takahira, M.; Sekiyama, N.; Kuroda, N.; Suzuki, T.; Iwaoka, M. Effects of ring size and polar functional groups on the glutathione peroxidase-like antioxidant activity of water-soluble cyclic selenides. J. Org. Chem. 2015, 80, 5633–5642. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with divinyl sulfone: Synthesis of novel four-and five-membered selenium heterocycles. Tetrahedron Lett. 2010, 51, 5258–5261. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Synthesis of bis (2-haloethyl) selenides by reaction of selenium dihalides with ethylene. Russ. J. Org. Chem. 2014, 50, 291–292. [Google Scholar] [CrossRef]
- Lautenschlaeger, F.K. Reaction of selenium monochloride with diolefins. J. Org. Chem. 1969, 34, 4002–4006. [Google Scholar] [CrossRef]
- Ma, Y.-T.; Liu, M.-C.; Zhou, Y.-B.; Wu, H.-Y. Synthesis of organoselenium compounds with elemental selenium. Adv. Synth. Catal. 2021, 363, 5386–5406. [Google Scholar] [CrossRef]
- Kurkutov, E.O.; Borodina, T.N. The iodine-assisted hydroxyselenenylation of alkenes with elemental selenium. Mendeleev Commun. 2020, 30, 760–761. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Kurkutov, E.O.; Kleinpeter, E.A. low-temperature dynamic 1H, 13C, and 77Se NMR study of 2, 2′-selenodicyclohexanol. Magn. Reson. Chem. 2022, 60, 165–171. [Google Scholar] [CrossRef]
- Kurkutov, E.O.; Shainyan, B.A. One-pot assembling of selenazolines from elemental selenium, alkenes and acetonitrile. Mendeleev Commun. 2022, 32, 395–396. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Chapter 16; Butterworth-Heinemann: Oxford, UK, 1997; p. 571. [Google Scholar]
- Baldwin, J.E. Rules for ring closure. J. Chem. Soc. Chem. Commun. 1976, 734–736. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Gopal, M.; Milne, J. Spectroscopic evidence for selenium iodides in carbon disulfide solution: Se3I2, Se2I2, and SeI2. Inorg. Chem. 1992, 31, 4530–4533. [Google Scholar] [CrossRef]
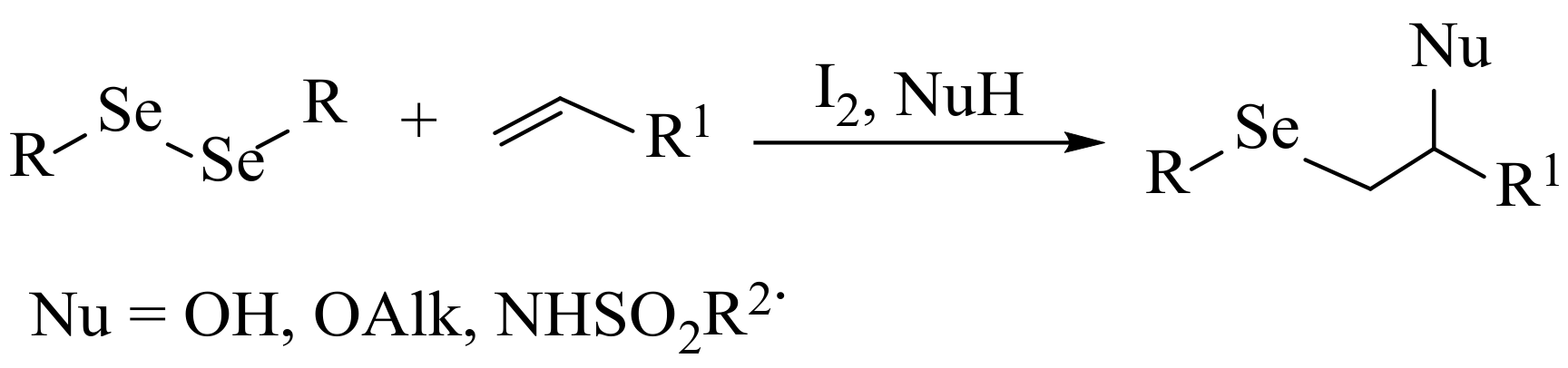
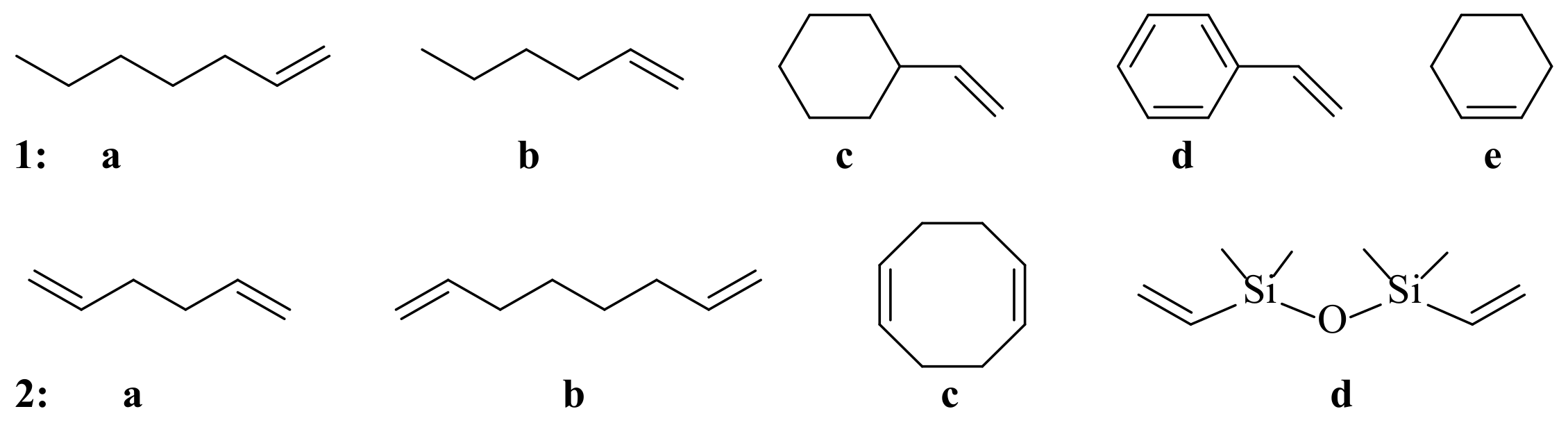
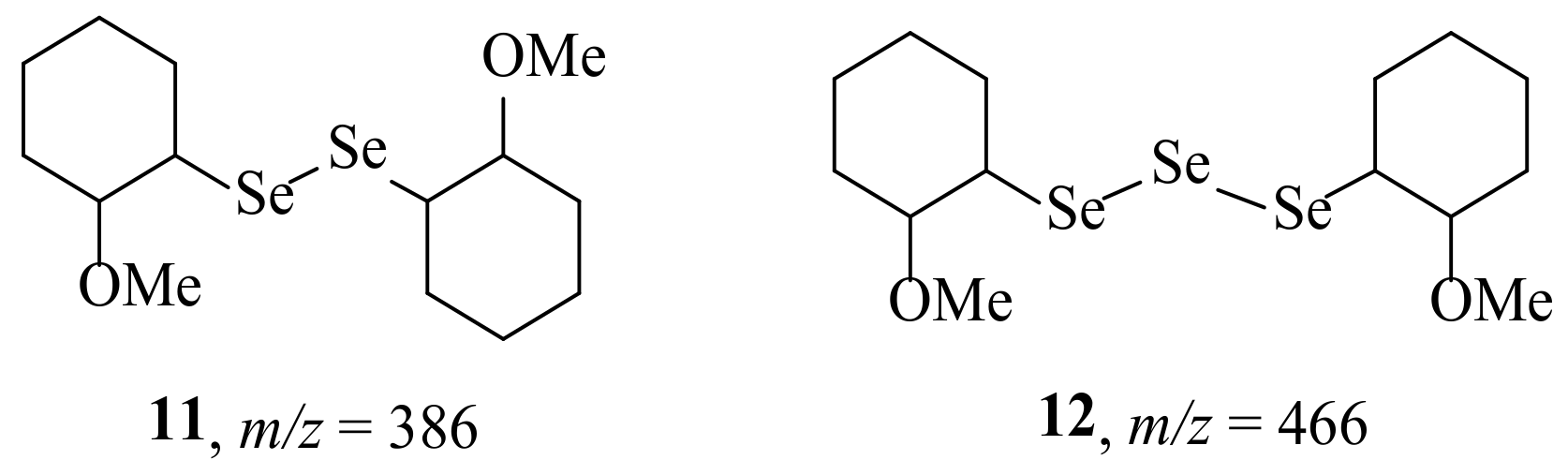
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Iodine Source (Equiv) | Solvent (mL) | Time, h | T, °C | Yield, % ** | Se Conversion, % |
| 1 | 1.0 I2 | MeCN:MeOH (1:0.1) | 40 | 25 | 71 | 54 |
| 2 | 1.0 I2 | MeOH (1) | 40 | 25 | 90 | 68 |
| 3 | 1.0 I2 | MeOH (1) | 120 | 25 | 97 | 88 |
| 4 | 1.5 I2 | MeOH (1) | 40 | 25 | 85 | 86 |
| 5 | 2.0 I2 | MeOH (1) | 40 | 25 | 87 | ~100 |
| 6 | 2.0 I2 | MeOH (1) | 20 | 25 | 88 | 69 |
| 7 | 1.0 I2 | MeOH (1) | 10 | 50 | 70 | 48 |
| 8 | 2.0 NIS | MeOH (1) | 20 | 25 | 70 | 63 |
| 9 | 0.2 I2 | DMSO:MeOH (0.15:0.1) | 20 | 25 | Mixture of adducts | 27 |
| 10 | 2.0 I2 | MeOH (0.2) (5 equiv) | 40 | 25 | 79 | 57 |
| 11 | – | MeOH (1) | 40 | 25 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkutov, E.O.; Shainyan, B.A. Iodine-Mediated Alkoxyselenylation of Alkenes and Dienes with Elemental Selenium. Molecules 2022, 27, 6169. https://doi.org/10.3390/molecules27196169
Kurkutov EO, Shainyan BA. Iodine-Mediated Alkoxyselenylation of Alkenes and Dienes with Elemental Selenium. Molecules. 2022; 27(19):6169. https://doi.org/10.3390/molecules27196169
Chicago/Turabian StyleKurkutov, Evgeny O., and Bagrat A. Shainyan. 2022. "Iodine-Mediated Alkoxyselenylation of Alkenes and Dienes with Elemental Selenium" Molecules 27, no. 19: 6169. https://doi.org/10.3390/molecules27196169
APA StyleKurkutov, E. O., & Shainyan, B. A. (2022). Iodine-Mediated Alkoxyselenylation of Alkenes and Dienes with Elemental Selenium. Molecules, 27(19), 6169. https://doi.org/10.3390/molecules27196169