Concurrent Determination of Tigecycline, Tetracyclines and Their 4-Epimer Derivatives in Chicken Muscle Isolated from a Reversed-Phase Chromatography System Using Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Stability of Standard Solutions
2.2. Optimization of Sample Preparation
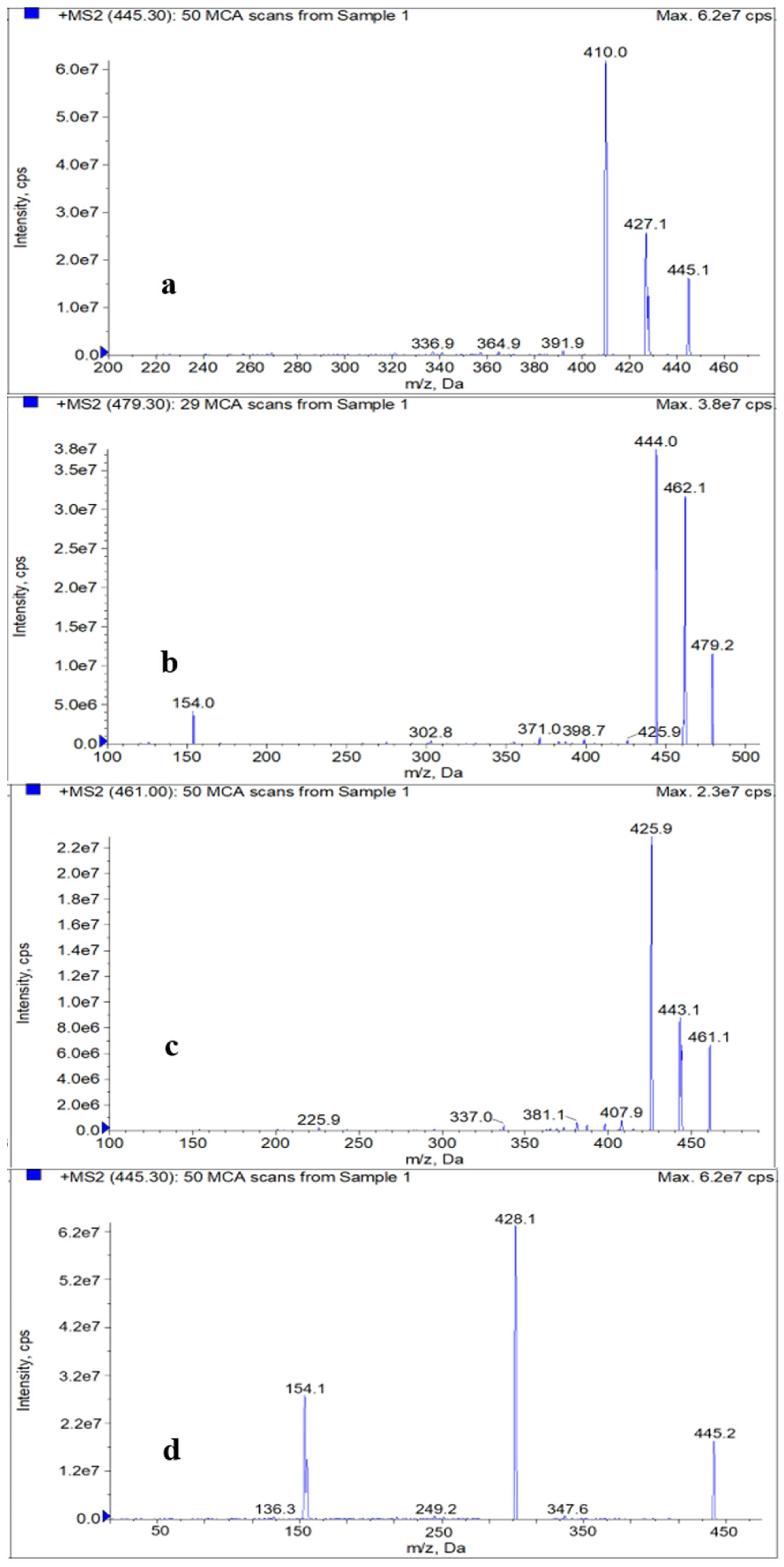
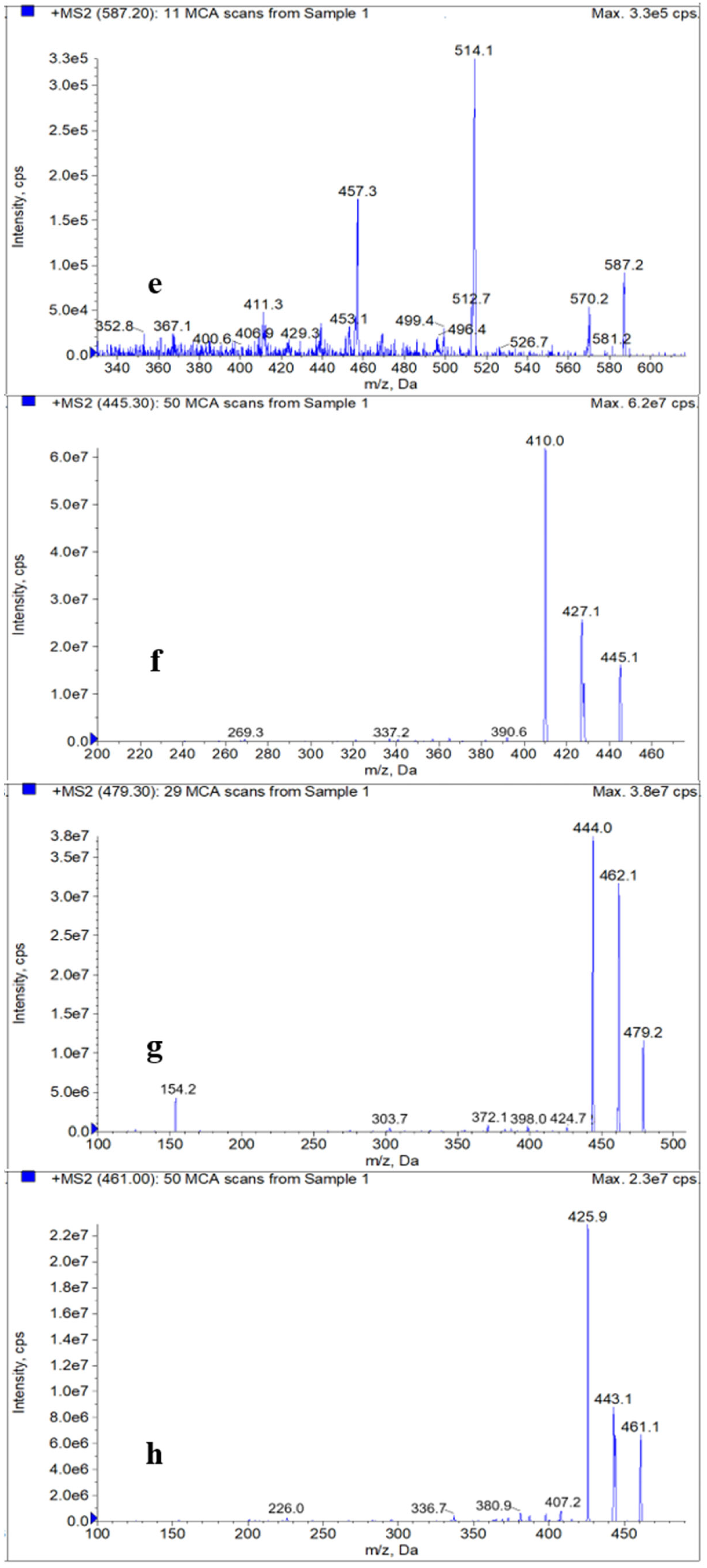
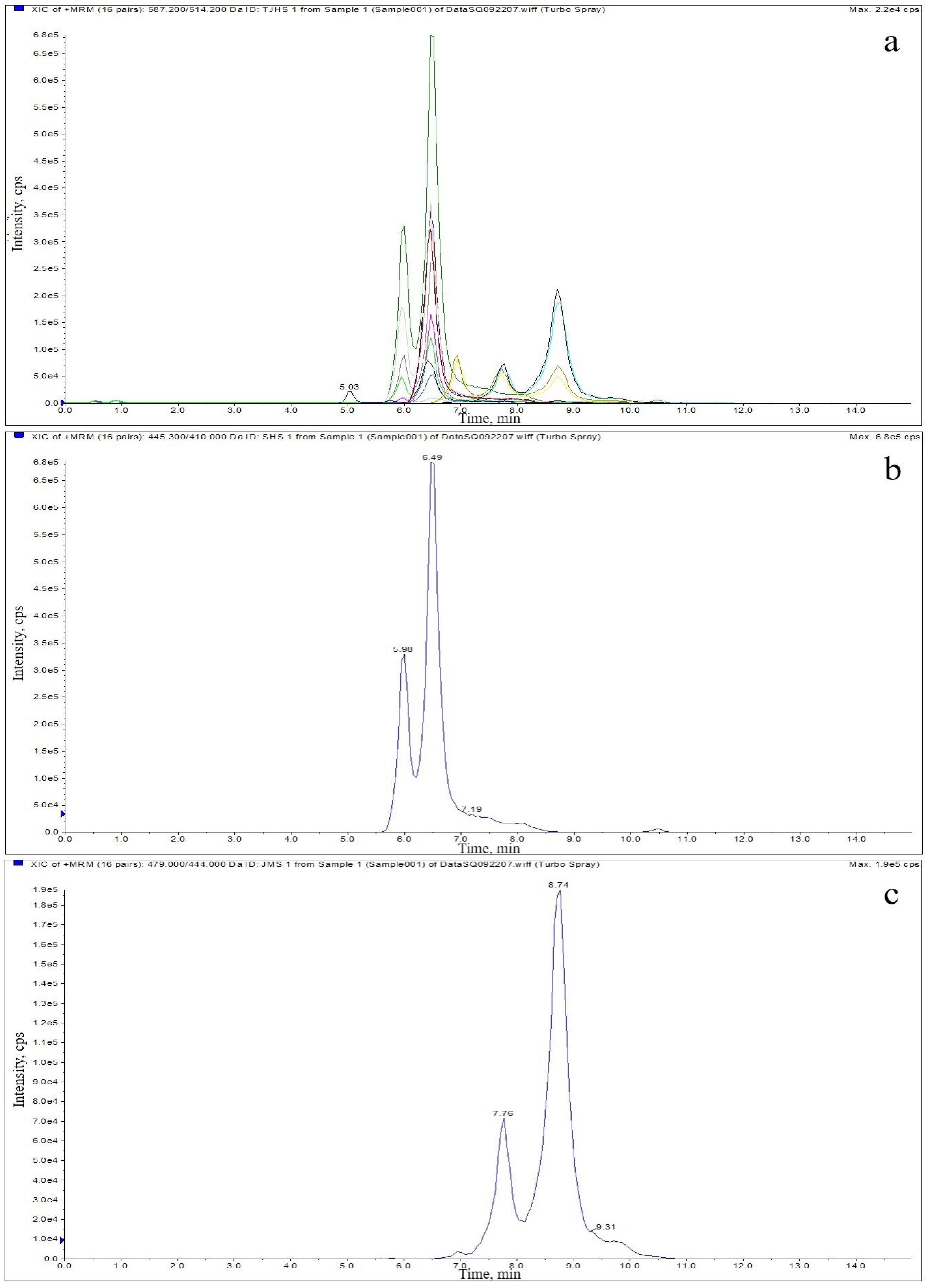
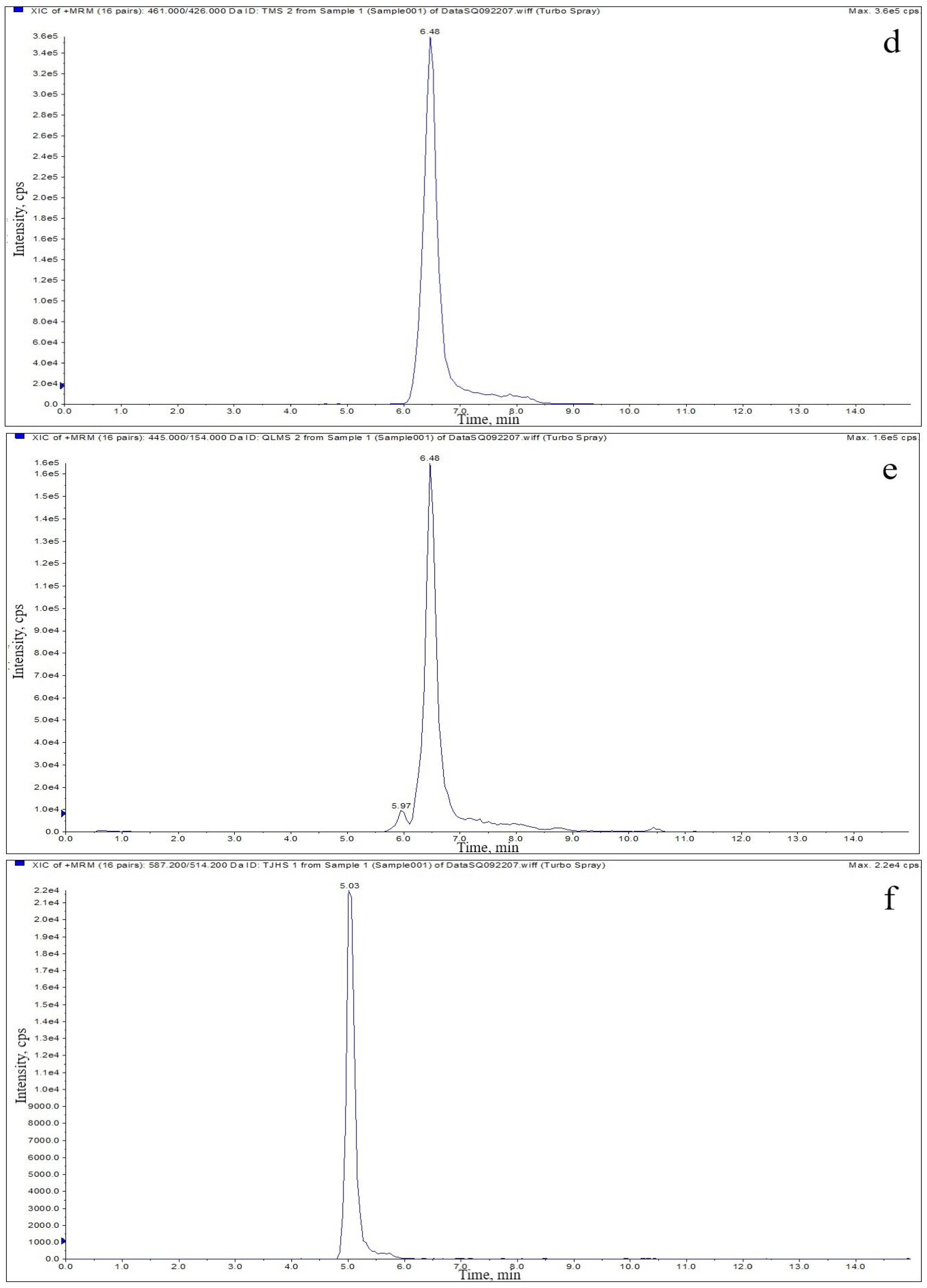
2.3. Optimization of HPLC–MS/MS
2.4. Analytical Method Validation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Stock and Working Solutions
3.3. LC and MS/MS Conditions
3.4. Animals and Sample Preparation
3.5. Analytical Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China; National Health Commission of the People’s Republic of China; State Administration of Market Regulation. National Food Safety Standard-Maximum Residue Limits of Veterinary Drugs in Foods; Standards Press of China: Beijing, China, 2019. [Google Scholar]
- The European Medicines Agency. Commission Regulation (EU) No. 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; European Union: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Guo, Y.; He, Z.; Chen, J.; Chen, L.; Xie, K.; Zhang, T.; Zhang, G.; Dai, G. Simultaneous determination of tetracyclines and fluoroquinolones in poultry eggs by UPLC integrated with dual-channel-fluorescence detection method. Molecules 2021, 26, 5684. [Google Scholar] [CrossRef] [PubMed]
- Odorici, G.; Monfrecola, G.; Bettoli, V. Tetracyclines and photosensitive skin reactions: A narrative review. Dermatol. Ther. 2021, 34, e14978. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistance genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Moon, D.; Kim, S.; Mechesso, A.; Song, H.; Kang, H.; Choi, J.; Yoon, S.; Lim, S. Nationwide surveillance on antimicrobial resistance profiles of Enterococcus faecium and Enterococcus faecalis isolated from healthy food animals in South Korea, 2010 to 2019. Microorganisms 2021, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, X.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of antibiotic resistance genes in the environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef] [PubMed]
- Kasbekar, N. Tigecycline: A new glycylcycline antimicrobial agent. Am. J. Health-Syst. Pharm. 2006, 63, 1235–1243. [Google Scholar] [CrossRef]
- Bergeron, J.; Ammirati, M.; Danley, D.; James, L.; Norcia, M.; Retsema, J.; Strick, C.A.; Su, W.; Sutcliffe, J.; Wondrack, L. Glycylcyclines bind to the high-affinity tetracycline ribosomal binding site and evade Tet(M)- and Tet(O)-mediated ribosomal protection. Antimicrob. Agents Chemother. 1996, 40, 2226–2228. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.Y.; Xi, L.; Gelfand, J.A.; Hamblin, M.R. Tetracyclines function as dual-action light-activated antibiotics. PLoS ONE 2018, 13, e0196485. [Google Scholar] [CrossRef]
- Ziókowski, H.; Jasiecka-Mikoajczyk, A.; Madej-Miechowska, H.; Janiuk, J.; Zygmuntowicz, A.; Dbrowski, M. Comparative pharmacokinetics of chlortetracycline, tetracycline, minocycline, and tigecycline in broiler chickens. Poult. Sci. 2020, 99, 4750–4757. [Google Scholar] [CrossRef]
- Jasiecka-Mikołajczyk, A.; Ziółkowski, H.; Jaroszewski, J.J. Pharmacokinetics of tigecycline in turkeys following different routes of administration. J. Vet. Pharmacol. Ther. 2018, 41, e22–e29. [Google Scholar] [CrossRef]
- He, T.; Wang, R.; Liu, D.; Walsh, T.R.; Wang, Y. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Much, P.; Sun, H.; Lassnig, H.; Koeberl-Jelovcan, H.; Schliessnig, H.; Stueger, H.P. Differences in antimicrobial resistance of commensal Escherichia coli isolated from caecal contents of organically and conventionally raised broilers in Austria, 2010–2014 and 2016. Prev. Vet. Med. 2019, 171, 104755. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, Y.; Liu, D.; Yang, D.; Jiang, H. Abundance of tigecycline resistance genes and association with antibiotic residues in chinese livestock farms. J. Hazard. Mater. 2021, 409, 124921. [Google Scholar] [CrossRef] [PubMed]
- Piperaki, E.T.; Tzouvelekis, L.S.; Miriagou, V.; Daikos, G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019, 25, 951–957. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Salgado, H.R.N. Rapid turbidimetric assay to potency evaluation of tigecycline in lyophilized powder. J. Microbiol. Methods 2015, 110, 49–53. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, X.; Song, S.; Wu, A.; Liu, L.; Kuang, H.; Xu, C. Development of a monoclonal antibody-based immunochromatographic strip for the rapid detection of tigecycline in human serum. Anal. Methods 2021, 13, 817–824. [Google Scholar] [CrossRef]
- Cairoli, S.; Simeoli, R.; Tarchi, M.; Dionisi, M.; Vitale, A.; Perioli, L.; Dionisi-Vici, C.; Goffredo, B.M. A new HPLC-DAD method for contemporary quantification of 10 antibiotics for therapeutic drug monitoring of critically ill pediatric patients. Biomed. Chromatogr. 2020, 34, e4880. [Google Scholar] [CrossRef]
- D’Avolio, A.; Peila, E.; Simiele, M.; Pensi, D.; Baietto, L.; Cusato, J.; Cinnirella, G.; De Rosa, F.; Di Perri, G. Ultra performance liquid chromatography PDA method for determination of tigecycline in human plasma. Ther. Drug Monit. 2013, 35, 853–858. [Google Scholar] [CrossRef]
- Li, C.; Sutherland, C.A.; Nightingale, C.H.; Nicolau, D.P. Quantitation of tigecycline, a novel glycylcycline, by liquid chromatography. J. Chromatogr. B 2004, 811, 225–229. [Google Scholar] [CrossRef]
- Zorpas, K.M.; Valsami, G.N.; Vryonis, E.V.; Skoutelis, A.T.; Archontaki, H.A. Robust and sensitive high-performance liquid chromatographic-UV detection technique for the determination of tigecycline in rabbit plasma. J. AOAC Int. 2011, 94, 847–856. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Salgado, H.R.N. Validation of a stability-indicating RP-LC method for the determination of tigecycline in lyophilized powder. J. Chromatogr. Sci. 2012, 51, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Granados-Chinchilla, F.; Sánchez, J.; García, F.; Rodríguez, C. A novel green chemistry method for nonaqueous extraction and high-performance liquid chromatography detection of first-, second-, and third-generation tetracyclines, 4-epitetracycline, and tylosin in animal feeds. J. Agric. Food Chem. 2012, 60, 7121–7128. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, T.; Wang, X.; Cheng, X.; Dong, H.; Wang, Y.; Zheng, X.; Zhou, L.; Xing, J.; Dong, Y. Quantitative analysis and pharmacokinetics study of tigecycline in human serum using a validated sensitive liquid chromatography with tandem mass spectrometry method. J. Sep. Sci. 2014, 37, 1396–1403. [Google Scholar] [CrossRef]
- Shao, R.; Li, X.; Hu, Y.; Chen, J.; Lou, H.; Dai, H. Determination of tigecycline in human plasma by LC–MS/MS and its application to population pharmacokinetics study in Chinese patients with hospital-acquired pneumonia. Biomed. Chromatogr. 2018, 32, e4045. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Wang, Y.; Hou, Y.; Wang, X.; Lan, J.; Wu, Z.; Wang, Y.; Chen, C. Establishment and validation of a liquid chromatography-tandem mass spectrometry method for the determination of tigecycline in critically ill patients. Int. J. Anal. Chem. 2020, 6671392. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Mesini, A.; Barbagallo, L.; Maffia, A.; Tripodi, G.; Pea, F.; Saffioti, C.; Castagnola, E.; Cangemi, G. A liquid chromatography-tandem mass spectrometry platform for the routine therapeutic drug monitoring of 14 antibiotics: Application to critically ill pediatric patients. J. Pharm. Biomed. Anal. 2020, 186, 113273. [Google Scholar] [CrossRef]
- Jasiecka-Mikoajczyk, A.; Jaroszewski, J.J. Determination of tigecycline in turkey plasma by LC–MS/MS: Validation and application in a pharmacokinetic study. Pol. J. Vet. Sci. 2017, 20, 241–249. [Google Scholar] [CrossRef]
- Mei, S.; Luo, X.; Li, X.; Li, Q.; Huo, J.; Yang, L.; Zhu, L.; Feng, W.; Zhou, J.; Shi, G.; et al. Development and validation of an LC–MS/MS method for the determination of tigecycline in human plasma and cerebrospinal fluid and its application to a pharmacokinetic study. Biomed. Chromatogr. 2020, 186, 113273. [Google Scholar] [CrossRef]
- Ji, A.J.; Saunders, J.P.; Amorusi, P.; Stein, G.; Wadgaonkar, N.P.; O’Leary, k.; Leal, M.; Dukart, G.; Marshall, B.; Fluhler, E.N. A sensitive human bone assay for quantitation of tigecycline using LC/MS/MS. J. Pharm. Biomed. Anal. 2008, 48, 866–875. [Google Scholar] [CrossRef]
- Ji, A.J.; Saunders, J.P.; Wadgaonkar, N.D.; Petersen, P.J.; O’Leary, K.; McWilliams, W.E.; Amorusi, P.; Leal, M.; Fluhler, E.N. A novel antibiotic bone assay by liquid chromatography/tandem mass spectrometry for quantitation of tigecycline in rat bone. J. Pharm. Biomed. Anal. 2007, 44, 970–979. [Google Scholar] [CrossRef]
- Ji, A.J.; Saunders, J.P.; Amorusi, P.; Stein, G.; Wadgaonkar, N.P.; O’Leary, k.; Leal, M.; Fluhler, E.N. Determination of tigecycline in human skin using a novel validated LC–MS/MS method. Bioanalysis 2010, 2, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xie, J.; Wang, H.; Zhang, Y.; Zhao, Y.; Li, S.; Zhang, K.; Dong, Y. Determination of tigecycline in human lung epithelial cells and polymorphonuclear neutrophils by LC–MS/MS and its application in cellular pharmacokinetic study. Rapid Commun. Mass Spectrom. 2021, 35, e9112. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Gotfried, M.H.; Cwik, M.; Korth-Bradley, J.M.; Dukart, G.; Ellis-Grosse, A.E.J. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 2007, 58, 1221–1229. [Google Scholar] [CrossRef]
- Munyeza, C.F.; Shobo, A.; Baijnath, S.; Bratkowska, D.; Naiker, S.; Bester, L.A.; Singh, S.D.; Maguire, G.E.M.; Kruger, H.G.; Naicker, T.; et al. Development and validation of a liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for the quantification of tigecycline in rat brain tissues. Biomed. Chromatogr. 2016, 30, 837–845. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Zhu, D.C.; Sun, J.Z. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- The European Communities. Commission Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results; European Communities: Brussels, Belgium, 2002. [Google Scholar]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research; Center for Veterinary Medicine. Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Da Silveira, V.G.; Oliveira, M.S.; de Almeida, C.A.A.; Hoff, R.B.; Mallmann, C.A. Liquid chromatography-tandem mass spectrometry determination and depletion profile of chlortetracycline, doxycycline, and oxytetracycline in broiler chicken muscle after oral administration. Food Anal. Method 2018, 11, 2181–2194. [Google Scholar] [CrossRef]
- Cetinkaya, F.; Yibar, A.; Soyutemiz, G.E.; Okutan, B.; Ozcan, A.; Karaca, M.Y. Determination of tetracycline residues in chicken meat by liquid chromatography-tandem mass spectrometry. Food Addit. Contam. B 2012, 5, 45–49. [Google Scholar] [CrossRef]
- Tölgyesi, A.; Tölgyesi, L.; Békési, K.; Sharma, V.K.; Fekete, J. Determination of tetracyclines in pig and other meat samples using liquid chromatography coupled with diode array and tandem mass spectrometric detectors. Meat Sci. 2014, 96, 1332–1339. [Google Scholar] [CrossRef]
- Uekane, T.M.; Neto, F.R.A.; Gomes, L.N.F. Development and validation of a method for the analysis of tetracyclines in chicken-muscle by liquid chromatography-electrospray-mass spectrometry in tandem (LC–ESI–MS/MS). Quím. Nova 2011, 34, 43–48. [Google Scholar] [CrossRef]
- Pokrant, E.V.; Maddaleno, A.E.; Araya, C.E.; San Martínb, B.V.; Cornejo, J. In-house validation of HPLC–MS/MS methods for detection and quantification of tetracyclines in edible tissues and feathers of broiler chickens. J. Braz. Chem. Soc. 2018, 29, 659–668. [Google Scholar] [CrossRef]
- Cherlet, M.; Schelkens, M.; Croubels, S.; De Backer, P. Quantitative multi-residue analysis of tetracyclines and their 4-epimers in pig tissues by high-performance liquid chromatography combined with positive-ion electrospray ionization mass spectrometry. Anal. Chim. Acta 2003, 492, 199–213. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Anizan, S.; Tien, M.M.; Savoy, M.C.; Bion, C. Determination of five tetracyclines and their epimers by LC–MS/MS based on a liquid–liquid extraction with low temperature partitioning. Food Addit. Contam. A 2018, 35, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, H.; Ino, S.; Kato, K.; Watanabe, T.; Ito, Y.; Oka, H. Simultaneous determination of residual tetracyclines in foods by high-performance liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. J. Chromatogr. B 1999, 732, 55–64. [Google Scholar] [CrossRef]
- Alanazi, F.; Almugbel, R.; Maher, H.M.; Alodaib, F.M.; Alzoman, N.Z. Determination of tetracycline, oxytetracycline and chlortetracycline residues in seafood products of Saudi Arabia using high-performance liquid chromatography-photo diode array detection. Saudi Pharm. J. 2021, 29, 566–575. [Google Scholar] [CrossRef]
- Hu, X.; Pan, J.; Hu, Y.; Huo, Y.; Li, G. Preparation and evaluation of solid-phase microextraction fiber based on molecularly imprinted polymers for trace analysis of tetracyclines in complicated samples. J. Chromatogr. B 2008, 1188, 97–107. [Google Scholar] [CrossRef]
- Bayliss, M.A.J.; Rigdova, K.; Kyriakides, M.; Grier, S.; Lovering, A.M.; Ellery, K.; Griffith, D.C.; MacGowan, A. Challenges in the bioanalysis of tetracyclines: Epimerisation and chelation with metals. J. Chromatogr. B 2019, 1134–1135, 121807. [Google Scholar] [CrossRef]
- Little, J.L.; Wempe, M.F.; Buchanan, C.M. Liquid chromatography–mass spectrometry/mass spectrometry method development for drug metabolism studies: Examining lipid matrix ionization effects in plasma. J. Chromatogr. B 2006, 833, 219–230. [Google Scholar] [CrossRef]
- SANTE. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed European Commission Document no SANTE/11945/2015; European Union: Brussels, Belgium, 2015. [Google Scholar]

| Analytes | Sample Preparation | LC Conditions | Detection Method | Linearity Range (μg/kg) | Sensitivity (μg/kg) | Recovery (%) |
|---|---|---|---|---|---|---|
| CTC, OTC, DOXY (chicken) [41] | Extracted with acetonitrile containing 0.025 mol/L formic acid; Low-temperature cleanup | Mobile phase: water containing 0.025 mol/L formic acid–methanol containing 0.025 mol/L formic acid; stationary phase: C18 column (50 mm × 4.6 mm, 1.8 μm) | HPLC–MS/MS | 20–400 | LODs: 10.0; LOQs: 10.0 | 98.4–103.2 |
| TC, CTC, OTC, DOXY (chicken) [42] | Extracted with methanol and 0.01 mol/L EDTA containing 1% formic acid | Mobile phase: water containing 0.1% formic acid–methanol containing 0.1% formic acid; stationary phase: C18 column (75 mm × 4.6 mm, 5 μm) | HPLC–MS/MS | 50–200 | LODs: 7.9-14.6; LOQs: 24.2–44.3 | 56.9–101.2 |
| TC, CTC, OTC, DOXY (pig meat) [43] | Extracted with McIlvaine buffer and 2% acetic acid (pH 2.0); SPE using Strata-XL column | Mobile phase: water containing 0.1% formic acid–acetonitrile; stationary phase: C18 column (150 mm × 4.6 mm, 2.6 μm); Mobile phase: water containing 0.01 mol/L oxalic acid–acetonitrile; stationary phase: C18 column (150 mm × 4.6 mm, 2.7 μm) | HPLC–MS/MS; HPLC–DAD | 25–200 | LODs: 5.0–10.0, LOQs: 17.0-33.0; LODs: 0.5, LOQs: 1.7 | 79.9–106.7; 51.9–80.1 |
| TC, CTC, OTC, DOXY (chicken) [44] | Extracted with 0.1 mol/L Na2EDTA–McIlvaine buffer; SPE using C18 column | Mobile phase: water containing 0.1% formic acid–acetonitrile containing 0.1% formic acid; stationary phase: C18 column (100 mm × 2.0 mm, 5 μm) | HPLC–MS/MS | 0–200 | LOQs: 7.0–35.0 | 89.4–106.3 |
| CTC, OTC, 4-epi-CTC, 4-epi-OTC (chicken) [45] | Extracted with EDTA–McIlvaine; SPE using C18 column | Mobile phase: water containing 0.1% formic acid–methanol containing 0.1% formic acid; stationary phase: C18 column (150 mm × 2.1 mm, 3.5 μm) | HPLC–MS/MS | 20–200 | LODs: 20.0; LOQs: 21.2–21.6 | 94.0–108.0 |
| TC, CTC, OTC, DOXY, 4-epi-TC, 4-epi-CTC, 4-epi-OTC (pig muscle) [46] | Extracted with 0.1 mol/L sodium succinate solution (adjusted to pH 4.0 with 10 mol/L NaOH); SPE using HLB column | Mobile phase: 3% tetrahydrofuran containing 0.001 mol/L oxalic acid and 0.5% formic acid–tetrahydrofuran; stationary phase: PLRP-S polymeric column (250 mm × 4.6 mm, 8 μm) | HPLC–MS/MS | 0–1000 | LODs: 0.5–4.5 | - |
| TC, CTC, OTC, DOXY, 4-epi-TC, 4-epi-CTC, 4-epi-OTC, 6-epi-DOXY; demeclocycline, 4-epi-demeclocycline (poultry and pig muscle, fish) [47] | Extracted with 0.1 mol/L EDTA and acetonitrile | Mobile phase: water containing 0.01 mol/L oxalic acid–methanol containing 0.1% formic acid; stationary phase: HSS T3 column (100 mm × 2.1 mm, 1.8 μm) | HPLC–MS/MS | 0–400 | - | - |
| TC, CTC, OTC, DOXY, demeclocycline (chicken and fish) [48] | Extracted with 0.1 mol/L Na2EDTA–McIlvaine buffer (pH 4.0); SPE using Bond Elut Env column | Mobile phase: methanol:acetonitrile:0.005 mol/L oxalic acid (18:27:55, V:V:V); stationary phase: C8 column (250 mm × 4.6 mm, 5 μm) | HPLC–APCI/MS/MS | 0–0.50 ppm | LODs: 0.001–0.004 ppm | 60.1–88.9 |
| TC, CTC, OTC (fish and shellfish) [49] | Extracted with 20% trifluoroacetic acid, EDTA and methanol:0.01 mol/L citrate (80:20, V:V, pH 4.0) | Mobile phase: 0.05 mol/L oxalic acid:acetonitrile:methanol (70:20:10, V:V:V); stationary phase: C18 column (250 mm × 4.6 mm, 3.5 μm) | HPLC–DAD | 12.5–1250 (fish) 17.5–2500 (shellfish) | LODs: 15.0–62.0; LOQs: 125.0 | 95.0–105.0 |
| TC, CTC, OTC, DOXY (chicken) [50] | Extracted with 0.1 mol/L citrate buffer (pH 5.0) and ethyl acetate; Solid-phase microextraction fiber based on molecularly imprinted polymers | Mobile phase: 0.1 mol/L malonate:0.05 mol/L magnesium chloride (30:70, V:V, adjusted to pH 6.5 with NH3·H2O); stationary phase: C18 column (250 mm × 4.6 mm, 5 μm) | HPLC–FLD | 5–200 μg/L | LODs: 1.02–2.31 μg/L | 72.6–92.8 |
| Analyte | Relative Molecular Mass | Transition (m/z) | Declustering Potential (V) | Collision Energy (eV) |
|---|---|---|---|---|
| TC | 444.4 | 445.1 > 410.0 * 445.1 > 427.1 | 130 130 | 21 21 |
| CTC | 478.9 | 479.2 > 444.0 * 479.2 > 462.1 | 135 135 | 30 30 |
| OTC | 460.4 | 461.1 > 425.9 * 461.1 > 443.1 | 121 121 | 23 23 |
| DOXY | 444.4 | 445.2 > 428.1 * 445.2 > 154.1 | 131 131 | 31 31 |
| TGC | 585.7 | 587.2 > 514.1 * 587.2 > 457.3 | 120 120 | 28 28 |
| 4-epi-TC | 480.9 | 445.1 > 410.0 * 445.1 > 427.1 | 130 130 | 21 21 |
| 4-epi-CTC | 515.3 | 479.2 > 444.0 * 479.2 > 462.1 | 134 136 | 28 29 |
| 4-epi-OTC | 460.4 | 461.1 > 425.9 * 461.1 > 443.1 | 121 121 | 20 21 |
| Analyte | Regression Equation | R2 | Linearity Range (μg/kg) |
|---|---|---|---|
| TC | y = 88,271 x + 8955.5 | 0.9999 | 0.15–200 |
| CTC | y = 22,813 x + 71,883 | 0.9997 | 0.13–200 |
| OTC | y = 30,672 x + 57,745 | 0.9998 | 0.16–200 |
| DOXY | y = 19,943 x + 48,367 | 0.9997 | 0.12–400 |
| TGC | y = 11,454 x + 32,725 | 0.9997 | 0.19–200 |
| 4-epi-TC | y = 150,673 x + 163,280 | 0.9998 | 0.17–200 |
| 4-epi-CTC | y = 48,531 x + 157,731 | 0.9998 | 0.16–200 |
| 4-epi-OTC | y = 39,020 x + 41,188 | 0.9999 | 0.18–200 |
| Analyte | Fortified Level (μg/kg) | Recovery (%) | RSD (%) | Within-Run RSD (%) | Between-Run RSD (%) |
|---|---|---|---|---|---|
| TC | 0.15 | 90 ± 2.9 | 3.3 | 3.0 | 6.0 |
| 0.45 | 92 ± 2.7 | 3.0 | 2.7 | 4.5 | |
| 100 α | 96 ± 3.3 | 3.4 | 3.8 | 5.9 | |
| 200 | 94 ± 3.7 | 4.0 | 3.5 | 5.0 | |
| CTC | 0.13 | 89 ± 3.7 | 4.2 | 3.6 | 5.9 |
| 0.39 | 93 ± 3.3 | 3.5 | 4.9 | 5.2 | |
| 100 α | 93 ± 2.3 | 2.5 | 3.7 | 5.7 | |
| 200 | 97 ± 3.5 | 3.6 | 3.2 | 6.9 | |
| OTC | 0.16 | 91 ± 2.7 | 2.9 | 3.4 | 4.6 |
| 0.48 | 95 ± 3.1 | 3.3 | 3.0 | 4.2 | |
| 100 α | 94 ± 4.0 | 4.1 | 3.4 | 4.8 | |
| 200 | 98 ± 4.3 | 4.4 | 4.0 | 5.9 | |
| DOXY | 0.12 | 91 ± 2.1 | 2.3 | 3.2 | 5.7 |
| 0.36 | 94 ± 3.2 | 3.4 | 4.3 | 5.9 | |
| 200 α | 95 ± 2.5 | 2.6 | 3.0 | 4.1 | |
| 400 | 92 ± 2.7 | 2.9 | 5.0 | 4.3 | |
| TGC | 0.19 | 89 ± 2.9 | 3.2 | 3.6 | 5.2 |
| 0.57 | 89 ± 2.6 | 3.0 | 3.8 | 5.8 | |
| 100 α | 93 ± 1.8 | 2.0 | 2.3 | 3.8 | |
| 200 | 94 ± 1.8 | 1.9 | 2.0 | 3.6 | |
| 4-epi-TC | 0.17 | 94 ± 2.6 | 2.8 | 3.7 | 5.7 |
| 0.51 | 93 ± 3.1 | 3.3 | 3.2 | 5.9 | |
| 100 α | 90 ± 2.8 | 3.1 | 3.2 | 5.3 | |
| 200 | 92 ± 2.9 | 3.1 | 4.1 | 6.2 | |
| 4-epi-CTC | 0.16 | 93 ± 2.9 | 3.1 | 4.0 | 6.5 |
| 0.48 | 96 ± 2.7 | 2.8 | 2.9 | 5.0 | |
| 100 α | 96 ± 1.8 | 1.9 | 2.1 | 3.6 | |
| 200 | 98 ± 2.1 | 2.1 | 3.1 | 4.5 | |
| 4-epi-OTC | 0.18 | 90 ± 3.1 | 3.5 | 3.8 | 6.4 |
| 0.54 | 91 ± 2.2 | 2.4 | 3.0 | 4.0 | |
| 100 α | 94 ± 3.2 | 3.4 | 4.5 | 6.9 | |
| 200 | 94 ± 3.0 | 3.2 | 2.9 | 4.3 |
| Analyte | LOD (µg/kg) | LOQ (µg/kg) | CCα (µg/kg) | CCβ (µg/kg) |
|---|---|---|---|---|
| TC | 0.09 | 0.15 | 104 | 108 |
| CTC | 0.06 | 0.13 | 119 | 129 |
| OTC | 0.08 | 0.16 | 104 | 109 |
| DOXY | 0.08 | 0.12 | 206 | 207 |
| TGC | 0.07 | 0.19 | 102 | 106 |
| 4-epi-TC | 0.08 | 0.17 | 101 | 102 |
| 4-epi-CTC | 0.06 | 0.16 | 102 | 104 |
| 4-epi-OTC | 0.07 | 0.18 | 103 | 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; He, Z.; Gao, P.; Liu, S.; Zhu, Y.; Xie, K.; Dong, Y. Concurrent Determination of Tigecycline, Tetracyclines and Their 4-Epimer Derivatives in Chicken Muscle Isolated from a Reversed-Phase Chromatography System Using Tandem Mass Spectrometry. Molecules 2022, 27, 6139. https://doi.org/10.3390/molecules27196139
Guo Y, He Z, Gao P, Liu S, Zhu Y, Xie K, Dong Y. Concurrent Determination of Tigecycline, Tetracyclines and Their 4-Epimer Derivatives in Chicken Muscle Isolated from a Reversed-Phase Chromatography System Using Tandem Mass Spectrometry. Molecules. 2022; 27(19):6139. https://doi.org/10.3390/molecules27196139
Chicago/Turabian StyleGuo, Yawen, Zhaoyuan He, Pengfei Gao, Shuyu Liu, Yali Zhu, Kaizhou Xie, and Yuhao Dong. 2022. "Concurrent Determination of Tigecycline, Tetracyclines and Their 4-Epimer Derivatives in Chicken Muscle Isolated from a Reversed-Phase Chromatography System Using Tandem Mass Spectrometry" Molecules 27, no. 19: 6139. https://doi.org/10.3390/molecules27196139
APA StyleGuo, Y., He, Z., Gao, P., Liu, S., Zhu, Y., Xie, K., & Dong, Y. (2022). Concurrent Determination of Tigecycline, Tetracyclines and Their 4-Epimer Derivatives in Chicken Muscle Isolated from a Reversed-Phase Chromatography System Using Tandem Mass Spectrometry. Molecules, 27(19), 6139. https://doi.org/10.3390/molecules27196139






