Zirconium Molybdate Nanocomposites’ Sensing Platform for the Sensitive and Selective Electrochemical Detection of Adefovir
Abstract
1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Apparatus
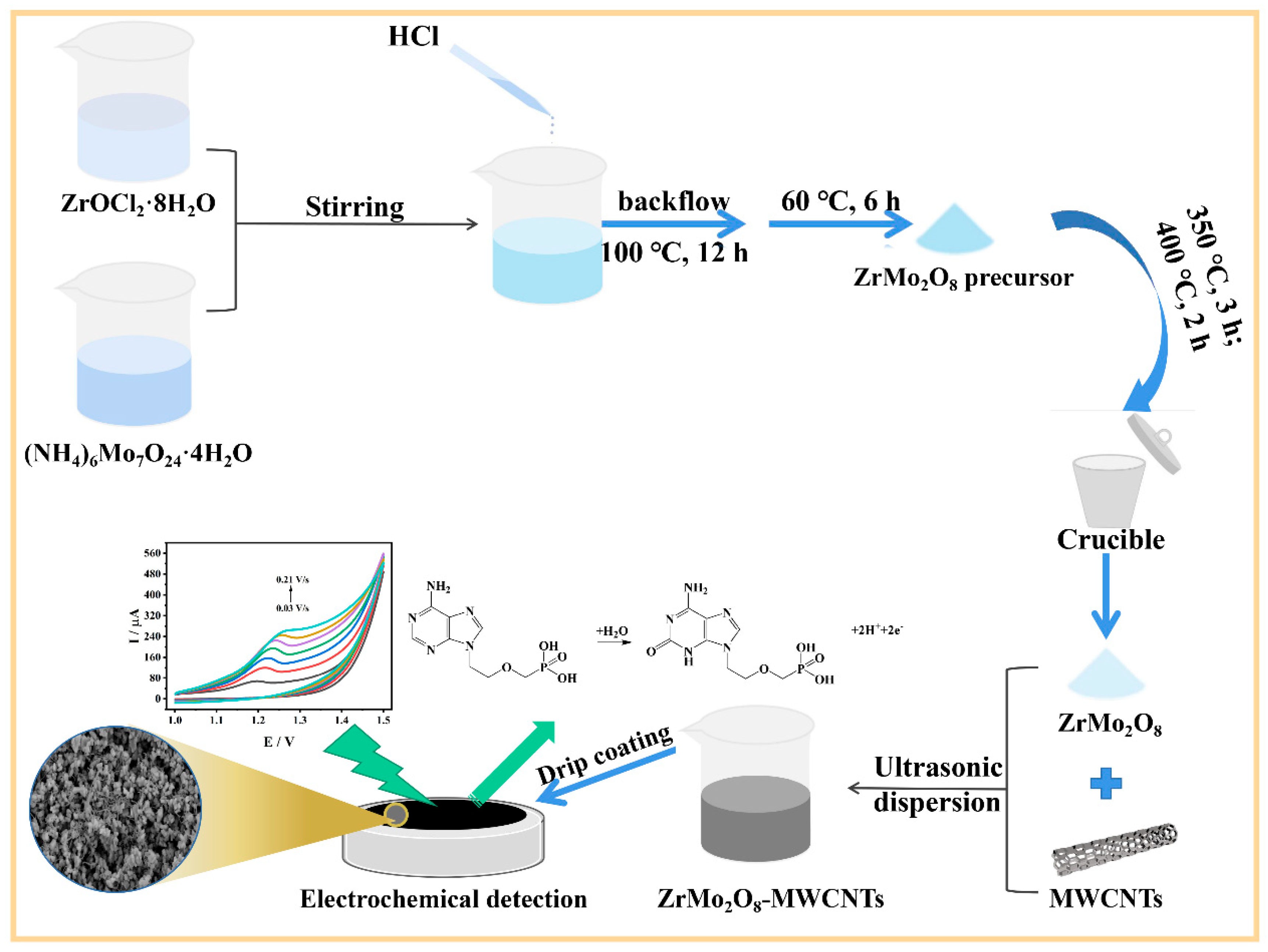
2.3. Preparation of ZrMo2O8 and ZrMo2O8-MWCNTs Composites
2.4. Fabrication of Modified Electrodes
2.5. Electrochemical Detection
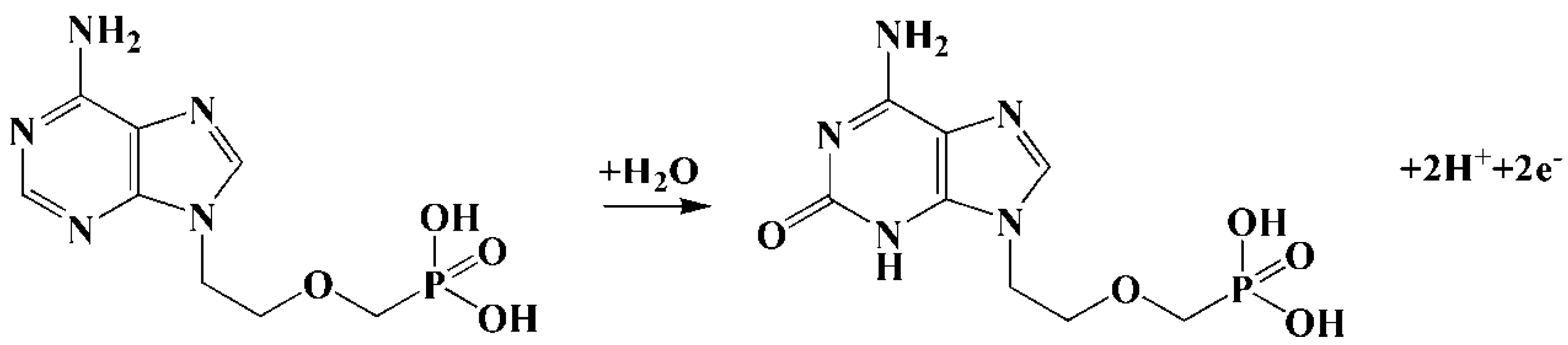
3. Results and Discussion
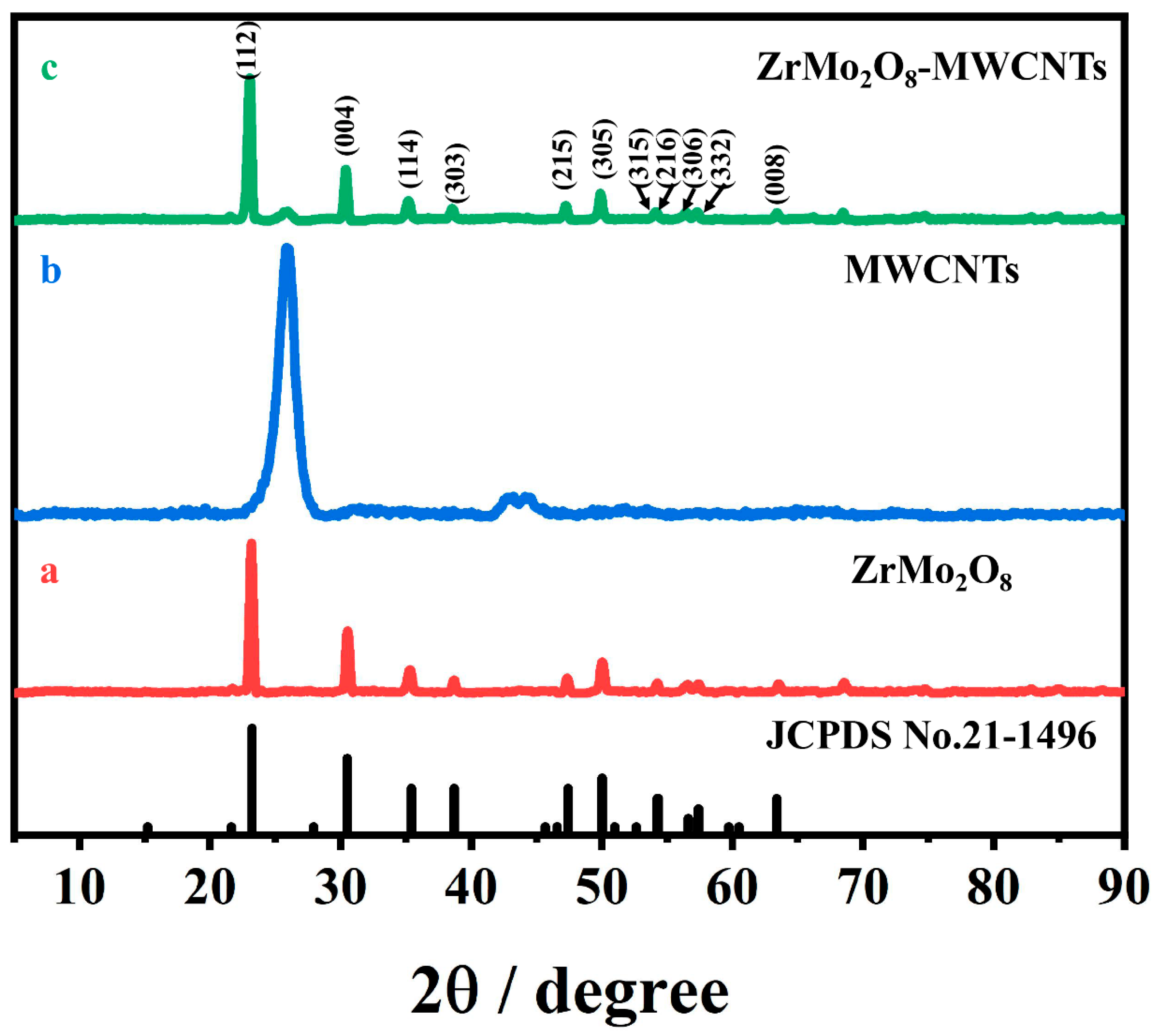
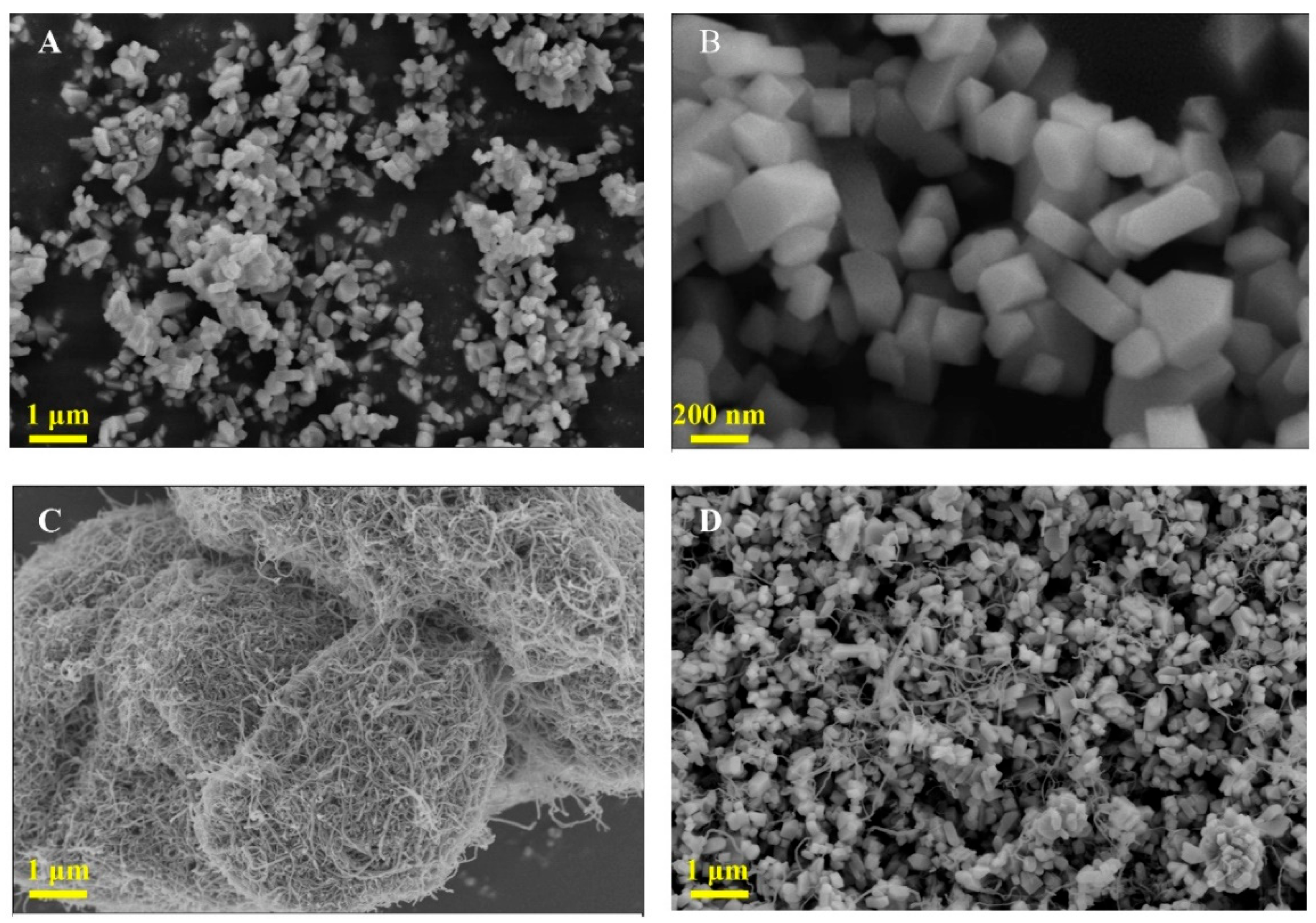
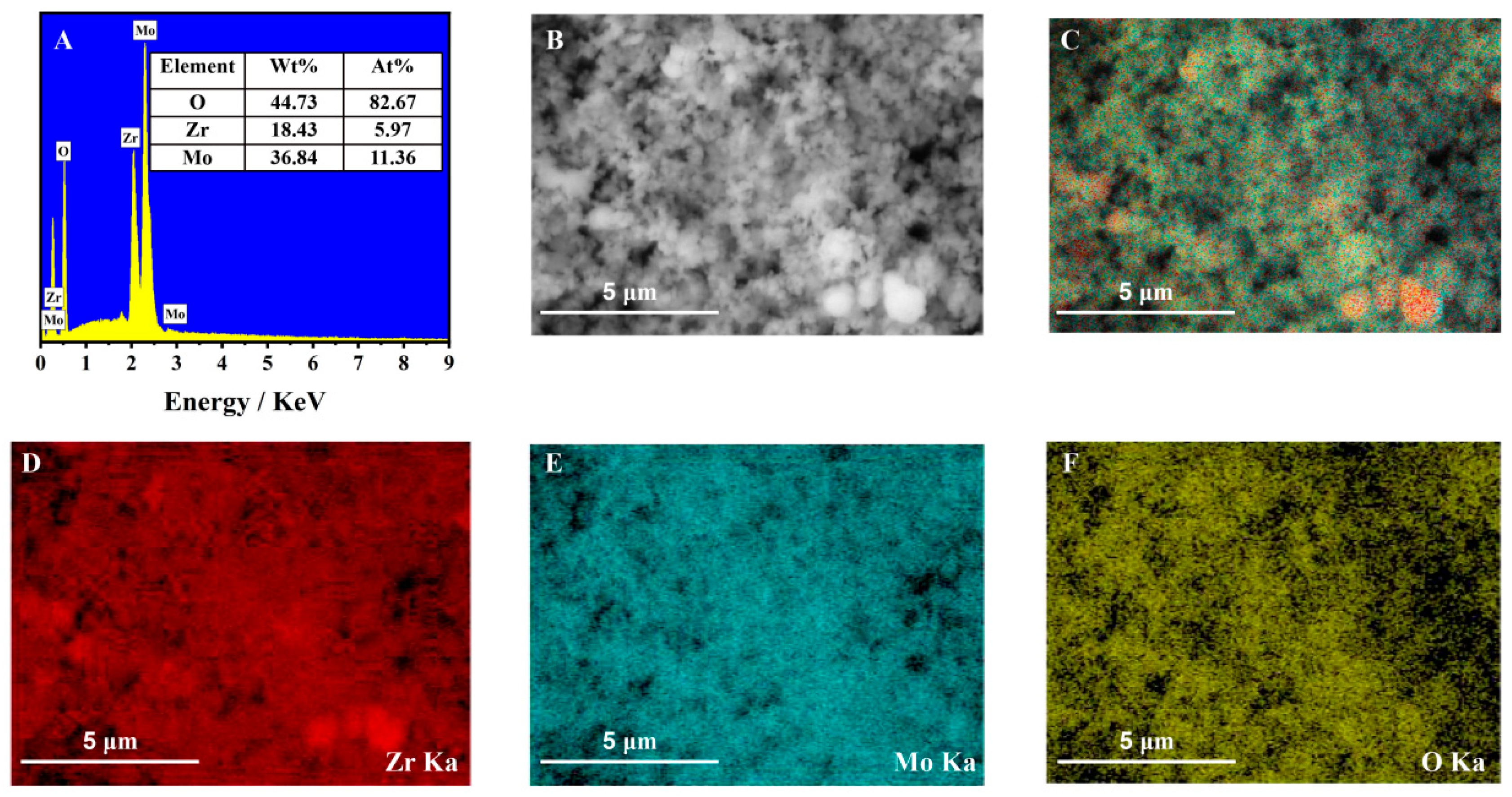
3.1. Crystal Phase and Morphology Characterization
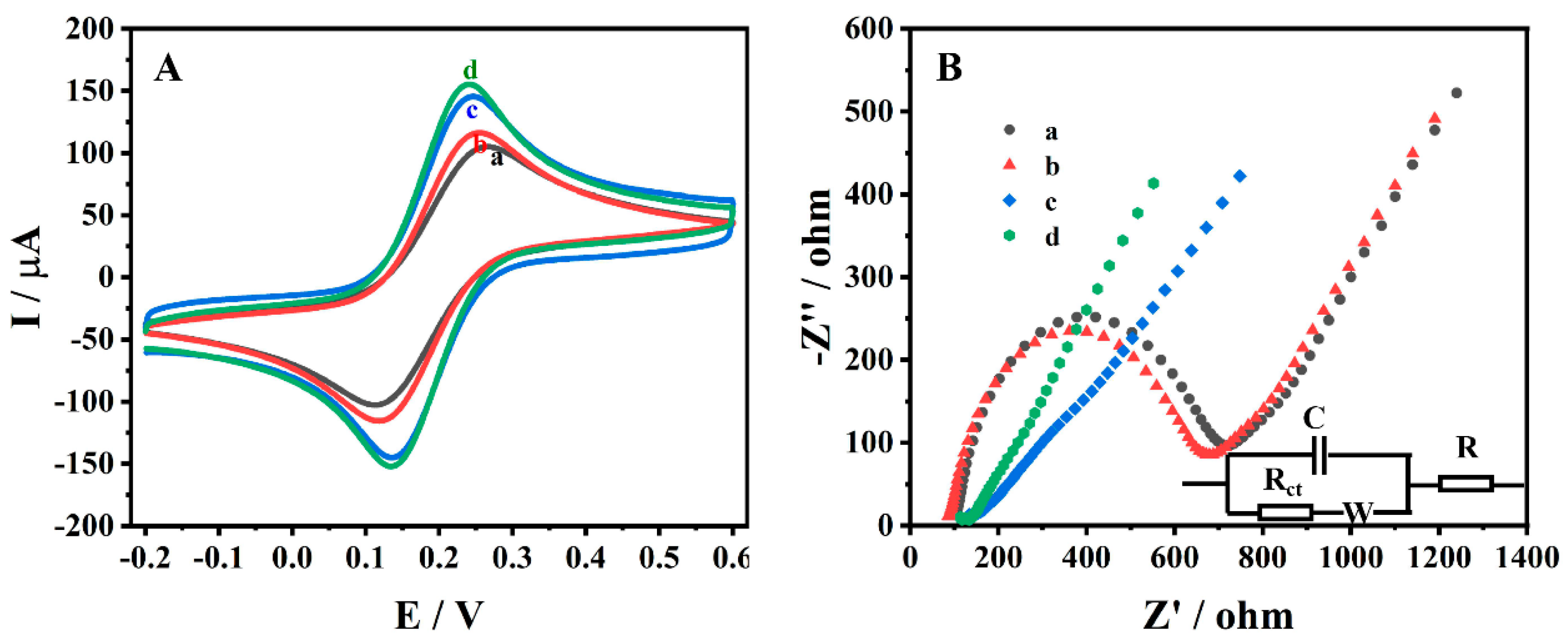
3.2. Electrochemical Characterisation of Different Electrodes
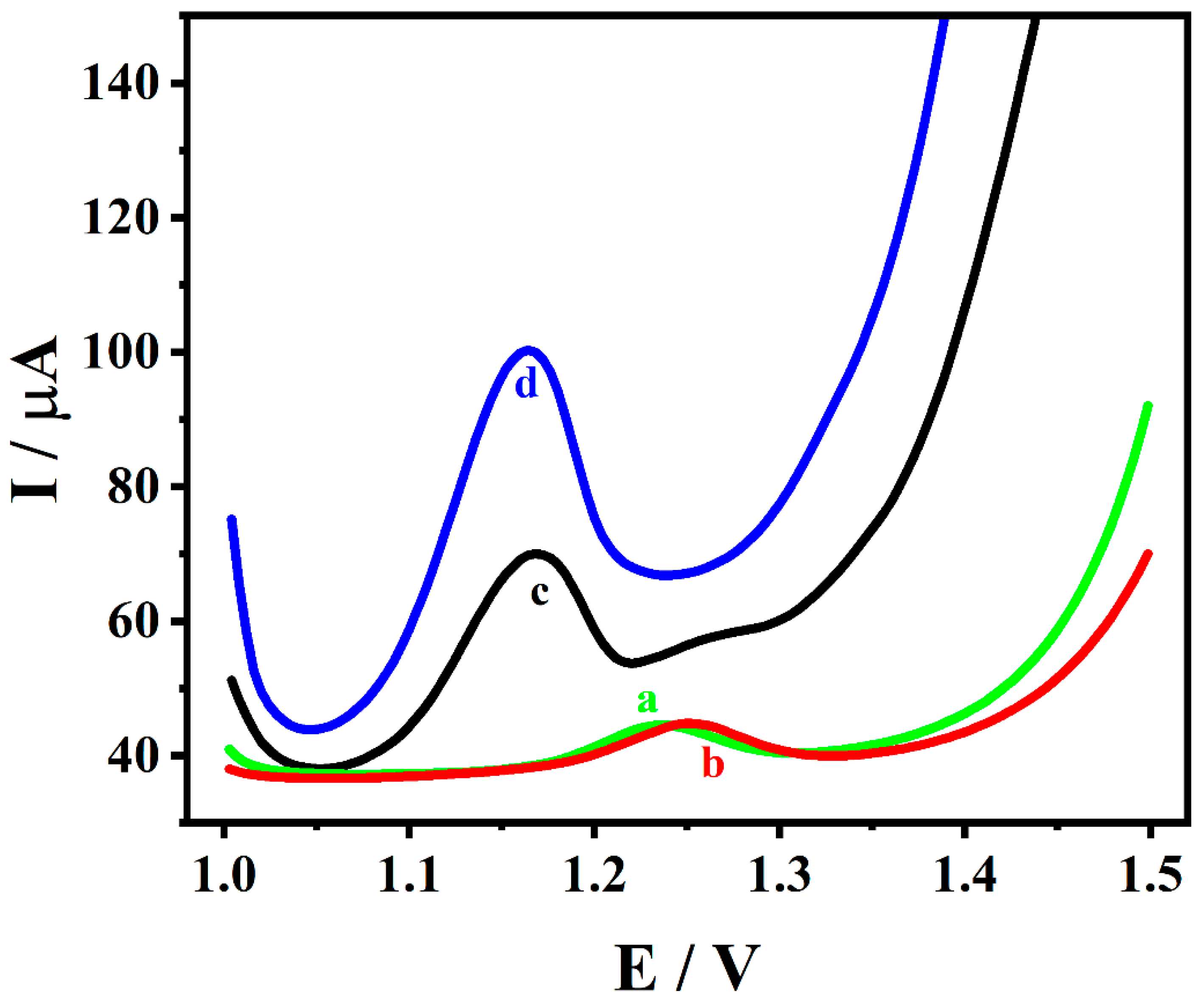
3.3. Electrochemical Behavior of Adefovir on Different Electrodes
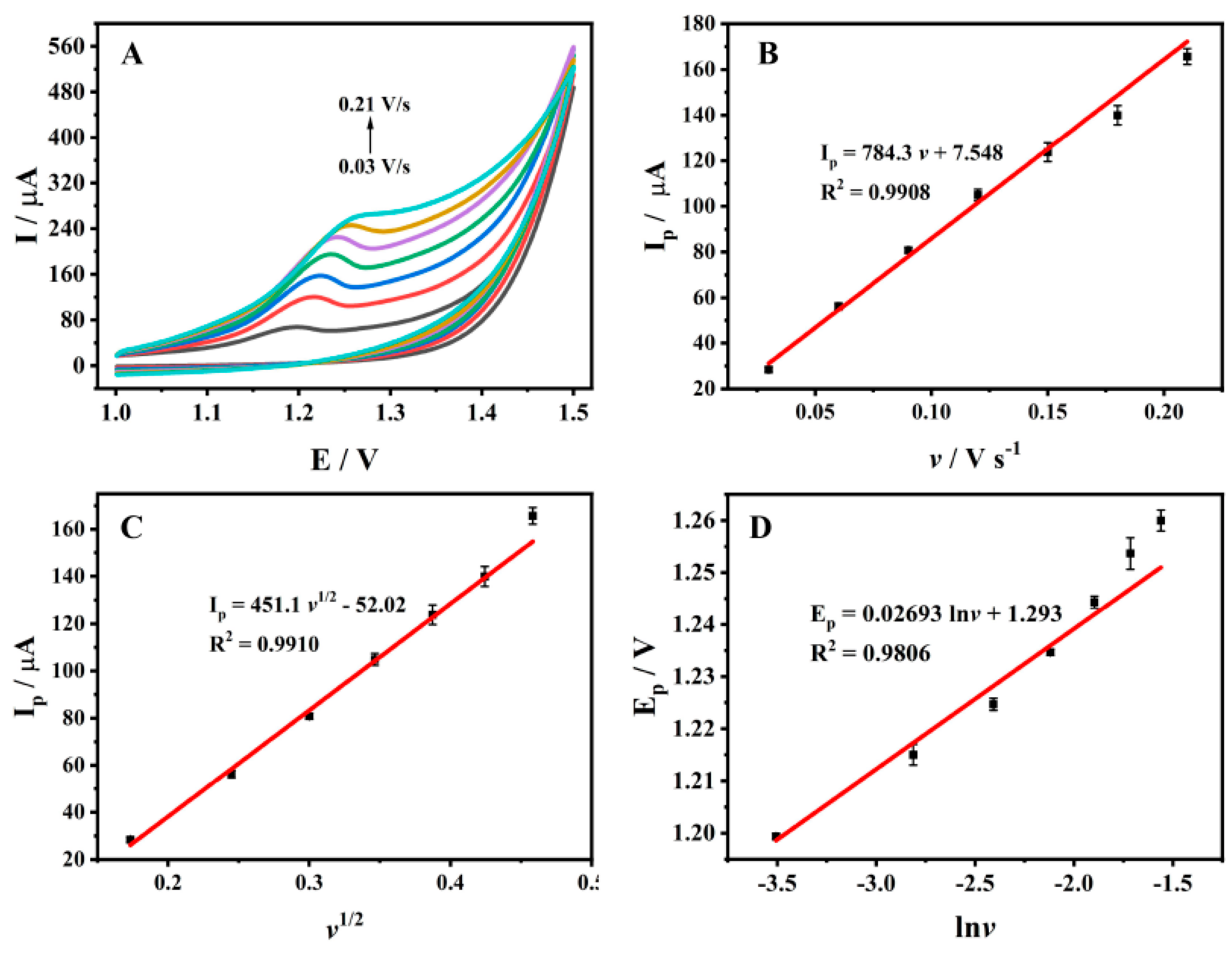
3.4. Effect of Scan Rate
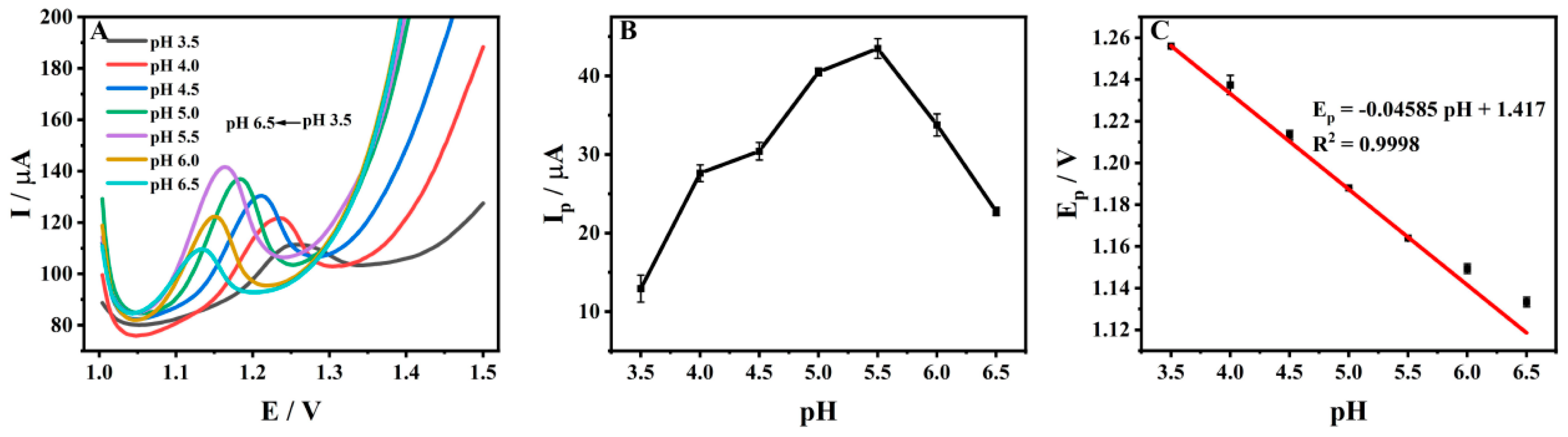
3.5. Effect of pH
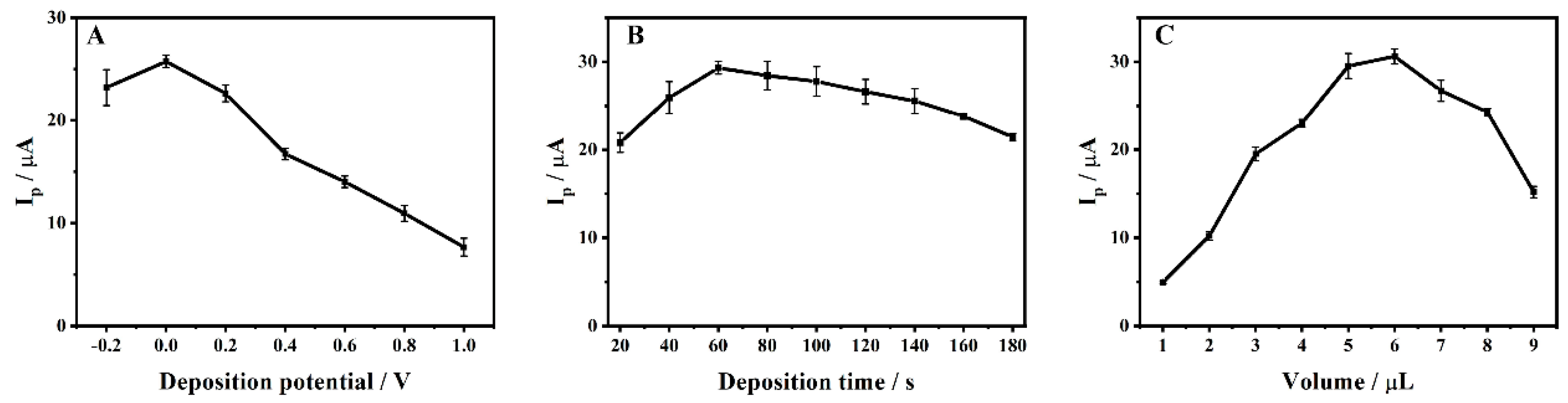
3.6. Effects of Deposition Conditions and Dropping Amount of ZrMo2O8-MWCNTs Composites
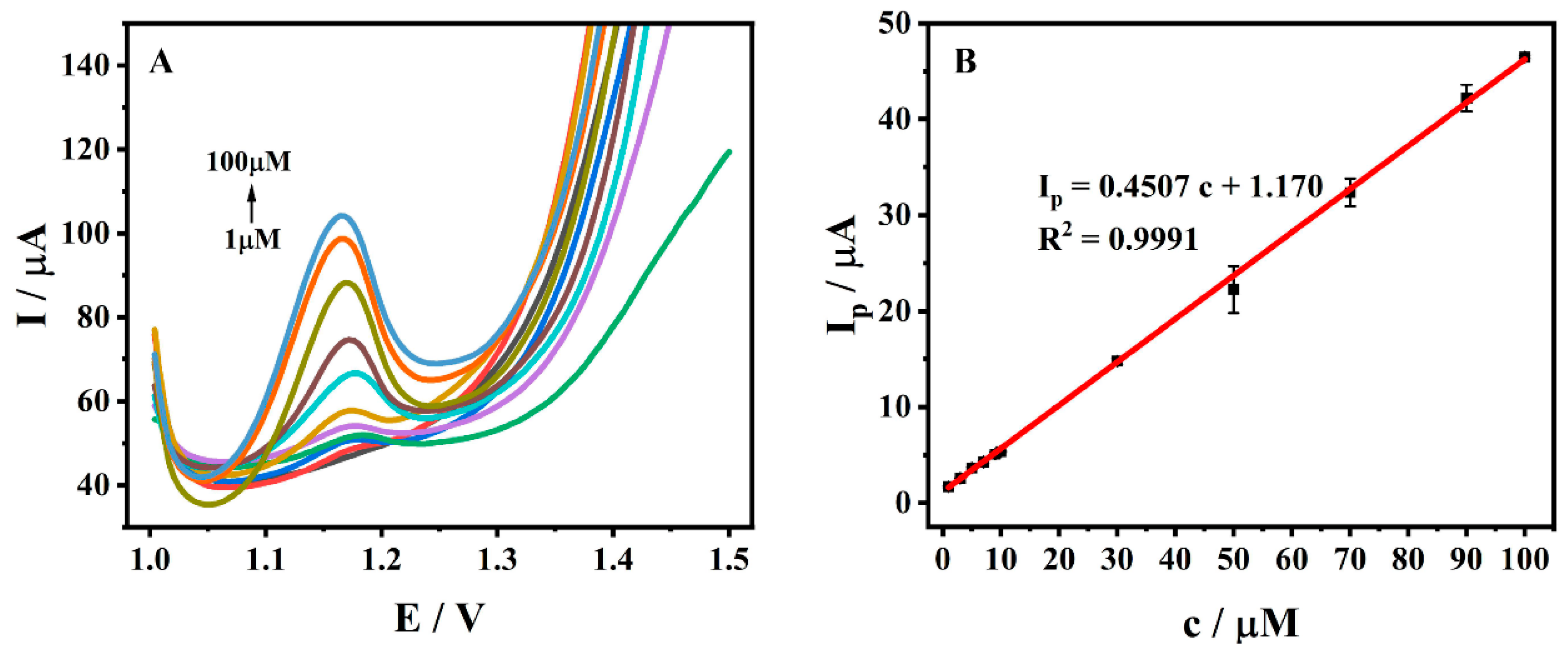
3.7. Standard Curve and Detection Limit
3.8. Investigation of Selectivity
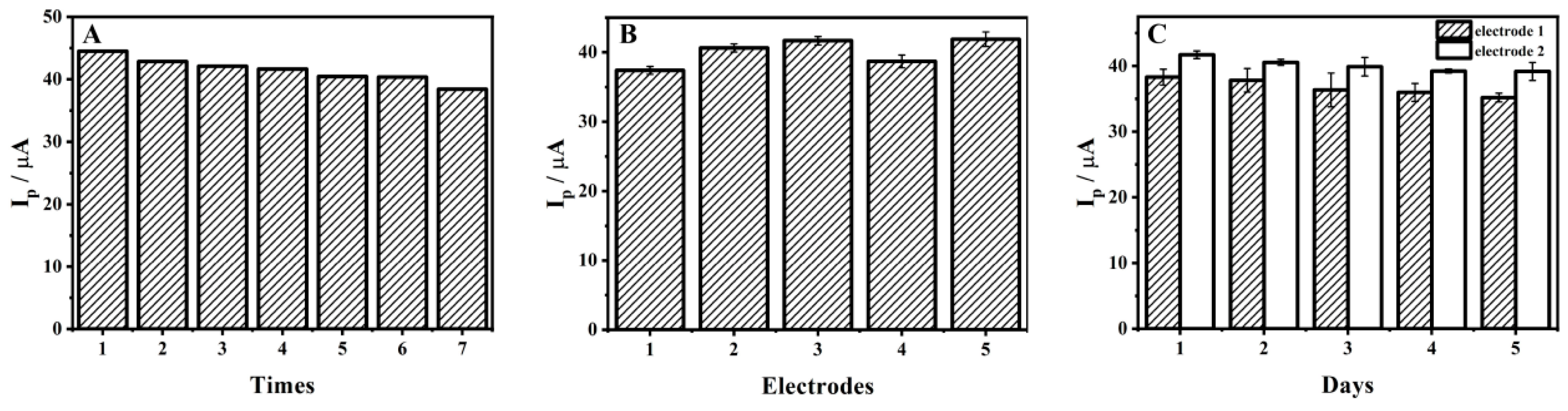
3.9. Repeatability, Reproducibility and Stability
3.10. Real Sample Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mulato, A.S.; Ho, E.S.; Cihlar, T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of Adefovir mediated by the human renal organic anion transporter 1. J. Pharmacol. Exp. Ther. 2000, 295, 10–15. [Google Scholar] [PubMed]
- Qi, X.; Xiong, S.; Yang, H.; Miller, M.; Delaney IV, W.E. In vitro susceptibility of Adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir. Ther. 2007, 12, 355–362. [Google Scholar] [CrossRef]
- Lada, O.; Benhamou, Y.; Cahour, A.; Katlama, C.; Poynard, T.; Thibault, V. In vitro susceptibility of Lamivudine-resistant hepatitis B virus to Adefovir and Tenofovir. Antivir. Ther. 2004, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.; Peng, C.Y.; Hann, H.W.; Sollano, J.; Lao-Tan, J.; Hsu, C.W.; Lesmana, L.; Yuen, M.F.; Jeffers, L.; Sherman, M.; et al. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of Entecavir versus Adefovir. Hepatology 2009, 49, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, S.S.; Ebru, B.; Serpil, P.; Aylin, D.; Seda, T.; Gonul, A.; Orhan, S.; Engin, A.; Gurol, E.; Enver, U. Detection of hepatitis B virus polymerase gene variants associated with Lamivudine, Adefovir and Entecavir resistance and some undefined mutations isolated from chronic hepatitis B patients in the south of Turkey. Mol. Genet. Microbiol. Virol. 2010, 25, 178–182. [Google Scholar] [CrossRef]
- Lsanska, L.O.; Ihlar, T.C.; Otruba, I.V.; Oly, A.H. Transport of Adefovir (PMEA) in human T-Lymphoblastoid 9-(2-Phosphonomethoxyethyl) adenine (PMEA; Adefovir) represents the simplest structural type of Acyclic nucleoside phosphonates, an important group of novel antiviral agents. Compound 1997, 62, 821–828. [Google Scholar]
- Naesens, L.; Snoeck, R.; Andrei, G.; Balzarini, J.; Neyts, J.; De Clercq, E. HPMPC (Cidofovir), PMEA (Adefovir) and related acyclic nucleoside phosphonate analogues. A review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 1997, 8, 1–23. [Google Scholar] [CrossRef]
- Vela, J.E.; Olson, L.Y.; Huang, A.; Fridland, A.; Ray, A.S. Simultaneous quantitation of the nucleotide analog Adefovir, its phosphorylated anabolites and 2′-deoxyadenosine triphosphate by ion-pairing LC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 848, 335–343. [Google Scholar] [CrossRef]
- Cundy, K.C. Clinical pharmacokinetics of the antiviral nucleotide analogues Cidofovir and Adefovir. Clin. Pharmacokinet. 1999, 36, 127–143. [Google Scholar] [CrossRef]
- Ho, E.S.; Lin, D.C.; Mendel, D.B.; Cihlar, T. Cytotoxicity of antiviral nucleotides Adefovir and Cidofovir is induced by the expression of human renal organic anion transporter 1. J. Am. Soc. Nephrol. 2000, 11, 383–393. [Google Scholar] [CrossRef]
- Goswami, D.; Gurule, S.; Saha, A.; Vats, P.; Khuroo, A.; Monif, T. Liquid chromatography-tandem mass spectrometry method for the estimation of Adefovir in human plasma: Application to a pharmacokinetic study. J. Pharm. Anal. 2015, 5, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, H.; Wang, B.; Guo, R. Development and validation of a sensitive LC-MS/MS method for the determination of Adefovir in human serum and urine. J. Pharm. Biomed. Anal. 2006, 42, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, L.; Zhan, Y.; Xiao, Q.Q.; Yang, J. Development and validation of a sensitive LC-MS-MS method for the determination of Adefovir in human serum and urine: Application to a clinical pharmacokinetic study. J. Chromatogr. Sci. 2016, 54, 507–515. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Duan, Y. Field-amplified on-line sample stacking for separation and determination of Adefovir and Tenofovir using capillary electrophoresis. Asian J. Chem. 2015, 27, 3143–3148. [Google Scholar] [CrossRef]
- Vávrová, K.; Lorencová, K.; Klimentová, J.; Novotný, J.; Hrabálek, A. HPLC method for determination of in vitro delivery through and into porcine skin of Adefovir (PMEA). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 853, 198–203. [Google Scholar] [CrossRef]
- Sparidans, R.W.; Veldkamp, A.; Hoetelmans, R.M.W.; Beijnen, J.H. Improved and simplified liquid chromatographic assay for Adefovir, a novel antiviral drug, in human plasma using derivatization with chloroacetaldehyde. J. Chromatogr. B Biomed. Sci. Appl. 1999, 736, 115–121. [Google Scholar] [CrossRef]
- Ding, Z.; Deng, P.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; He, Q. A novel modified electrode for detection of the food colorant sunset yellow based on nanohybrid of MnO2 nanorods-decorated electrochemically reduced graphene oxide. Molecules 2019, 24, 1174. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Xia, Y.; Tuo, D.; Deng, P.; Tian, Y.; Wu, Y.; Li, G.; Chen, D. Rapid and sensitive voltammetric detection of Rhodamine B in chili-containing foodstuffs using MnO2 nanorods/electro-reduced graphene oxide composite. J. Electrochem. Soc. 2019, 166, B805–B813. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Liu, J.; Li, J.; Li, G.; He, Q. High sensitive voltammetric sensor for nanomolarity vanillin detection in food samples via manganese dioxide nanowires hybridized electrode. Microchem. J. 2020, 157, 104885. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, Z.; Wu, Z.; Xie, M.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. Ta2O5/rGO nanocomposite modified electrodes for detection of tryptophan through electrochemical route. Nanomaterials 2019, 9, 811. [Google Scholar] [CrossRef]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Liu, J.; Li, G. Rapid and sensitive determination of vanillin based on a glassy carbon electrode modified with Cu2O-electrochemically reduced graphene oxide nanocomposite film. Sensors 2018, 18, 2762. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.M.; Buza, J.; He, Q.; Tan, Y. Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 2019, 23, e00064. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Ding, Z.; Li, G.; Liu, J.; He, Q. A simple and efficient molecularly imprinted electrochemical sensor for the selective determination of tryptophan. Biomolecules 2019, 9, 294. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Feng, J.; Wu, Y.; Tian, Y.; Li, G.; Chen, D. Sensitive voltammetric sensor for tryptophan detection by using polyvinylpyrrolidone functionalized graphene/GCE. Nanomaterials 2020, 10, 125. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of tartrazine based on TiO2-electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef]
- Ozkan, S.A.; Uslu, B. From mercury to nanosensors: Past, present and the future perspective of electrochemistry in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2016, 130, 126–140. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, R. Voltammetric quantification of anti-hepatitis drug Adefovir in biological matrix and pharmaceutical formulation. J. Pharm. Anal. 2012, 2, 98–104. [Google Scholar] [CrossRef]
- Zaabal, M.; Bakirhan, N.K.; Doulache, M.; Kaddour, S.; Saidat, B.; Ozkan, S.A. A new approach on sensitive assay of Adefovir in pharmaceutical and biological fluid samples using polypyrrole modified glassy carbon electrode. Sens. Actuators B Chem. 2020, 323, 128657. [Google Scholar] [CrossRef]
- Li, J.H.; Wu, J.; Yu, Y.X. DFT exploration of sensor performances of two-dimensional WO3 to ten small gases in terms of work function and band gap changes and I-V responses. Appl. Surf. Sci. 2021, 546, 149104. [Google Scholar] [CrossRef]
- Chen, R.; Luo, S.; Xie, D.; Yu, Y.; Xiang, L. Highly dispersive palladium loading on ZnO by galvanic replacements with improved methane sensing performance. Chemosensors 2022, 10, 329. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently enhancing electrocatalytic activity of α-MnO2 nanorods/N-doped ketjenblack carbon for oxygen reduction reaction and oxygen evolution reaction using facile regulated hydrothermal treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef]
- Chen, K.; Wang, M.; Li, G.; He, Q.; Liu, J.; Li, F. Spherical α-MnO2 supported on N-KB as efficient electrocatalyst for oxygen reduction in Al-air battery. Materials 2018, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P.; Chen, D. Novel electrochemical sensors based on cuprous oxide-electrochemically reduced graphene oxide nanocomposites modified electrode toward sensitive detection of sunset yellow. Molecules 2018, 23, 2130. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Magesa, F.; Liu, J.; Li, G.; He, Q. Construction of effective electrochemical sensor for the determination of quinoline yellow based on different morphologies of manganese dioxide functionalized graphene. J. Food Compos. Anal. 2019, 84, 103280. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Li, G.; Hu, J.; He, Q. Ultrasensitive detection of dopamine via electrochemical route on spindle-like α-Fe2O3 Mesocrystals/rGO modified GCE. Mater. Res. Bull. 2021, 133, 111050. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Jin, H.; Xia, Y.; Liu, J.; He, Q.; Chen, D. Titania/electro-reduced graphene oxide nanohybrid as an efficient electrochemical sensor for the determination of allura red. Nanomaterials 2020, 10, 307. [Google Scholar] [CrossRef]
- Liu, J.; Dong, S.; He, Q.; Yang, S.; Xie, M.; Deng, P.; Xia, Y.; Li, G. Facile preparation of Fe3O4/C nanocomposite and its application for cost-effective and sensitive detection of tryptophan. Biomolecules 2019, 9, 245. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles- coated reduced graphene oxide nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef]
- Chen, T.W.; Sivasamy Vasantha, A.; Chen, S.M.; Al Farraj, D.A.; Soliman Elshikh, M.; Alkufeidy, R.M.; Al Khulaifi, M.M. Sonochemical synthesis and fabrication of honeycomb like zirconium dioxide with chitosan modified electrode for sensitive electrochemical determination of anti-tuberculosis (TB) drug. Ultrason. Sonochem. 2019, 59, 104718. [Google Scholar] [CrossRef]
- Pacchioni, G. Role of nanostructuring on the properties of oxide materials: The case of zirconia nanoparticles. Eur. J. Inorg. Chem. 2019, 2019, 751–761. [Google Scholar] [CrossRef]
- Sahoo, P.P.; Sumithra, S.; Madras, G.; Row, T.G. Synthesis, characterization, and photocatalytic properties of ZrMo2O8. J. Phys. Chem. C 2009, 113, 2107–2113. [Google Scholar] [CrossRef]
- Sahoo, P.P.; Sumithra, S.; Madras, G.; Row, G.T.N. Synthesis, structure and photocatalytic properties of β-ZrMo2O8. Bull. Mater. Sci. 2009, 32, 337–342. [Google Scholar] [CrossRef]
- Gül, G.Ç.; Kurtuluş, F. Conventional synthesis and characterization of zirconium molybdate, Nd2Zr3(MoO4)9. Int. J. Metall. Mater. Eng. 2016, 10, 1506–1509. [Google Scholar]
- Nguyen, T.D.; Nguyen, A.S.; Tran, B.A.; Ke, O.V.; To, T. Molybdate intercalated hydrotalcite/graphene oxide composite as corrosion inhibitor for carbon steel. Surf. Coat. Technol. 2020, 399, 126165. [Google Scholar] [CrossRef]
- Mohamed, S.N.; Thomas, N.; Tamilmani, J.; Boobalan, T.; Matheswaran, M.; Kalaichelvi, P.; Alagarsamy, A.; Pugazhendhi, A. Bioelectricity generation using iron (II) molybdate nanocatalyst coated anode during treatment of sugar wastewater in microbial fuel cell. Fuel 2020, 277, 118119. [Google Scholar] [CrossRef]
- Liu, X.; He, J.; Sakthivel, R.; Chung, R. Rare earth erbium molybdate nanoflakes decorated functionalized carbon nanofibers: An affordable and potential catalytic platform for the electrooxidation of phenothiazine. Electrochim. Acta. 2020, 358, 136885. [Google Scholar] [CrossRef]
- Boopathy, G.; Keerthi, M.; Chen, S.; Umapathy, M.J.; Kumar, B.N. Highly porous nickel molybdate@graphene oxide nanocomposite for the ultrasensitive electrochemical detection of environmental toxic pollutant catechol. Mater. Chem. Phys. 2020, 239, 121982. [Google Scholar] [CrossRef]
- Karthik, R.; Vinoth Kumar, J.; Chen, S.M.; Karuppiah, C.; Cheng, Y.H.; Muthuraj, V. A study of electrocatalytic and photocatalytic activity of cerium molybdate nanocubes decorated graphene oxide for the sensing and degradation of antibiotic drug chloramphenicol. ACS Appl. Mater. Interfaces 2017, 9, 6547–6559. [Google Scholar] [CrossRef]
- Sheha, R.R.; El-Khouly, S.H. Adsorption and diffusion of cesium ions in zirconium(IV) iodomolybdate exchanger. Chem. Eng. Res. Des. 2013, 91, 942–954. [Google Scholar] [CrossRef]
- Gupta, A.P.; Verma, G.L.; Ikram, S. Studies on a new heteropolyacid-based inorganic ion exchanger; Zirconium(IV) selenomolybdate. React. Funct. Polym. 2000, 43, 33–41. [Google Scholar] [CrossRef]
- Lind, C.; VanDerveer, D.G.; Wilkinson, A.P.; Chen, J.; Vaughan, M.T.; Weidner, D.J. New high-pressure form of the negative thermal expansion materials zirconium molybdate and hafnium molybdate. Chem. Mater. 2001, 13, 487–490. [Google Scholar] [CrossRef]
- Krogh Andersen, A.M.; Carlson, S. High-pressure structures of a- and δ-ZrMo2O8. Acta Crystallogr. Sect. B. 2001, 57, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lind, C.; Wilkinson, A.P.; Hu, Z.; Short, S.; Jorgensen, J.D. Synthesis and properties of the negative thermal expansion material cubic ZrMo2O8. Chem. Mater. 1998, 10, 2335–2337. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthik, R.; Chen, S.M.; Raja, N.; Selvam, V.; Muthuraj, V. Evaluation of a new electrochemical sensor for selective detection of non-enzymatic hydrogen peroxide based on hierarchical nanostructures of zirconium molybdate. J. Colloid Interface Sci. 2017, 500, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mancheva, M.N.; Iordanova, R.S.; Dimitriev, Y.B.; Tyuliev, G.G.; Iliev, T.C. Influence of heat treatment on the phase transition of ZrMo2O8 and photocatalytic activity. Cent. Eur. J. Chem. 2011, 9, 460–465. [Google Scholar] [CrossRef]
- Iordanova, R.; Mancheva, M.; Dimitriev, Y.; Klissurski, D.; Tyuliev, G.; Kunev, B. Synthesis of ZrMo2O8 polymorphs by a melt quenching method and mechanochemical activation. J. Alloys Compd. 2009, 485, 104–109. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P.; Rawn, C.J.; Payzant, E.A. Preparation of the negative thermal expansion material cubic ZrMo2O8. J. Mater. Chem. 2001, 11, 3354–3359. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P. Seeding and the non-hydrolytic sol-gel synthesis of ZrW2O8 and ZrMo2O8. J. Sol-Gel Sci. Technol. 2002, 25, 51–56. [Google Scholar] [CrossRef]
- Shivanekar, A.; Chudasama, U. Catalytic behaviour of a Cu(II) complex sorbed on zirconium molybdate in the decomposition of hydrogen peroxide. Transit. Met. Chem. 1990, 15, 226–230. [Google Scholar] [CrossRef]
- Nataraj, N. An efficient electrochemical sensor based on zirconium molybdate decorated reduced graphene oxide for the detection of Hydroquinone. Int. J. Electrochem. Sci. 2020, 15, 8321–8335. [Google Scholar] [CrossRef]
- Bui, T.H.; Hong, S.P.; Yoon, J. Enhanced selective removal of arsenic(V) using a hybrid nanoscale zirconium molybdate embedded anion exchange resin. Environ. Sci. Pollut. Res. 2019, 26, 37046–37053. [Google Scholar] [CrossRef]
- Bui, T.H.; Hong, S.P.; Yoon, J. Development of nanoscale zirconium molybdate embedded anion exchange resin for selective removal of phosphate. Water Res. 2018, 134, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mphuthi, N.G.; Adekunle, A.S.; Ebenso, E.E. Electrocatalytic oxidation of Epinephrine and Norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef] [PubMed]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter dependence of interwall separation and strain in multiwalled carbon nanotubes probed by X-ray diffraction and Raman scattering studies. Diam. Relat. Mater. 2010, 19, 1281–1288. [Google Scholar] [CrossRef]
- Feng, J.; Deng, P.; Xiao, J.; Li, J.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. New voltammetric method for determination of tyrosine in foodstuffs using an oxygen-functionalized multi-walled carbon nanotubes modified acetylene black paste electrode. J. Food Compos. Anal. 2020, 96, 103708. [Google Scholar] [CrossRef]
- Deng, P.; Xiao, J.; Feng, J.; Tian, Y.; Wu, Y.; Li, J.; He, Q. Highly sensitive electrochemical sensor for tyrosine detection using a sub-millimeter electrode. Microchem. J. 2021, 165, 106106. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, L.; Wu, Y.; Liu, X.; Feng, J.; Ding, J.; Li, K.; He, Q. Ultrasensitive electrochemical detection for nanomolarity Acyclovir at ferrous molybdate nanorods and graphene oxide composited glassy carbon electrode. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128601. [Google Scholar] [CrossRef]

| Method | Material | Linear Range (μM) | LOD (μM) | References |
|---|---|---|---|---|
| capillary electrophoresis | hydroxypropyl methyl cellulose | 0.0366~1.83 | 0.00977 | [14] |
| liquid chromatography | / | 0.366~18.3 | 0.366 | [15] |
| liquid chromatography mass spectrometry | / | 0.0458~0.586 (Serum) | 0.00366 (Serum) | [12] |
| / | 0.000183~0.0293 (Urine) | / | [12] | |
| SWV | a HMDE | 1.830~18.30 (Plasma) | 0.6956 (Plasma) | [28] |
| HMDE | 0.9151~8.236 (drug) | 0.2928 (drug) | [28] | |
| DPV | b PPy/GCE | 0.25~50 | 0.0031 | [29] |
| DPV | ZrMo2O8-MWCNTs/GCE | 1~100 | 0.253 | this work |
| Sample | Measured Value (μM) | Amount Added (μM) | Total Determination (μM) | RSD (%) | Recovery Rate (%) |
|---|---|---|---|---|---|
| Serum 1 | a ND | 5 | 4.908 | 2.98 | 98.16 |
| ND | 10 | 9.996 | 3.08 | 99.96 | |
| ND | 15 | 15.00 | 3.43 | 100.0 | |
| Serum 2 | ND | 5 | 4.835 | 4.83 | 96.70 |
| ND | 10 | 9.605 | 5.03 | 96.05 | |
| ND | 15 | 14.56 | 4.36 | 97.07 | |
| Urine 1 | ND | 5 | 4.793 | 5.32 | 95.86 |
| ND | 10 | 9.787 | 1.60 | 97.87 | |
| ND | 15 | 15.35 | 1.40 | 102.3 | |
| Urine 2 | ND | 5 | 4.884 | 2.93 | 97.68 |
| ND | 10 | 10.38 | 1.26 | 103.8 | |
| ND | 15 | 14.87 | 1.85 | 99.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xiao, J.; Yao, L.; Wei, Y.; Zuo, J.; Zeng, W.; Ding, J.; He, Q. Zirconium Molybdate Nanocomposites’ Sensing Platform for the Sensitive and Selective Electrochemical Detection of Adefovir. Molecules 2022, 27, 6022. https://doi.org/10.3390/molecules27186022
Li W, Xiao J, Yao L, Wei Y, Zuo J, Zeng W, Ding J, He Q. Zirconium Molybdate Nanocomposites’ Sensing Platform for the Sensitive and Selective Electrochemical Detection of Adefovir. Molecules. 2022; 27(18):6022. https://doi.org/10.3390/molecules27186022
Chicago/Turabian StyleLi, Wenming, Jingyun Xiao, Liangyuan Yao, Yanping Wei, Jinsong Zuo, Weili Zeng, Jianhua Ding, and Quanguo He. 2022. "Zirconium Molybdate Nanocomposites’ Sensing Platform for the Sensitive and Selective Electrochemical Detection of Adefovir" Molecules 27, no. 18: 6022. https://doi.org/10.3390/molecules27186022
APA StyleLi, W., Xiao, J., Yao, L., Wei, Y., Zuo, J., Zeng, W., Ding, J., & He, Q. (2022). Zirconium Molybdate Nanocomposites’ Sensing Platform for the Sensitive and Selective Electrochemical Detection of Adefovir. Molecules, 27(18), 6022. https://doi.org/10.3390/molecules27186022






