The Free Radical Scavenging Property of the Leaves, Branches, and Roots of Mansoa hirsuta DC: In Vitro Assessment, 3D Pharmacophore, and Molecular Docking Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Metabolic Content of Mansoa hirsuta
2.2. Chromatographic Profile of Mansoa hirsuta
2.2.1. Chromatographic Analysis
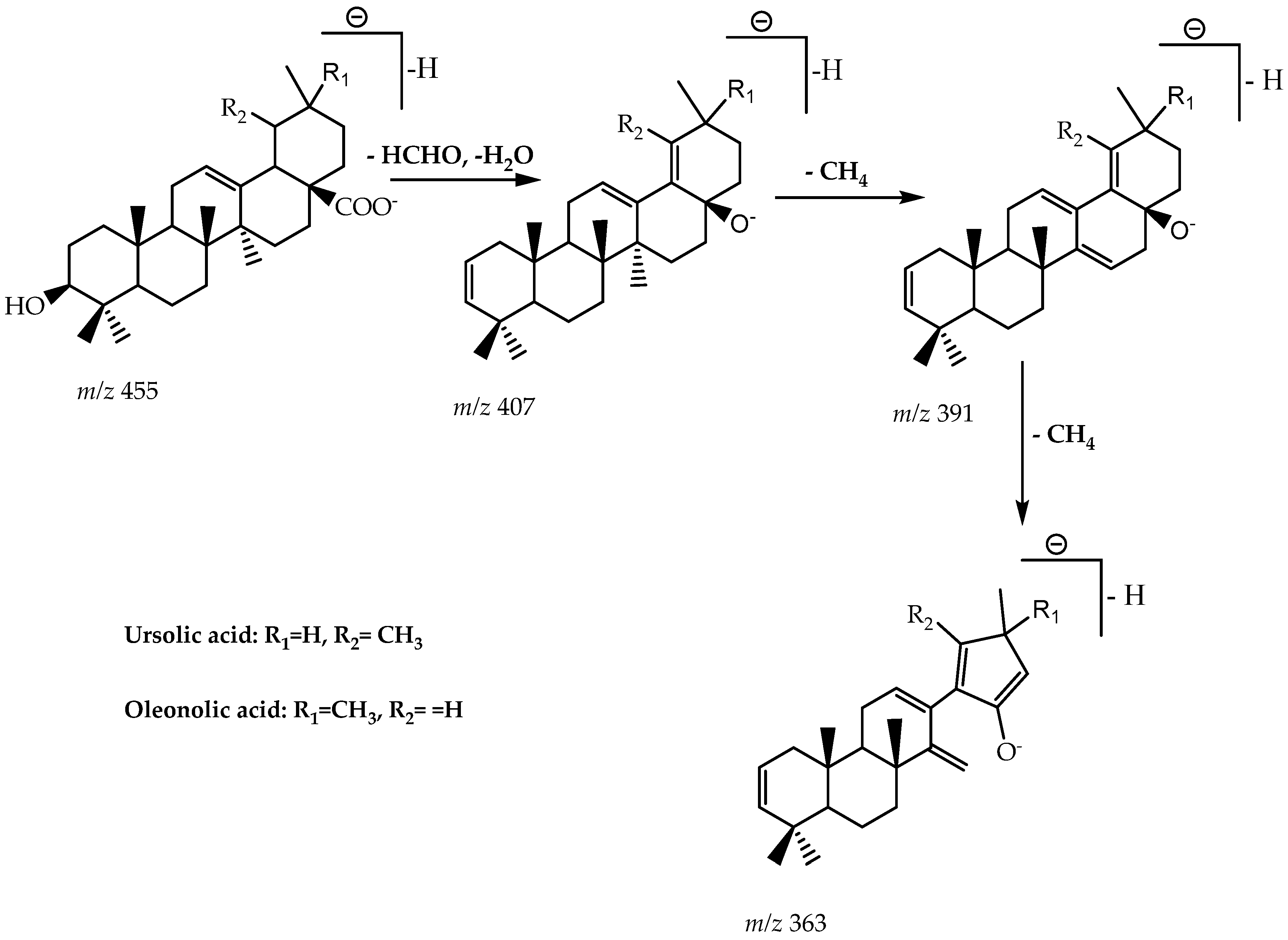
2.2.2. LC-MS Profiling
2.2.3. H-NMR
2.3. Antioxidant Activity by DPPH and ABTS Tests
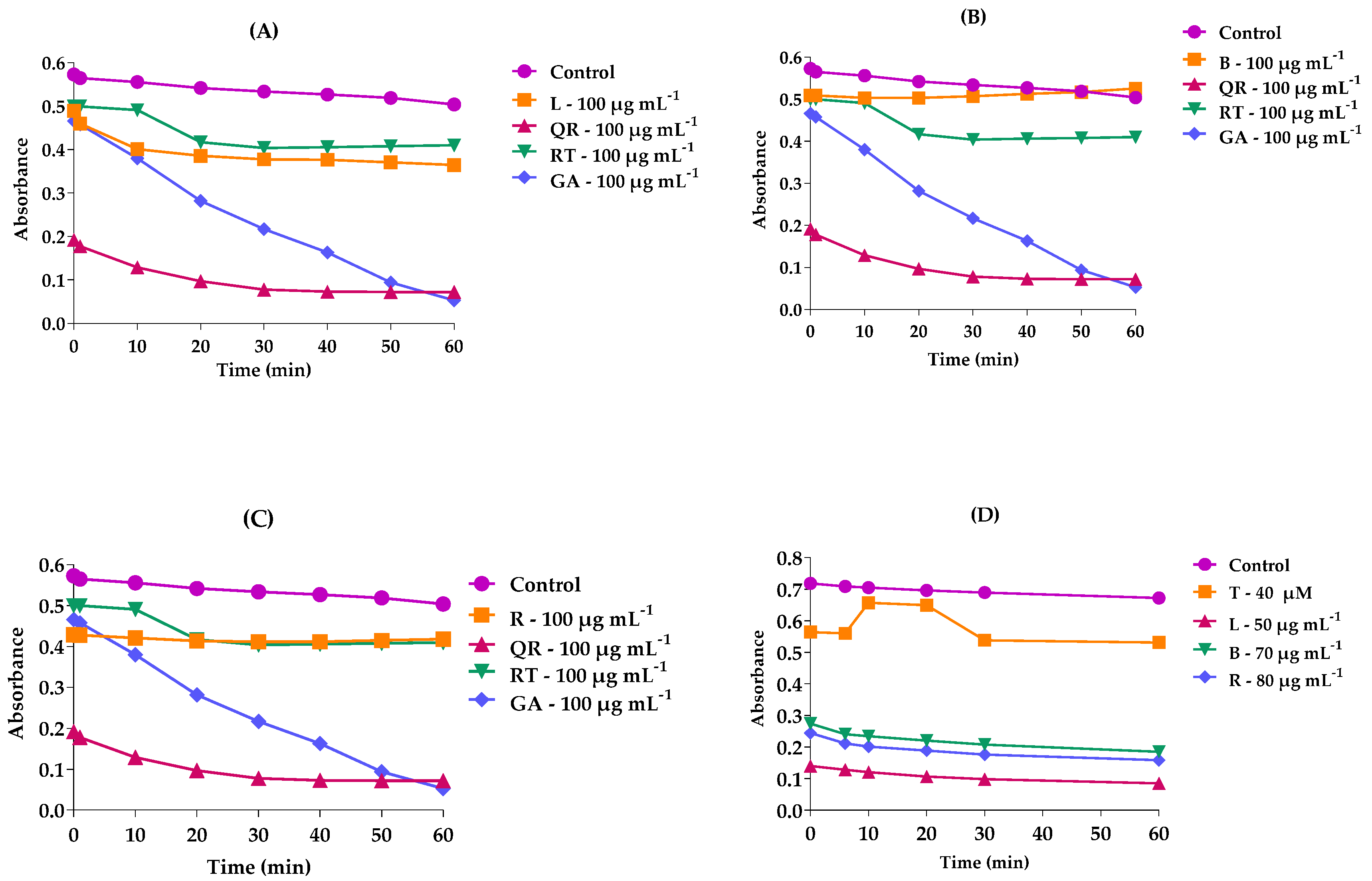
2.4. Chemical Kinetics of DPPH and ABTS
2.5. Total Phenols and Total Flavonoids Content
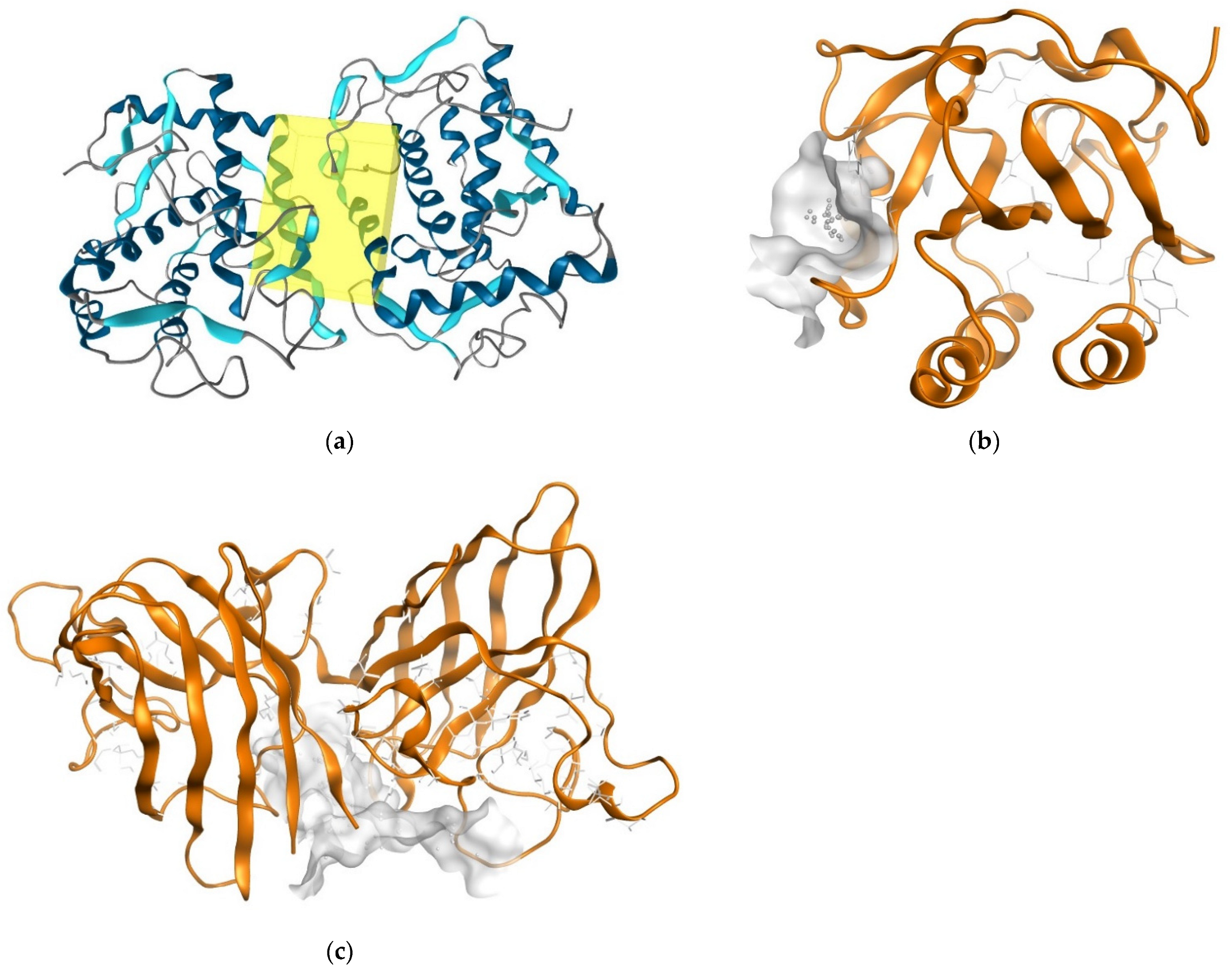
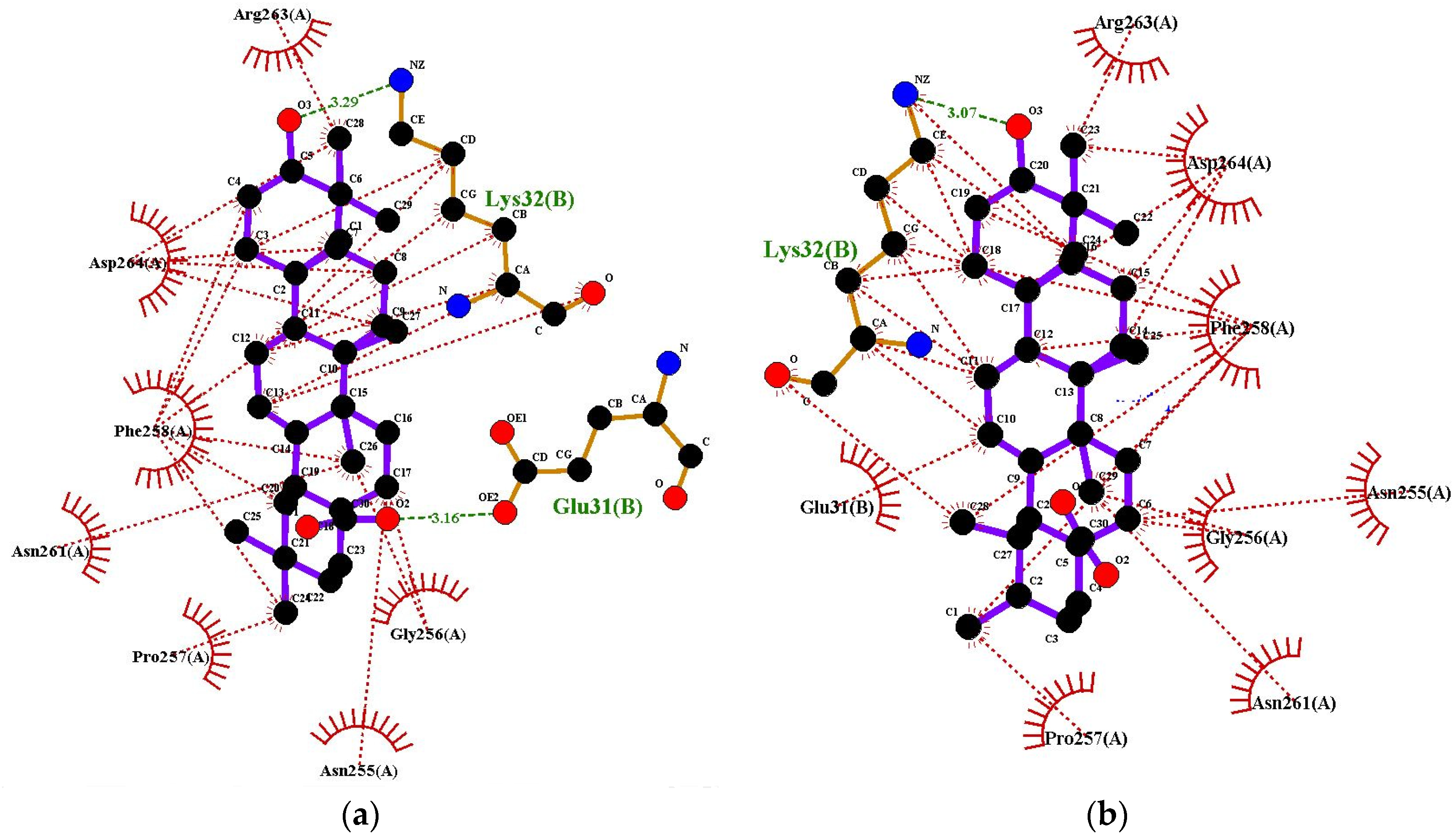
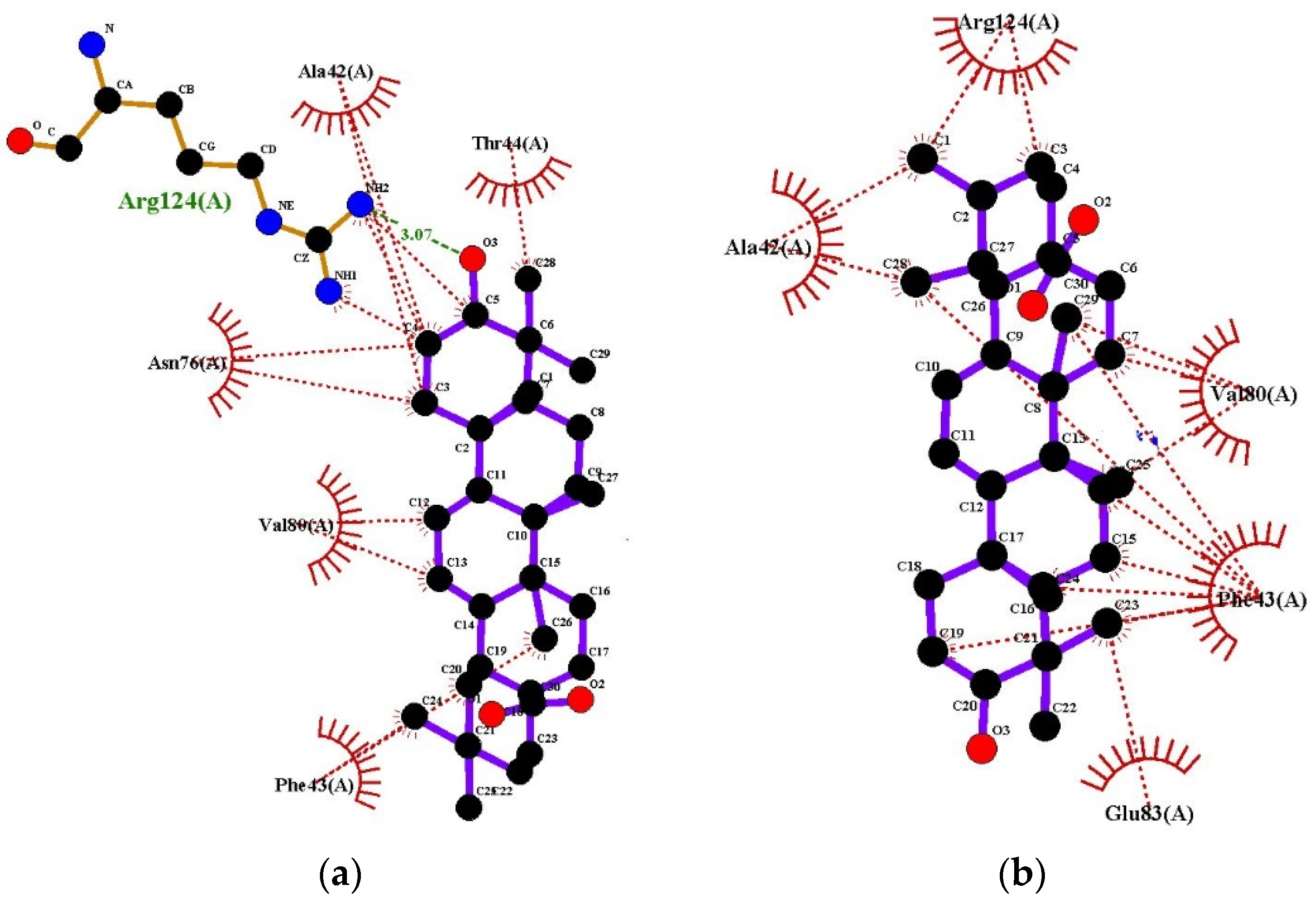
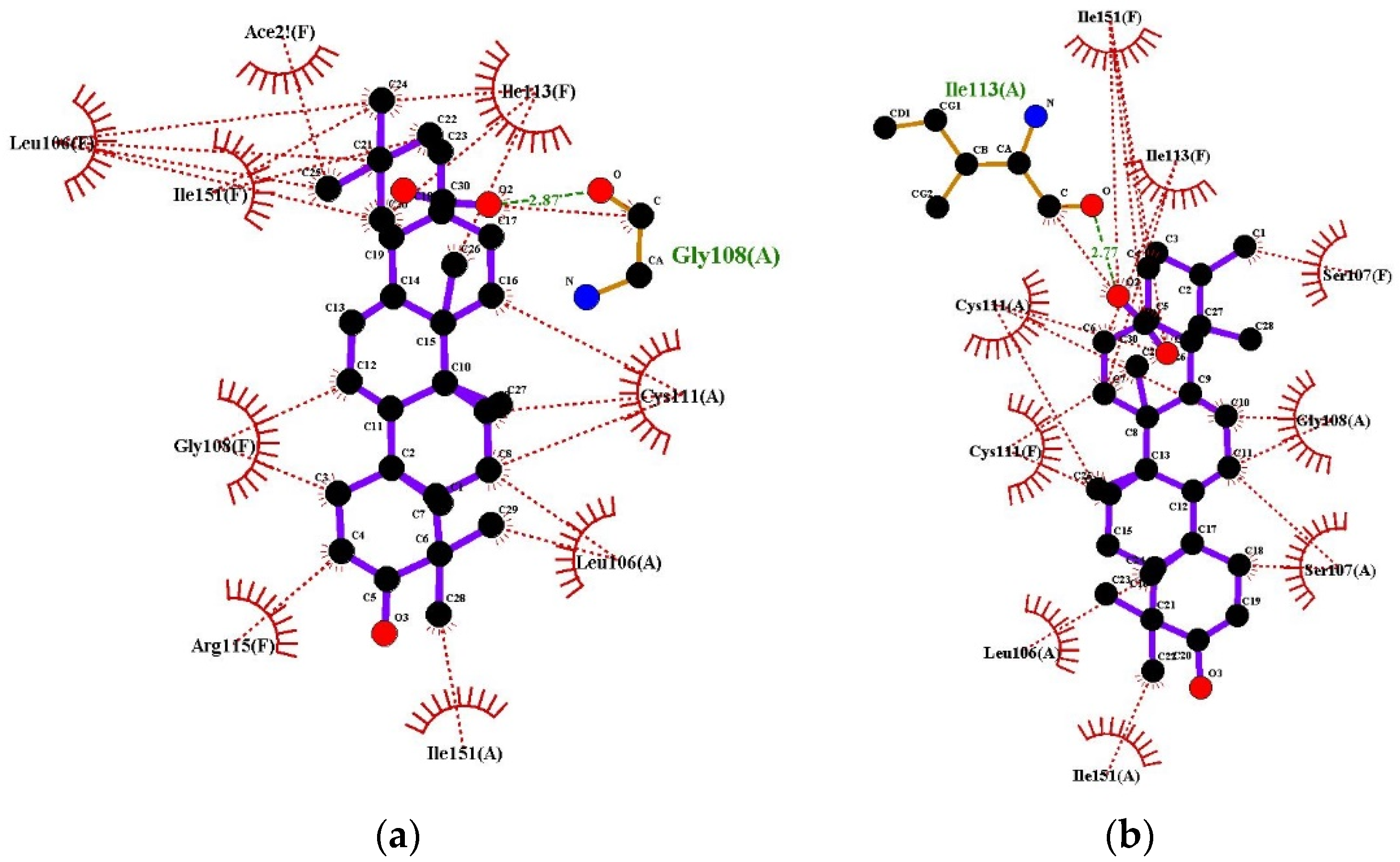
2.6. Determination of Antioxidant Activity Using Molecular Docking
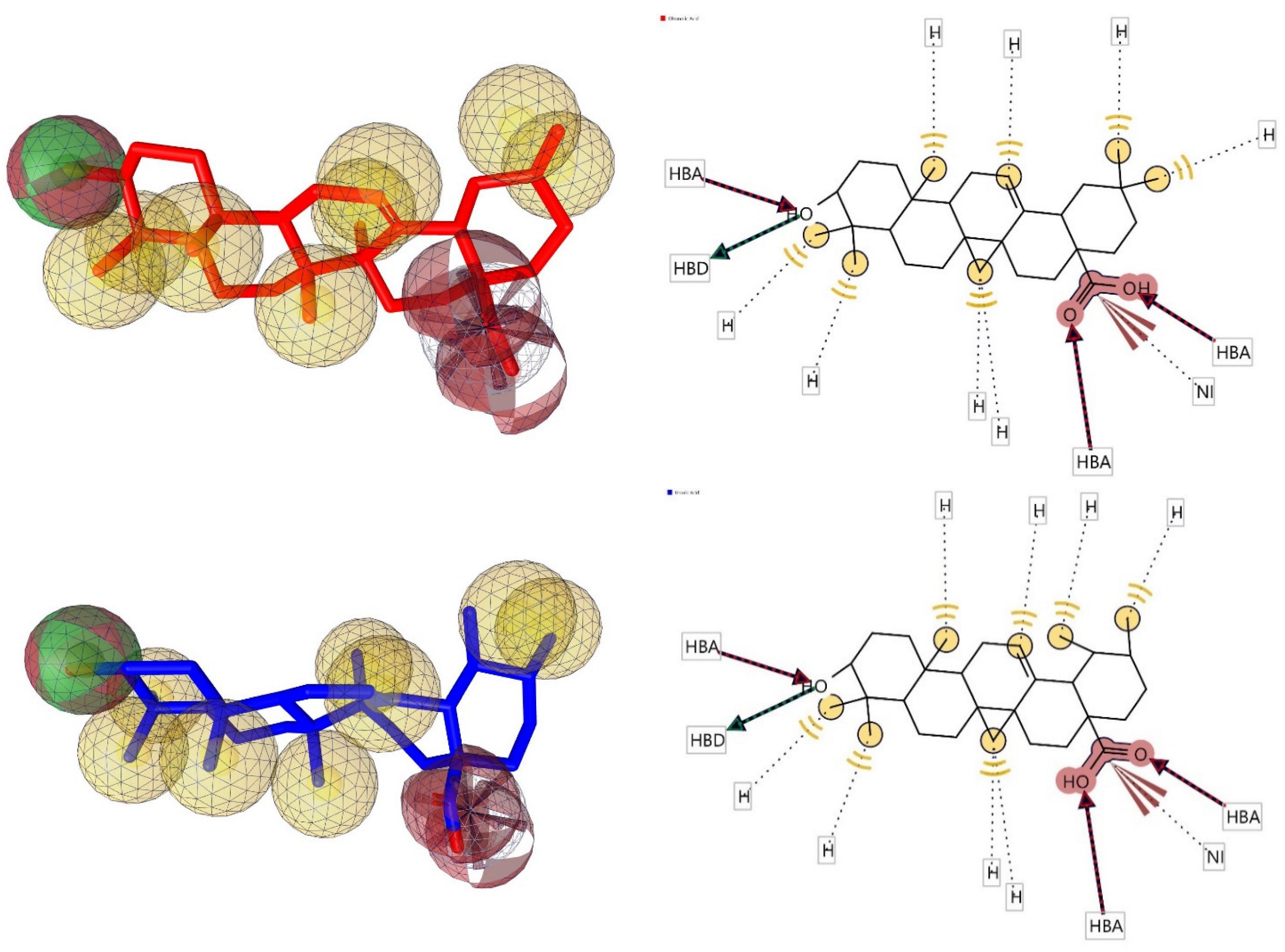
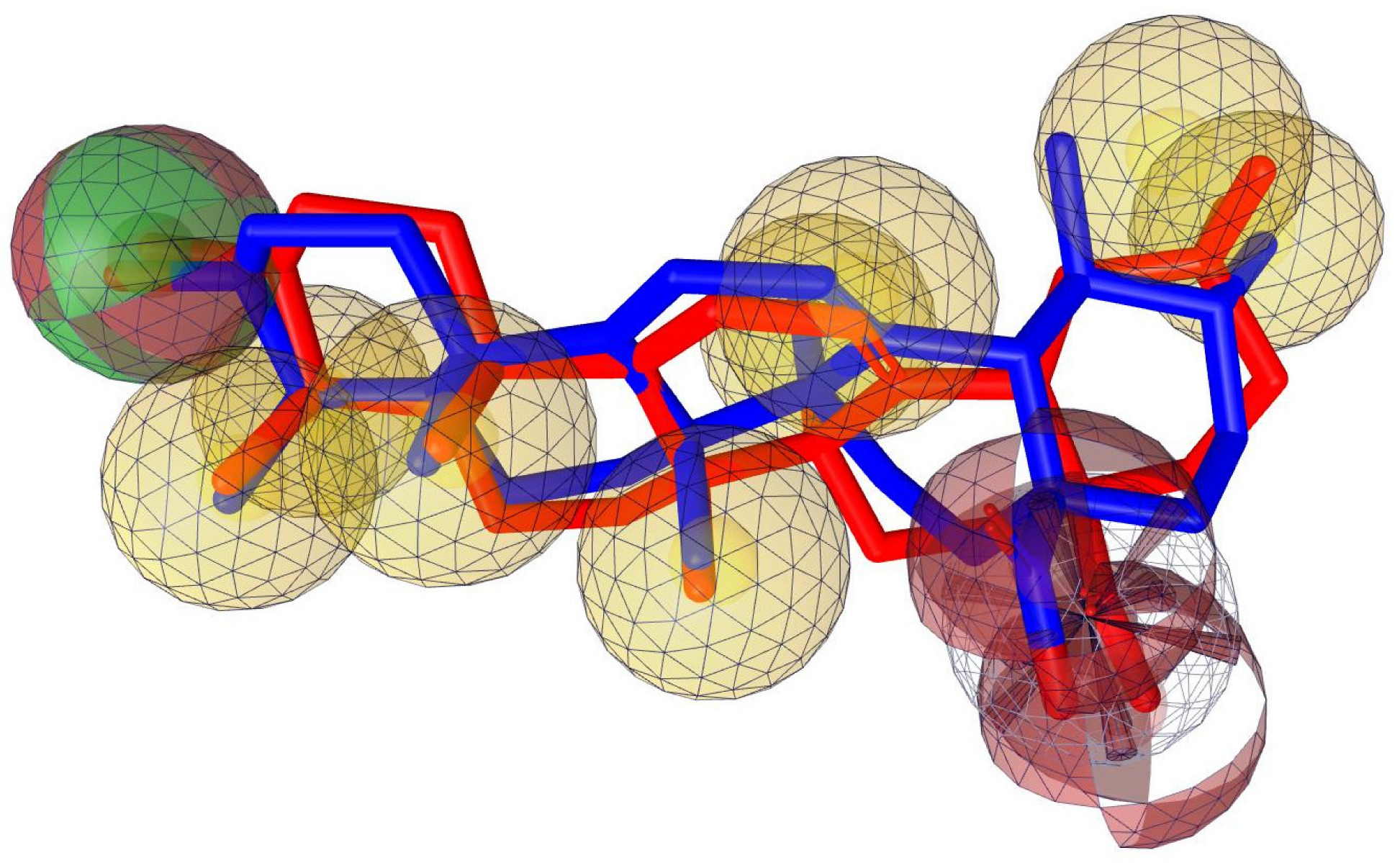
2.7. Pharmacophore Evaluation
3. Materials and Methods
3.1. Chemicals and Equipment
3.2. Plant Sampling, Identification, and Extraction
3.3. Secondary Metabolites Content Assessment from M. hirsuta Extracts
3.3.1. Phytochemical Screening
3.3.2. Determination of Chemical Composition by HPLC-PDA, LC-MS, and 1H-NMR
3.4. Antioxidant Activity Assessment
3.4.1. Antiradical Potential towards DPPH• (2,2-Diphenyl-1-picrylhydrazyl)
3.4.2. Antiradical Potential towards ABTS•+ (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic Acid)
3.4.3. DPPH and ABTS Chemical Kinetics
3.4.4. Determination of Total Phenols Content
3.4.5. Total Flavonoid Content
3.5. Statistical Analysis
3.6. Molecular Docking
3.7. 3D Pharmacophore Model Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Santos, V.H.M.; Minatel, I.O.; Lima, G.P.P.; Silva, R.M.G.; Chen, C.Y.O. Antioxidant capacity and phytochemical characterization of Spathodea campanulata growing in different climatic zones in Brazil. Biocatal. Agric. Biotechnol. 2020, 24, 101536. [Google Scholar] [CrossRef]
- Bingol, Z.; Kızıltas, H.; Goren, A.C.; Kose, L.P.; Topal, M.; Durmaz, L.; Alwasel, S.H.; Gulcin, I. Antidiabetic, anticholinergic and antioxidant activities of aerial parts of shaggy bindweed (Convulvulus betonicifolia Miller subsp.)—Profiling of phenolic compounds by LC-HRMS. Heliyon 2021, 7, 06986. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Seelan, J.S.S.; Iqbal, M. Phytochemical investigation and antioxidant activities of methanol extract, methanol fractions and essential oil of Dillenia suffruticosa leaves. Arab. J. Chem. 2020, 13, 7170–7182. [Google Scholar] [CrossRef]
- Adusei, S.; Otchere, J.K.; Oteng, P.; Mensah, R.Q.; Tei-Mensah, E. Phytochemical analysis, antioxidant and metal chelating capacity of Tetrapleura tetraptera. Heliyon 2019, 5, 02762. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Anouar, E.H.; Marzouk, M.; Taie, H.A.; Abuelizz, H.A. Screening and evaluation of antioxidant activity of some 1,2,4-triazolo[1,5-a]quinazoline derivatives. Future Med. Chem. 2017, 10, 379–390. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J. Am. Coll. Nutr. 2015, 34, 10–13. [Google Scholar] [CrossRef]
- Engwa, G.A. Free Radicals and the Role of Plant Phytochemicals as Antioxidants Against Oxidative Stress-Related Diseases. Phytochem. Source Antioxid. Role Dis. Prev. BoD–Books Demand 2018, 7, 49–74. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, K.S.; Yokozawa, T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem. Toxicol. 2008, 46, 2466–2471. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, T.Y.; Chang, L.Z.; Wang, H.F.; Yih, K.H.; Hsieh, W.Y.; Chang, T.M. Inhibition of melanogenesis versus antioxidant properties of essential oil extracted from leaves of Vitex negundo Linn and chemical composition analysis by GC-MS. Molecules 2012, 17, 3902–3916. [Google Scholar] [CrossRef] [PubMed]
- Pintus, F.; Matos, M.J.; Vilar, S.; Hripcsak, G.; Varela, C.; Uriarte, E.; Santana, L.; Borges, F.; Medda, R.; Di Petrillo, A.; et al. New insights into highly potent tyrosinase inhibitors based on 3-heteroarylcoumarins: Antimelanogenesis and antioxidant activities, and computational molecular modeling studies. Bioorg. Med. Chem. 2017, 25, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Danah, S.; Al-Shamary, M.A.; Al-Alshaikh, N.A.; Kheder, Y.; Nasser, M.; Syed, L.B. Molecular docking and biological evaluation of some thioxoquinazolin-4(3H)-one derivatives as anticancer, antioxidant and anticonvulsant agents. Chem. Cent. J. 2017, 11, 48. [Google Scholar] [CrossRef]
- Khurana, R.K.; Jain, A.; Jain, A.; Sharma, T.; Singh, B. Administration of antioxidants in cancer: Debate of the decade. Drug Disc. Today 2018, 23, 763–770. [Google Scholar] [CrossRef]
- Pereira, J.R.; Queiroz, R.F.; Siqueira, E.A.; Brasileiro-Vidal, A.C.; Sant’ana, A.E.G.; Silva, D.M. Evaluation of cytogenotoxicity, antioxidant and hypoglycemiant activities of isolate compounds from Mansoa hirsuta D.C. (Bignoniaceae). An. Da Acad. Bras. De Ciências 2017, 89, 317–331. [Google Scholar] [CrossRef]
- Chaves, S.A.D.M.; Reinhard, K.J. Paleopharmacology and pollen: Theory, method, and application. Mem. Inst. Oswaldo Cruz. 2003, 98, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Endringer, D.C.; Valadares, Y.M.; Campana, P.R.V.; Campos, J.J.; Guimarães, K.G.; Pezzuto, J.M.; Braga, F.C. Evaluation of Brazilian Plants on Cancer Chemoprevention Targets In Vitro. Phytother. Res. 2010, 24, 928–933. [Google Scholar] [CrossRef]
- Campana, P.R.; Coleman, C.M.; Sousa, L.P.; Teixeira, M.M.; Ferreira, D.; Braga, F.C. Mansoins C−F, Oligomeric Flavonoid Glucosides Isolated from Mansoa hirsuta Fruits with Potential Anti-inflammatory Activity. J. Nat. Prod. 2016, 79, 2279–2286. [Google Scholar] [CrossRef]
- Braga, F.C.; Wagner, H.; Lombardi, J.Á.; Oliveira, A.B. Screening the Brazilian flora for anti-hypertensive plant species for in vitro angiotensin-I converting enzyme inhibiting activity. Phytomedicine 2000, 7, 245–250. [Google Scholar] [CrossRef]
- Rocha, A.D. Estudo Fitoquímico de Clytostoma ramentaceum Bureau & k. Schum. e Mansoa hirsuta D.C. (Bignoniaceae) Biomonitorado por Ensaios In Vitro de Atividade Antifúngica. Master’s Dissertation, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2002. [Google Scholar]
- Kaneria, M.J.; Rakholiya, K.D.; Marsonia, L.R.; Dave, R.A.; Golakiya, B.A. Nontargeted metabolomics approach to determine metabolites profile and antioxidant study of Tropical Almond (Terminalia catappa L.) fruit peels using GC-QTOF-MS and LC-QTOF-MS. J. Pharm. Biomed. 2018, 160, 415–427. [Google Scholar] [CrossRef]
- Kaur, R.; Matta, T.; Kaur, H. Plant derived alkaloids. Saudi J. Life Sci. 2019, 2, 158–189. [Google Scholar] [CrossRef]
- Raju, S.; Kavimani, S.; Uma, M.V.; Sreeramulu, R.K. Tecomastans (L.) Juss. ex Kunth (Bignoniaceae): Ethnobotany, phytochemistry and pharmacology. JPBMS 2011, 8, 1–5. [Google Scholar]
- Choudhury, S.; Datta, S.; Talukdar, A.D.; Choudhury, M.D. Phytochemistry of the family Bignoniaceae—A review. Environ. Sci. Technol. 2011, 7, 145–150. [Google Scholar]
- Morais, S.K.R.; Silva, S.G.; Portela, C.N.; Nunomura, M.N.; Quignard, E.L.J.; Pohlit, A.M. Bioactive dihydroxy furanona phthoquinones from the bark of Tabebuia incana A.H. Gentry (Bignoniaceae) and HPLC analysis of commercial pau d’arco and certified T. incana bark infusions. Acta Amazon. 2007, 37, 99–102. [Google Scholar] [CrossRef]
- Lenta, B.N.; Weniger, B.; Antheaune, C.; Noungoue, D.T.; Ngouela, S.; Assob, J.C.N. Anthraquinones from the stem bark of Stereospermum zenkeri with antimicrobial activity. Phytochemistry 2007, 68, 1595–1599. [Google Scholar] [CrossRef]
- Leite, J.P.V.; Oliveira, A.B.; Lombardi, J.A.; Souza-Filho, J.D.; Chiari, E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol. Pharm. Bull. 2006, 29, 2307–2309. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Oliveira, J.; Skelding, G.M. The genus Mansoa (Bignoniaceae): A source of organosulfur compounds. Rev. Bras. Farmacogn. 2008, 19, 795–804. [Google Scholar] [CrossRef]
- Rogers, R.W.; Stevens, G.N. The Usnea baileyi complex (Parmeliaceae, Lichenised Ascomycetes) in Australia. Aust. Syst. Bot. 1998, 1, 355–361. [Google Scholar] [CrossRef]
- Gwatidzo, L.; Dzomba, P.; Mangena, M. TLC separation and antioxidant activity of flavonoids from Carissa bispinosa, Ficus sycomorus, and Grewia bicolar fruits. Nutrire 2018, 43, 1–7. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Structure Analysis of Flavonoids by Ultraviolet Spectroscopy. In The Systematic Identification of Flavonoids, 2nd ed.; Mabry, T.J., Markham, K.R., Thomas, M.B., Eds.; Springer: Berlin/Heidelderg, Germany; New York, NY, USA, 1970; Volume 1, pp. 35–40. [Google Scholar]
- Campana, P.R.V. Fitoquímica e Atividade Vasodilatadora “In Vitro de” “Mansoa hirsuta DC”. Master’s Dissertation, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2008. [Google Scholar]
- Svedstrom, U.; Vuorela, H.; Kostiainen, R.; Laakso, I.; Hiltunen, R. Fractionation of polyphenols in hawthorn into polymeric procyanidins, phenolic acids and flavonoids prior to high-performance liquid chromatographic analysis. J. Chromatogr. A 2006, 1112, 103–111. [Google Scholar] [CrossRef]
- Tian, S.; Shi, Y.; Yu, Q.; Upur, H. Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam. by HPLC method. Pharmacogn. Mag. 2010, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Pachú, C.O. Processamento de Plantas Medicinais Para Obtenção de Extratos Secos e Líquidos. Ph.D. Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2007. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introdução à Espectroscopia, 4th ed.; Cengage Learning: São Paulo, Brazil, 2012; pp. 394–395. [Google Scholar]
- Pimentel, C.V.M.B.; Francki, V.M.; Gallucke, A.P.B. Alimentos Funcionais: Introdução as Principais Substancias Bioativas em Alimentos; Varela: São Paulo, Brazil, 2005. [Google Scholar]
- Queiroz, M.M.F. Identificação dos Inibidores de Acetilcolinesterase em Tetrapterys mucronata Cav. (Malpighiaceae) e Comparação Quali e Quantitativa dos Derivados Triptamícos Presentes na Espécie em Estudo e Ayahuasca. Doctoral Thesis, Paulista State University, São Paulo, Brazil, 2013. [Google Scholar]
- Kamiya, K.; Watanabe, C.; Endang, H.; Umar, M.; Satake, T. Studies on the constituents of bark of Parameria laevigata Moldenke. Chem. Pharm. Bull. 2001, 49, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, W.; Li, Y.; Xu, H.; Lv, L.; Wang, X.; Chai, Y.; Zhang, G. Simultaneous determination of oleanolic and ursolic acids in rat plasma by HPLC–MS: Application to a pharmacokinetic study after oral administration of different combinations of QingGanSanJie decoction extracts. J. Chromatogr. Sci. 2015, 53, 1185–1192. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Zhang, W.; Chen, Z. Identification and quantification of oleanolic acid and ursolic acid in Chinese herbs by liquid chromatography–ion trap mass spectrometry. Biomed. Chromatogr. 2011, 25, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.M.; Falcoski, T.O.; Silveira, R.S.; Vieira, H.S.; Santos, V.N.C.; Ramos, R.R.; Silva, J.C.P.; Andrade, T.J.A.S.; Carli, A.P.; Costa, P.I.; et al. Effectiveness of different methods for the extraction of principle actives and phytochemicals content in medicinal herbals. BLACPMA 2021, 20, 324–338. [Google Scholar] [CrossRef]
- Sultana, M.; Verma, P.K.; Raina, R.; Prawez, S.; Dar, M. Quantitative analysis of total phenolic, flavonoids and tannin contents in acetone and n-hexane extracts of Ageratum conyzoides. Int. J. Chemtech. Res. 2012, 4, 996–999. [Google Scholar]
- Faqueti, L.G.; Sandjo, L.P.; Biavatti, W. Simultaneous identification and quantification of polymethoxyflavones, coumarin and phenolic acids in Ageratum conyzoides by UPLC-ESI-QToF-MS and UPLC-PDA. J. Pharm. Biomed. 2017, 145, 621–628. [Google Scholar] [CrossRef]
- Mantovani, A.C.G.; Chendynski, L.T.; Galvan, D.; Borsato, D.; Mauro, E.D. Evaluation of the Oxidation Degradation Process of Biodiesel via 1H NMR Spectroscopy. J. Braz. Chem. Soc. 2020, 31, 1661–1667. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, A.K. Antibacterial lead compounds and their targets for drug development. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–292. [Google Scholar]
- El Aziz, M.M.A.; Ashour, A.S.; Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. Nanomed. Res. 2019, 8, 6–12. [Google Scholar] [CrossRef]
- Kluger, R.H.; Clayton, R.B. Steroid. In Encyclopedia Britannica; 1 April 2021. Available online: https://www.britannica.com/science/steroid (accessed on 6 June 2021).
- Britannica, T. Editors of Encyclopaedia. “Fatty Acid”. Encyclopedia Britannica. Available online: https://www.britannica.com/science/fatty-acid (accessed on 22 March 2021).
- Vera, R. Chemical composition of the essential oil of Ageratum conyzoides L. (Asteraceae) from Réunion. Flavour Fragr. J. 1993, 8, 257–260. [Google Scholar] [CrossRef]
- Sousa, N.M.S.; Alves, P.S.; Junior, P.S.; Costa, J.S.C.; Melo, C.R.S.; Lima, N.M.; Citó, A.M.G.L.; Andrade, T.J.A.S. GC–MS determination of major volatile compounds in Mansoa hirsuta branches and leaves. Atena Ed. 2021, 2, 96–103. [Google Scholar] [CrossRef]
- Vilhena-Potiguara, R.C.; Aguiar-Dias, A.C.A.; Kikuchi, T.Y.S.; Santos, A.C.F.; Silva, R.J.F. Estruturas secretoras em Cipó-de-alho (Mansoa standleyi Steyerm.) A. H. Gentry, (Bignoniaceae): Ocorrência e morfologia. Acta Amazon. 2012, 42, 321–328. [Google Scholar] [CrossRef]
- Silva, D.M. Perfil Metabolômico e Farmacológico da Mansoa hirsuta DC (Bignoniaceae). Doctoral Thesis, Federal University of Alagoas, Maceió, Brazil, 2009. [Google Scholar]
- Munikishore, R.; Padmaja, A.; Gunasekar, D.; Blond, A.; Bodo, B. Two new flavonoids from Ageratum conyzoides. Indian J. Chem. Sect. B 2013, 52, 1479–1482. [Google Scholar] [CrossRef]
- Milan, C.; Hana, C.; Petko, D.; Maria, K.; Anton, S.; Antonín, L. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant activity and protecting health effects of common medicinal plants. Adv. Food Nutr. Res. 2012, 67, 75–139. [Google Scholar] [CrossRef]
- Bianchi, J.; Montovani, M.S.; Marin Morales, M.A. Analysis of the genotoxic potential of low concentrations of Malathion on the Allium cepa cells and rat hepatoma tissue culture. J. Environ. Sci. 2015, 16, 102–111. [Google Scholar] [CrossRef]
- Chirunthorn, R.; Supavita, T.; Intaraksa, N.; Kummee, S.; Junkong, N.; Chisorn, B.; Itharat, A. Study on biological activities of Mansao hymenaea (DC.) A. Gentry leaf extracts. J. Sci. Technol. 2005, 27, 489–495. [Google Scholar]
- Da Silva, A.F.G.; Feitosa, B.H.; Pezenti, L.; Abel, M.C.N.; Silva, N.F.A. Triagem fitoquímica e atividade antioxidante de Mansoa difficilis e Hippocratea volubilis. Atena Ed. 2019, 1, 388–416. [Google Scholar]
- Kurt, A.R.; Margaret, J.B.; Edward, J.K. Antioxidant potential of seven myrtaceous fruits. Ethnobot. Res. Appl. 2005, 3, 25–35. [Google Scholar]
- Lee, K.J.; You Chang, O.H.; Won Kyung, C.H.O.; Jin Yeul, M.A. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. eCAM 2015, 2015, 165457. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, I.B.; Sader, H.S.; Gonçalves, A.G.; Reis, A.O.; Gales, A.C.; Varella, A.D.; Younes, R.N. Screening of antibacterial extracts from plants native to the Brazilian Amazon Rain Forest and Atlantic Forest. Braz. J. Med. Biol. Res. 2004, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Giada, M.D.L.R. Uma abordagem sobre a capacidade antioxidante in vitro de alimentos vegetais e bebidas. Demetra 2014, 9, 137–146. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Reis, S.; Segundo, M.A. Kinetic matching approach applied to ABTS assay for high-throughput determination of total antioxidant capacity of food products. J. Food Compos. Anal. 2014, 33, 187–194. [Google Scholar] [CrossRef]
- Labrinea, E.P.; Georgiou, C.A. Stopped-flow method for assessment of pH and timing effect on the ABTS total antioxidant capacity assay. Anal. Chim. Acta. 2004, 526, 63–68. [Google Scholar] [CrossRef]
- Simões, C.M.O. Farmacognosia da Planta ao Medicamento, 2nd ed.; Ed Universidade/UFRGS/Ed; Universidade/UFSC: Florianópolis, Brazil, 2000. [Google Scholar]
- Sahu, N.; Saxena, J. Total Phenolic & total flavonoid content of Bougainvillea Glabra choisy and Calforina gold flower Extracts. Int. J. Pharm. 2013, 5, 5581–5585. [Google Scholar]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Abel, M.C.N.; Pezenti, L.; Silva, N.F.A.; Feitosa, B.H.; Silva, A.F.G. Phytochemical screening and antioxidant activity of Mansoa difficilis and Hippocratea volubilis. Atena Ed. 2019, 1, 225–231. [Google Scholar]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complementary Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef]
- Kouki, M.; Manetas, Y. Resource availability affects differentially the levels of gallotannins and condensed tannins in Ceratonia siliqua. Biochem. Syst. Ecol. 2002, 30, 631–639. [Google Scholar] [CrossRef]
- Monteiro, J.M.; Albuquerque, U.P.; Lins Neto, E.M.F.; Araújo, E.L.; Albuquerque, M.M.; Amorim, E.L.C. The effects of seasonal climate changes in the Caatinga on tanninlevel. Rev. Bras. Farmacogn. 2006, 16, 338–344. [Google Scholar] [CrossRef]
- Burri, S.C.M.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Haldar, P.K.; Ghosh, A.K. Antioxidant and free-radical-scavenging effects of fruits of Dregea volubilis. J. Nat. Sci. Biol. Med. 2010, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- John, B.; Sulaiman, C.T.; Satheesh, G.; Redd, V.R.K. Total phenolics and flavonoids in selected medicinal plants from Kerala. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–408. [Google Scholar]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef]
- Declercq, J.P.; Evrard, C.; Clippe, A.; Stricht, D.V.; Bernard, A.; Knoops, B. Crystal structure of human peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 A resolution. J. Mol. Biol. 2001, 311, 751–759. [Google Scholar] [CrossRef]
- Strange, R.W.; Antonyuk, S.V.; Hough, M.A.; Doucette, P.A.; Valentine, J.S.; Hasnain, S.S. Variable metallation of human superoxide dismutase: Atomic resolution crystal structures of Cu-Zn, Zn-Zn and as-isolated wild-type enzymes. J. Mol. Biol. 2006, 356, 1152–1162. [Google Scholar] [CrossRef]
- Wolber, G.; Thierry, L. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef]
- De Menezes Filho, A.C.P.; de Souza Castro, C.F. Identificação das classes de metabólitos secundários em extratos etanólicos foliares de Campomanesia adamantium, Dimorphandra mollis, Hymenaea stigonocarpa, Kielmeyera lathrophytum e Solanum lycocarpum. Ens. E Cienc. 2019, 23, 16–23. [Google Scholar] [CrossRef]
- Do Santos, R.C.; De Souza, A.V.; Andrade-Silva, M.; Kassuya, C.A.L.; Cardoso, C.A.L.; Vieira, M.D.C.; Formagio, A.S.N. Antioxidant, anti-rheumatic and anti-inflammatory investigation of extract and dicentrinone from Duguetia furfuracea (A. St.-Hil.) Benth. & Hook. f. J. Ethnopharmacol. 2018, 211, 9–16. [Google Scholar] [CrossRef]
- Boroski, M.; Visentainer, J.V.; Cottica, S.M.; Morais, D.R. Antioxidants: Principles and Analytical Methods, 1st ed.; Appris: Curitiba, Brazil, 2015. [Google Scholar]
- Cotelle, N.; Bernier, J.L.; Catteau, J.P.; Pommery, J.; Wallet, J.C.; Gaydou, E.M. Antioxidant Properties of hydroxy-flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybidic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Nunes, P.H.; Martins, M.D.C.C.; Oliveira, R.D.C.M.; Chaves, M.H.; Sousa, E.A.; Leite, J.R.; Véras, L.M.; Almeida, F.R. Gastric antiulcerogenic and hypokinetic activities of Terminalia fagifolia Mart. & Zucc. (Combretaceae). Biomed. Res. Int. 2014, 2014, 261745. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera- a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- SAMSON: Software for Adaptive Modeling and Simulation of Nanosystems. Available online: https://www.samson-connect.net (accessed on 18 June 2022).
| Saponins | Organic Acids | Phenols and Tannins | Flavonoids | Alkaloids | Catechins | |
|---|---|---|---|---|---|---|
| EMFMh | + | + | + | - | + | - |
| EMGMh | - | + | + | + | + | - |
| EMRMh | - | + | + | - | + | - |
| M. hirsuta | Rt (Min) | λ Max | Classes of Compounds | Reference |
|---|---|---|---|---|
| EMFMh, EMGMh and EMRMh | 2.69 | 260/269 | Flavonoids (isoflavone) | [31] |
| 17.24 | 273 | Proanthocyanidins | [32,33] | |
| 18.27 | 284/350 | |||
| 19.49 | 282/350 | |||
| 20.70 | 285/327 | |||
| 21.18 | 204/283 | Flavonoids (flavanones or dihydroflavonols) | [31] | |
| 21.24 | 214 | Triterpenes (Oleanolic acid) | [34] | |
| 22.65 | 214 | Triterpenes (Ursolic acid) | [34] |
| Extract | Rt (Min) | Ionization Mode | Fragments/% Abundance | Compound | Reference |
|---|---|---|---|---|---|
| EMFMh | 43.9 | [M-H]—negative | m/z 363 (4.4%), 407 (3.4%), m/z 455 (0.8%) | Oleanolic acid and Ursolic acid | [40,41] |
| EMGMh | 45.6 | [M-H]—negative | m/z 363 (19.4%), 391 (5.6%), m/z 407 (16.7%) | Oleanolic acid and Ursolic acid | [41] |
| EMRMh | 27.0 | [M-H]—negative | m/z 363 (29.1%), 407 (16.7%), m/z 455 (5.8%) | Oleanolic acid and Ursolic acid | [40,41] |
| Samples | DPPH (µg mL−1)—IC50 | ABTS (µg mL−1)—IC50 |
|---|---|---|
| EMFMh | 78.9 ± 0.05 | 43.5 ± 0.14 |
| EMGMh | 77.3 ± 0.03 | 63.6 ± 0.54 |
| EMRMh | 82.7 ± 0.1 | 56.1 ± 0.05 |
| Quercetin | 41.0 | - |
| Trolox | - | 73.2 |
| Compounds | Docking Score (-) (kcal/mol) | Docking Score (-) (kcal/mol) | Docking Score (-) (kcal/mol) |
|---|---|---|---|
| PDB ID: 3NM8 (Bacterial Tyrosinase) | PDB ID: 1HD2 (Human Peroxiredoxin 5) | PDB ID: 2C9V (Human Superoxide Dismutase) | |
| Quercetin (Standard) | 9.2 | 3.3 | 7.1 |
| Oleanolic Acid | 8.3 | 6.4 | 8.6 |
| Ursolic Acid | 8.2 | 6.3 | 8.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.e.S.; Preet, G.; Dias, L.; Oliveira, M.; Silva, R.; Castro, I.; Silva, G.; Júnior, J.; Lima, N.; Silva, D.H.; et al. The Free Radical Scavenging Property of the Leaves, Branches, and Roots of Mansoa hirsuta DC: In Vitro Assessment, 3D Pharmacophore, and Molecular Docking Study. Molecules 2022, 27, 6016. https://doi.org/10.3390/molecules27186016
Alves PeS, Preet G, Dias L, Oliveira M, Silva R, Castro I, Silva G, Júnior J, Lima N, Silva DH, et al. The Free Radical Scavenging Property of the Leaves, Branches, and Roots of Mansoa hirsuta DC: In Vitro Assessment, 3D Pharmacophore, and Molecular Docking Study. Molecules. 2022; 27(18):6016. https://doi.org/10.3390/molecules27186016
Chicago/Turabian StyleAlves, Patrícia e Silva, Gagan Preet, Leandro Dias, Maria Oliveira, Rafael Silva, Isione Castro, Giovanna Silva, Joaquim Júnior, Nerilson Lima, Dulce Helena Silva, and et al. 2022. "The Free Radical Scavenging Property of the Leaves, Branches, and Roots of Mansoa hirsuta DC: In Vitro Assessment, 3D Pharmacophore, and Molecular Docking Study" Molecules 27, no. 18: 6016. https://doi.org/10.3390/molecules27186016
APA StyleAlves, P. e. S., Preet, G., Dias, L., Oliveira, M., Silva, R., Castro, I., Silva, G., Júnior, J., Lima, N., Silva, D. H., Andrade, T., Jaspars, M., & Feitosa, C. (2022). The Free Radical Scavenging Property of the Leaves, Branches, and Roots of Mansoa hirsuta DC: In Vitro Assessment, 3D Pharmacophore, and Molecular Docking Study. Molecules, 27(18), 6016. https://doi.org/10.3390/molecules27186016









