Polygonatum sibiricum Polysaccharides Attenuate Lipopoly-Saccharide-Induced Septic Liver Injury by Suppression of Pyroptosis via NLRP3/GSDMD Signals
Abstract
:1. Introduction
2. Results
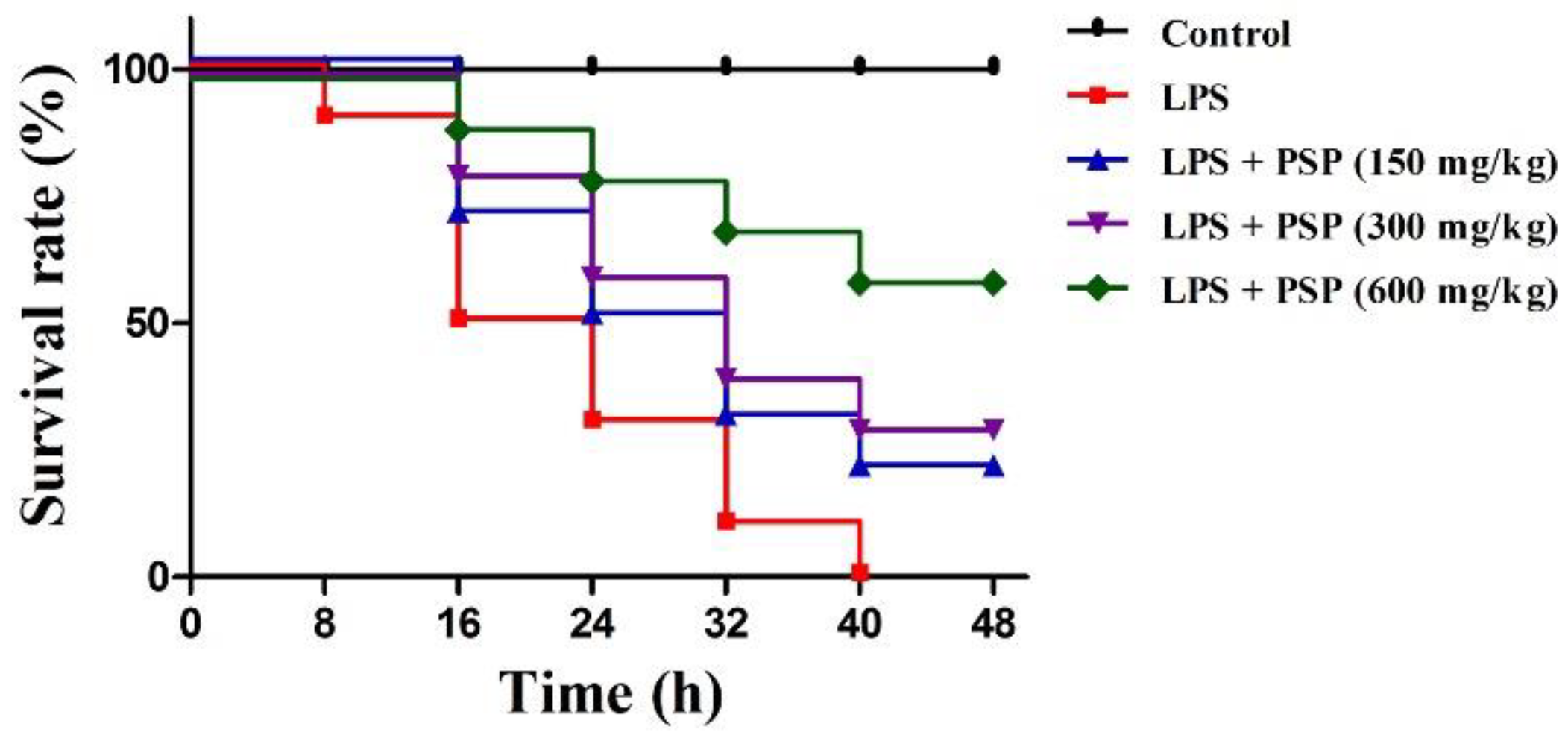
2.1. PSP Increased Survival Rates of Septic Mice
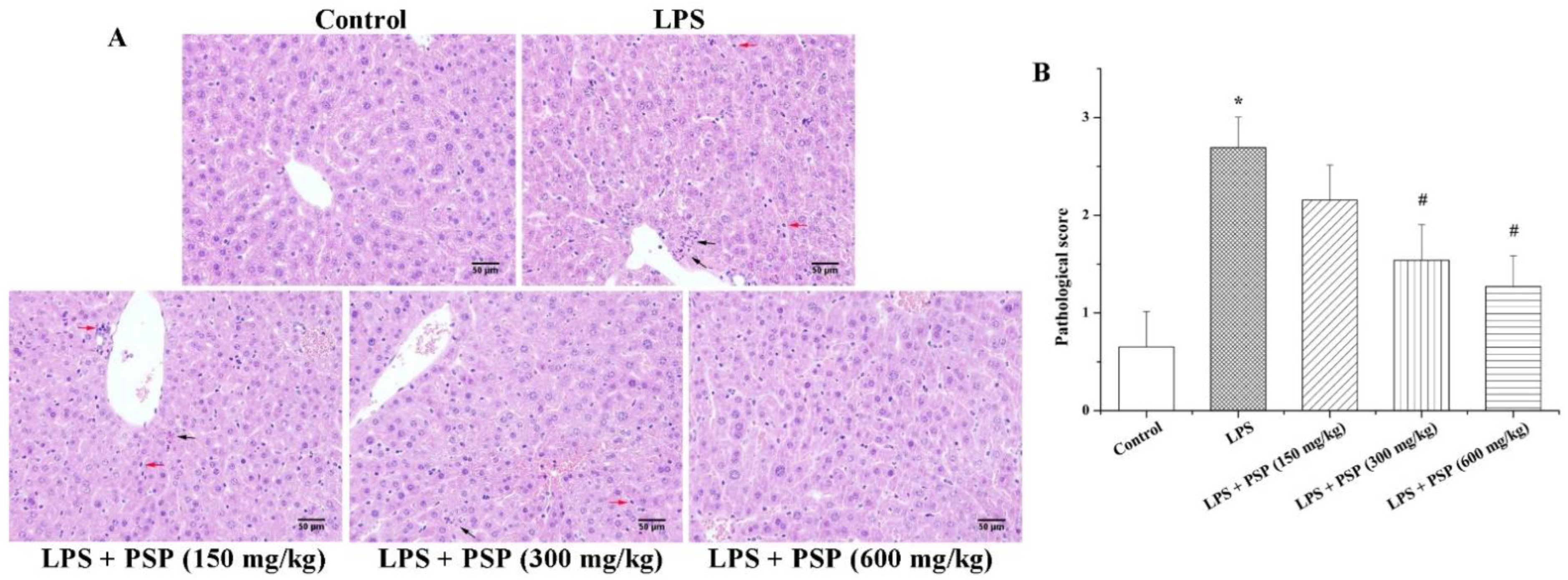
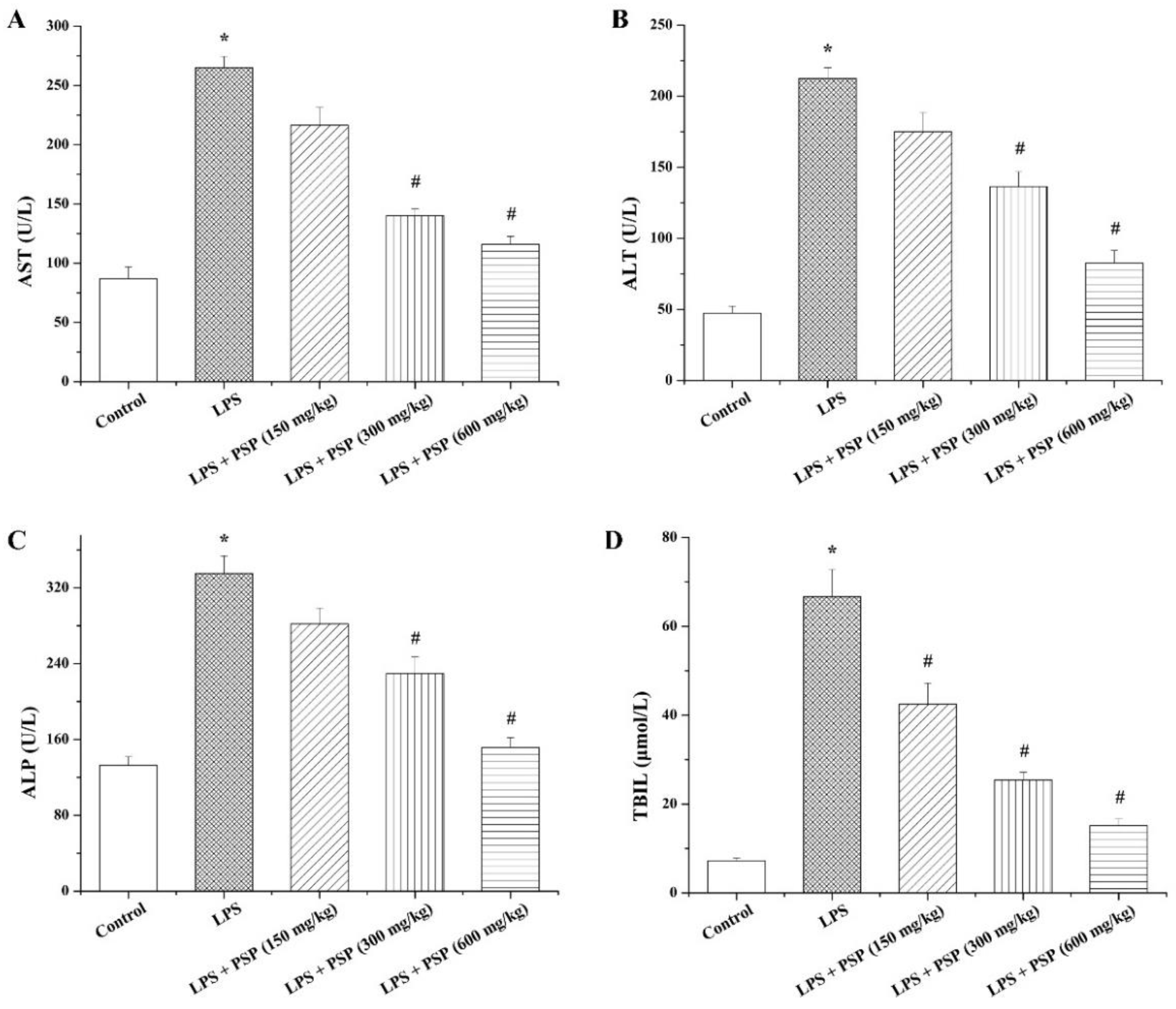
2.2. PSP Alleviated Acute Liver Injury of Septic Mice
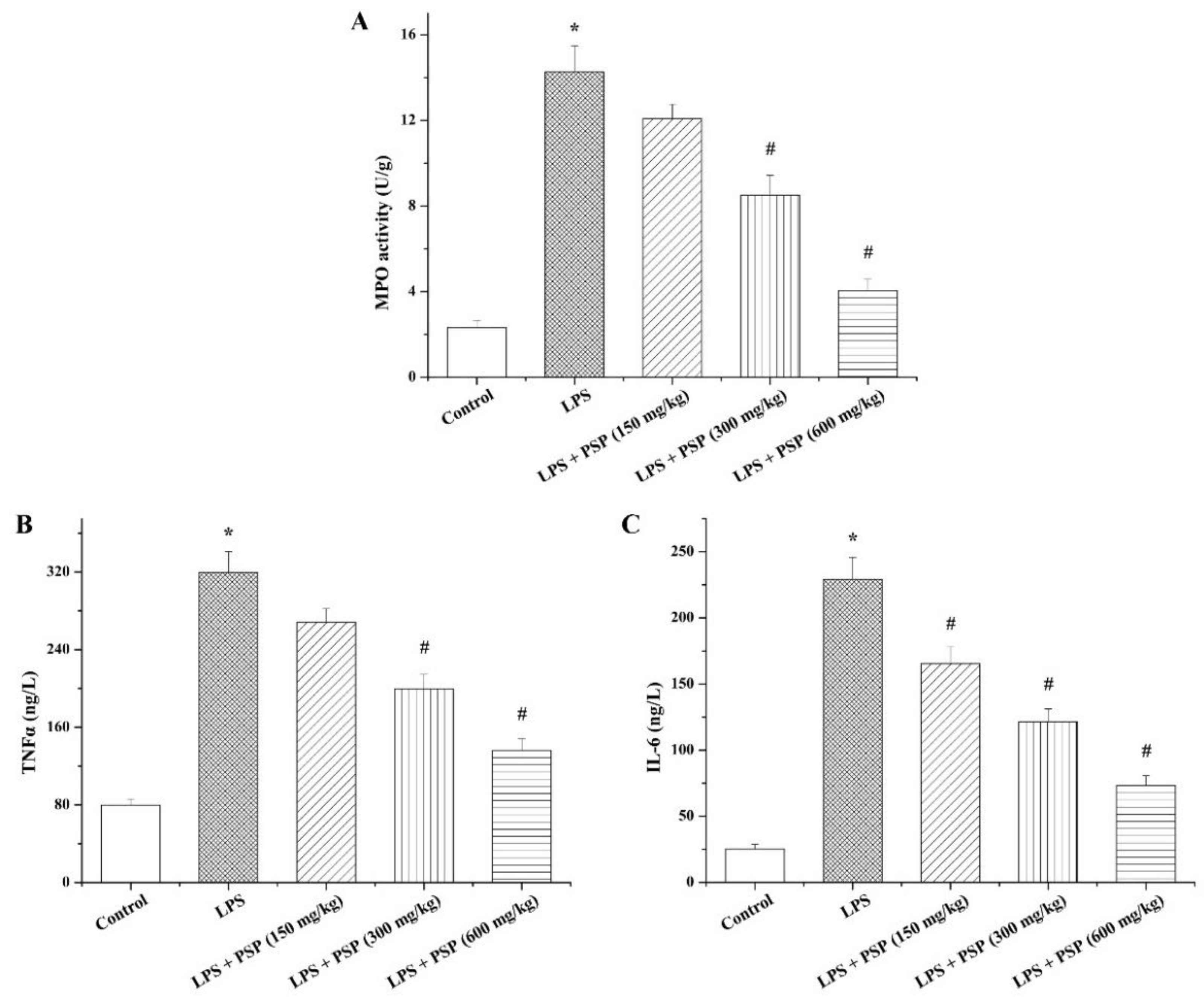
2.3. PSP Suppressed MPO Activity and Inflammatory Cytokine Secretion of Septic Mice
2.4. PSP Ameliorated Hepatocyte Pyroptosis of Septic Mice
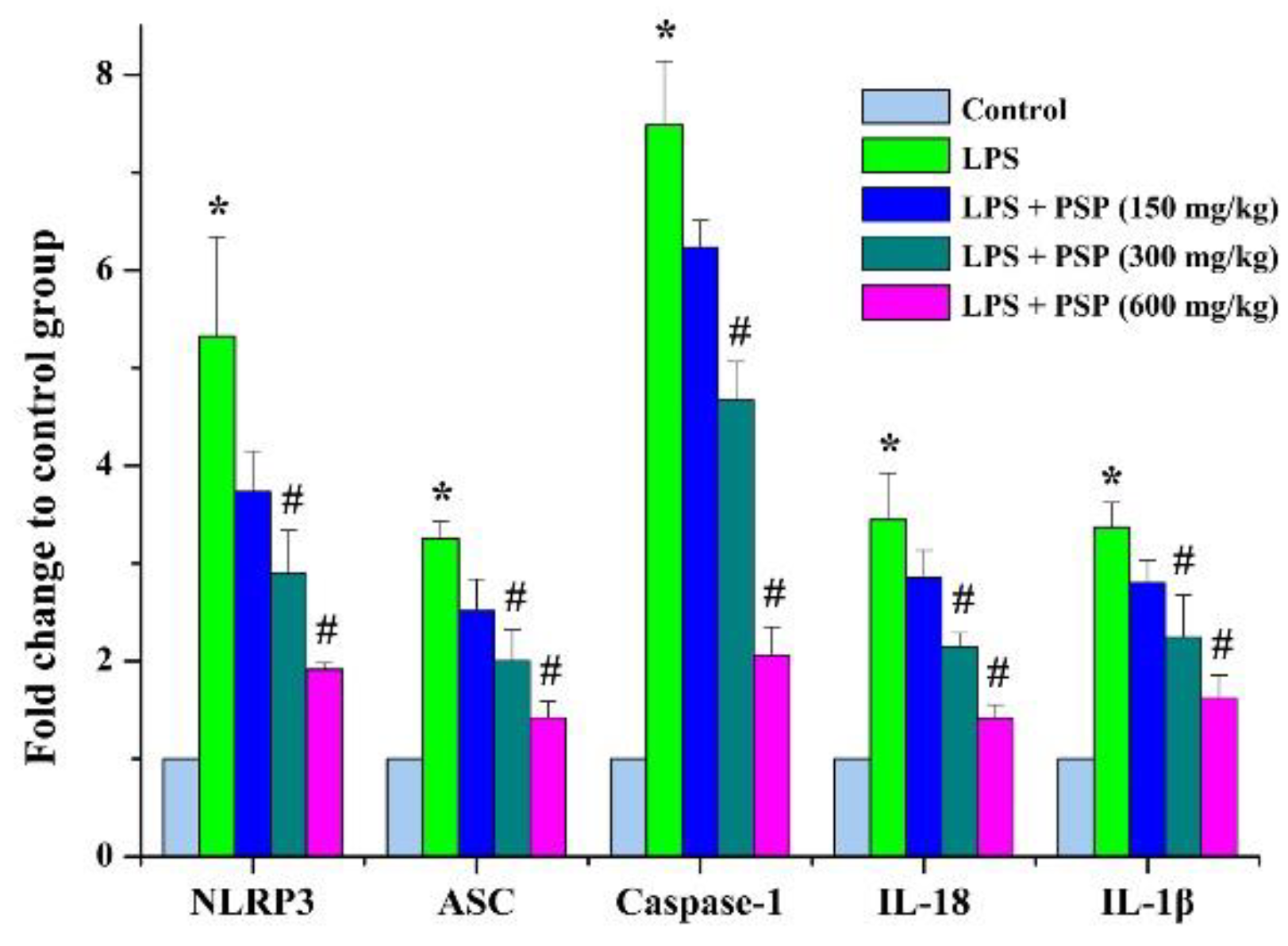
2.5. PSP Mitigated the Pyroptosis by Inhibiting NLRP3 Inflammasome Signaling
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Specimen Collection
4.3. Histological Analysis
4.4. TUNEL Assay
4.5. Biochemical Measurements
4.6. Total RNA Extraction and Quantitative RT-PCR Assay
4.7. Western Blot Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, J.; Wei, H.M. Immune intervention in sepsis. Front. Pharmacol. 2021, 12, 718089. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; Lagu, T.; Lindenauer, P.K. Trends in Sepsis and infection sources in the United States a population-based study. Ann. Am. Thorac. Soc. 2015, 12, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, H.; Kang, Y.; Zhou, L.; Liu, Z.; Qin, B.; Ma, X.; Cao, X.; Chen, D.; Lu, W.; et al. The epidemiology of sepsis in Chinese ICUs: A national cross-sectional survey. Crit. Care Med. 2020, 48, e209–e218. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Jentzer, J.C.; Geske, J.B.; Kumar, M.; Sakhuja, A.; Singhal, A.; Poterucha, J.T.; Kashani, K.; Murphy, J.G.; Gajic, O.; et al. New-onset heart failure and mortality in hospital survivors of sepsis-related left ventricular dysfunction. Shock 2018, 49, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, Y.; Yao, Y. Advances in sepsis-associated liver dysfunction. Burns Trauma 2014, 2, 97–105. [Google Scholar]
- Wu, G.J.; Lin, Y.W.; Chuang, C.Y.; Tsai, H.C.; Chen, R.M. Liver nitrosation and inflammation in septic rats were suppressed by propofol via downregulating TLR4/NF-κB-mediated iNOS and IL-6 gene expressions. Life Sci. 2018, 195, 25–32. [Google Scholar] [CrossRef]
- Nesseler, N.; Launey, Y.; Aninat, C.; Morel, F.; Mallédant, Y.; Seguin, P. Clinical review: The liver in sepsis. Crit. Care 2012, 16, 235. [Google Scholar] [CrossRef]
- Savio, L.E.; de Andrade Mello, P.; Figliuolo, V.R.; de Avelar Almeida, T.F.; Santana, P.T.; Oliveira, S.D.; Silva, C.L.M.; Feldbrügge, L.; Csizmadia, E.; Minshall, R.D.; et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J. Hepatol. 2017, 67, 716–726. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Xia, Q. Inhibition of miR-103a-3p suppresses lipopolysaccharide-induced sepsis and liver injury by regulating FBXW7 expression. Cell Biol. Int. 2020, 44, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, W.; Gong, F.; Chen, Y.; Chen, E. The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: A review. Front. Immunol. 2021, 12, 711939. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Lan, Z.; Xin, Z.; He, C.; Guo, Z.; Xia, X.; Hu, T. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell. Physiol. 2020, 235, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Hua, S.; Ma, M.; Fei, X.; Zhang, Y.; Gong, F.; Fang, M. Glycyrrhizin attenuates hepatic ischemia-reperfusion injury by suppressing HMGB1-dependent GSDMD-mediated kupffer cells pyroptosis. Int. Immunopharmacol. 2019, 68, 145–155. [Google Scholar] [CrossRef]
- Liu, L.; Sun, B. Neutrophil pyroptosis: New perspectives on sepsis. Cell. Mol. Life Sci. 2019, 76, 2031–2042. [Google Scholar]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Russo, A.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.; Rathinam, V. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef]
- Pai, M.H.; Wu, J.M.; Yang, P.J.; Lee, P.C.; Huang, C.C.; Yeh, S.L.; Lin, M.T. Antecedent dietary glutamine supplementation benefits modulation of liver pyroptosis in mice with polymicrobial sepsis. Nutrients 2020, 12, 1086. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.H.; Zhu, X.S.; Xu, H.Y.; Zhao, Z.X. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, J. Chemical constituents of the genus Polygonatum and their role in medicinal treatment. Nat. Prod. Commun. 2015, 10, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, S.; Cao, H.; Guo, H.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Han, C. A review: The bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides. Molecules 2018, 23, 1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Li, Q.; Lu, F.; Wang, H.; Yan, S.; Wang, Q.; Zhu, W. Antiatherosclerotic potential of rhizoma Polygonati polysaccharide in hyperlipidemia-induced atherosclerotic hamsters. Drug Res. 2014, 65, 479–483. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.Y.; Song, Q.Q.; Xie, J.H. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohyd. Polym. 2018, 183, 91–101. [Google Scholar]
- Wang, H.; Yuan, D.P.; Zeng, C.H.; Chen, Z. Advances in pharmacological effects and clinical application of Polygonatum. J. Hubei Minzu Univ. 2017, 34, 58–60. [Google Scholar]
- Li, Y.Y.; Deng, H.B.; Xiang, D.X.; Chen, L.L. The effect of Polygonatic rhizome on hyperlipoidemia and anti-atherosclerosis. Chin. J. Arterioscler. 2005, 13, 429–431. [Google Scholar]
- Wang, W.; Li, S.; Song, M. Polygonatum sibiricum polysaccharide inhibits high glucose-induced oxidative stress, inflammatory response, and apoptosis in RPE cells. J. Recept. Sig. Transd. 2022, 42, 189–196. [Google Scholar] [CrossRef]
- Shen, F.; Xie, P.; Li, C.; Bian, Z.; Wang, X.; Peng, D.; Zhu, G. Polysaccharides from Polygonatum cyrtonema Hua reduce depression-like behavior in mice by inhibiting oxidative stress-calpain-1-NLRP3 signaling axis. Oxid. Med. Cell. Longev. 2022, 2022, 2566917. [Google Scholar] [CrossRef]
- Han, C.Y.; Sun, T.T.; Liu, Y.W.; Fan, G.T.; Zhang, W.J.; Liu, C.Y. Protective effect of Polygonatum sibiricum polysaccharides on gentamicin-induced acute kidney injury in rats via inhibiting p38MAPK/ATF2 pathway. Int. J. Biol. Macromol. 2020, 151, 595–601. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Wang, B.; You, G.; Zhao, J.; Xia, S.; Zhang, Y.; Zhao, L.; Zhou, H. Carboxyfullerene nanoparticles alleviate acute hepatic injury in severe hemorrhagic shock. Biomaterials 2017, 112, 72–81. [Google Scholar] [CrossRef]
- Luo, X.; Yin, Y.; You, G.; Chen, G.; Wang, Y.; Zhao, J.; Wang, B.; Zhao, L.; Zhou, H. Gradually increased oxygen administration improved oxygenation and mitigated oxidative stress after resuscitation from severe hemorrhagic shock. Anesthesiology 2015, 123, 1122–1132. [Google Scholar] [CrossRef]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef]
- Cookson, B.; Brennan, M. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Karmakar, M.; Minns, M.; Greenberg, E.N.; Diaz-Aponte, J.; Pestonjamasp, K.; Johnson, J.L.; Rathkey, J.K.; Abbott, D.W.; Wang, K.; Shao, F.; et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun. 2020, 11, 2212. [Google Scholar] [CrossRef]
- Wree, A.; Eguchi, A.; McGeough, M.D.; Pena, C.A.; Johnson, C.D.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2013, 59, 898–910. [Google Scholar] [CrossRef]
- Gao, J.; Cui, J.Z.; To, E.; Cao, S.; Matsubara, J.A. Evidence for the activation of pyroptotic and apoptotic pathways in RPE cells associated with NLRP3 inflammasome in the rodent eye. J. Neuroinflamm. 2018, 15, 15. [Google Scholar] [CrossRef]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Gene ID |
|---|---|---|---|
| IL-1β | TCGCAGCAGCACATCAACAAGAG | TGCTCATGTCCTCATCCTGGAAGG | NM_008361.4 |
| IL-18 | ATGCTTTCTGGACTCCTGCC | AGTCTTCTGACATGGCAGCC | NM_008360.2 |
| NLRP3 | GAGCTGGACCTCAGTGACAATGC | ACCAATGCGAGATCCTGACAACAC | NM_001359638.1 |
| ASC | GCAACTGCGAGAAGGCTATG | GTGAGCTCCAAGCCATACGA | NM_023258.4 |
| Caspase-1 | ACAACCACTCGTACACGTCTTGC | CCAGATCCTCCAGCAGCAACTTC | NM_009807.2 |
| β-actin | CCTAGGCACCAGGGTGTGAT | TCCATGTCGTCCCAGTTGGT | NM_007393.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Qi, L.; Zhang, G.; Liu, H.; Gu, Y.; Zhang, L.; Zhang, M.; Wu, H. Polygonatum sibiricum Polysaccharides Attenuate Lipopoly-Saccharide-Induced Septic Liver Injury by Suppression of Pyroptosis via NLRP3/GSDMD Signals. Molecules 2022, 27, 5999. https://doi.org/10.3390/molecules27185999
Xiao L, Qi L, Zhang G, Liu H, Gu Y, Zhang L, Zhang M, Wu H. Polygonatum sibiricum Polysaccharides Attenuate Lipopoly-Saccharide-Induced Septic Liver Injury by Suppression of Pyroptosis via NLRP3/GSDMD Signals. Molecules. 2022; 27(18):5999. https://doi.org/10.3390/molecules27185999
Chicago/Turabian StyleXiao, Linxia, Liang Qi, Guozhe Zhang, Hongxia Liu, Yaqin Gu, Lihu Zhang, Mingguang Zhang, and Hongyan Wu. 2022. "Polygonatum sibiricum Polysaccharides Attenuate Lipopoly-Saccharide-Induced Septic Liver Injury by Suppression of Pyroptosis via NLRP3/GSDMD Signals" Molecules 27, no. 18: 5999. https://doi.org/10.3390/molecules27185999
APA StyleXiao, L., Qi, L., Zhang, G., Liu, H., Gu, Y., Zhang, L., Zhang, M., & Wu, H. (2022). Polygonatum sibiricum Polysaccharides Attenuate Lipopoly-Saccharide-Induced Septic Liver Injury by Suppression of Pyroptosis via NLRP3/GSDMD Signals. Molecules, 27(18), 5999. https://doi.org/10.3390/molecules27185999





