Effect and Mechanism of Pharmaceutical Excipients on Berberine to Alleviate Ulcerative Colitis via Regulating Gut Microbiota
Abstract
:1. Introduction
2. Results
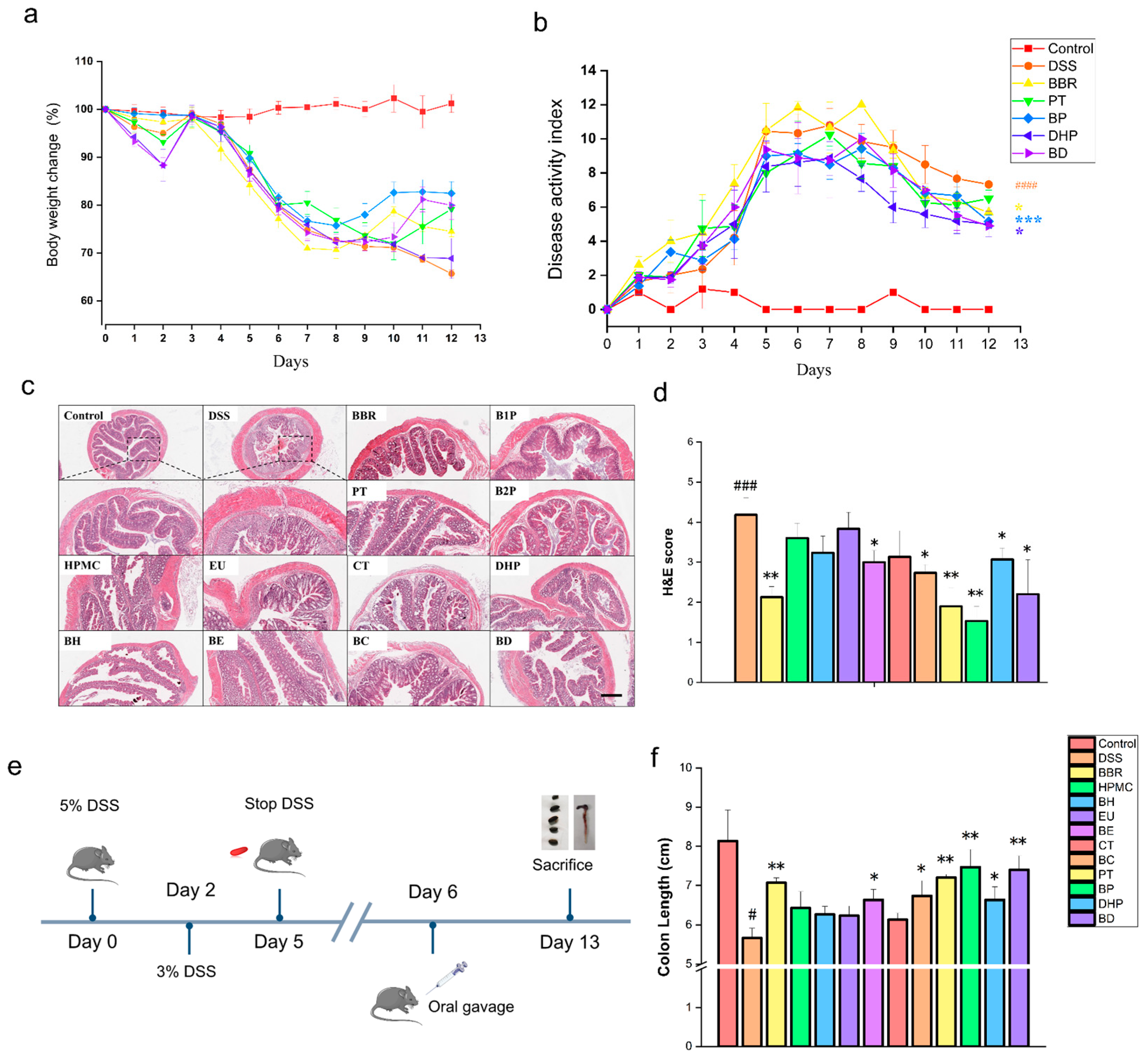
2.1. Effect of PT and DHP Supplement on Colonic Damage by DSS
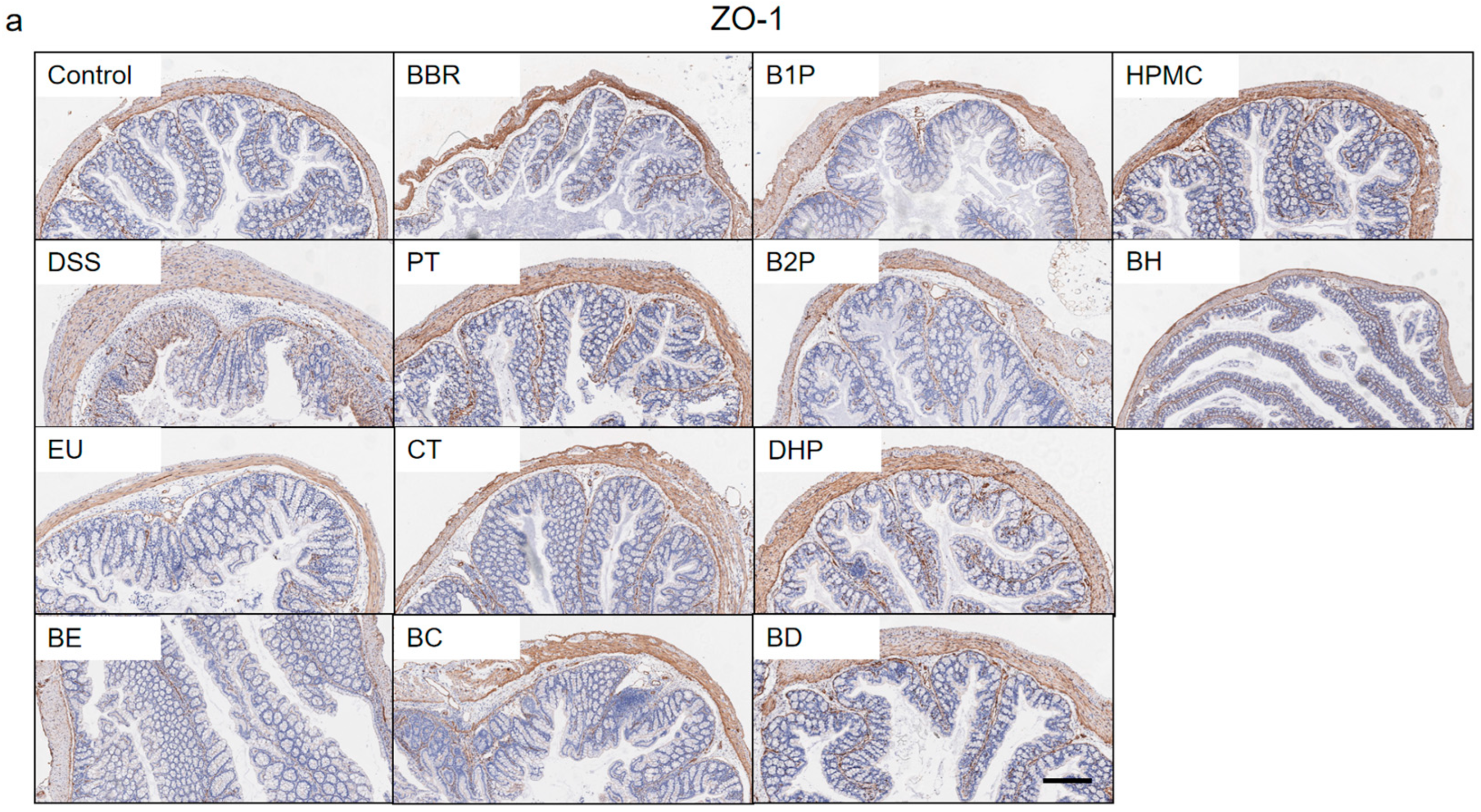
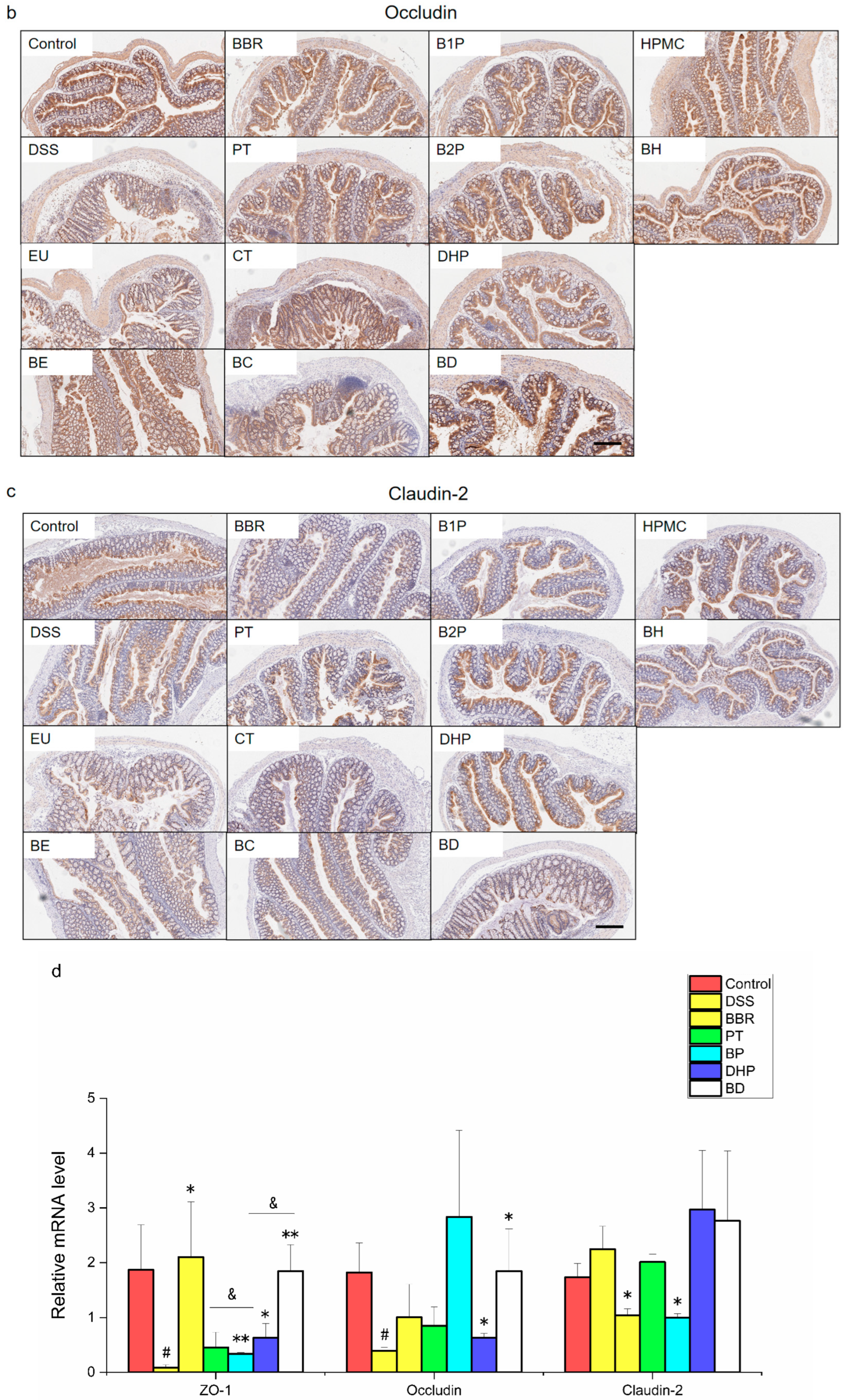
2.2. Effect of PT and DHP Supplement on the Expression of Tight Junction
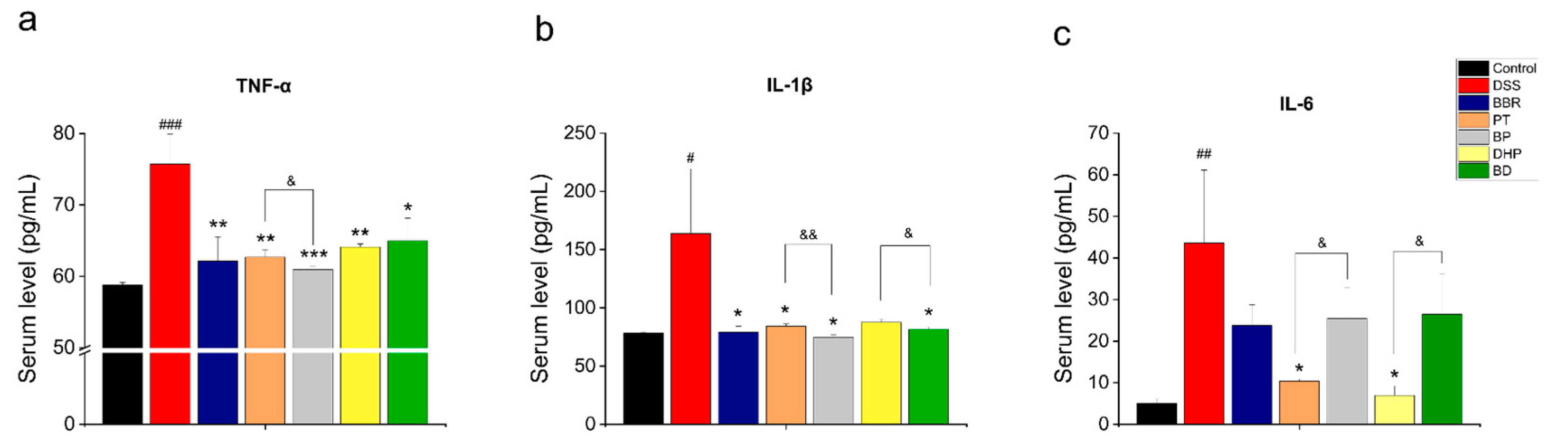
2.3. Effect of PT and DHP Supplement on the Serum Inflammation
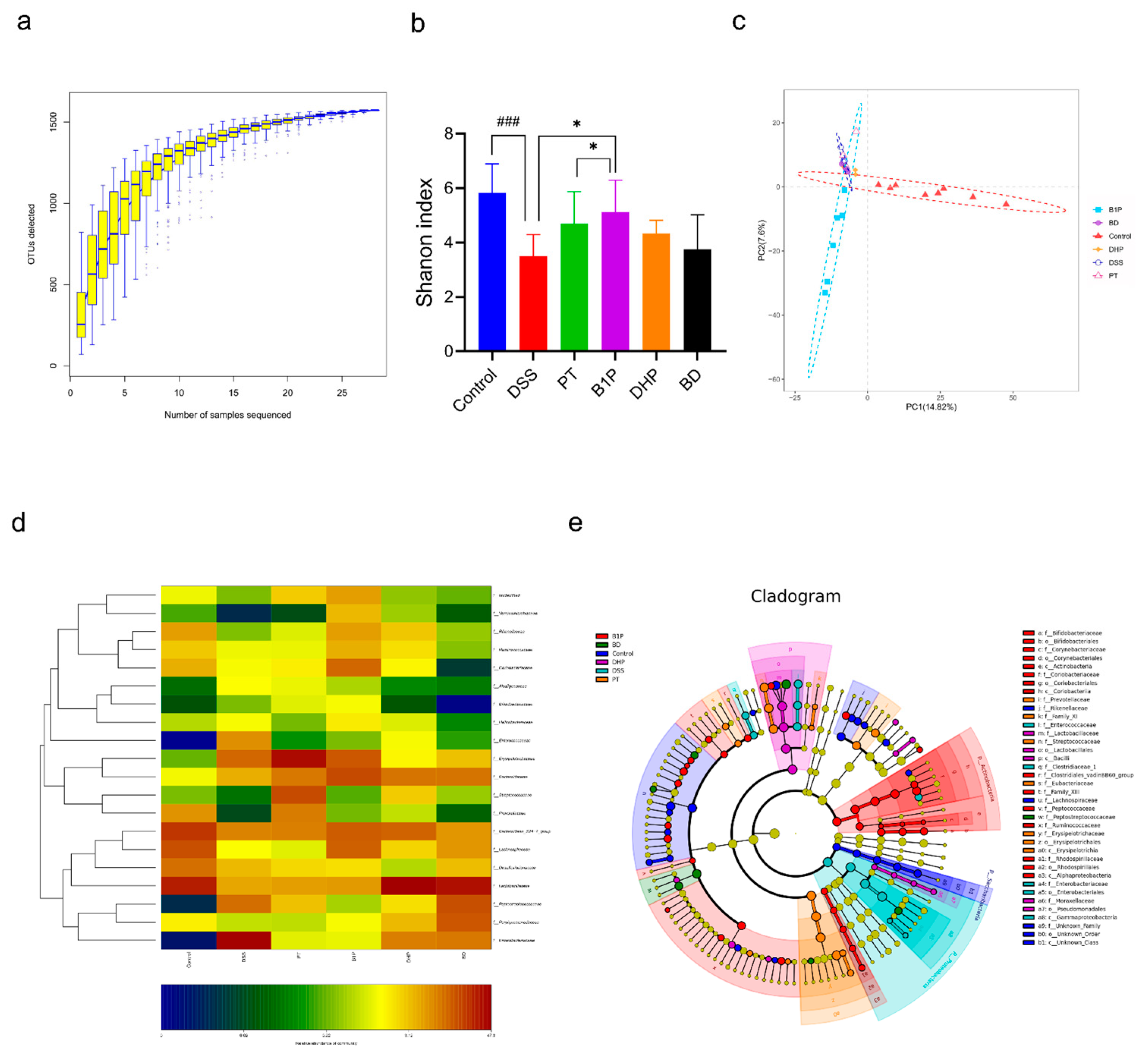
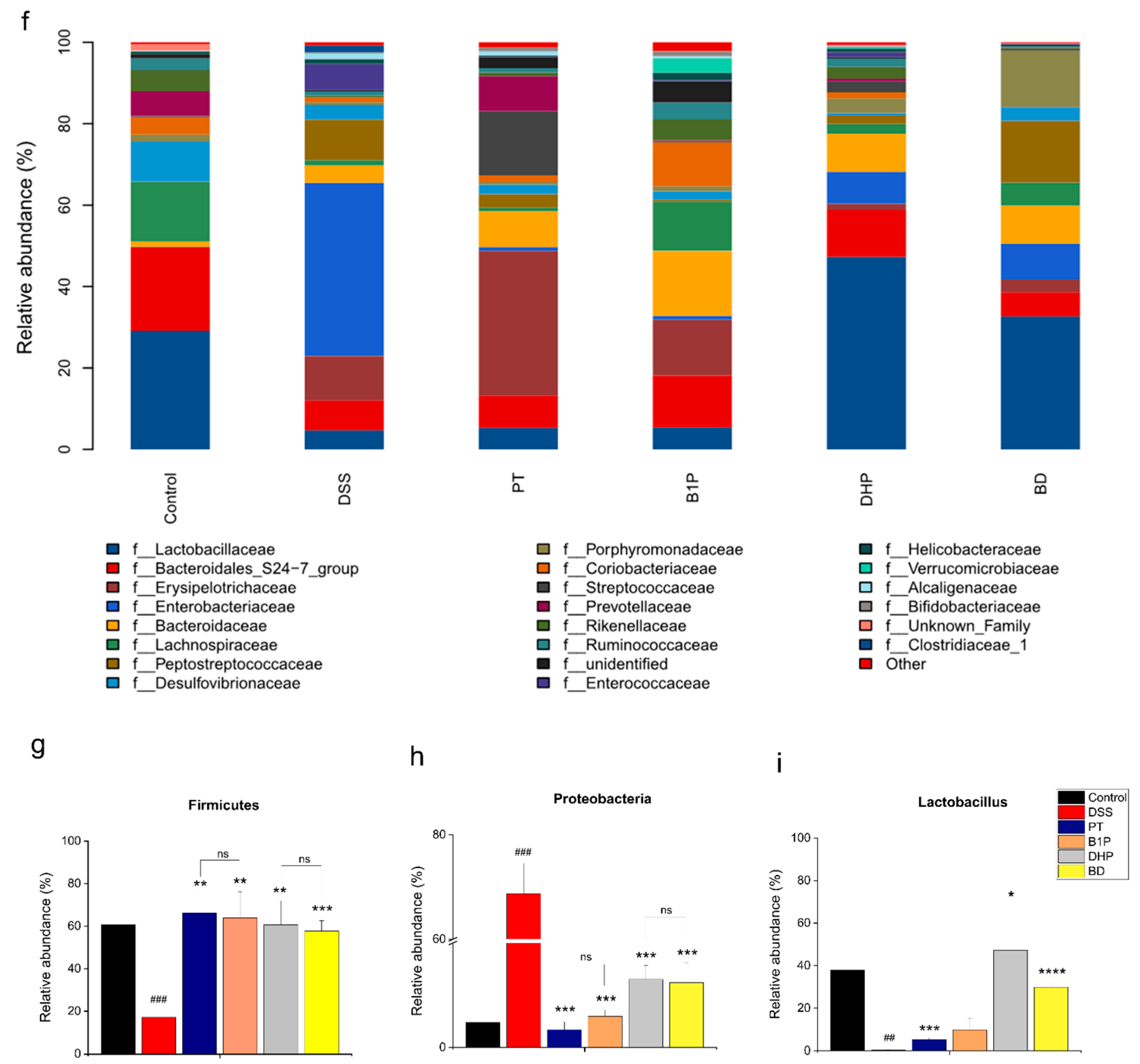
2.4. Effect of PT and DHP Supplement on the Microbial Imbalance
2.5. Screen of the Ratio of BBR to PT via Evaluating the Abundance of the Gut Community
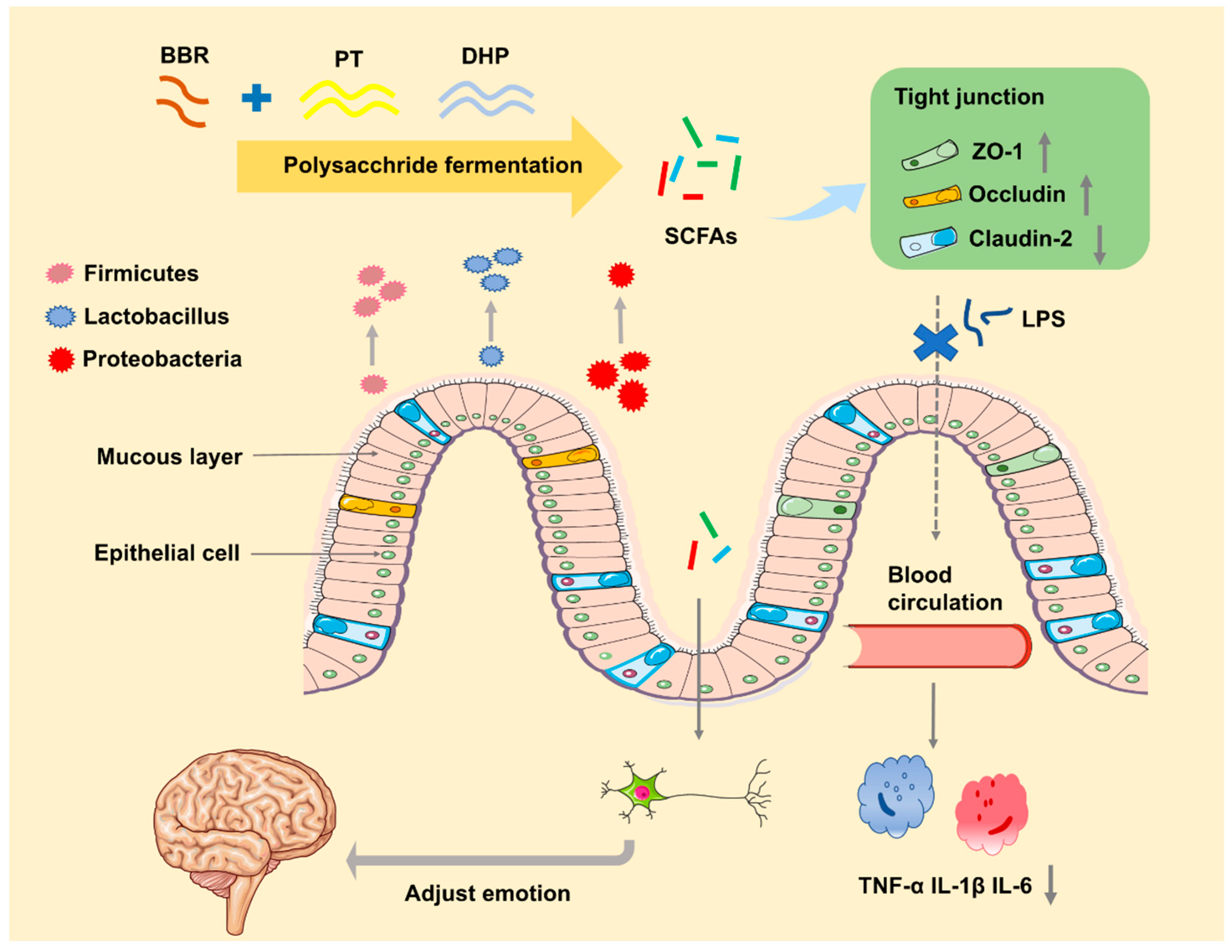
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Experimental Animals
4.3. Induction and Assessment of DSS Colitis
4.4. Drug Intervention
4.5. Histological Analysis
4.6. Immunohistochemistry Analysis
4.7. Serum Supernatant Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
4.8. RNA Extraction and RT-qPCR
4.9. Microbiome Analysis by 16S rRNA Gene Sequencing
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Gálvez, E.J.; Iljazovic, A.; Amend, L.; Lesker, T.R.; Renault, T.; Thiemann, S.; Hao, L.; Roy, U.; Gronow, A.; Charpentier, E.; et al. Distinct Polysaccharide Utilization Determines Interspecies Competition between Intestinal Prevotella spp. Cell Host Microbe 2020, 28, 838–852. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]
- Khorasani, H.M.; Usefi, H.; Peña-Castillo, L. Detecting ulcerative colitis from colon samples using efficient feature selection and machine learning. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Yan, L.; Wang, W.; Wang, D. Berberine alleviates type 2 diabetic symptoms by altering gut mi-crobiota and reducing aromatic amino acids. Biomed. Pharmacother. 2020, 131, 110669. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.; Zhao, Z.; Pan, L.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, S.; Liu, W.; Zou, B.; Lin, L.; Chen, M.; Liu, D.; Wang, M.; Li, L.; Cai, Y.; et al. LC-MS-based metabolomics analysis of Berberine treatment in ulcerative colitis rats. J. Chromatogr. B 2019, 1133, 121848. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, C.; Fan, C.; Yang, Y.; Yang, X.; Lu, H.; Lu, Q.; Zhu, F.; Xiang, C.; Zhang, Z.; et al. Intervention of oncostatin M-driven mucosal inflammation by berberine exerts therapeutic property in chronic ulcerative colitis. Cell Death Dis. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cai, Y.; Jia, B.; Li, J.; Zhao, S.; Chu, X.; Lin, J.; Zhang, X.; Bian, Y.; Zhang, P.; et al. Berberine Regulates Treg/Th17 Balance to Treat Ulcerative Colitis Through Modulating the Gut Microbiota in the Colon. Front. Pharmacol. 2018, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Wu, Z.; Zhao, Z.; Wu, C.; Yuan, M.; Su, Z.; Wang, Y.; Wang, Z. Berberine-Loaded Nanostructured Lipid Carriers Enhance the Treatment of Ulcerative Colitis. Int. J. Nanomed. 2020, 15, 3937–3951. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2020, 11, 1789–1812. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Silva, Y.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Chen, X.; D’Souza, R.; Hong, S. The role of gut microbiota in the gut-brain axis: Current challenges and perspec-tives. Protein Cell 2013, 4, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, H.; Qin, N.; Ren, X.; Zhu, B.; Xia, X. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci. Technol. 2020, 99, 295–310. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z.; et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Vamanu, E.; Pelinescu, D.; Gatea, F.; Sârbu, I. Altered in Vitro Metabolomic Response of the Human Microbiota to Sweeteners. Genes 2019, 10, 535. [Google Scholar] [CrossRef]
- Fu, Z.; Han, L.; Zhang, P.; Mao, H.; Zhang, H.; Wang, Y.; Gao, X.; Liu, E. Cistanche polysaccharides enhance echinacoside absorption in vivo and affect the gut microbiota. Int. J. Biol. Macromol. 2020, 149, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; de Santana-Filho, A.P.; Watanabe, P.D.S.; Sant´ana, D.D.M.G.; Luciano, F.B.; Bocate, K.C.P.; Wijngaard, R.M.V.D.; Werner, M.F.D.P.; et al. Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2020, 12, 331–345. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wang, C.; Lou, Y.; Xia, X.; Xu, H. Whole and polysaccharide powdered Sporisorium reilianum improves DSS-induced colitis in BALB/c mice by modulating gut microbiota. J. Funct. Foods 2021, 79, 104409. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Hao, J. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, J.; Yi, J.; Wu, X.; Ma, Y.; Li, R. Pectin and homogalacturonan with small molecular mass modulate microbial community and generate high SCFAs via in vitro gut fermentation. Carbohydr. Polym. 2021, 269, 118326. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, Y.; Marion, T.; Tong, Y.; Zaiss, M.M.; Tang, Z.; Zhang, Q.; Liu, Y.; Luo, Y. Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. J. Autoimmun. 2020, 116, 102564. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.J.; Rico, C.W.; Um, I.C.; Kang, M.Y. Comparative evaluation of the hypolipidemic effects of hydroxyethyl methylcellulose (HEMC) and hydroxypropyl methylcellulose (HPMC) in high fat-fed mice. Food Chem. Toxicol. 2012, 50, 130–134. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory com-mensal bacteria in the human colon. EMS Microbiol. Ecol. 2017, 93, 127. [Google Scholar]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Wu, X.; Kim, M.J.; Yang, H.J.; Park, S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 2021, 60, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Pourjafar, H.; Jodat, V.; Sahebi, J.; Ataei, A. Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus. AMB Express 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Gao, X.; Li, T.; Jing, W.; Han, B.; Jia, Y.; Hu, N.; Yan, Z.; Li, S.; Yan, R. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J. Ethnopharmacol. 2018, 216, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mao, Y.; Zhang, Z.; Li, Z.; Wang, L. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice human-ized with gut microbiota from patients with colorectal cancer. Theranostics 2021, 11, 4155. [Google Scholar] [CrossRef] [PubMed]
- Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, N.; Clark, A.; Cuthill, A.; Dirnagl, U. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 40, 1769–1777. [Google Scholar]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulate gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef]
- Henrich Lobo, R. Enzymatic Immunohistochemistry. Methods Mol. Biol. 2022, 2422, 125–129. [Google Scholar]
- Li, A.; Yang, D. Application of Immunohistochemistry in Basic and Clinical Studies. Methods Mol. Biol. 2020, 2108, 43–55. [Google Scholar]
- Kapoor, B.; Gulati, M.; Singh, S.K.; Khatik, G.L.; Gupta, R.; Kumar, R.; Kumar, R.; Gowthamarajan, K.; Mahajan, S.; Gupta, S. Fail-safe Nano-formulation of Prodrug of Sulfapyridine: Preparation and Evaluation for Treatment of Rheumatoid Arthritis. Mater. Sci. Eng. C 2021, 118, 111332. [Google Scholar] [CrossRef] [PubMed]
- Kirdak, T.; Uysal, E.; Sezgin, E.; Cecen, G.; Cavun, S. Inflammatory response markers in rats undergoing abdominal surgical procedures. Ann. Gastroenterol. 2020, 33, 528–535. [Google Scholar] [CrossRef]
- Bachman, J. Reverse-Transcription PCR (RT-PCR). Methods Enzymol. 2013, 530, 67–74. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zheng, T.; Chen, H.; Zou, P.; Zhang, M.; Wang, J.; Li, N.; Zhang, Y.; Li, Y.; Dong, Z. Effect and Mechanism of Pharmaceutical Excipients on Berberine to Alleviate Ulcerative Colitis via Regulating Gut Microbiota. Molecules 2022, 27, 5997. https://doi.org/10.3390/molecules27185997
Wu C, Zheng T, Chen H, Zou P, Zhang M, Wang J, Li N, Zhang Y, Li Y, Dong Z. Effect and Mechanism of Pharmaceutical Excipients on Berberine to Alleviate Ulcerative Colitis via Regulating Gut Microbiota. Molecules. 2022; 27(18):5997. https://doi.org/10.3390/molecules27185997
Chicago/Turabian StyleWu, Chenyang, Tingting Zheng, Huan Chen, Peizhi Zou, Mengxue Zhang, Jinrui Wang, Nan Li, Yun Zhang, Ying Li, and Zhengqi Dong. 2022. "Effect and Mechanism of Pharmaceutical Excipients on Berberine to Alleviate Ulcerative Colitis via Regulating Gut Microbiota" Molecules 27, no. 18: 5997. https://doi.org/10.3390/molecules27185997
APA StyleWu, C., Zheng, T., Chen, H., Zou, P., Zhang, M., Wang, J., Li, N., Zhang, Y., Li, Y., & Dong, Z. (2022). Effect and Mechanism of Pharmaceutical Excipients on Berberine to Alleviate Ulcerative Colitis via Regulating Gut Microbiota. Molecules, 27(18), 5997. https://doi.org/10.3390/molecules27185997





