Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Contents
2.2. Total Flavonoids Contents
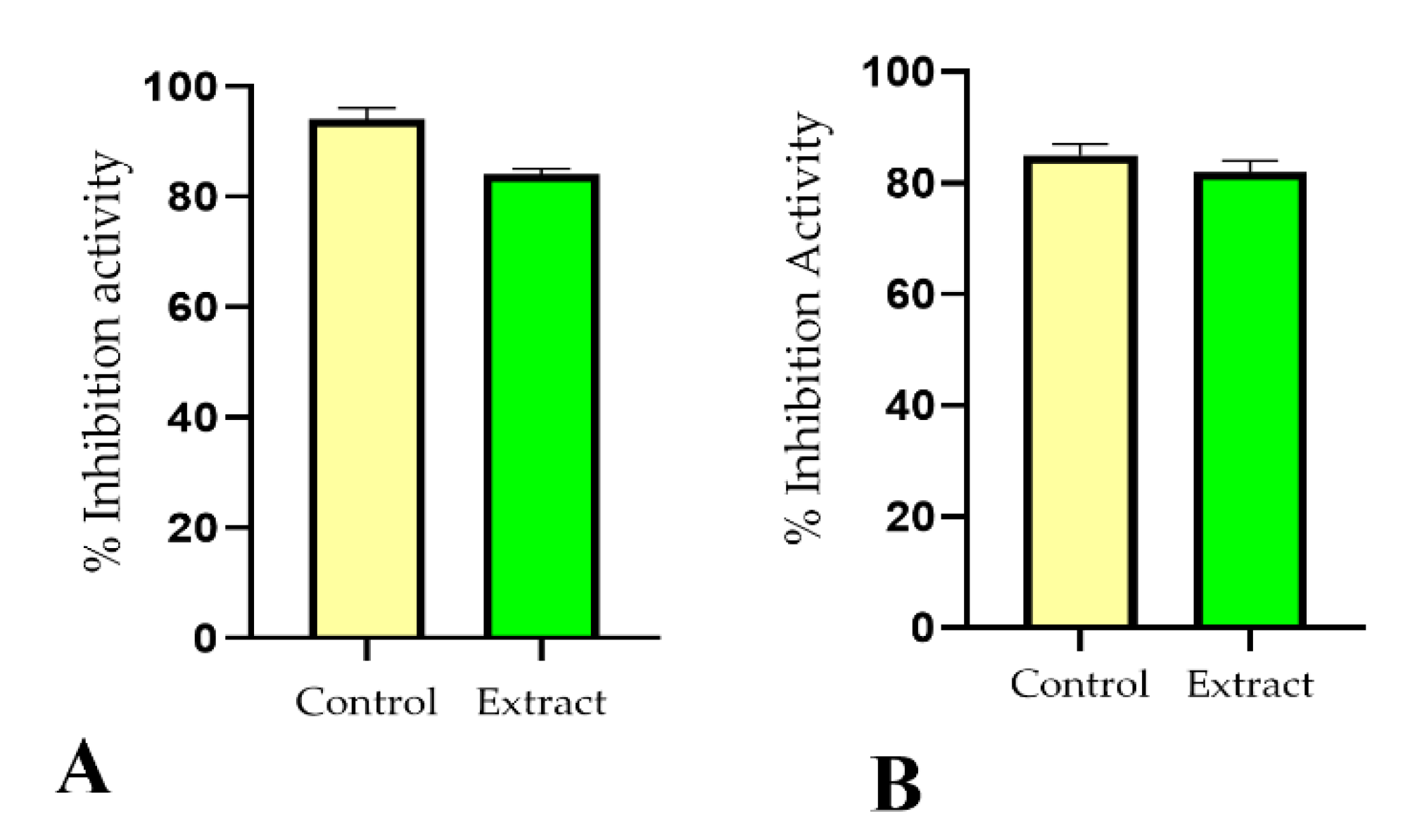
2.3. Antioxidant Potential
2.4. Tyrosinase Enzyme Inhibition Activity
2.5. Gas Chromatography-Mass Spectroscopy (GC-MS)
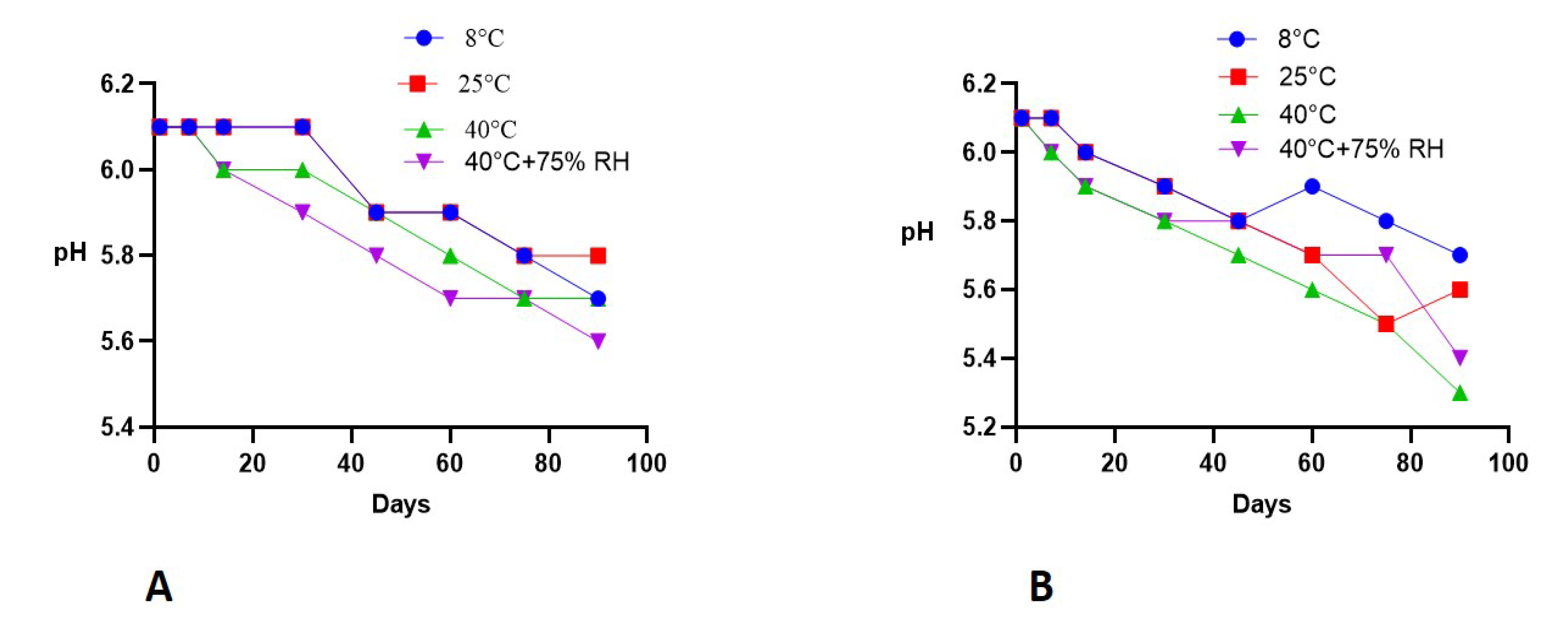
2.6. Stability Studies
2.6.1. Organoleptic Properties
2.6.2. pH
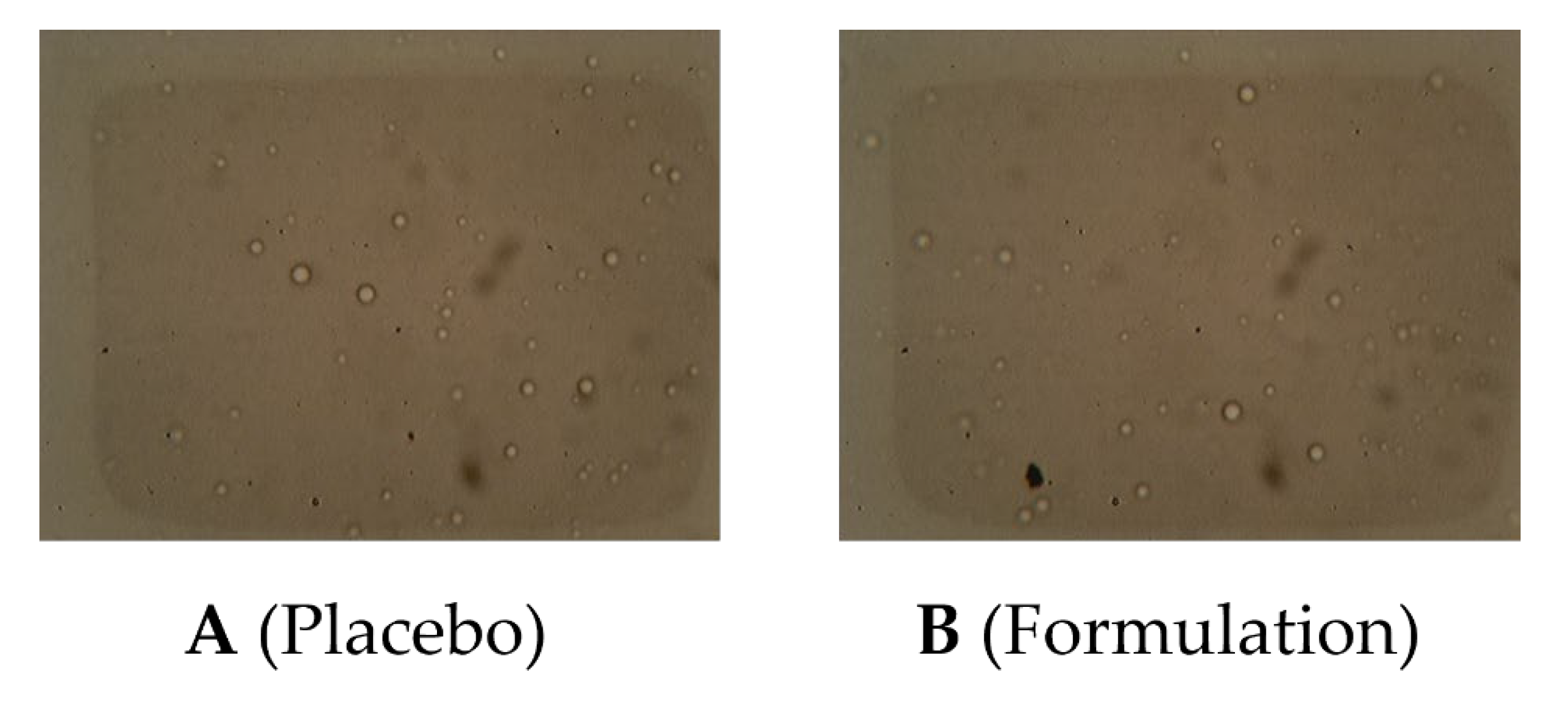
2.6.3. Microscopy
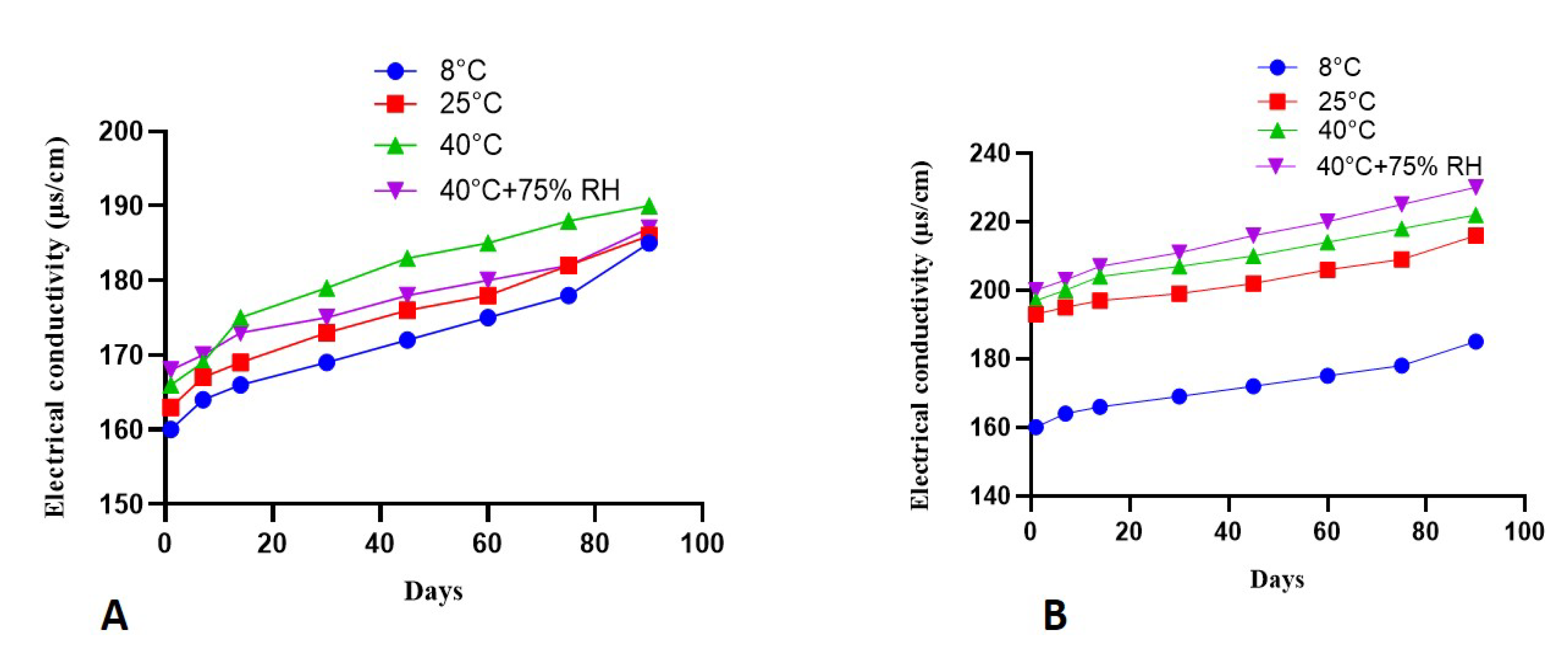
2.7. Electrical Conductivity
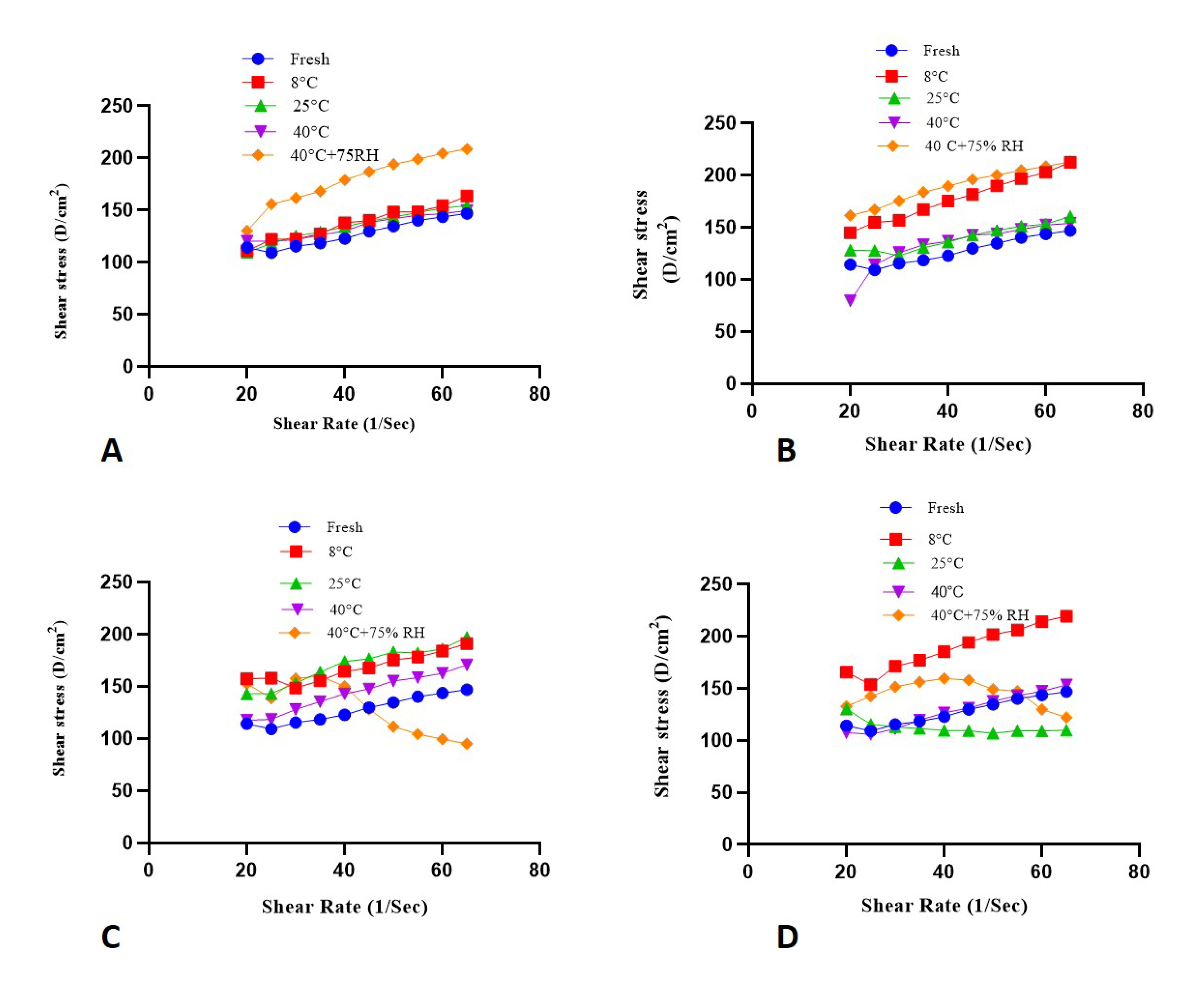
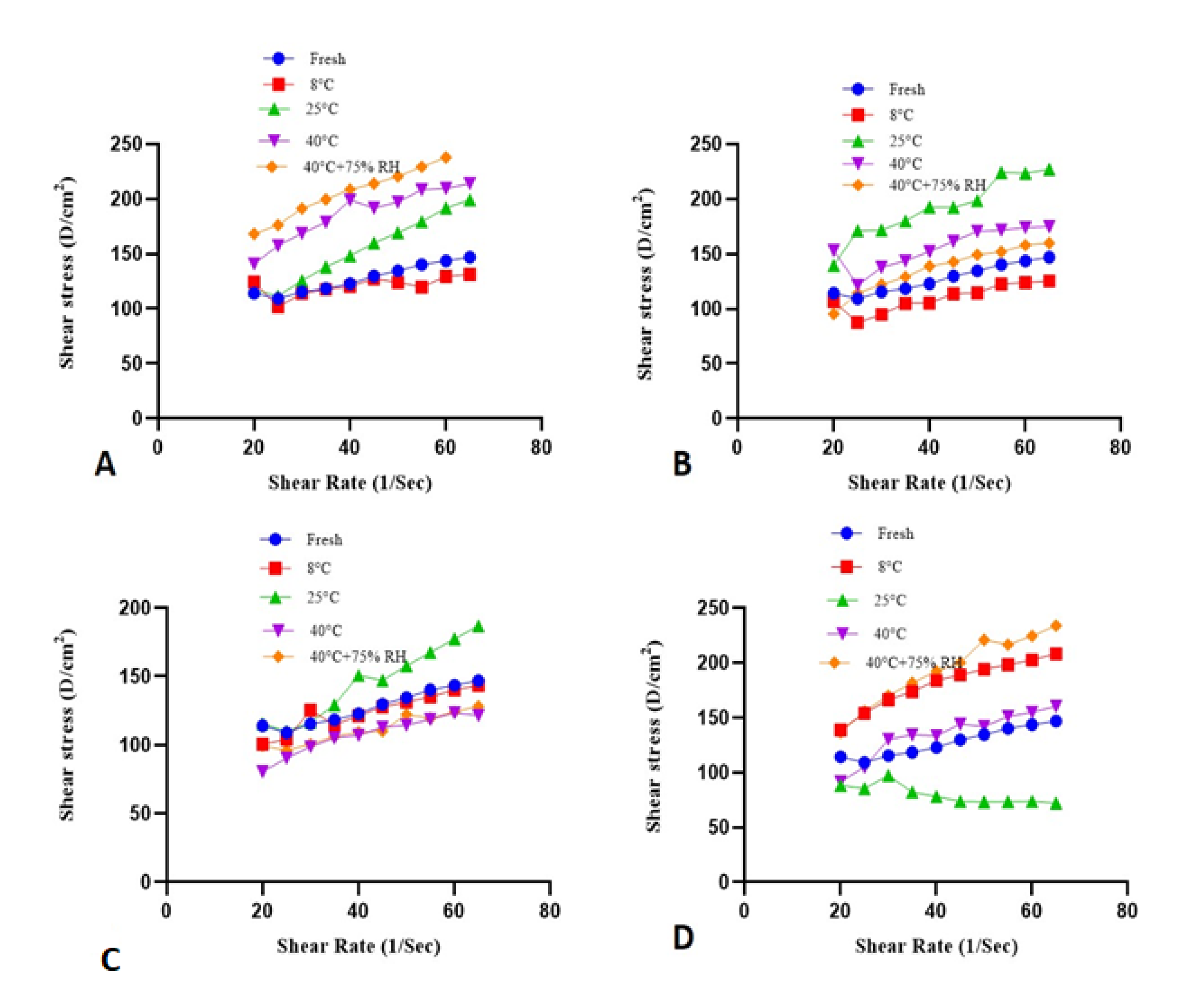
2.8. Rheological Evaluation
2.9. Centrifugation Test
2.10. Sun Protection Factor (SPF)
2.11. Zeta Potential and Globule Size
2.12. Skin Irritation (Tolerance) Test
2.13. Spreadability
2.14. Drug Content Determination
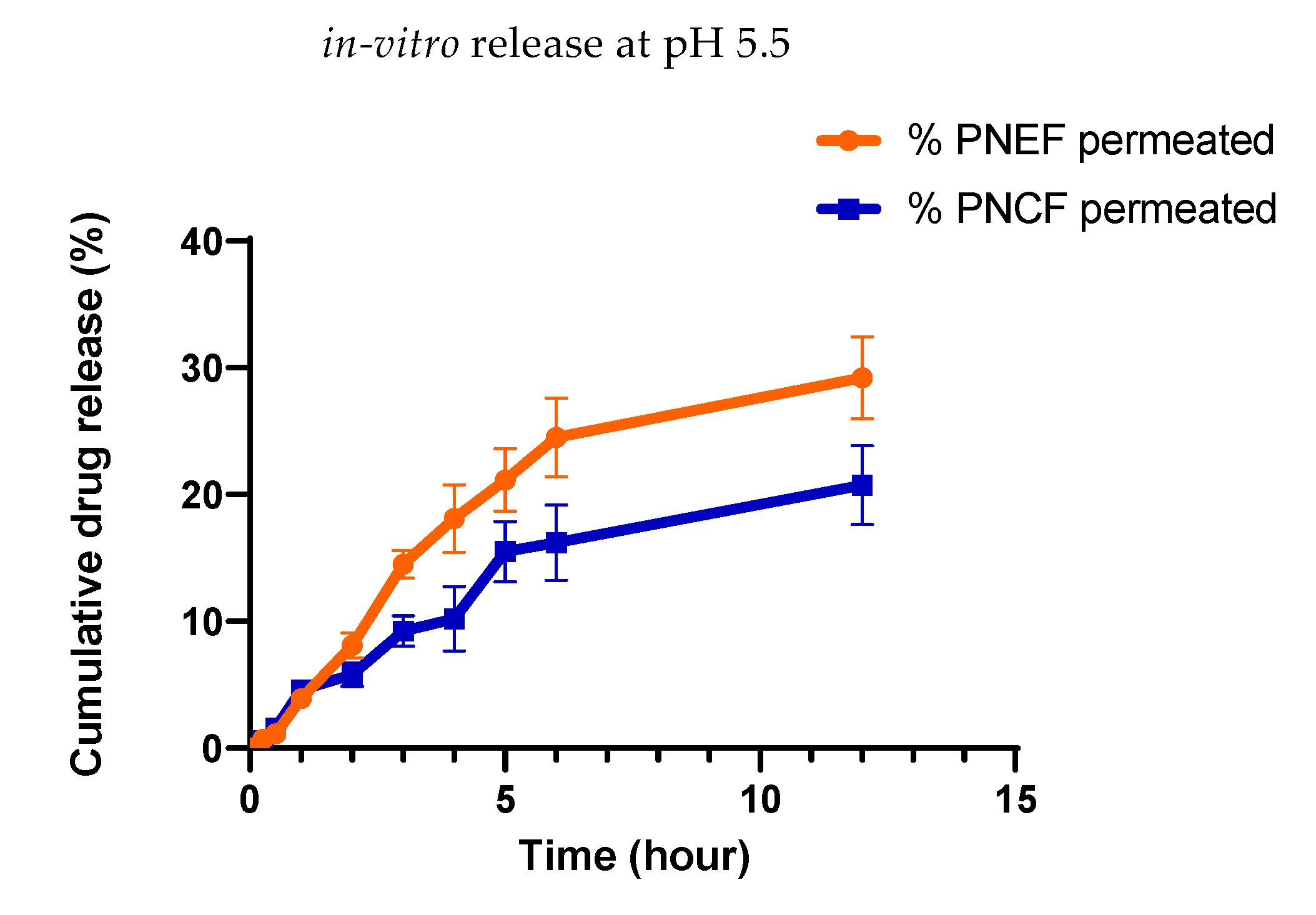
2.15. In Vitro Release
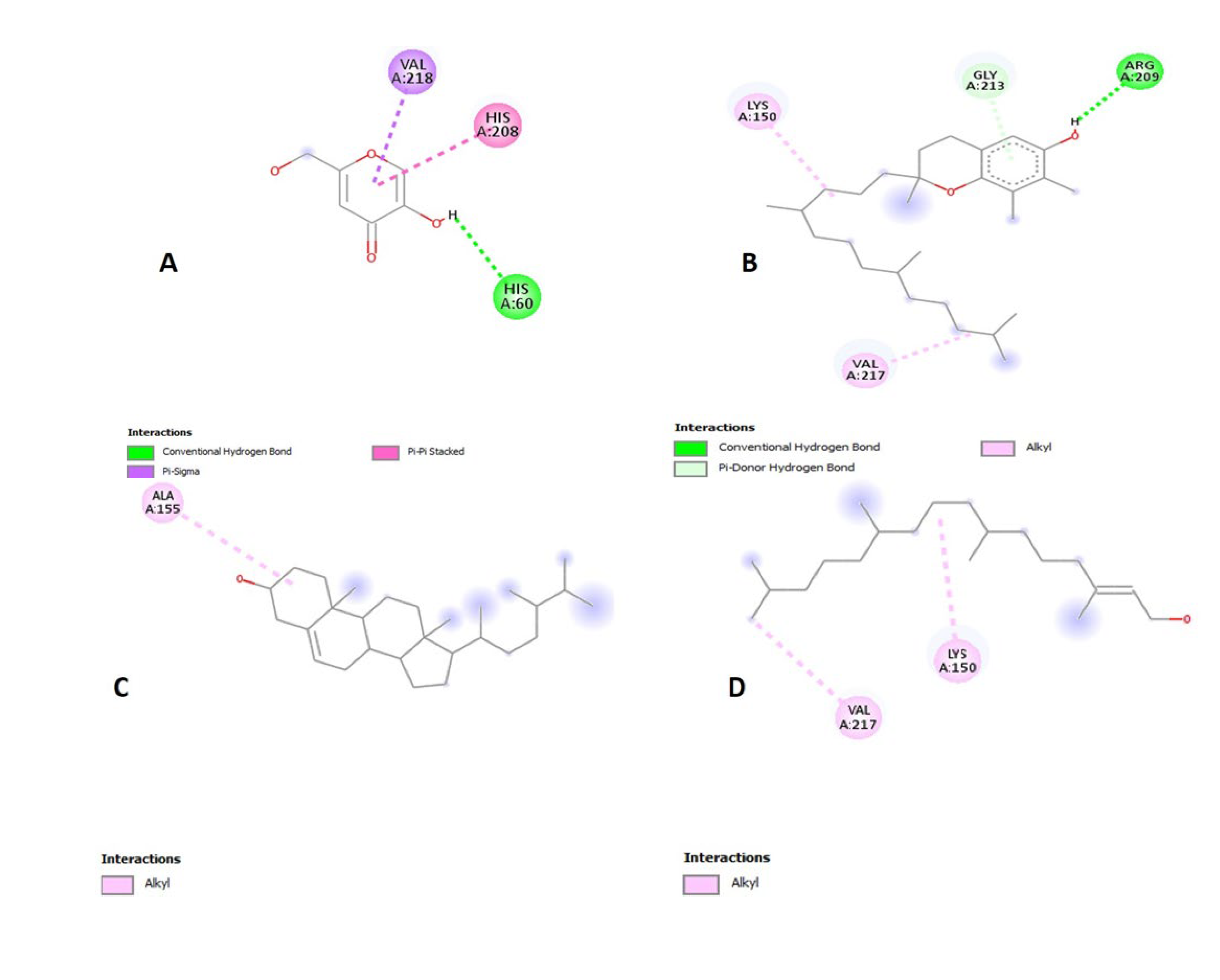
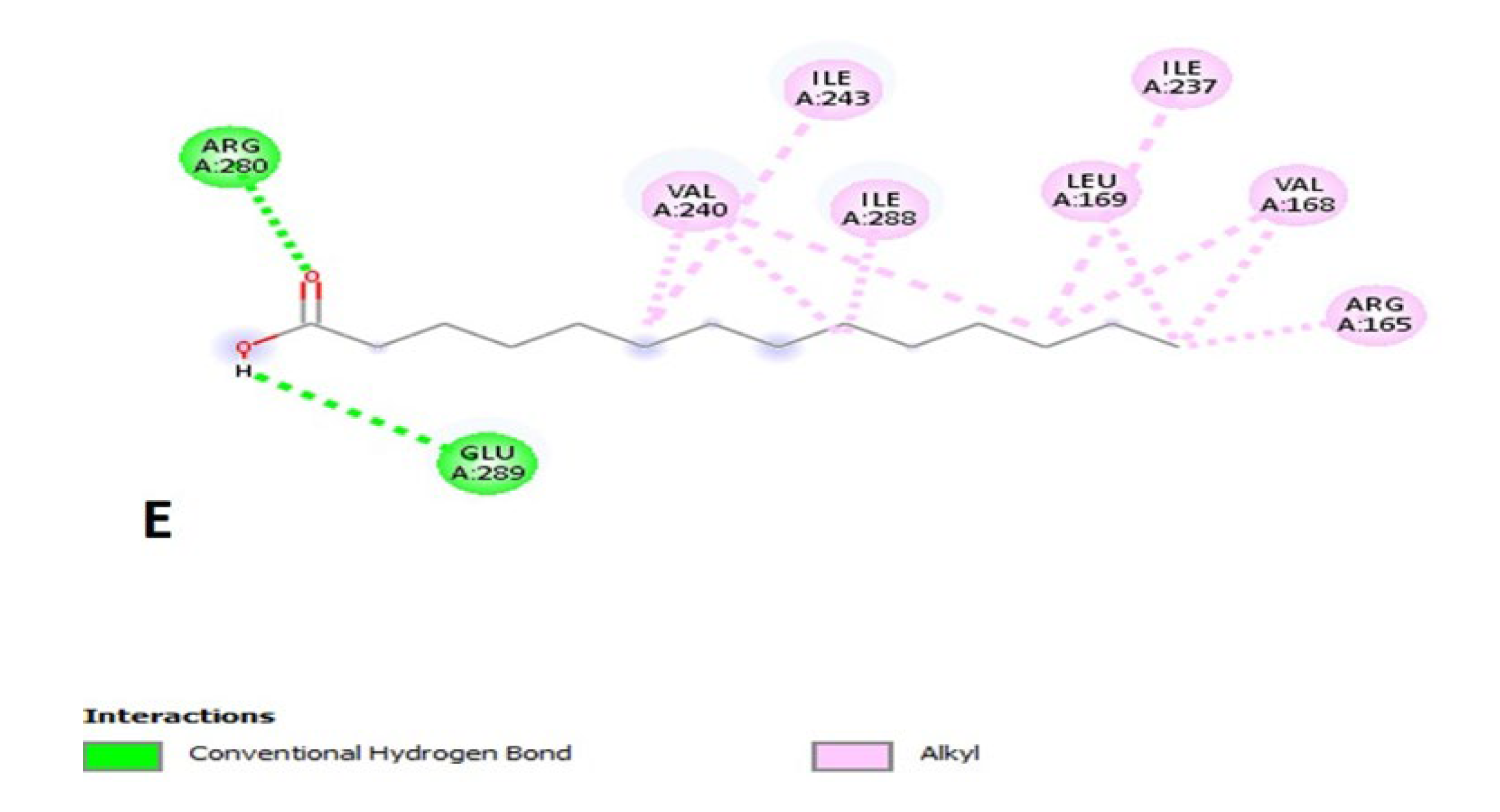
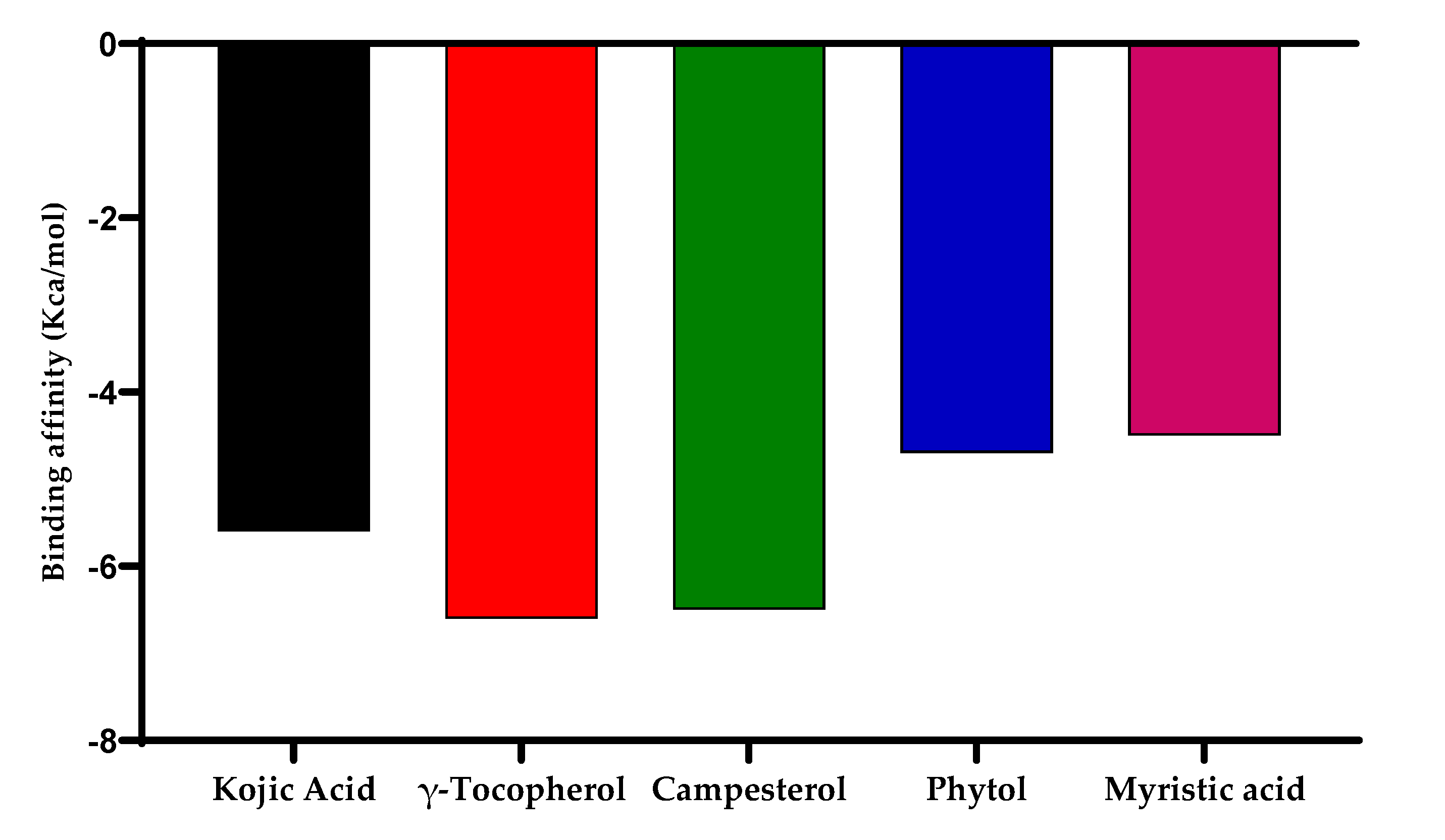
2.16. Molecular Docking for Tyrosinase Enzyme
3. Discussion
4. Materials and Methods
4.1. Chemicals and Equipment
4.2. Plant Material
4.3. Preparation of Extract
4.4. Phytochemical Analysis
4.4.1. Total Phenolic Content (TPC)
4.4.2. Total Flavonoids Contents (TFC)
4.5. Antioxidant Activity by DPPH Method
4.6. Tyrosinase Enzyme Inhibition Activity
4.7. Gas Chromatography-Mass Spectroscopy
4.8. Preparation of Test and Placebo Formulation
4.9. Emulgel In Vitro Characterization
4.9.1. Organoleptic Evaluation
4.9.2. pH
4.9.3. Microscopy
4.9.4. Electrical Conductivity
4.9.5. Rheological Evaluation
4.10. Centrifugation
4.11. In Vitro Sun Protection Factor (SPF)
4.12. Particle Size and Zeta Potential
4.13. Skin Irritation (Tolerance) Test
4.14. Spreadability
4.15. Drug Content Determination
4.16. In Vitro Release
4.17. In-Silico Molecular Docking Studies
4.18. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talha, M.; Islam, N.U.; Zahoor, M.; Sadiq, A.; Nawaz, A.; Khan, F.A.; Gulfam, N.; Alshamrani, S.A.; Nahari, M.H.; Alshahrani, M.A. Biological evaluation, phytochemical screening, and fabrication of Indigofera linifolia leaves extract-loaded nanoparticles. Molecules 2022, 27, 4707. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.U.; Abbas, S.Q.; Ali, F.; Ishaq, M.; Bano, I.; Hassan, M.; Jin, H.-Z.; Bungau, S.G. A Comprehensive in silico exploration of pharmacological properties, bioactivities, molecular docking, and anticancer potential of vieloplain F from Xylopia vielana targeting B-Raf kinase. Molecules 2022, 27, 917. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Aldawsari, H.M.; Bahmdan, R.H.; Sindi, A.M.; Kurakula, M.; Alrobaian, M.M.; Aldryhim, A.Y.; Alkhalidi, H.M.; Bahmdan, H.H.; Khallaf, R.A. Preparation, optimization, and evaluation of hyaluronic acid-based hydrogel loaded with miconazole self-nanoemulsion for the treatment of oral thrush. AAPS Pharm. Sci. Tech. 2019, 20, 297. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Alruwaili, N.K.; Alhakamy, N.A.; Abualsunun, W.A.; Bakhaidar, R.B.; Bahmdan, R.H.; Rizg, W.Y. Oral gel loaded with penciclovir–lavender oil nanoemulsion to enhance bioavailability and alleviate pain associated with herpes labialis. Drug Deliv. 2021, 28, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Madaan, V.; Chanana, A.; Kataria, M.; Bilandi, A. Emulsion technology and recent trends in emulsion applications. Int. Res. J. Pharm. 2014, 5, 533–542. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mishra, M.K.; Tiwari, A.; Shukla, A. Emulgel: A new approach for enhanced topical drug delivery. Int. J. Curr. Pharm. Res. 2016, 9, 15–19. [Google Scholar] [CrossRef]
- Isaac, V.L.B.; Chiari, B.G.; Miglioli, K.; Moreira, R.; Oliveira, J.R.S.; Salgado, H.; Relkin, P.; Correa, M.A.; Salgado, A.; Ribeiro, H.M. Development of a topical formulation containing S. lutea extract: Stability, in vitro studies and cutaneous permeation. J. Appl. Pharm. Sci. 2012, 2, 174–179. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Alhadrami, H.A.A.; Bhandari, A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum linn fruits. Asian. Pac. J. Trop. Biomed. 2015, 5, 101–107. [Google Scholar] [CrossRef]
- Vadgama, R.N.; Odaneth, A.A.; Lali, A.M. Green synthesis of isopropyl myristate in novel single phase medium part I: Batch optimization studies. Biotechnol. Rep. 2015, 8, 133–137. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Premathilaka, R.; Silva, M. Bioactive compounds and antioxidant activity of Bunchosia armenica. World J. Pharm. Sci. 2016, 5, 1237–1247. [Google Scholar]
- Abubakar, M.N.; Majinda, R.R. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, J.; de Oliveira, R.N.; Costa, J.P.; Junior, A.L.; de Sousa, D.P.; Freitas, R.M.; Allegretti, S.M.; Pinto, P.L. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2617. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.d.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. J. Neurosci. 2013, 2013, 949452. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.S.; Sofian-Seng, N.-S.; Mustapha, W.W. Functional properties of oleoresin extracted from white pepper (Piper nigrum L.) retting waste water. Sains Malays. 2018, 47, 2009–2015. [Google Scholar] [CrossRef]
- Caldas, H. Leading order corrections to the free energy and phase separation in two-component fermion systems. J. Stat. Mech. Theory Exp. 2019, 2019, 103102. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against oxidative stress: From molecular mechanisms to clinical applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Markowska, A.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Antioxidant and anti-inflammatory effect of cannabidiol contributes to the decreased lipid peroxidation of keratinocytes of rat skin exposed to UV radiation. Oxid. Med. Cell. Longev. 2021, 2021, 6647222. [Google Scholar] [CrossRef]
- Noriega, P. Terpenes in essential oils: Bioactivity and applications. In Terpenes and Terpenoids-Recent Advances; IntechOpen: London, UK, 2020. [Google Scholar]
- Dos Santos, S.M.; de Oliveira Junior, P.C.; de Matos Balsalobre, N.; Kassuya, C.A.L.; Cardoso, C.A.L.; Pereira, Z.V.; Silva, R.M.M.F.; Formagio, A.S.N. Variation in essential oil components and anti-inflammatory activity of Allophylus edulis leaves collected in central-western Brazil. J. Ethnopharmacol. 2021, 267, 113495. [Google Scholar] [CrossRef]
- Xian, D.; Song, J.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Emerging roles of redox-mediated angiogenesis and oxidative stress in dermatoses. Oxid. Med. Cell. Longev. 2019, 2019, 2304018. [Google Scholar] [CrossRef]
- Tarshish, E.; Hermoni, K.; Sharoni, Y.; Muizzuddin, N. Effect of Lumenato oral supplementation on plasma carotenoid levels and improvement of visual and experiential skin attributes. J. Cosmet. Dermatol. 2022, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, I.S.; Khan, H.M.S.; Arshad, A.I.; Ijaz, H.; Banerjee, P.; Khan, A.U.; Juthi, A. In vitro characterization and assessment of cosmetic potentials of w/o emulsion cream containing 2% prosopis cineraria extract. Acta. Pol. Pharm. Drug Res. 2015, 72, 1233–1238. [Google Scholar]
- Blaak, J.; Staib, P. The relation of pH and skin cleansing. In pH of the Skin: Issues and Challenges; Karger Publishers: Berlin, Germany, 2018; Volume 54, pp. 132–142. [Google Scholar] [CrossRef]
- Huma, S.; Khan, H.M.S.; Sohail, M.; Akhtar, N.; Rasool, F.; Majeed, F.; Daniyal, M. Development, in-vitro characterization and assessment of cosmetic potential of Beta vulgaris extract emulsion. J. Herb. Med. 2020, 23, 100372. [Google Scholar] [CrossRef]
- Ijaz, S.; Khan, H.M.S.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Bocharova, V.; Sokolov, A.P. Perspectives for polymer electrolytes: A view from fundamentals of ionic conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Ethier, A.; Bansal, P.; Baxter, J.; Langley, N.; Richardson, N.; Patel, A.M. The role of excipients in the microstructure of topical semisolid drug products. In The Role of Microstructure in Topical Drug Product Development; Springer: Berlin/Heidelberg, Germany, 2019; pp. 155–193. [Google Scholar]
- Li, D.; Xie, H.; Liu, Z.; Li, A.; Li, J.; Liu, B.; Liu, X.; Zhou, D. Shelf life prediction and changes in lipid profiles of dried shrimp (Penaeus vannamei) during accelerated storage. Food Chem. 2019, 297, 124951. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant secondary metabolites against skin photodamage: Mexican plants, a potential source of uv-radiation protectant molecules. Plants 2022, 11, 220. [Google Scholar] [CrossRef]
- Plohl, O.; Zemljič, L.F.; Potrč, S.; Luxbacher, T. Applicability of electro-osmotic flow for the analysis of the surface zeta potential. RSC Adv. 2020, 10, 6777–6789. [Google Scholar] [CrossRef]
- Guter, M.; Breunig, M. Hyaluronan as a promising excipient for ocular drug delivery. Eur. J. Pharm. Biopharm. 2017, 113, 34–49. [Google Scholar] [CrossRef]
- Umeyor, C.E.; Okonkwo, A.U.; Ejielo, O.D.; Umeyor, I.C.; Uronnachi, E.M.; Nwakile, C.D.; Okeke, I.J.; Attama, A.A. Formulation design and preclinical evaluations of surface modified lipid nanoparticles-coupled gel encapsulating dihydroartemisinin for treatment of localized inflammation. Lett. Appl. NanoBioSci. 2021, 11, 3745–3769. [Google Scholar] [CrossRef]
- Navaneetha, K.; Asma, B.; Sumalatha, P.; Vinita, D.; Sravan, J.; Chinnala, M. Formulation and in-vitro evaluation of capsaicin emulgel for topical delivery. Sch. Acad. J. Pharm. 2017, 6, 281–287. [Google Scholar]
- Moghimipour, E.; Salimi, A.; Eftekhari, S. Design and characterization of microemulsion systems for naproxen. Adv. Pharm. Bull. 2013, 3, 63. [Google Scholar] [PubMed]
- Cojocaru, V.; Ranetti, A.E.; Hinescu, L.G.; Ionescu, M.; Cosmescu, C.; Poștoarcă, A.G.; Cinteză, L.O. Formulation and evaluation of in vitro release kinetics of Na3CaDTPA decorporation agent embedded in microemulsion-based gel formulation for topical delivery. Farmacia 2015, 63, 656–664. [Google Scholar]
- Hassan, S.S.U.; Zhang, W.-D.; Jin, H.-Z.; Basha, S.H.; Priya, S.S. In-silico anti-inflammatory potential of guaiane dimers from Xylopia vielana targeting COX-2. J. Biomol. 2022, 40, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.N.; Ahmad, S.; Tousif, M.I.; Ahmad, I.; Rao, H.; Ahmad, B.; Basit, A. Profiling of phytochemicals from aerial parts of Terminalia neotaliala using LC-ESI-MS2 and determination of antioxidant and enzyme inhibition activities. PLoS ONE 2022, 17, e0266094. [Google Scholar] [CrossRef]
- Dilshad, R.; Ahmad, S.; Aati, H.; Al-qahtani, J.H.; Sherif, A.E.; Hussain, M.; Ghalloo, B.A.; Tahir, H.; Basit, A.; Ahmed, M. Phytochemical profiling, in vitro biological activities, and in-silico molecular docking studies of Typha domingensis. Arab. J. Chem. 2022, 27, 104133. [Google Scholar] [CrossRef]
- Dilshad, R.; Khan, K.-U.-R.; Saeed, L.; Sherif, A.E.; Ahmad, S.; Ovatlarnporn, C.; Nasim, J.; Hussain, M.; Ghalloo, B.A.; Basit, A.; et al. Chemical composition and biological evaluation of Typha domingensis pers. to ameliorate health pathologies: In vitro and in silico approaches. BioMed Res. Int. 2022, 2022, 8010395. [Google Scholar] [CrossRef]
- Arshad, W.; Khan, H.M.S.; Akhtar, N.; Mohammad, I.S. Polymeric emulgel carrying Cinnamomum tamala extract: Promising delivery system for potential topical applications. Braz. J. Pharm. Sci. 2020, 56, e18318. [Google Scholar] [CrossRef]
- Mollik, M.; Rahman, M.; Al-Shaeri, M.; Ashraf, G.M.; Alexiou, A.; Gafur, M. Isolation, characterization and in vitro antioxidant activity screening of pure compound from black pepper (Piper nigrum). Environ. Sci. Pollut. Res. Int. 2022, 29, 52220–52232. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Famous, E.; Pan, S.; Peng, X.; Tian, J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021, 346, 128845. [Google Scholar] [CrossRef]
- Rajkumar, V.; Gunasekaran, C.; Dharmaraj, J.; Chinnaraj, P.; Paul, C.A.; Kanithachristy, I. Structural characterization of chitosan nanoparticle loaded with Piper nigrum essential oil for biological efficacy against the stored grain pest control. Pestic. Biochem. Physiol. 2020, 166, 104566. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Xu, P.-H.; Shen, B.-D.; Min, H.-Y.; Li, X.-R.; Han, J.; Yuan, H.-L. Nanogel for dermal application of the triterpenoids isolated from Ganoderma lucidum (GLT) for frostbite treatment. Drug Deliv. 2016, 23, 610–618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huma, S.; Khan, H.M.S.; Ijaz, S.; Sarfraz, M.; Zaka, H.S.; Ahmad, A. Development of niacinamide/ferulic acid-loaded multiple emulsion and its in vitro/in vivo investigation as a cosmeceutical product. BioMed Res. Int. 2022, 2022, 1725053. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.U.; Muhammad, I.; Abbas, S.Q.; Hassan, M.; Majid, M.; Jin, H.-Z.; Bungau, S. Stress driven discovery of natural products from actinobacteria with anti-oxidant and cytotoxic activities including docking and admet properties. Int. J. Mol. Sci. 2021, 22, 11432. [Google Scholar] [CrossRef]
- Ghalloo, B.A.; Khan, K.-U.-R.; Ahmad, S.; Aati, H.Y.; Al-Qahtani, J.H.; Ali, B.; Mukhtar, I.; Hussain, M.; Shahzad, M.N.; Ahmed, I. Phytochemical profiling, in vitro biological activities, and in silico molecular docking studies of Dracaena reflexa. Molecules 2022, 27, 913. [Google Scholar] [CrossRef]
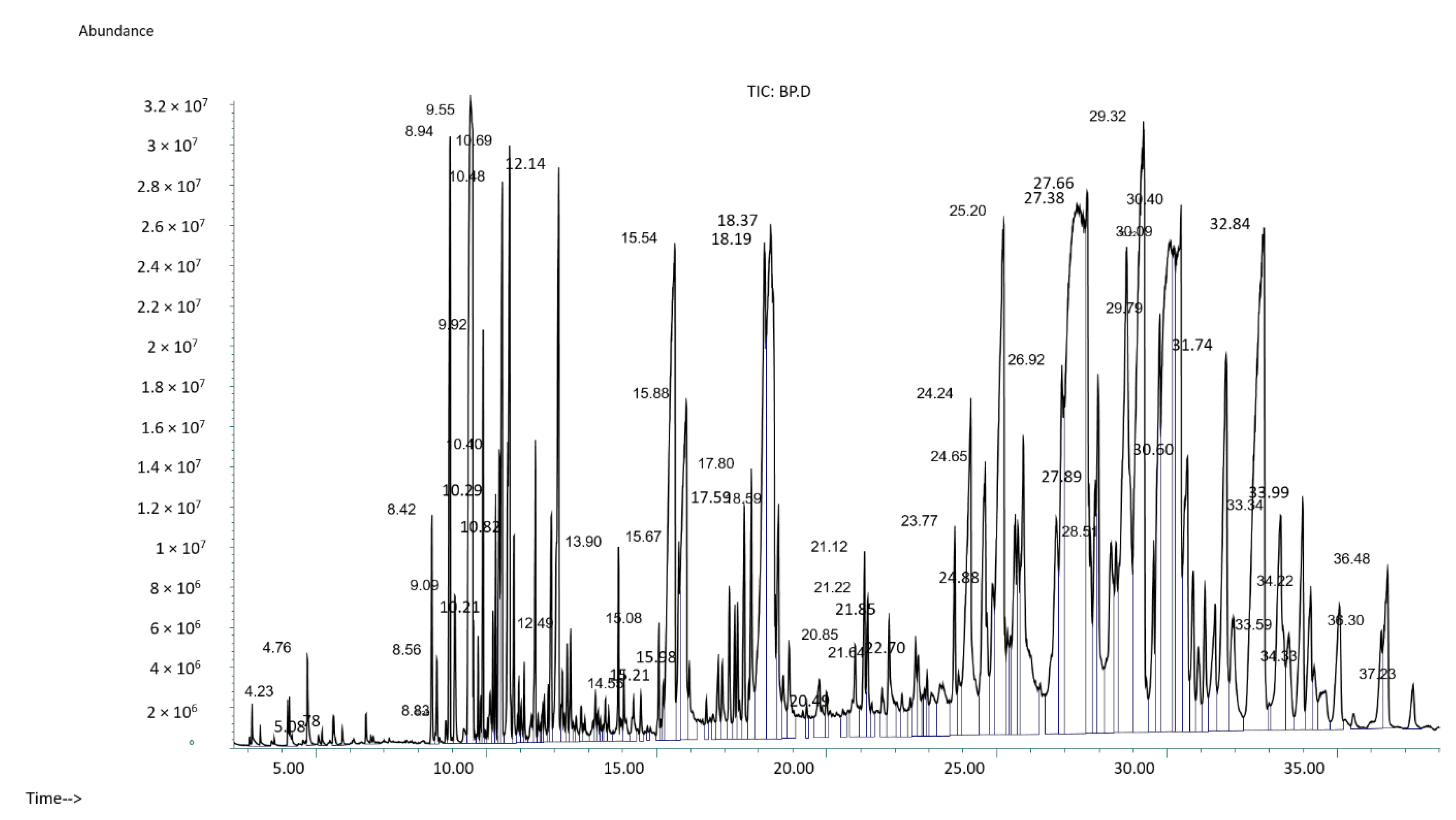

| Sr. | Rt (min) | Peak Area (%) | Name of Compound | Molecular Formula | MW | Nature of Compound | SI | Biological Activity |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.76 | 0.11 | D-limonene | C10H16 | 136.23 | Natural cyclic monoterpene | 97 | * Anticancer, Chemopreventive |
| 2 | 5.54 | 0.05 | Terpineol | C10H18O | 154.25 | Terpineol | 94 | * Insectifuge, Pesticide |
| 3 | 6.63 | 0.02 | 3-cyclohexene-1 methanol | C12H20O2 | 196.29 | Menthane monoterpenoids | 92 | * ACE-Inhibitor, Aldose-Reductase, Antibacterial, Antiinflammatory |
| 4 | 9.55 | 2.87 | Caryophyllene | C15H24 | 204.35 | Bicyclic sesquiterpenoid | 99 | * Antitumor, Anti-inflammatory, Antibacterial, Antidermatitic, Antiacne |
| 5 | 10.69 | 1.22 | Delta-cadinene | C15H24 | 204.35 | Bicyclic sesquiterpenoid | 96 | * Antibacterial, Anticariogenic, |
| 6 | 10.97 | 0.08 | (1S,2S,4R)-(−)-alpha,alpha-dimethyl-1-vinyl-o-menth-8-ene-4-methanol)/Elemol | C15H26O | 222.37 | Sesquiterpenoids, Tertiary alcohol | 91 | * Anti acetylcholinesterase, Antiulcer |
| 7 | 12.14 | 1.22 | Copaene | C15H24 | 204.35 | Sesquiterpene | 96 | * Carminative |
| 8 | 13.22 | 0.07 | Myristic acid | C14H28O2 | 228.37 | Long-chain fatty acids | 99 | * Antioxidant, Cosmetic, Cancer-Preventive, Lubricant |
| 9 | 13.90 | 0.29 | Isopropyl myristate | C17H34O2 | 270.50 | Fatty acid ester | 96 | Emollient, Thickening agent, Lubricant [9] |
| 10 | 15.08 | 0.14 | Hexadecanoic acid methyl ester | C17H34O2 | 270.45 | Fatty acid methyl esters | 99 | Anti-inflammatory [10], Flavor [11], Antioxidant [11], Antibacterial [12] |
| 11 | 15.88 | 1.77 | N-hexadecanoic acid | C16H32O2 | 256.42 | Long-chain fatty acids | 96 | * Antialopecic, Antioxidant, Flavor, |
| 12 | 17.39 | 0.26 | 8,11-octadecadienoic acid, methyl ester | C19H34O2 | 294.50 | Ester | 99 | Not activity reported |
| 13 | 17.59 | 0.46 | Phytol | C20H40O | 296.50 | Acyclic diterpenoids | 93 | It produces anxiolytic and sedative effects [13], Antioxidant [14] |
| 14 | 18.74 | 0.20 | 2-cis,6-trans-farnesol | C15H26O | 222.36 | Sesquiterpenoids | 93 | Not activity reported |
| 15 | 26.76 | 1.37 | Piperine | C17H19NO3 | 285.34 | Alkaloids | 99 | * Analgesic, Antibacterial, Antiinflammatory |
| 16 | 31.12 | 0.42 | γ-tocopherol | C28H48O2 | 416.70 | Methylated phenol | 94 | * Antioxidant, Anticancer, Antiinflammatory |
| 17 | 34.33 | 0.24 | Campesterol | C28H48O | 400.70 | Phytosterol | 94 | * Antioxidant, Hypocholesterolemic |
| Features | Base | Test Formulation | ||
|---|---|---|---|---|
| Fresh (0 h) | After 3 Months | Fresh (0 h) | After 3 Months | |
| Color | White | White | Off white | Off white |
| Liquification | No | No | No | No |
| Microbial growth | No | No | No | No |
| Phase separation | No | No | No | No |
| Centrifugation | Stable | Stable | Stable | Stable |
| Stability Characteristics | Zeta Potential (mV) |
|---|---|
| Maximum agglomeration and precipitation | 0 to +3 |
| Range of strong agglomeration and precipitation | +5 to −5 |
| Threshold of agglomeration | −10 to −15 |
| Threshold of delicate depression | −16 to −30 |
| Moderate stability | −31 to −40 |
| Fairly good stability | −41 to −60 |
| Very good stability | −61 to −80 |
| Extremely good stability | −81 to −100 |
| Compound Name | Binding Energy (kcal/mol) | Interacting Ligands at Binding Site of Enzyme | |
|---|---|---|---|
| Bonding Type | Binding Amino Acid | ||
| Kojic acid | −5.4 | Hydrogen bonding Pi-Pi stacked Pi-Sigma | His60 His208 Val218 |
| γ-tocopherol | −6.6 | Hydrogen bonding Alkyl | Arg209, Gly213 Lys150, Val217 |
| Campesterol | −6.5 | Alkyl | Ala155 |
| Phytol | −4.7 | Alkyl | Lys150, Val217 |
| Myristic acid | −4.5 | Hydrogen bonding Alkyl | Arg280, Glu289, Arg165, Val168, Ley169, Ile237, Val240, Ile243, Ile288 |
| Components | Control (w/w) | Test (with Plant Extract) (w/w) | Role of Ingredients |
|---|---|---|---|
| Cetyl alcohol | 1.50%, | 1.50%, | Emulsifier |
| Liquid paraffin | 9.00% | 9.00% | Dispersing agent |
| Span 80 | 1.20% | 1.20% | Surfactant |
| Propyl paraben | 0.06% | 0.06% | Preservative |
| Tween 80 | 1.92% | 1.92% | Co-surfactant |
| Propylene glycol | 10.00% | 10.00% | Permeation enhancer |
| Methyl paraben | 0.11% | 0.11% | Preservative (prevent germ growth) |
| Double distilled water | 76.21% | 76.21% | Solvent |
| Piper nigrum extract | 0.00% | 4.00% | Active ingredient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousuf, M.; Khan, H.M.S.; Rasool, F.; Khan, K.u.R.; Usman, F.; Ghalloo, B.A.; Umair, M.; Babalghith, A.O.; Kamran, M.; Aadil, R.M.; et al. Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging. Molecules 2022, 27, 5990. https://doi.org/10.3390/molecules27185990
Yousuf M, Khan HMS, Rasool F, Khan KuR, Usman F, Ghalloo BA, Umair M, Babalghith AO, Kamran M, Aadil RM, et al. Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging. Molecules. 2022; 27(18):5990. https://doi.org/10.3390/molecules27185990
Chicago/Turabian StyleYousuf, Muhammad, Haji Muhammad Shoaib Khan, Fatima Rasool, Kashif ur Rehman Khan, Faisal Usman, Bilal Ahmad Ghalloo, Muhammad Umair, Ahmad O. Babalghith, Muhammad Kamran, Rana Muhammad Aadil, and et al. 2022. "Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging" Molecules 27, no. 18: 5990. https://doi.org/10.3390/molecules27185990
APA StyleYousuf, M., Khan, H. M. S., Rasool, F., Khan, K. u. R., Usman, F., Ghalloo, B. A., Umair, M., Babalghith, A. O., Kamran, M., Aadil, R. M., Al Jaouni, S. K., Selim, S., Korma, S. A., & Conte-Junior, C. A. (2022). Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper nigrum Seeds Extract Loaded Emulgel for Anti-Aging. Molecules, 27(18), 5990. https://doi.org/10.3390/molecules27185990












