Design, Synthesis, and Evaluation of a Set of Carboxylic Acid and Phosphate Prodrugs Derived from HBV Capsid Protein Allosteric Modulator NVR 3-778
Abstract
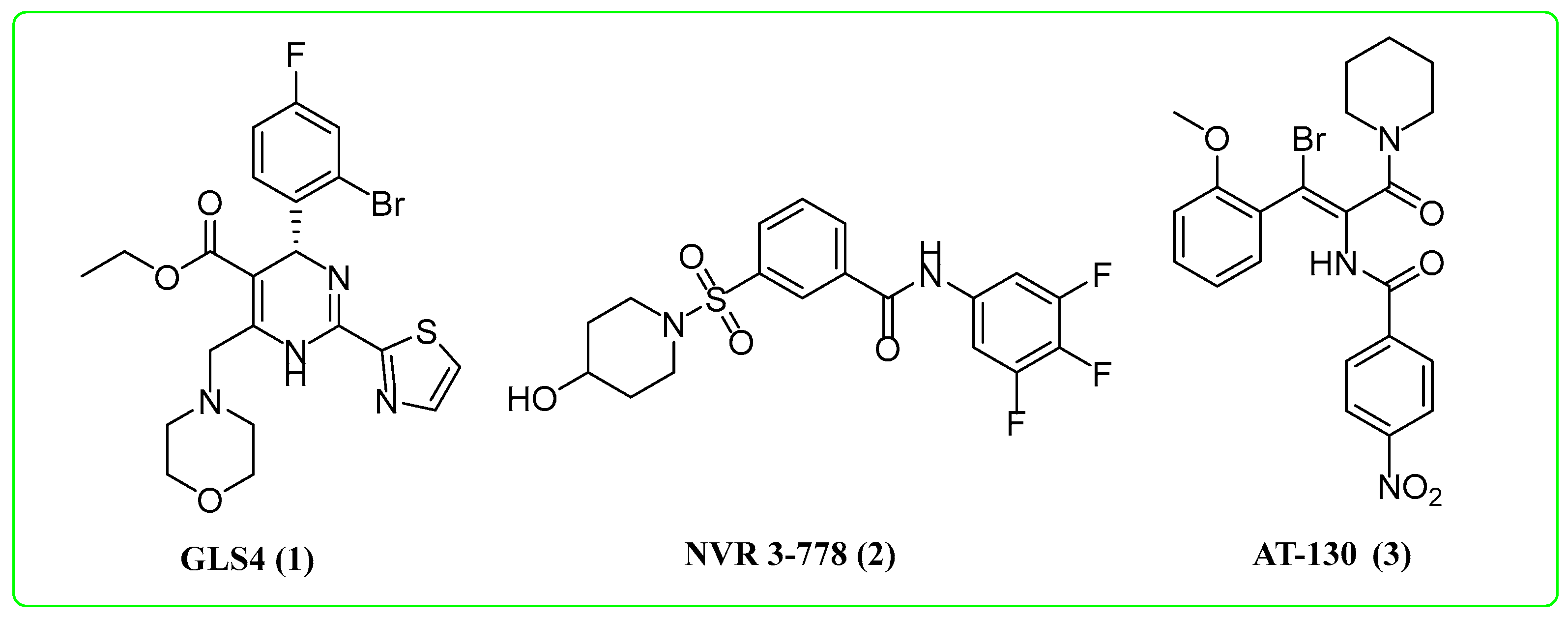
:1. Introduction
2. Results and Discussion
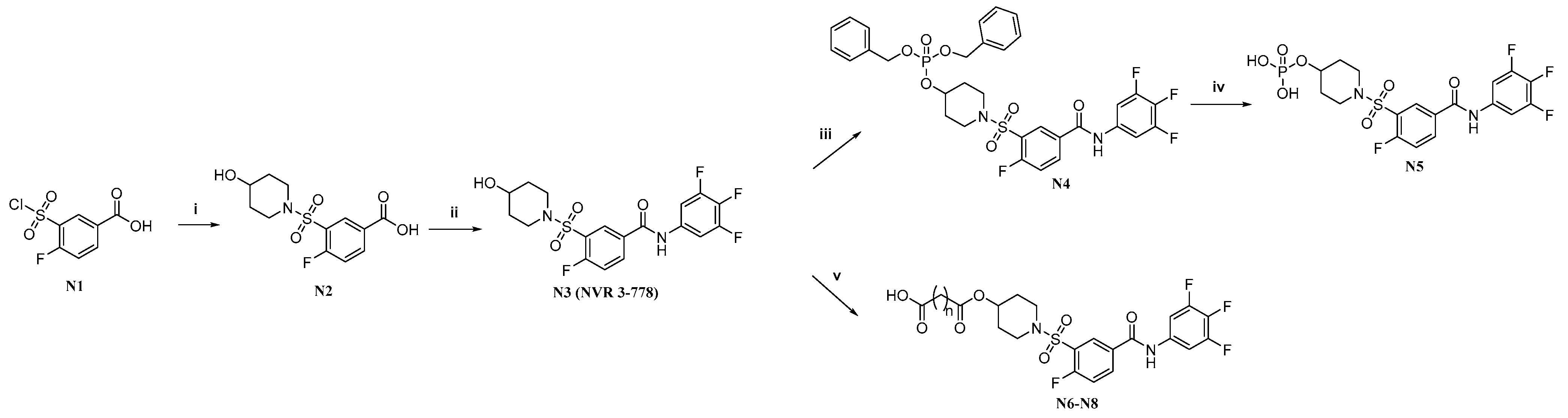
2.1. Synthesis of the Target Molecules
2.2. Biological Activity
2.3. Water Solubility
2.4. Plasma and Whole Blood Stability
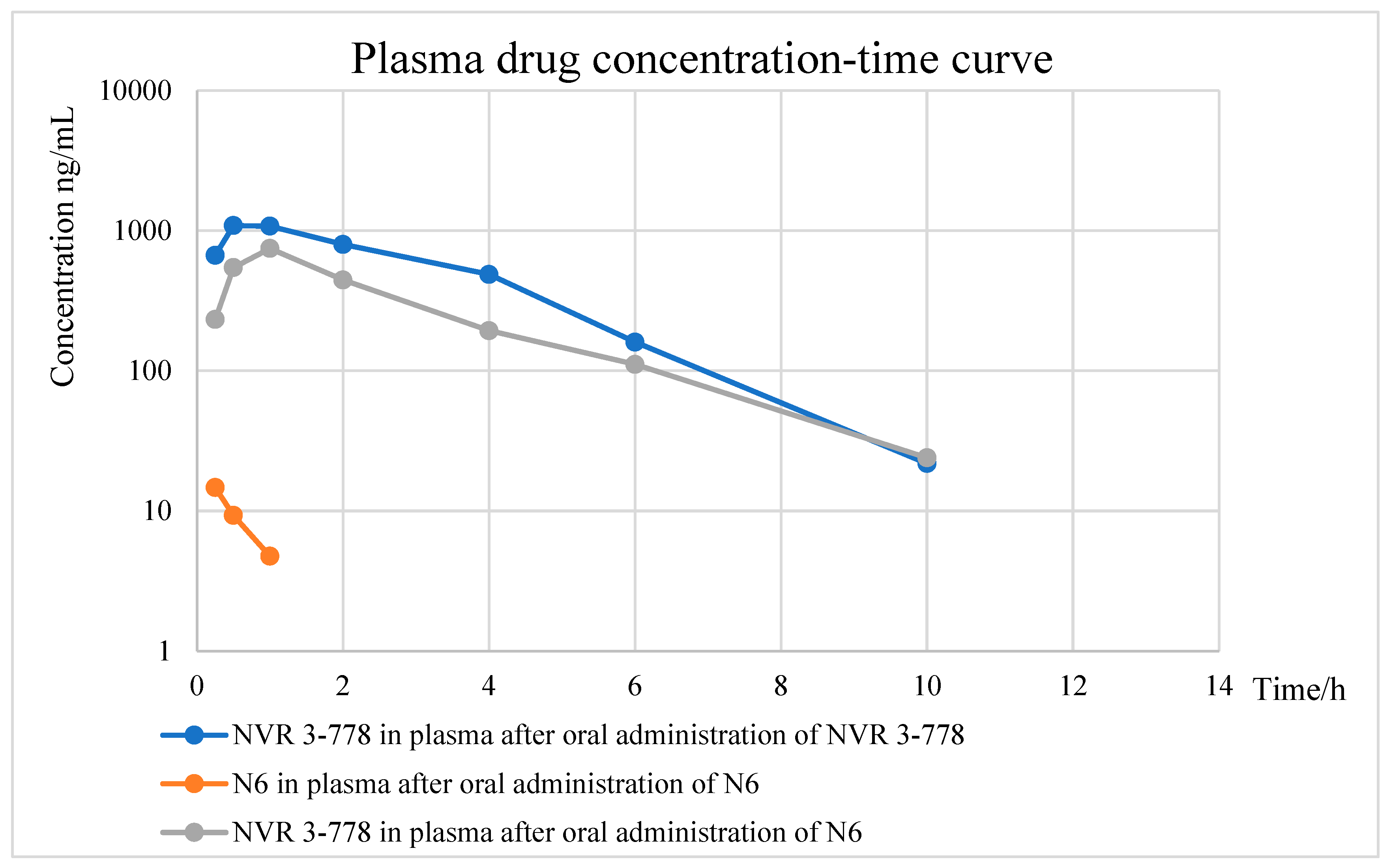
2.5. Pharmacokinetic Experiments of N6
3. Conclusions
4. Experimental Section
4.1. Chemistry
4.1.1. 4-Fluoro-3-((4-hydroxypiperidin-1-yl)sulfonyl)benzoic Acid (N2)
4.1.2. 4-Fluoro-3-((4-hydroxypiperidin-1-yl)sulfonyl)-N-(3,4,5-trifluorophenyl)benzamide (N3)
4.1.3. Dibenzyl (1-((2-Fluoro-5-((3,4,5-trifluorophenyl)carbamoyl)phenyl)sulfonyl)piperidin-4-yl) Phosphate (N4)
4.1.4. 1-((2-Fluoro-5-((3,4,5-trifluorophenyl)carbamoyl)phenyl)sulfonyl)piperidin-4-yl Dihydrogen Phosphate (N5)
4.1.5. General Procedure for the Synthesis of Target Compounds N6–N8
4.2. In Vitro Anti-HBV Assay
4.2.1. Assessment of Inhibitory Activity on HBV Replication in Hep38.7-Tet Cells
4.2.2. Cytotoxicity Assay
4.3. Water Solubility Test of N6
4.4. Plasma and Whole Blood Stability Experiments of N6
4.5. Pharmacokinetic Experiment of N6
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Férir, G.; Kaptein, S.; Neyts, J.; De Clercq, E. Antiviral treatment of chronic hepatitis B virus infections: The past, the present and the future. Rev. Med. Virol. 2008, 18, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, S.; Ren, Y.; Cherukupalli, S.; Li, Q.; Woodson, M.E.; Bradley, D.P.; Tavis, J.E.; Liu, X.; Zhan, P. Design, synthesis and evaluation of heteroaryldihydropyrimidine analogues bearing spiro ring as hepatitis B virus capsid protein inhibitors. Eur. J. Med. Chem. 2021, 225, 113780. [Google Scholar] [CrossRef] [PubMed]
- European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J. Hepatol. 2012, 57, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Schall, R.A.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Michailidis, E.; Kirby, K.A.; Hachiya, A.; Yoo, W.; Hong, S.P.; Kim, S.; Folk, W.R.; Sarafianos, S.G. Antiviral therapies: Focus on hepatitis B reverse transcriptase. Int. J. Biochem. Cell Biol. 2012, 44, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Protzer, U.; Siddiqui, A. Revisiting Hepatitis B Virus: Challenges of Curative Therapies. J. Virol. 2019, 93, e01032-19. [Google Scholar] [CrossRef] [PubMed]
- Nassal, M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J. Virol. 1992, 66, 4107–4116. [Google Scholar] [CrossRef]
- Basagoudanavar, S.H.; Perlman, D.H.; Hu, J. Regulation of hepadnavirus reverse transcription by dynamic nucleocapsid phosphorylation. J. Virol. 2007, 81, 1641–1649. [Google Scholar] [CrossRef]
- Durantel, D.; Zoulim, F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J. Hepatol. 2016, 64 (Suppl. S1), S117–S131. [Google Scholar] [CrossRef]
- Katen, S.P.; Chirapu, S.R.; Finn, M.G.; Zlotnick, A. Trapping of hepatitis B virus capsid assembly intermediates by phenylpropenamide assembly accelerators. ACS Chem. Biol. 2010, 5, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Hu, T.; Zhou, X.; Wildum, S.; Garcia-Alcalde, F.; Xu, Z.; Wu, D.; Mao, Y.; Tian, X.; Zhou, Y.; et al. Heteroaryldihydropyrimidine (HAP) and Sulfamoylbenzamide (SBA) Inhibit Hepatitis B Virus Replication by Different Molecular Mechanisms. Sci. Rep. 2017, 7, 42374. [Google Scholar] [CrossRef]
- Nair, S.; Li, L.; Francis, S.; Turner, W.W.; VanNieuwenhze, M.; Zlotnick, A. Use of a Fluorescent Analogue of a HBV Core Protein-Directed Drug To Interrogate an Antiviral Mechanism. J. Am. Chem. Soc. 2018, 140, 15261–15269. [Google Scholar] [CrossRef]
- Schlicksup, C.J.; Wang, J.C.Y.; Francis, S.; Venkatakrishnan, B.; Turner, W.W.; VanNieuwenhze, M.; Zlotnick, A. Hepatitis B virus core protein allosteric modulators can distort and disrupt intact capsids. Elife 2018, 7, 31473. [Google Scholar] [CrossRef]
- Lam, A.M.; Espiritu, C.; Vogel, R.; Ren, S.; Lau, V.; Kelly, M.; Kuduk, S.D.; Hartman, G.D.; Flores, O.A.; Klumpp, K. Preclinical Characterization of NVR 3-778, a First-in-Class Capsid Assembly Modulator against Hepatitis B Virus. Antimicrob. Agents Chemother. 2019, 63, e01734-18. [Google Scholar] [CrossRef]
- Klumpp, K.; Shimada, T.; Allweiss, L.; Volz, T.; Lütgehetmann, M.; Hartman, G.; Flores, O.A.; Lam, A.M.; Dandri, M. Efficacy of NVR 3-778, Alone and In Combination With Pegylated Interferon, vs Entecavir In uPA/SCID Mice With Humanized Livers and HBV Infection. Gastroenterology 2018, 154, 652–662.e8. [Google Scholar] [CrossRef]
- Taverniti, V.; Ligat, G.; Debing, Y.; Kum, D.B.; Baumert, T.F.; Verrier, E.R. Capsid Assembly Modulators as Antiviral Agents against HBV: Molecular Mechanisms and Clinical Perspectives. J. Clin. Med. 2022, 11, 1349. [Google Scholar] [CrossRef]
- Testa, B. Prodrugs: Bridging pharmacodynamic/pharmacokinetic gaps. Curr. Opin. Chem. Biol. 2009, 13, 338–344. [Google Scholar] [CrossRef]
- Aungst, B.J. Intestinal permeation enhancers. J. Pharm. Sci. 2000, 89, 429–442. [Google Scholar] [CrossRef]
- Testa, B. Prodrug research: Futile or fertile? Biochem. Pharmacol. 2004, 68, 2097–2106. [Google Scholar] [CrossRef]
- Derendorf, H.; Möllmann, H.; Rohdewald, P.; Rehder, J.; Schmidt, E.W. Kinetics of methylprednisolone and its hemisuccinate ester. Clin. Pharmacol. Ther. 1985, 37, 502–507. [Google Scholar] [CrossRef]
- Melby, J.C.; St Cyr, M. Comparative studies on absorption and metabolic disposal of water-soluble corticosteroid esters. Metabolism 1961, 10, 75–82. [Google Scholar] [PubMed]
- Clas, S.D.; Sanchez, R.I.; Nofsinger, R. Chemistry-enabled drug delivery (prodrugs): Recent progress and challenges. Drug Discov. Today 2014, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Na, H.G.; Imran, A.; Kim, K.; Han, H.S.; Lee, Y.J.; Kim, M.; Yun, C.; Jung, Y.; Lee, J.; Han, S.B. Discovery of a New Sulfonamide Hepatitis B Capsid Assembly Modulator. ACS Med. Chem. Lett. 2020, 11, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tropp, J.; Qiao, B.; Pink, M.; Azoulay, J.D.; Flood, A.H. Tunable Adhesion from Stoichiometry-Controlled and Sequence-Defined Supramolecular Polymers Emerges Hierarchically from Cyanostar-Stabilized Anion-Anion Linkages. J. Am. Chem. Soc. 2020, 142, 2579–2591. [Google Scholar] [CrossRef]
- Iwamoto, M.; Cai, D.; Sugiyama, M.; Suzuki, R.; Aizaki, H.; Ryo, A.; Ohtani, N.; Tanaka, Y.; Mizokami, M.; Wakita, T.; et al. Functional association of cellular microtubules with viral capsid assembly supports efficient hepatitis B virus replication. Sci. Rep. 2017, 7, 10620. [Google Scholar] [CrossRef]
- Ogura, N.; Watashi, K.; Noguchi, T.; Wakita, T. Formation of covalently closed circular DNA in Hep38.7-Tet cells, a tetracycline inducible hepatitis B virus expression cell line. Biochem. Biophys. Res. Commun. 2014, 452, 315–321. [Google Scholar] [CrossRef]
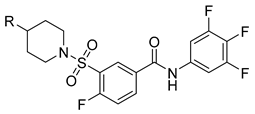
| Compds | R | EC50 (µM) a | CC50 (µM) b | SI c |
|---|---|---|---|---|
| N5 |  | 0.42 ± 0.069 | 13.66 ± 1.45 | 33 |
| N6 | 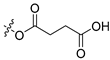 | 0.35 ± 0.048 | 12.62 ± 1.05 | 36 |
| N7 |  | 0.28 ± 0.024 | 12.98 ± 1.74 | 46 |
| N8 | 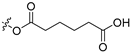 | 0.35 ± 0.032 | >256 | >731 |
| NVR 3-778 | -OH | 0.38 ± 0.047 | 13.65 ± 0.21 | 36 |
| Compds | pH = 2.0 (µg/mL) | pH = 7.0 (µg/mL) | pH = 7.4 (µg/mL) |
|---|---|---|---|
| NVR 3-778 | 3.82 | 24.02 | 4.84 |
| N6 | 695 | 7500 | 4416 |
| Compds | Plasma (4 °C) | Whole Blood (37 °C) | ||||
|---|---|---|---|---|---|---|
| 0 h | 2 h | Residual | 0 h | 2 h | Residual | |
| N6 | 0.88 ± 0.052 | 0.89 ± 0.016 | 101% | 0.65 ± 0.048 | 0.70 ± 0.026 | 108% |
| NVR 3-778 | 0.12 ± 0.0078 | 0.10 ± 0.0066 | 85% | 0.18 ± 0.016 | 0.16 ± 0.0026 | 91% |
| PK Parameters | N6 (18.8 nmol/kg) | NVR 3-778 (23.1 nmol/kg) | |
|---|---|---|---|
| N6 | NVR 3-778 | ||
| T1/2 (h) a | NDb | 1.92 ± 0.227 | 1.40 ± 0.250 |
| Tmax (h) c | 0.25 | 1.00 ± 0.00 | 0.833 ± 0.289 |
| Tlast (h) d | 1.00 | 10.0 | 10.0 |
| Cmax (ng/mL) e | 14.7 ± 1.31 | 747 ± 104 | 1161 ± 261 |
| AUC0-last (ng.h/mL) f | 8.13 ± 1.50 | 2153 ± 877 | 3893 ± 948 |
| AUC0-inf (ng.h/mL) f | ND b | 2222 ± 935 | 3937 ± 948 |
| MRT0-last (h) g | 0.487 | 2.67 ± 0.489 | 2.67 ± 0.106 |
| MRT0-inf (h) g | ND b | 2.95 ± 0.610 | 2.78 ± 0.144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Jiang, X.; Kobayashi, C.; Ren, Y.; Hu, L.; Gao, Z.; Kang, D.; Jia, R.; Zhang, X.; Zhao, S.; et al. Design, Synthesis, and Evaluation of a Set of Carboxylic Acid and Phosphate Prodrugs Derived from HBV Capsid Protein Allosteric Modulator NVR 3-778. Molecules 2022, 27, 5987. https://doi.org/10.3390/molecules27185987
Ji X, Jiang X, Kobayashi C, Ren Y, Hu L, Gao Z, Kang D, Jia R, Zhang X, Zhao S, et al. Design, Synthesis, and Evaluation of a Set of Carboxylic Acid and Phosphate Prodrugs Derived from HBV Capsid Protein Allosteric Modulator NVR 3-778. Molecules. 2022; 27(18):5987. https://doi.org/10.3390/molecules27185987
Chicago/Turabian StyleJi, Xiangkai, Xiangyi Jiang, Chisa Kobayashi, Yujie Ren, Lide Hu, Zhen Gao, Dongwei Kang, Ruifang Jia, Xujie Zhang, Shujie Zhao, and et al. 2022. "Design, Synthesis, and Evaluation of a Set of Carboxylic Acid and Phosphate Prodrugs Derived from HBV Capsid Protein Allosteric Modulator NVR 3-778" Molecules 27, no. 18: 5987. https://doi.org/10.3390/molecules27185987
APA StyleJi, X., Jiang, X., Kobayashi, C., Ren, Y., Hu, L., Gao, Z., Kang, D., Jia, R., Zhang, X., Zhao, S., Watashi, K., Liu, X., & Zhan, P. (2022). Design, Synthesis, and Evaluation of a Set of Carboxylic Acid and Phosphate Prodrugs Derived from HBV Capsid Protein Allosteric Modulator NVR 3-778. Molecules, 27(18), 5987. https://doi.org/10.3390/molecules27185987









