Separation of Mandelic Acid by a Reactive Extraction Method Using Tertiary Amine in Different Organic Diluents
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cma | Molar concentration of acid in the aqueous phase (mol.L−1) |
| Cma0 | Initial molar concentration of acid in the aqueous phase (mol.L−1) |
| Cma,org | Molar concentration of acid in the organic phase (mol.L−1) |
| CTOA,org | Molar concentration of amine in the organic phase (mol.L−1) |
| D | Distribution coefficient |
| DMP | Dimethyl phthalate |
| E | Extraction efficiency |
| MA | Mandelic acid |
| MIBK | Methyl isobutyl ketone |
| TBA | Tri-n-butylamine |
| TBP | Tri-n-butyl phosphate |
| TOA | Tri-n-octylamine |
| TOPO | Tri-n-octyl phosphine oxide |
| TPA | Tri-n-propylamine |
| Z | Loading factor |
References
- Brittain, H.G. Mandelic acid. In Analytical Profiles of Drug Substances and Excipients; Academic Press: Cambridge, UK, 2002; Volume 29, pp. 179–211. [Google Scholar]
- Sun, Z.; Ning, Y.; Liu, L.; Liu, Y.; Sun, B.; Jiang, W.; Yang, C.; Yang, S. Metabolic engineering of the L-phenylalanine pathway in Escherichia coli for the production of S- or R-mandelic Acid. Microb. Cell Fact. 2011, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.P.; Cheng, H.M.; Cui, S.M.; Wang, X.R.; Song, L.Y.; Zhou, W.; Li, S.J. DL-mandelic acid intercalated Zn-Al layered double hydroxide: A novel antimicrobial layered material. Colloids Surf. B Biointerfaces 2018, 165, 111–117. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Shen, K.; Fu, Y.; Zhang, M.; Zhu, C.; Cheng, Y. Enantioselective fluorescent recognition of mandelic acid by unsymmetrical salalen and salan sensors. Org. Biomol. Chem. 2011, 9, 6011–6021. [Google Scholar] [CrossRef] [PubMed]
- Jartarkar, S.R.; Gangadhar; Manjunatha. Mandelic acid chemical peel in acne vulgaris: A Boon or a Bane? IOSR J. Dent. Med. Sci. Ver. VII 2015, 14, 32–35. [Google Scholar] [CrossRef]
- Taylor, M.B. Summary of mandelic acid for the improvement of skin conditions. Cosmet. Dermatol. 1999, 12, 26–28. [Google Scholar]
- Hussain, S.; Rahim, S.A.; Farooqui, M. Studies of binary complexes of metal ions with mandelic acid by potentiometry. Chem. J. 2012, 2, 206–209. [Google Scholar] [CrossRef][Green Version]
- Green, B.A.; Yu, R.J.; Van Scott, E.J. Clinical and cosmeceutical uses of hydroxyacids. Clin. Dermatol. 2009, 27, 495–501. [Google Scholar] [CrossRef]
- Salam, A.; Dadzie, O.E.; Galadari, H. Chemical peeling in ethnic skin: An Update. Br. J. Dermatol. 2013, 169, 82–90. [Google Scholar] [CrossRef]
- Sharon, M.; Durve, A.; Pandey, A.; Pathak, M. Mandelic Acid: Aha; Partridge Publishing: Bloomington, India, 2018. [Google Scholar]
- Mori, T.; Masakatsu, F.; Nakamichi, K.; Takashashi, E. The Process for Producing D-Mandelic Acid. European Patent 0596466A2, 11 May 1994. [Google Scholar]
- Wang, S.P.; Liao, C.S. Comparison of ion-pair chromatography and capillary zone electrophoresis for the assay of organic acids as markers of abnormal metabolism. J. Chromatogr. A 2004, 1051, 213–219. [Google Scholar] [CrossRef]
- Husson, S.M.; King, C.J. Multiple-acid equilibria in adsorption of carboxylic acids from dilute aqueous solution. Ind. Eng. Chem. Res. 1999, 38, 502–511. [Google Scholar] [CrossRef]
- Boyaval, P.; Corre, C.; Terre, S. Continuous lactic acid fermentation with concentrated product recovery by ultrafiltration and electrodialysis. Biotechnol. Lett. 1987, 9, 207–212. [Google Scholar] [CrossRef]
- Cao, X.; Yun, H.S.; Koo, Y.M. Recovery of L-(+)-lactic acid by anion exchange resin amberlite IRA-400. Biochem. Eng. J. 2002, 11, 189–196. [Google Scholar] [CrossRef]
- Wardell, J.M.; King, C.J. Solvent equilibriums for extraction of carboxylic acids from water. J. Chem. Eng. Data 1978, 23, 144–148. [Google Scholar] [CrossRef]
- Juang, R.S.; Huang, R.H.; Wu, R.T. Separation of citric and lactic acids in aqueous solutions by solvent extraction and liquid membrane processes. J. Membr. Sci. 1997, 136, 89–99. [Google Scholar] [CrossRef]
- Timmer, J.M.K.; Kromkamp, J.; Robbertsen, T. Lactic acid separation from fermentation broths by reverse osmosis and nanofiltration. J. Membr. Sci. 1994, 92, 185–197. [Google Scholar] [CrossRef]
- Pazouki, M.; Panda, T. Recovery of citric acid—A Review. Bioprocess Eng. 1998, 19, 435–439. [Google Scholar] [CrossRef]
- Djas, M.; Henczka, M. Reactive extraction of carboxylic acids using organic solvents and supercritical fluids: A Review. Sep. Purif. Technol. 2018, 201, 106–119. [Google Scholar] [CrossRef]
- Antony, F.M.; Wasewar, K.L. Reactive separation of protocatechuic acid using tri-n-octyl amine and di-(2-ethylhexyl) phosphoric acid in methyl isobutyl ketone. Sep. Purif. Technol. 2018, 207, 99–107. [Google Scholar] [CrossRef]
- Hong, Y.K.; Hong, W.H. Removal of acetic acid from aqueous solutions containing succinic acid and acetic acid by tri-n-octylamine. Sep. Purif. Technol. 2005, 42, 151–157. [Google Scholar] [CrossRef]
- Yunhai, S.; Houyong, S.; Deming, L.; Qinghua, L.; Dexing, C.; Yongchuan, Z. Separation of glycolic acid from glycolonitrile hydrolysate by reactive extraction with tri-n-octylamine. Sep. Purif. Technol. 2006, 49, 20–26. [Google Scholar] [CrossRef]
- Marti, M.E.; Zeidan, H.; Uslu, H. Reactive extraction of pimelic (heptanedioic) acid from dilute aqueous solutions using trioctylamine in decan-1-ol. Fluid Phase Equilib. 2016, 417, 197–202. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Girisuta, B.; Van de Bovenkamp, H.H.; Winkelman, J.G.M.; Leijenhorst, E.J.; Venderbosch, R.H.; Windt, M.; Meier, D.; Heeres, H.J. Recovery of acetic acid from an aqueous pyrolysis oil phase by reactive extraction using tri-n-octylamine. Chem. Eng. J. 2011, 176, 244–252. [Google Scholar] [CrossRef]
- Jun, Y.S.; Lee, E.Z.; Huh, Y.S.; Hong, Y.K.; Hong, W.H.; Lee, S.Y. Kinetic Study for the extraction of succinic acid with toa in fermentation broth; effects of ph, salt and contaminated acid. Biochem. Eng. J. 2007, 36, 8–13. [Google Scholar] [CrossRef]
- Krzyzaniak, A.; Leeman, M.; Vossebeld, F.; Visser, T.J.; Schuur, B.; De Haan, A.B. Novel extractants for the recovery of fermentation derived lactic acid. Sep. Purif. Technol. 2013, 111, 82–89. [Google Scholar] [CrossRef]
- Uslu, H.; Kirbaşlar, Ş.I. Extraction of aqueous of malic acid by trioctylamine extractant in various diluents. Fluid Phase Equilib. 2010, 287, 134–140. [Google Scholar] [CrossRef]
- Keshav, A.; Wasewar, K.L.; Chand, S. Extraction of propionic acid with tri-n-octyl amine in different diluents. Sep. Purif. Technol. 2008, 63, 179–183. [Google Scholar] [CrossRef]
- Hong, Y.K.; Hong, W.H. Extraction of succinic acid with 1-octanol/n-heptane solutions of mixed tertiary amine. Bioprocess Eng. 2000, 23, 535–538. [Google Scholar] [CrossRef]
- Caşcaval, D.; Kloetzer, L.; Galaction, A.I. Influence of organic phase polarity on interfacial mechanism and efficiency of reactive extraction of acetic acid with tri-n-octylamine. J. Chem. Eng. Data 2011, 56, 2521–2526. [Google Scholar] [CrossRef]
- Thakre, N.; Datta, D.; Prajapati, A.K.; Chaudhari, P.K.; Pal, D. Reactive Extraction of citric acid using different extractants: Equilibrium, kinetics and modeling. Chem. Biochem. Eng. Q. 2018, 31, 437–446. [Google Scholar] [CrossRef]
- Datta, D.; Aşçı, Y.S.; Tuyun, A.F. Extraction equilibria of glycolic acid using tertiary amines: Experimental data and theoretical predictions. J. Chem. Eng. Data 2015, 60, 3262–3267. [Google Scholar] [CrossRef]
- Tamada, J.A.; Kertes, A.S.; King, C.J. Extraction of carboxylic acids with amine extractants. 1. Equilibria and law of mass action modeling. Ind. Eng. Chem. Res. 1990, 29, 1319–1326. [Google Scholar] [CrossRef]
- Marti, M.E.; Gurkan, T.; Doraiswamy, L.K. Equilibrium and kinetic studies on reactive extraction of pyruvic acid with trioctylamine in 1-octanol. Ind. Eng. Chem. Res. 2011, 50, 13518–13525. [Google Scholar] [CrossRef]
- Uslu, H.; Inci, I. (Liquid + liquid) equilibria of the (water + propionic acid + aliquat 336 + organic solvents) at T = 298.15 K. J. Chem. Thermodyn. 2007, 39, 804–809. [Google Scholar] [CrossRef]
- Aşçı, Y.S.; Inci, I. Extraction equilibria of acrylic acid from aqueous solutions by amberlite LA-2 in various diluents. J. Chem. Eng. Data 2010, 55, 2385–2389. [Google Scholar] [CrossRef]
- Bayazit, Ş.S.; Uslu, H.; Inci, I. Comparative equilibrium studies for citric acid by amberlite LA-2 or tridodecylamine (TDA). J. Chem. Eng. Data 2009, 54, 1991–1996. [Google Scholar] [CrossRef]
- Uslu, H. Extraction of gibberellic acid from aqueous solution by amberlite LA-2 in different diluents. J. Chem. Eng. Data 2012, 57, 3685–3689. [Google Scholar] [CrossRef]
- Aşcı, Y.S.; Inci, I. Extraction of glycolic acid from aqueous solutions by amberlite LA-2 in different diluent solvents. J. Chem. Eng. Data 2009, 54, 2791–2794. [Google Scholar] [CrossRef]
- Uslu, H.; Kırbaşlar, Ş.İ. Solvent effects on the extraction of malic acid from aqueous solution by secondary amine extractant. Sep. Purif. Technol. 2010, 71, 22–29. [Google Scholar] [CrossRef]
- Caşcaval, D.; Blaga, A.C.; Cămăruţ, M.; Galaction, A.I. Comparative study on reactive extraction of nicotinic acid with amberlite LA-2 and D2EHPA. Sep. Sci. Technol. 2007, 42, 389–401. [Google Scholar] [CrossRef]
- Uslu, H.; Datta, D.; Kumar, S. Reactive extraction of oxoethanoic acid (glyoxylic acid) using amberlite-LA2 in different diluents. J. Chem. Eng. Data 2014, 59, 2623–2629. [Google Scholar] [CrossRef]
- Uslu, H.; Bamufleh, H.S.; Keshav, A.; Pal, D.; Demir, G. Extractive separation of pentanedioic acid by amberlite LA-2 in various solvents. J. Chem. Eng. Data 2016, 61, 2450–2457. [Google Scholar] [CrossRef]
- Uslu, H.; Marti, M.E. Equilibrium data on the reactive extraction of picric acid from dilute aqueous solutions using amberlite LA-2 in ketones. J. Chem. Eng. Data 2017, 62, 2132–2135. [Google Scholar] [CrossRef]
- Kloetzer, L.; Poştaru, M.; Galaction, A.I.; Blaga, A.C.; Caşcaval, D. Comparative study on rosmarinic acid separation by reactive extraction with Amberlite LA-2 and D2EHPA. 1. Interfacial reaction mechanism and influencing factors. Ind. Eng. Chem. Res. 2013, 52, 13785–13794. [Google Scholar] [CrossRef]
- Aşci, Y.S.; İncί, I. Extraction equilibria of succinic acid from aqueous solutions by amberlite LA-2 in various diluents. J. Chem. Eng. Data 2009, 55, 847–851. [Google Scholar] [CrossRef]
- Inci, I.; Asci, Y.S.; Tuyun, A.F. Reactive extraction of L (+) tartaric acid by amberlite LA-2 in different solvents. E-J. Chem. 2011, 8, 509–515. [Google Scholar] [CrossRef]
- Keshav, A.; Wasewar, K.L.; Chand, S. Reactive extraction of propionic acid using tri-n-butyl phosphate in petroleum ether: Equilibrium study. Chem. Biochem. Eng. 2008, 22, 433–437. [Google Scholar]
- Kumar, S.; Mavely, T.R.; Babu, B.V. Reactive extration of carboxylic acids (butyric-, lactic-, tartaric-, itaconic-, succinic-and citric acids) using tri-nbutylphosphate (tbp) dissolved in 1-dodecanol and n-octane (1:1 v/v). In Proceedings of the International Symposium & 63rd Annual Session of IIChE in Association with International Partners (CHEMCON-2010), Annamalainagar, India, 27–29 December 2010. [Google Scholar]
- Labbaci, A.; Kyuchoukov, G.; Albet, J.; Molinier, J. Detailed investigation of lactic acid extraction with tributylphosphate dissolved in dodecane. J. Chem. Eng. Data 2010, 55, 228–233. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Keshav, A.; Agarwal, V.K.; Sonawane, S.S. Reactive extraction of citric acid from aqueous solutions using tri-n-octylamine in MIBK. IUP J. Chem. 2010, 3, 7–19. [Google Scholar]
- Pehlivanoglu, N.; Uslu, H.; Kırbaşlar, S.I. Experimental and modeling studies on the extraction of glutaric acid by trioctylamine. J. Chem. Eng. Data 2009, 54, 3202–3207. [Google Scholar] [CrossRef]
- Juang, R.S.; Huang, R.H. Equilibrium studies on reactive extraction of lactic acid with an amine extractant. Chem. Eng. J. 1997, 65, 47–53. [Google Scholar] [CrossRef]
- Datta, D.; Kumar, S. Equilibrium and kinetic studies of the reactive extraction of nicotinic acid with tri-n-octylamine dissolved in MIBK. Ind. Eng. Chem. Res. 2013, 52, 14680–14686. [Google Scholar] [CrossRef]
- Datta, D.; Kumar, S. Reactive extraction of picolinic acid using tri-n-octylamine dissolved in different diluents: Effect of solvent polarity. J. Chem. Eng. Data 2015, 60, 2709–2716. [Google Scholar] [CrossRef]
- Pal, D.; Tripathi, A.; Shukla, A.; Gupta, K.R.; Keshav, A. Reactive extraction of pyruvic acid using tri-n-octylamine diluted in decanol/kerosene: Equilibrium and effect of temperature. J. Chem. Eng. Data 2015, 60, 860–869. [Google Scholar] [CrossRef]
- Kumar, S.; Wasewar, K.L.; Babu, B.V. Intensification of nicotinic acid separation using organophosphorous solvating extractants by reactive extraction. Chem. Eng. Technol. 2008, 31, 1584–1590. [Google Scholar] [CrossRef]
- Fahim, M.A.; Qader, A.; Hughes, M.A. Extraction equilibria of acetic and propionic acids from dilute aqueous solution by several solvents. Sep. Sci. Technol. 1992, 27, 1809–1821. [Google Scholar] [CrossRef]
- Hong, Y.K.; Hong, W.H. Reactive extraction of lactic acid with mixed tertiary amine extractants. Biotechnol. Tech. 1999, 13, 915–918. [Google Scholar] [CrossRef]
- Uslu, H. Liquid+ liquid equilibria of the (water+tartaric acid + alamine 336 + organic solvents) at 298.15 K. Fluid Phase Equilib. 2007, 253, 12–18. [Google Scholar] [CrossRef]
- Biźek, V.; Horáček, J.; Koušová, M.; Heyberger, A.; Procházka, J. Mathematical model of extraction of citric acid with amine. Chem. Eng. Sci. 1992, 47, 1433–1440. [Google Scholar] [CrossRef]
| Type of Extractant | Type of Carboxylic Acid | Ref. |
|---|---|---|
| Alamine 336 | Acetic acid, lactic acid, succinic acid, malonic acid, fumaric acid, maleic acid | [34] |
| Pyruvic acid | [35] | |
| Aliquat 336 | Propionic acid | [36] |
| Amberlite LA-2 | Acrylic acid | [37] |
| Citric acid | [38] | |
| Gibberellic acid | [39] | |
| Glycolic acid | [40] | |
| Malic acid | [41] | |
| Nicotinic acid | [42] | |
| Oxoethanoic acid | [43] | |
| Pentanedioic acid | [44] | |
| Picric acid | [45] | |
| Rosmarinic acid | [46] | |
| Succinic acid | [47] | |
| Tartaric acid | [48] | |
| Tri-n-butylamine | Acetic acid | [16] |
| Tri-n-butyl phosphate | Propionic acid | [49] |
| Butyric acid, lactic acid, tartaric acid, itaconic acid, succinic acid, citric acid | [50] | |
| Lactic acid | [51] | |
| Tri-n-octylamine | Acetic acid | [22] |
| Citric acid | [52] | |
| Glutaric acid | [53] | |
| Glycolic acid | [33] | |
| Lactic acid | [54] | |
| Malic acid | [28] | |
| Nicotinic acid | [55] | |
| Picolinic acid | [56] | |
| Propionic acid | [29] | |
| Pyruvic acid | [57] | |
| Succinic acid | [26] | |
| Tri-n-octylphosphine oxide | Nicotinic acid | [58] |
| Propionic acid | [59] | |
| Tri-n-propylamine | Acetic acid | [60] |
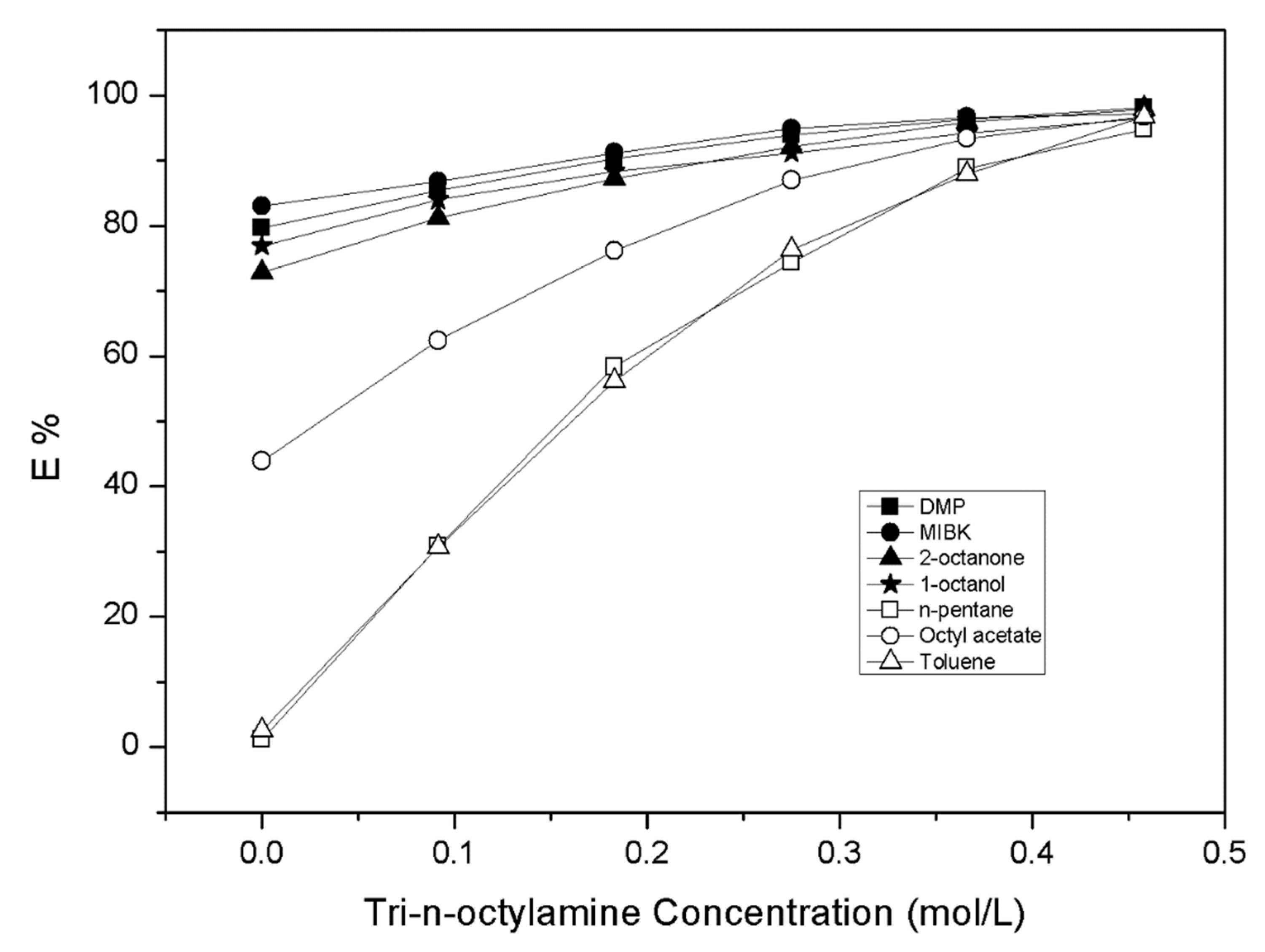
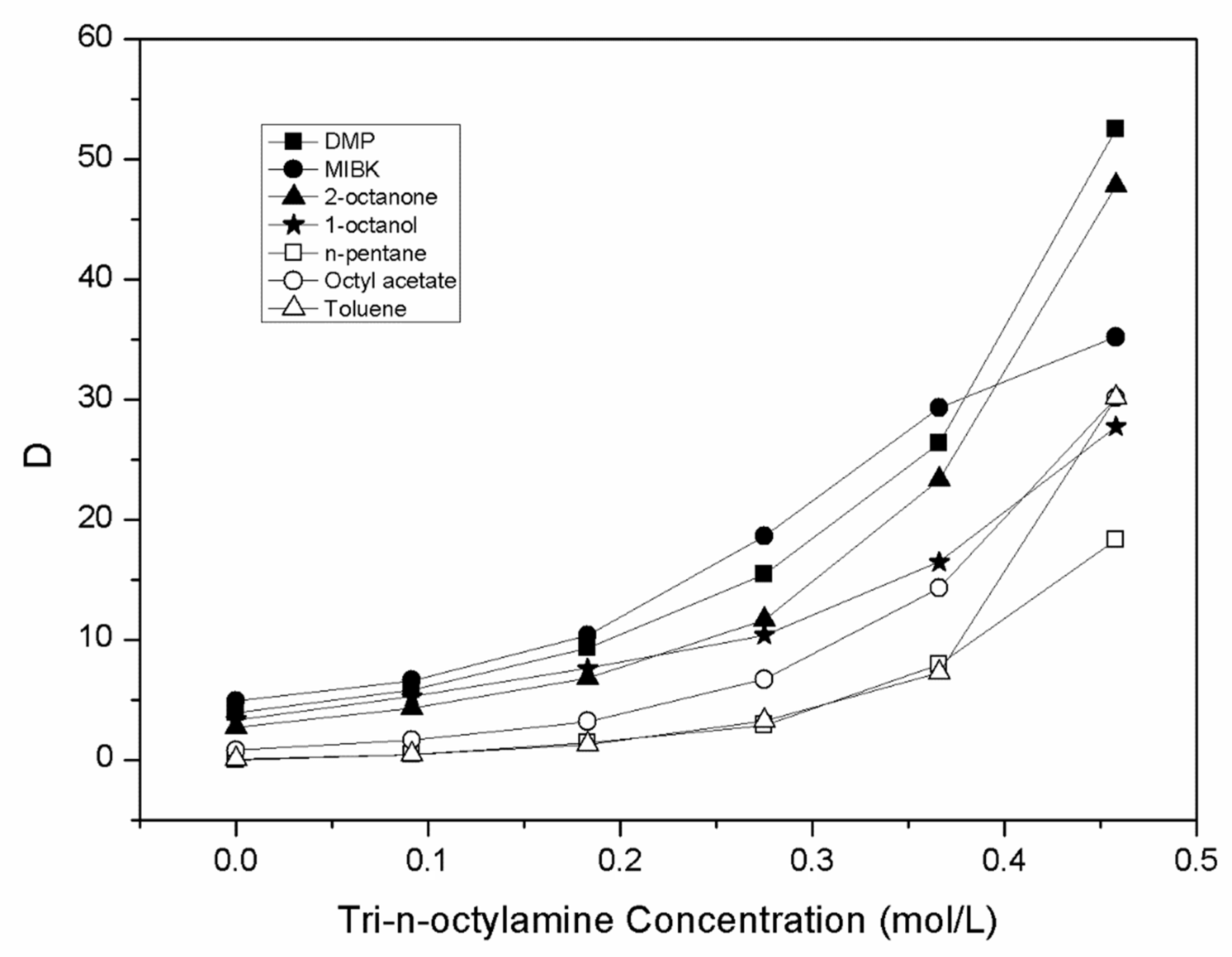
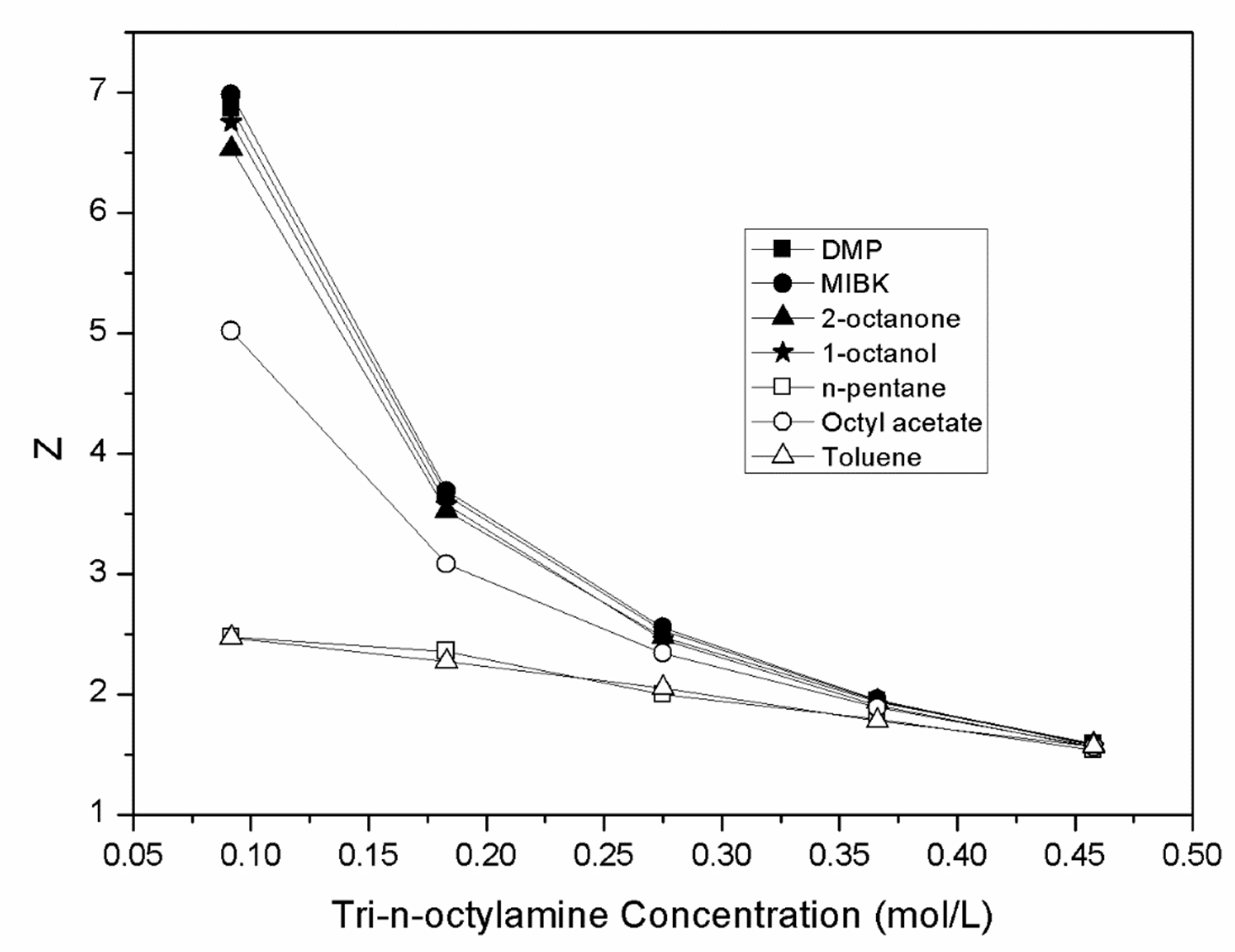
| Diluent | CTOA,org (mol.L−1) | Cma (mol.L−1) | Cma,org (mol.L−1) | D | Z | E (%) |
|---|---|---|---|---|---|---|
| DMP | 0.000 | 0.151 | 0.589 | 3.91 | - | 79.65 |
| 0.092 | 0.108 | 0.632 | 5.84 | 6.87 | 85.37 | |
| 0.183 | 0.072 | 0.668 | 9.31 | 3.65 | 90.30 | |
| 0.275 | 0.045 | 0.695 | 15.44 | 2.53 | 93.92 | |
| 0.366 | 0.027 | 0.713 | 2635 | 1.95 | 96.34 | |
| 0.458 | 0.014 | 0.726 | 52.50 | 1.59 | 98.13 | |
| MIBK | 0.000 | 0.125 | 0.615 | 4.91 | 83.07 | |
| 0.092 | 0.097 | 0.643 | 6.60 | 6.99 | 86.84 | |
| 0.183 | 0.065 | 0.675 | 10.38 | 3.69 | 91.21 | |
| 0.275 | 0.038 | 0.702 | 18.64 | 2.55 | 94.91 | |
| 0.366 | 0.024 | 0.716 | 29.32 | 1.96 | 96.70 | |
| 0.458 | 0.020 | 0.720 | 35.20 | 1.57 | 97.24 | |
| 2-Octanone | 0.000 | 0.201 | 0.539 | 2.68 | 72.81 | |
| 0.092 | 0.139 | 0.601 | 4.33 | 6.53 | 81.23 | |
| 0.183 | 0.095 | 0.645 | 6.82 | 3.53 | 87.21 | |
| 0.275 | 0.058 | 0.682 | 11.68 | 2.48 | 92.11 | |
| 0.366 | 0.030 | 0.710 | 23.36 | 1.94 | 95.90 | |
| 0.458 | 0.015 | 0.725 | 47.84 | 1.58 | 97.95 | |
| 1-Octanol | 0.000 | 0.171 | 0.569 | 3.34 | 76.95 | |
| 0.092 | 0.118 | 0.622 | 5.25 | 6.76 | 84.00 | |
| 0.183 | 0.086 | 0.654 | 7.61 | 3.57 | 88.39 | |
| 0.275 | 0.065 | 0.675 | 10.38 | 2.45 | 91.21 | |
| 0.366 | 0.042 | 0.698 | 16.48 | 1.91 | 94.28 | |
| 0.458 | 0.026 | 0.714 | 27.76 | 1.56 | 96.52 | |
| n-Pentane | 0.000 | 0.732 | 0.008 | 0.01 | 1.09 | |
| 0.092 | 0.512 | 0.228 | 0.44 | 2.48 | 30.78 | |
| 0.183 | 0.308 | 0.432 | 1.40 | 2.36 | 58.34 | |
| 0.275 | 0.190 | 0.550 | 2.89 | 2.00 | 74.32 | |
| 0.366 | 0.083 | 0.657 | 7.97 | 1.80 | 88.85 | |
| 0.458 | 0.038 | 0.702 | 18.30 | 1.53 | 94.82 | |
| Octyl acetate | 0.000 | 0.415 | 0.325 | 0.78 | 43.91 | |
| 0.092 | 0.278 | 0.462 | 1.66 | 5.02 | 62.41 | |
| 0.183 | 0.176 | 0.564 | 3.20 | 3.08 | 76.20 | |
| 0.275 | 0.096 | 0.644 | 6.71 | 2.34 | 87.02 | |
| 0.366 | 0.048 | 0.692 | 14.31 | 1.89 | 93.47 | |
| 0.458 | 0.024 | 0.716 | 30.16 | 1.56 | 96.79 | |
| Toluene | 0.000 | 0.721 | 0.019 | 0.03 | 2.51 | |
| 0.092 | 0.513 | 0.227 | 0.44 | 2.47 | 30.67 | |
| 0.183 | 0.324 | 0.416 | 1.28 | 2.27 | 56.19 | |
| 0.275 | 0.175 | 0.565 | 3.22 | 2.05 | 76.29 | |
| 0.366 | 0.089 | 0.651 | 7.29 | 1.78 | 87.94 | |
| 0.458 | 0.024 | 0.716 | 30.16 | 1.56 | 96.79 |
| Diluent | CTOA,org (mol.L−1) | K11 (mol.L−1) | K12 (L2.mol−2) | K23 (L4.mol−4) |
|---|---|---|---|---|
| DMP | 0.092 | 63.45 | 586.19 | |
| 0.183 | 50.88 | 709.07 | ||
| 0.275 | 56.16 | 1247.94 | ||
| 0.366 | 71.99 | 2660.25 | ||
| 0.458 | 114.63 | 8286.93 | ||
| MIBK | 0.092 | 71.72 | 736.49 | |
| 0.183 | 56.70 | 871.57 | ||
| 0.275 | 67.78 | 1798.76 | ||
| 0.366 | 80.10 | 3281.31 | ||
| 0.458 | 76.87 | 3760.70 | ||
| 2-Octanone | 0.092 | 47.02 | 338.46 | |
| 0.183 | 37.25 | 393.38 | ||
| 0.275 | 42.48 | 727.90 | ||
| 0.366 | 63.83 | 2101.43 | ||
| 0.458 | 104.45 | 6893.06 | ||
| 1-Octanol | 0.092 | 57.04 | 620.05 | |
| 0.183 | 41.61 | 227.38 | ||
| 0.275 | 37.73 | 137.20 | ||
| 0.366 | 45.02 | 123.02 | ||
| 0.458 | 60.60 | 132.32 | ||
| n-Pentane | 0.092 | 4.83 | 9.43 | |
| 0.183 | 7.65 | 24.82 | ||
| 0.275 | 10.52 | 55.38 | ||
| 0.366 | 21.77 | 263.76 | ||
| 0.458 | 39.95 | 1041.92 | ||
| Octyl acetate | 0.092 | 18.05 | 64.89 | |
| 0.183 | 17.49 | 99.30 | ||
| 0.275 | 24.38 | 253.92 | ||
| 0.366 | 39.10 | 808.89 | ||
| 0.458 | 65.85 | 2773.00 | ||
| Toluene | 0.092 | 4.81 | 9.37 | |
| 0.183 | 7.01 | 21.62 | ||
| 0.275 | 11.70 | 66.68 | ||
| 0.366 | 19.92 | 223.09 | ||
| 0.458 | 65.85 | 2773.00 |
| Molecular Formula | C6H5CH(OH)COOH |
|---|---|
| Molecular weight | 152.147 g/mol |
| Appearance | White crystalline solid |
| Density | 1.3 g/cm3 |
| Melting point | 131–135 °C |
| Solubility (in water) | 158.7 g/cm3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiriş, B.; Aşçı, Y.S.; Zahoor, M.; Hassan, S.S.u.; Bungau, S. Separation of Mandelic Acid by a Reactive Extraction Method Using Tertiary Amine in Different Organic Diluents. Molecules 2022, 27, 5986. https://doi.org/10.3390/molecules27185986
Kiriş B, Aşçı YS, Zahoor M, Hassan SSu, Bungau S. Separation of Mandelic Acid by a Reactive Extraction Method Using Tertiary Amine in Different Organic Diluents. Molecules. 2022; 27(18):5986. https://doi.org/10.3390/molecules27185986
Chicago/Turabian StyleKiriş, Barış, Yavuz Selim Aşçı, Muhammad Zahoor, Syed Shams ul Hassan, and Simona Bungau. 2022. "Separation of Mandelic Acid by a Reactive Extraction Method Using Tertiary Amine in Different Organic Diluents" Molecules 27, no. 18: 5986. https://doi.org/10.3390/molecules27185986
APA StyleKiriş, B., Aşçı, Y. S., Zahoor, M., Hassan, S. S. u., & Bungau, S. (2022). Separation of Mandelic Acid by a Reactive Extraction Method Using Tertiary Amine in Different Organic Diluents. Molecules, 27(18), 5986. https://doi.org/10.3390/molecules27185986












