Dimethylaminoethyl Methacrylate/Diethylene Glycol Dimethacrylate Grafted onto Folate-Esterified Bagasse Xylan/Andrographolide Composite Nanoderivative: Synthesis, Molecular Docking and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influences of Reaction Conditions on Grafting Rate (GR) and Grafting Efficiency (GE) of Bagasse Xylan/Andrographolide Folate-g-Dimethylaminoethyl Methacrylate/Diethylene Glycol Dimethacrylate (FA-BX/AD-g-DMAEMA/DEGDMA)
2.1.1. Influence of Reaction Temperature on GR and GE
2.1.2. Influence of Reaction Time on GR and GE
2.1.3. Influence of Initiator Concentration on GR and GE
2.1.4. Influence of the Mass Ratio of FA-BX/AD to Mixed Monomers on GR and GE
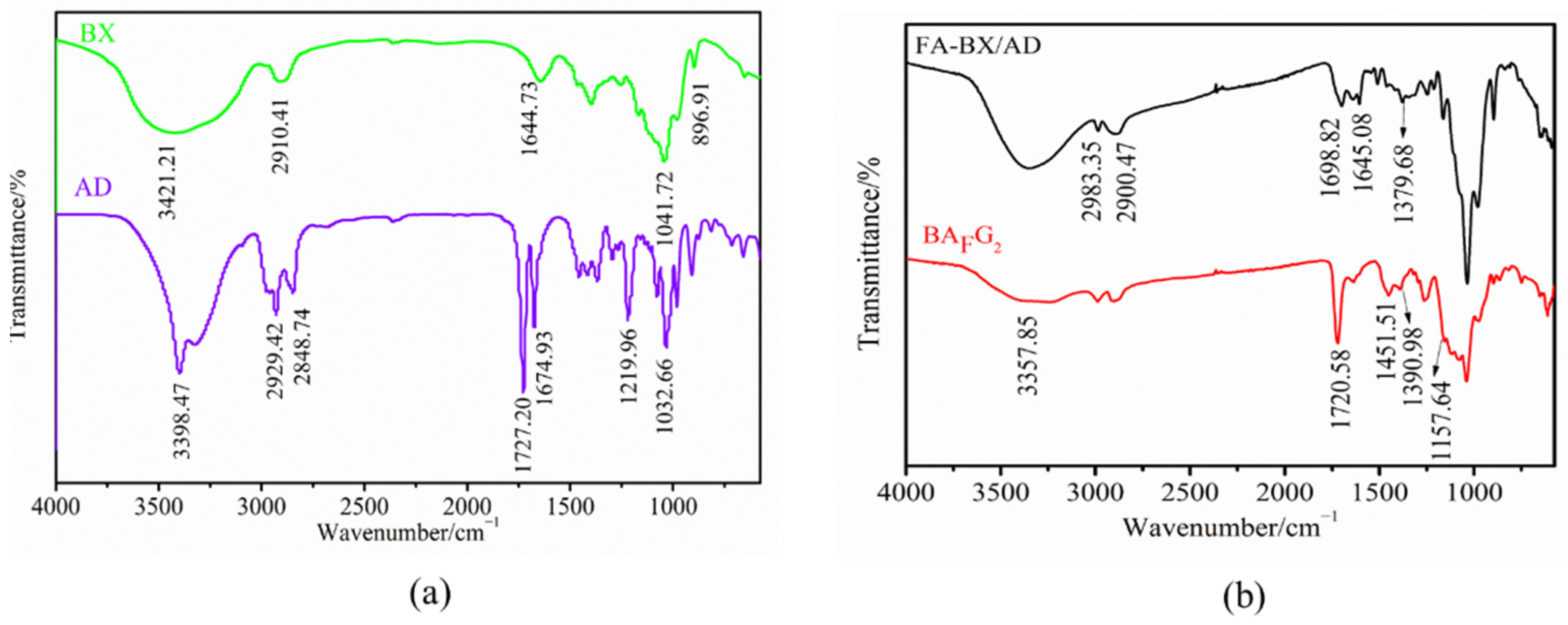
2.2. FTIR Analysis
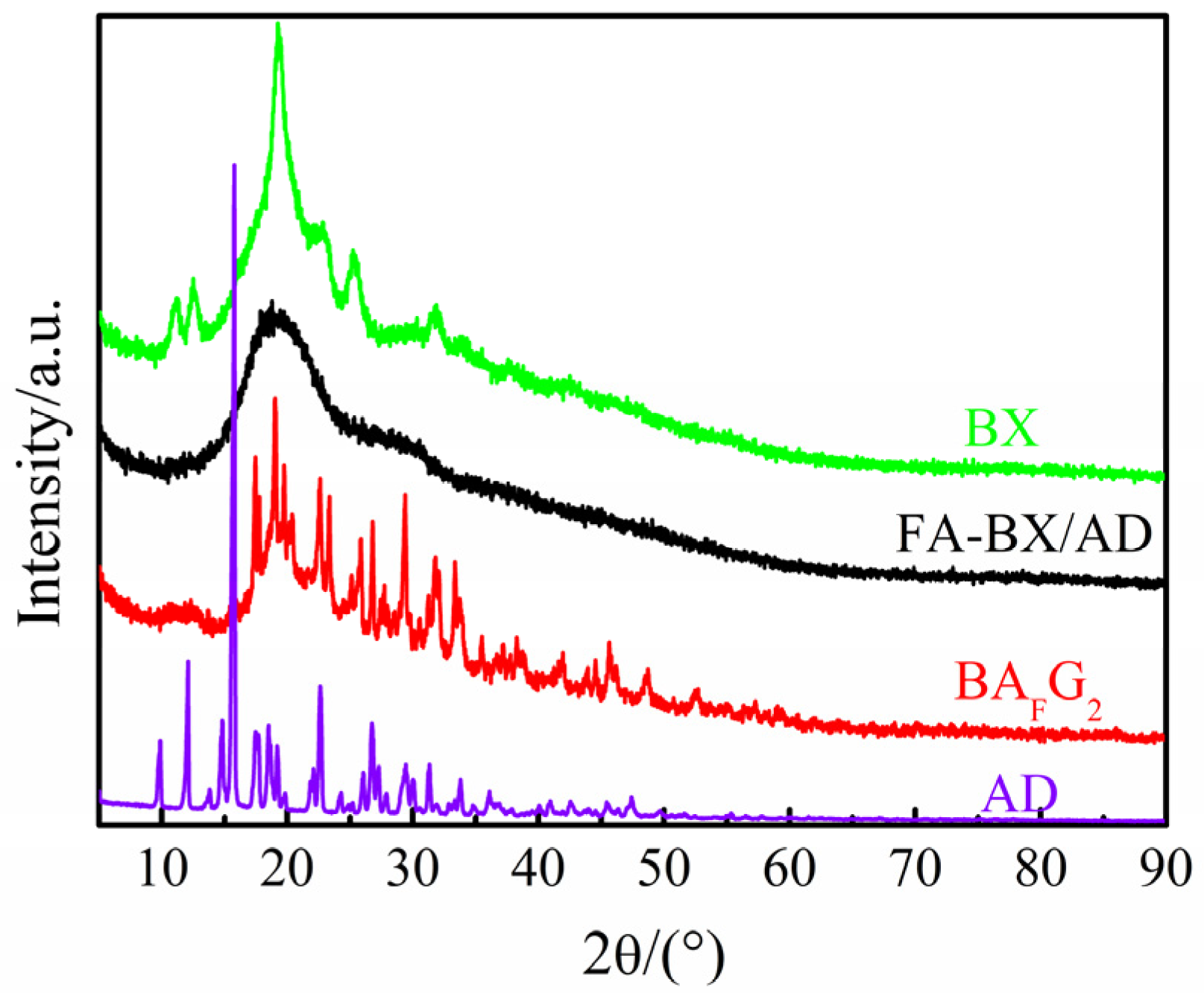
2.3. XRD Analysis
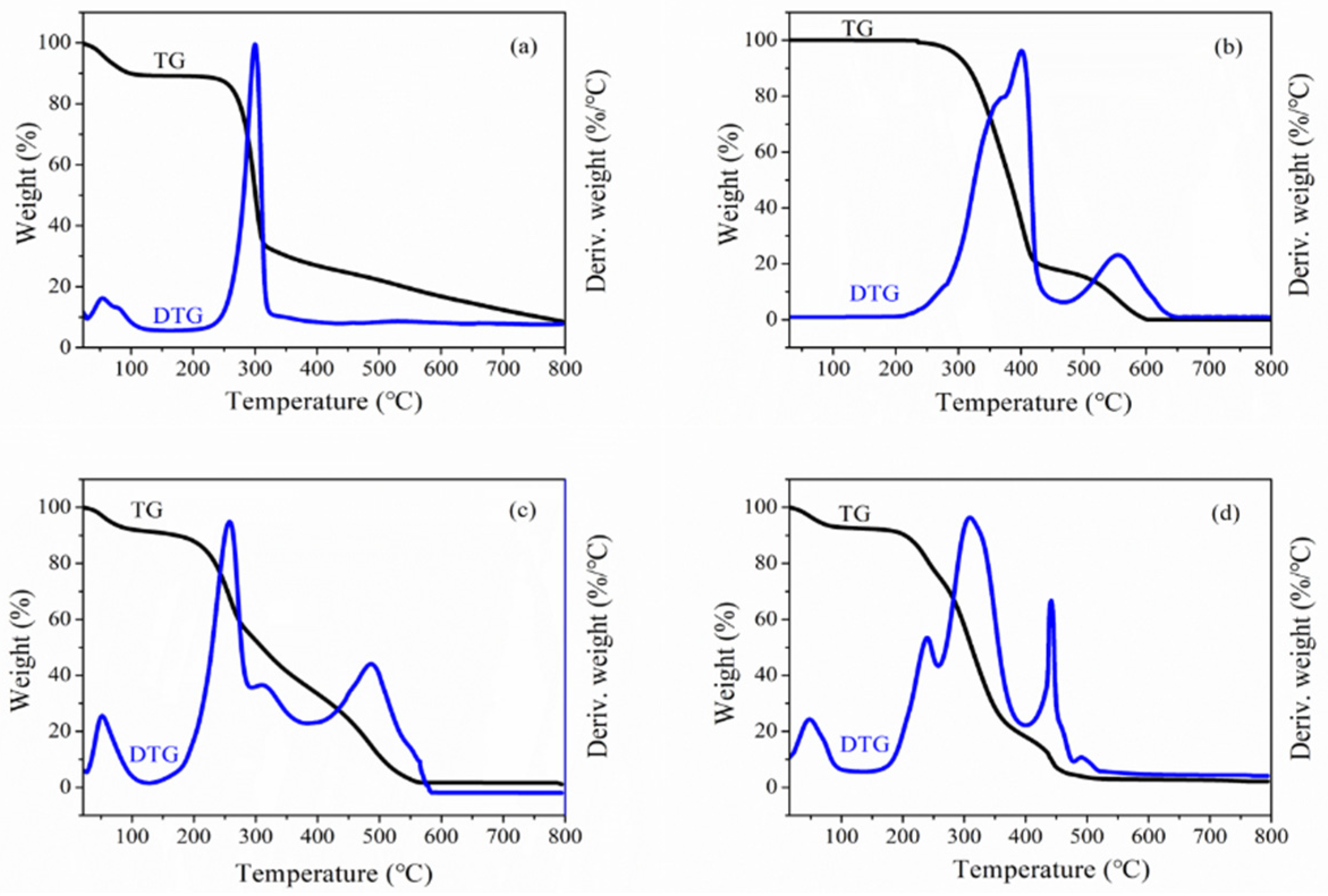
2.4. Derivative Thermogravimetric (DTG) Analysis
2.5. SEM Analysis
2.6. Molecular Docking
2.7. Inhibition Analysis of Tumor Cell
3. Materials and Methods
3.1. Materials
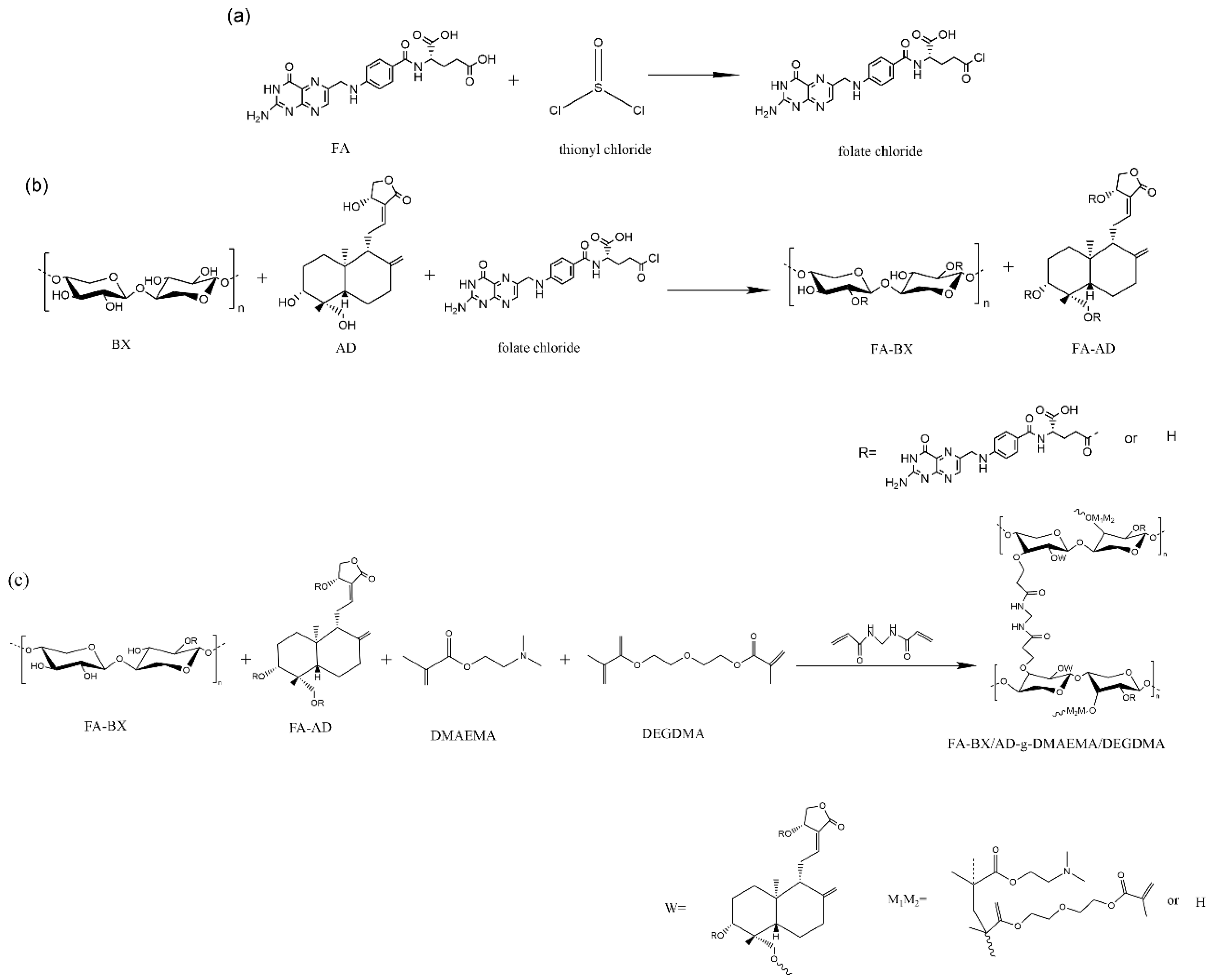
3.2. Acyl Chlorination of Folic Acid
3.3. Synthesis of Folate-Esterified BX/AD
3.4. Synthesis of Folate-Esterified BX/AD-g-DMAEMA/DEGDMA
3.5. Preparation of Folate-Esterified BX/AD-g-DMAEMA/DEGDMA Nanoparticles
3.6. Calculation Method for Grafting Rate (GR) and Grafting Efficiency (GE)
3.7. Characterization Methods
3.7.1. Fourier Transform Infrared (FTIR) Analysis
3.7.2. Scanning Electron Microscopy (SEM) Analysis
3.7.3. X-ray Diffraction (XRD) Analysis
3.7.4. DTG Analysis
3.8. Molecular Docking
3.9. Tumor Cell Proliferation Inhibitory Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jiang, D.; Zhang, L.; Liu, W.; Ding, Y.; Yin, J.; Ren, R.; Li, Q.; Chen, Y.; Shen, J.; Tan, X. Trends in cancer mortality in China from 2004 to 2018: A nationwide longitudinal study. Cancer Commun. 2021, 41, 1024–1036. [Google Scholar] [CrossRef]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Chitosan-based nanoscale and non-nanoscale delivery systems for anticancer drugs: A review. Eur. Polym. J. 2021, 154, 110533. [Google Scholar] [CrossRef]
- Ding, B.; Yu, Y.; Geng, S.; Liu, B.; Hao, Y.; Liang, G. Computational Methods for the Interaction between Cyclodextrins and Natural Compounds: Technology, Benefits, Limitations, and Trends. J. Agric. Food Chem. 2022, 70, 2466–2482. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, A.; Zhang, L. An overview of scoring functions used for protein–ligand interactions in molecular docking. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Serseg, T.; Benarous, K.; Yousfi, M. Hispidin and Lepidine E: Two Natural Compounds and Folic acid as Potential Inhibitors of 2019-novel coronavirus Main Protease (2019-nCoVMpro), molecular docking and SAR study. Curr. Comput.-Aided Drug Des. 2021, 17, 469–479. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, B.; Kulshreshtha, A.; Negi, Y.S. Redox-sensitive nanoparticles based on xylan-lipoic acid conjugate for tumor targeted drug delivery of niclosamide in cancer therapy. Carbohydr. Res. 2021, 499, 108222. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial cellulose-based composite scaffolds for biomedical applications: A review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M. Review on polysaccharides used in coatings for food packaging papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.; Zhong, L.; Chua, W.; Xiang, Z.; Sun, R. Lignocellulosic biomass derived functional materials: Synthesis and applications in biomedical engineering. Curr. Med. Chem. 2019, 26, 2456–2474. [Google Scholar] [CrossRef] [PubMed]
- Cheon, C. Synergistic effects of herbal medicines and anticancer drugs: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e27918. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Y.; Zhang, L.-L.; Ding, J.; Lu, J.-J. Anticancer drug discovery from Chinese medicinal herbs. Chin. Med. 2018, 13, 35. [Google Scholar] [CrossRef]
- Kaushik, S.; Dar, L.; Kaushik, S.; Yadav, J.P. Identification and characterization of new potent inhibitors of dengue virus NS5 proteinase from Andrographis paniculata supercritical extracts on in animal cell culture and in silico approaches. J. Ethnopharmacol. 2021, 267, 113541. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Chang, H.-H.; Chung, Y.-H.; Lee, T.-Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264. 7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef]
- Sa-Ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Anwar, D.M.; El-Sayed, M.; Reda, A.; Fang, J.-Y.; Khattab, S.N.; Elzoghby, A.O. Recent advances in herbal combination nanomedicine for cancer: Delivery technology and therapeutic outcomes. Expert Opin. Drug Deliv. 2021, 18, 1609–1625. [Google Scholar] [CrossRef]
- Shu, Y.; Sun, J.; Cai, P.; Wang, W.; Han, X.; Gu, Y. An open-label, randomized, controlled clinical trial to explore the curative effects between the treatment of capecitabine and andrographolide and the single capecitabine in the patients with pathological and/or histologic diagnosed unresectable, advanced, recurrent, and metastatic colorectal cancer. Am. Soc. Clin. Oncol. 2017, 35, 4. [Google Scholar] [CrossRef]
- Thingale, A.D.; Shaikh, K.S.; Channekar, P.R.; Galgatte, U.C.; Chaudhari, P.D.; Bothiraja, C. Enhanced hepatoprotective activity of andrographolide complexed with a biomaterial. Drug Deliv. 2015, 22, 117–124. [Google Scholar] [CrossRef]
- Xu, J.; Xia, R.; Yuan, T.; Sun, R. Use of xylooligosaccharides (XOS) in hemicelluloses/chitosan-based films reinforced by cellulose nanofiber: Effect on physicochemical properties. Food Chem. 2019, 298, 125041. [Google Scholar] [CrossRef]
- Hettrich, K.; Drechsler, U.; Loth, F.; Volkert, B. Preparation and characterization of water-soluble xylan ethers. Polymers 2017, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef]
- Peng, X.-w.; Ren, J.-l.; Sun, R.-c. Homogeneous esterification of xylan-rich hemicelluloses with maleic anhydride in ionic liquid. Biomacromolecules 2010, 11, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Smith, M.W.; Pecha, B.; Helms, G.; Scudiero, L.; Garcia-Perez, M. Chemical and morphological evaluation of chars produced from primary biomass constituents: Cellulose, xylan, and lignin. Biomass Bioenergy 2017, 104, 17–35. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165. [Google Scholar] [CrossRef]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int. J. Biol. Macromol. 2020, 156, 1217–1233. [Google Scholar] [CrossRef]
- Lim, J.C.W.; Chan, T.K.; Ng, D.S.; Sagineedu, S.R.; Stanslas, J.; Wong, W.F. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H. Nanoparticle-based drug delivery systems for enhanced tumor-targeting treatment. J. Biomed. Nanotechnol. 2019, 15, 1–27. [Google Scholar] [CrossRef]
- Casamonti, M.; Risaliti, L.; Vanti, G.; Piazzini, V.; Bergonzi, M.C.; Bilia, A.R. Andrographolide loaded in micro-and nano-formulations: Improved bioavailability, target-tissue distribution, and efficacy of the “king of bitters”. Engineering 2019, 5, 69–75. [Google Scholar] [CrossRef]
- Oseni, B.A.; Azubuike, C.P.; Okubanjo, O.O.; Igwilo, C.I.; Panyam, J. Encapsulation of Andrographolide in poly (lactide-co-glycolide) Nanoparticles: Formulation Optimization and in vitro Efficacy Studies. Front. Bioeng. Biotechnol. 2021, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Sun, R.-C. Nanocomposite films based on xylan-rich hemicelluloses and cellulose nanofibers with enhanced mechanical properties. Biomacromolecules 2011, 12, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhan, A.; Ye, Y.; Liu, C.; Hang, F.; Li, K.; Li, J. Molecular modification, structural characterization, and biological activity of xylans. Carbohydr. Polym. 2021, 269, 118248. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gu, J.; Wang, Y.; Yuan, H.; Chen, Y. Kinetics modeling of co-pyrolytic decomposition of binary system of cellulose, xylan and lignin. J. Energy Inst. 2022, 102, 278–288. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S. The structural and thermal characteristics of wheat straw hemicellulose. J. Anal. Appl. Pyrolysis 2010, 88, 134–139. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG–FTIR and Py–GC–FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Tao, W.; Yang, X.; Li, Y.; Zhu, R.; Si, X.; Pan, B.; Xing, B. Components and persistent free radicals in the volatiles during pyrolysis of lignocellulose biomass. Environ. Sci. Technol. 2020, 54, 13274–13281. [Google Scholar] [CrossRef]
- Brienzo, M.; Siqueira, A.F.; Milagres, A.M. Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochem. Eng. J. 2009, 46, 199–204. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Quick, A.; Taha, M.; Mignard, N.; Becquart, F.; Ayoub, A. Hemicellulose extraction and characterization for applications in paper coatings and adhesives. Ind. Crops Prod. 2017, 107, 370–377. [Google Scholar] [CrossRef]
- Łukajtis, R.; Rybarczyk, P.; Kucharska, K.; Konopacka-Łyskawa, D.; Słupek, E.; Wychodnik, K.; Kamiński, M. Optimization of saccharification conditions of lignocellulosic biomass under alkaline pre-treatment and enzymatic hydrolysis. Energies 2018, 11, 886. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Li, H.; Zuo, K.; Feng, X.; Hu, Y.; Zhang, S. Methacrylic acid/butyl acrylate onto feruloylated bagasse xylan: Graft copolymerization and biological activity. Mater. Sci. Eng. C 2019, 98, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, M.; Zhang, H.; Ren, J. Fabrication of bentonite reinforced dopamine grafted carboxymethyl xylan cross-linked with polyacrylamide hydrogels with adhesion properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129024. [Google Scholar] [CrossRef]
- Gao, B.; Cha, X.; Chen, T.; Fan, L. Designing and preparing of acid dye surface-imprinted material for effective removal of acid dyes from water. J. Environ. Chem. Eng. 2015, 3, 277–285. [Google Scholar] [CrossRef]
- Hansson, S.; Trouillet, V.; Tischer, T.; Goldmann, A.S.; Carlmark, A.; Barner-Kowollik, C.; Malmström, E. Grafting efficiency of synthetic polymers onto biomaterials: A comparative study of grafting-from versus grafting-to. Biomacromolecules 2013, 14, 64–74. [Google Scholar] [CrossRef]
- Işıklan, N.; Kurşun, F.; Inal, M. Graft copolymerization of itaconic acid onto sodium alginate using ceric ammonium nitrate as initiator. J. Appl. Polym. Sci. 2009, 114, 40–48. [Google Scholar] [CrossRef]
- Gysi, D.M.; Do Valle, Í.; Zitnik, M.; Ameli, A.; Gan, X.; Varol, O.; Ghiassian, S.D.; Patten, J.; Davey, R.A.; Loscalzo, J. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2025581118. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ke, J.; Zhou, X.E.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.; Xu, H.; Melcher, K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500, 486–489. [Google Scholar] [CrossRef]
- Akkoç, S.; Tüzün, B.; İlhan, İ.Ö.; Akkurt, M. Investigation of structural, spectral, electronic, and biological properties of 1, 3-disubstituted benzimidazole derivatives. J. Mol. Struct. 2020, 1219, 128582. [Google Scholar] [CrossRef]
- Rose, Y.; Duarte, J.M.; Lowe, R.; Segura, J.; Bi, C.; Bhikadiya, C.; Li, C.; Alexander, S.R.; Sebastian, B.; Stephen, K.B.; et al. RCSB Protein Data Bank: Architectural advances towards integrated searching and efficient access to macromolecular structure data from the PDB archive. J. Mol. Biol. 2021, 433, 166704. [Google Scholar] [CrossRef]
- Peter, W.R.; Andreas, P.; Ali, A.; Chunxiao, B.; Anthony, R.B.; Cole, H.C.; Di Luigi, C.; Jose, M.D.; Shuchismita, D.; Zukang, F.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017, 45, D271–D281. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Aslantürk, Ö.S.; Larramendy, M.; Soloneski, S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; Intechopen: London, UK, 2018; pp. 1–19. [Google Scholar] [CrossRef]
- Ciapetti, G.; Cenni, E.; Pratelli, L.; Pizzoferrato, A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 1993, 14, 359–364. [Google Scholar] [CrossRef]
- Stone, V.; Johnston, H.; Schins, R.P. Development of in vitro systems for nanotoxicology: Methodological considerations. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef]
- Ganot, N.; Meker, S.; Reytman, L.; Tzubery, A.; Tshuva, E.Y. Anticancer metal complexes: Synthesis and cytotoxicity evaluation by the MTT assay. J. Vis. Exp. 2013, 81, e50767. [Google Scholar] [CrossRef]
- Bano, Z.; Begum, S.; Ali, S.S.; Kiran, Z.; Siddiqui, B.S.; Ahmed, A.; Khawaja, S.; Fatima, F.; Jabeen, A. Phytochemicals from Carissa carandas with potent cytotoxic and anti-inflammatory activities. Nat. Prod. Res. 2022, 36, 1587–1592. [Google Scholar] [CrossRef]
- Sun, X.; Hou, L.; Qiu, C.; Kong, B. MiR-501 promotes tumor proliferation and metastasis by targeting HOXD10 in endometrial cancer. Cell. Mol. Biol. Lett. 2021, 26, 20. [Google Scholar] [CrossRef]


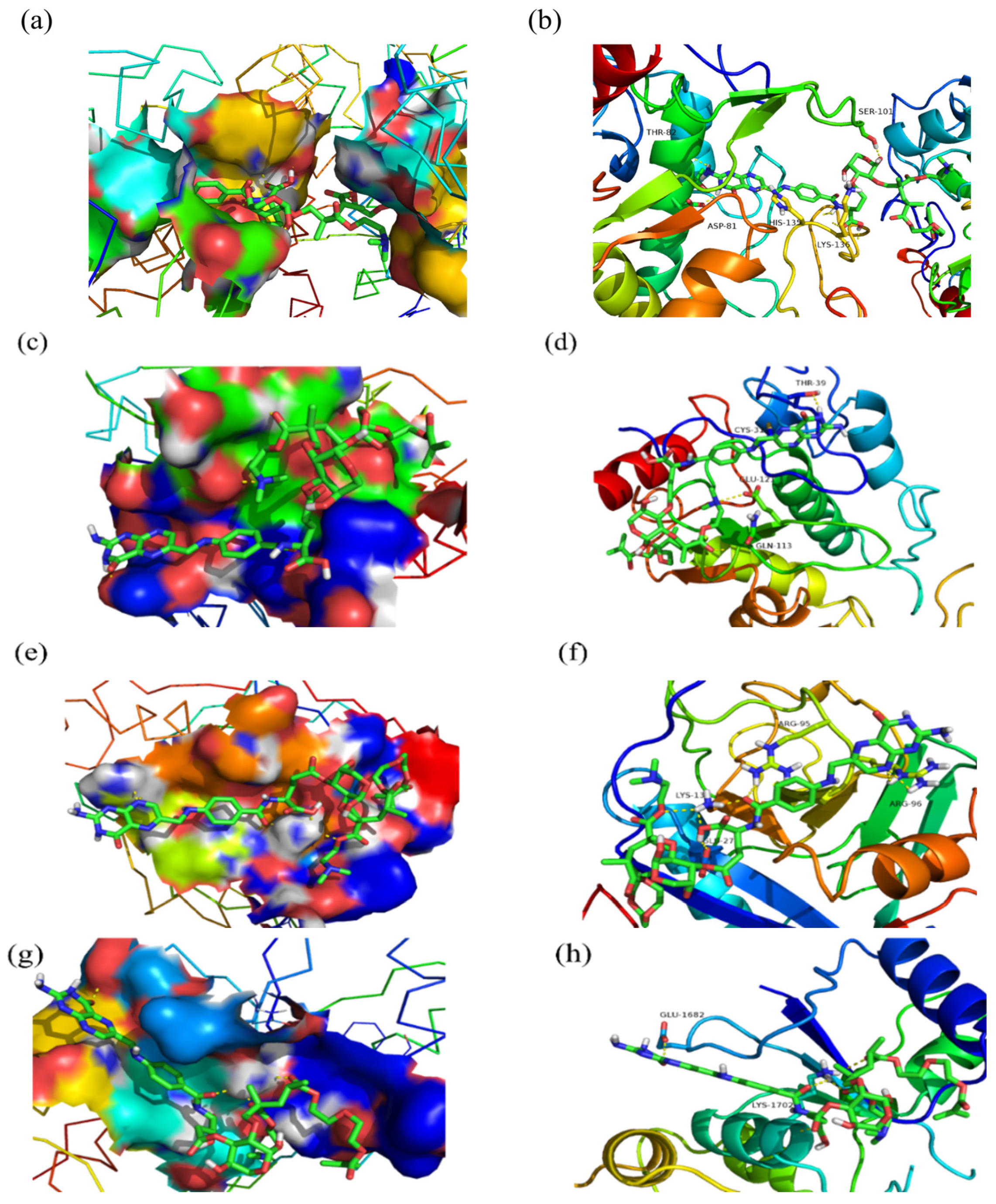
| PBD Code | Estimated Free Energy of Binding (kcal/mol) | Ki (μM) | Final Intermolecular Energy (kcal/mol) | Final Total Internal Energy (kcal/mol) |
|---|---|---|---|---|
| 4LRH | −4.12 | 958.82 | −14.56 | −7.14 |
| 4KMZ | −0.62 | 351,290 | −11.06 | −6.55 |
| 2HQ6 | −1.77 | 50,300 | −12.21 | −7.28 |
| 1JNX | −0.76 | 276,190 | −11.20 | −8.22 |
| Sample | Mass Concentration (μg/mL) | Inhibition Ratio (%) | |||
|---|---|---|---|---|---|
| BEAS-2B | NCI-H460 | MGC80-3B | BEL-7407 | ||
| BX | 100 | 1.93 ± 0.48 | 4.62 ± 2.79 | 2.02 ± 0.57 | 1.07 ± 0.71 |
| 50 | 1.72 ± 0.76 | 0.71 ± 0.22 | 0.24 ± 0.08 | 1.18 ± 0.34 | |
| 20 | −0.26 ± 0.57 | 0.24 ± 0.19 | −0.15 ± 0.13 | 0.35 ± 0.26 | |
| 10 | −2.94 ± 0.35 | −2.97 ± 1.43 | −2.99 ± 1.11 | 0.47 ± 0.29 | |
| 1 | −5.61 ± 0.23 | −4.33 ± 2.03 | −3.27 ± 1.61 | −0.45 ± 0.31 | |
| BX/AD | 100 | 1.26 ± 0.79 | 2.37 ± 0.73 | 5.62 ± 1.43 | 4.28 ± 1.26 |
| 50 | 0.83 ± 0.61 | 2.42 ± 0.81 | 3.85 ± 0.61 | 2.35 ± 0.71 | |
| 20 | −1.75 ± 1.02 | 1.28 ± 0.65 | 2.08 ± 0.29 | 1.73 ± 0.49 | |
| 10 | −5.21 ± 2.23 | 0.93 ± 0.34 | 1.67 ± 0.50 | 0.68 ± 0.35 | |
| 1 | −7.49 ± 0.38 | −0.74 ± 0.69 | 0.83 ± 0.26 | −1.23 ± 0.84 | |
| FA-BX/AD-g- DMAEMA/DEGDMA NPs | 100 | 3.67 ± 1.38 | 39.77 ± 5.62 | 34.87 ± 5.11 | 31.65 ± 3.79 |
| 50 | 2.83 ± 0.67 | 35.82 ± 5.03 | 30.46 ± 3.73 | 27.45 ± 3.60 | |
| 20 | 2.13 ± 0.45 | 31.91 ± 3.62 | 27.38 ± 2.96 | 24.81 ± 2.75 | |
| 10 | 1.46 ± 0.56 | 28.79 ± 3.19 | 24.59 ± 2.58 | 20.29 ± 2.02 | |
| 1 | 0.24 ± 0.18 | 22.64 ± 2.33 | 19.78 ± 3.25 | 16.52 ± 2.94 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Zhang, S.; Li, H.; Zhao, B.; Tian, K.; Zou, Z. Dimethylaminoethyl Methacrylate/Diethylene Glycol Dimethacrylate Grafted onto Folate-Esterified Bagasse Xylan/Andrographolide Composite Nanoderivative: Synthesis, Molecular Docking and Biological Activity. Molecules 2022, 27, 5970. https://doi.org/10.3390/molecules27185970
Su Y, Zhang S, Li H, Zhao B, Tian K, Zou Z. Dimethylaminoethyl Methacrylate/Diethylene Glycol Dimethacrylate Grafted onto Folate-Esterified Bagasse Xylan/Andrographolide Composite Nanoderivative: Synthesis, Molecular Docking and Biological Activity. Molecules. 2022; 27(18):5970. https://doi.org/10.3390/molecules27185970
Chicago/Turabian StyleSu, Yue, Shufen Zhang, Heping Li, Bin Zhao, Kexin Tian, and Zhiming Zou. 2022. "Dimethylaminoethyl Methacrylate/Diethylene Glycol Dimethacrylate Grafted onto Folate-Esterified Bagasse Xylan/Andrographolide Composite Nanoderivative: Synthesis, Molecular Docking and Biological Activity" Molecules 27, no. 18: 5970. https://doi.org/10.3390/molecules27185970
APA StyleSu, Y., Zhang, S., Li, H., Zhao, B., Tian, K., & Zou, Z. (2022). Dimethylaminoethyl Methacrylate/Diethylene Glycol Dimethacrylate Grafted onto Folate-Esterified Bagasse Xylan/Andrographolide Composite Nanoderivative: Synthesis, Molecular Docking and Biological Activity. Molecules, 27(18), 5970. https://doi.org/10.3390/molecules27185970






