Genus Smenospongia: Untapped Treasure of Biometabolites—Biosynthesis, Synthesis, and Bioactivities
Abstract
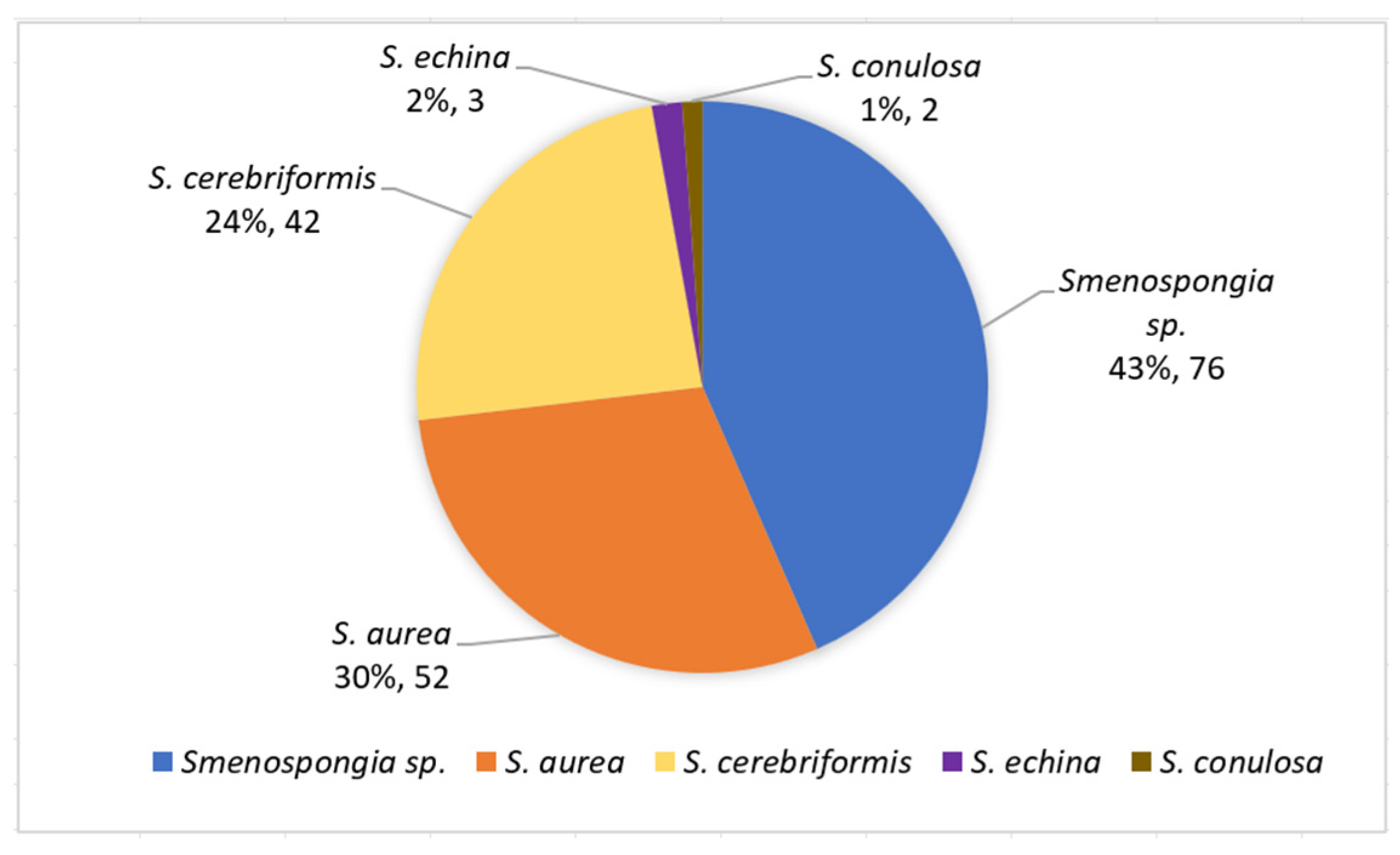
1. Introduction
2. Methodology
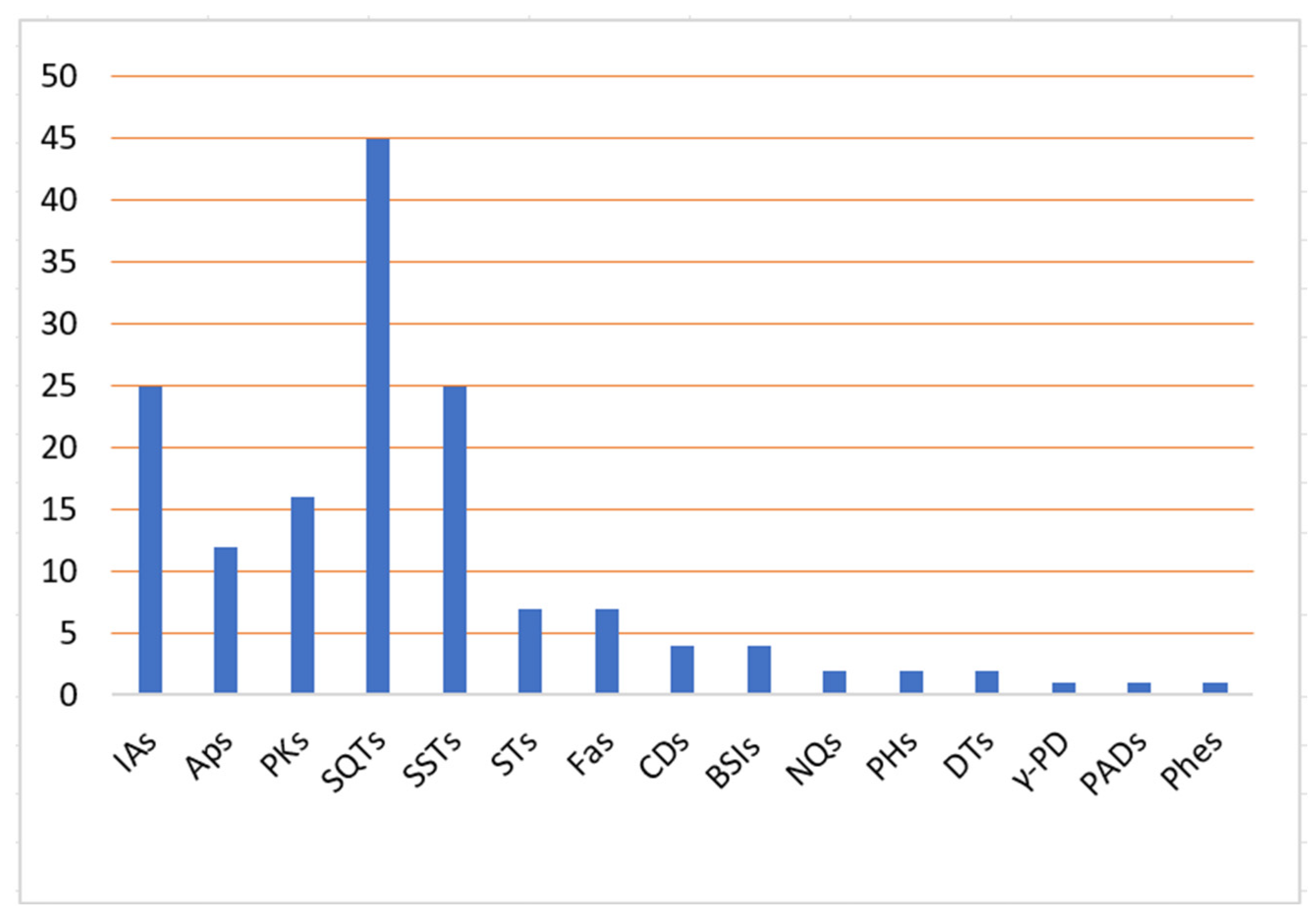
3. Smenospongia Metabolites and Their Bioactivities
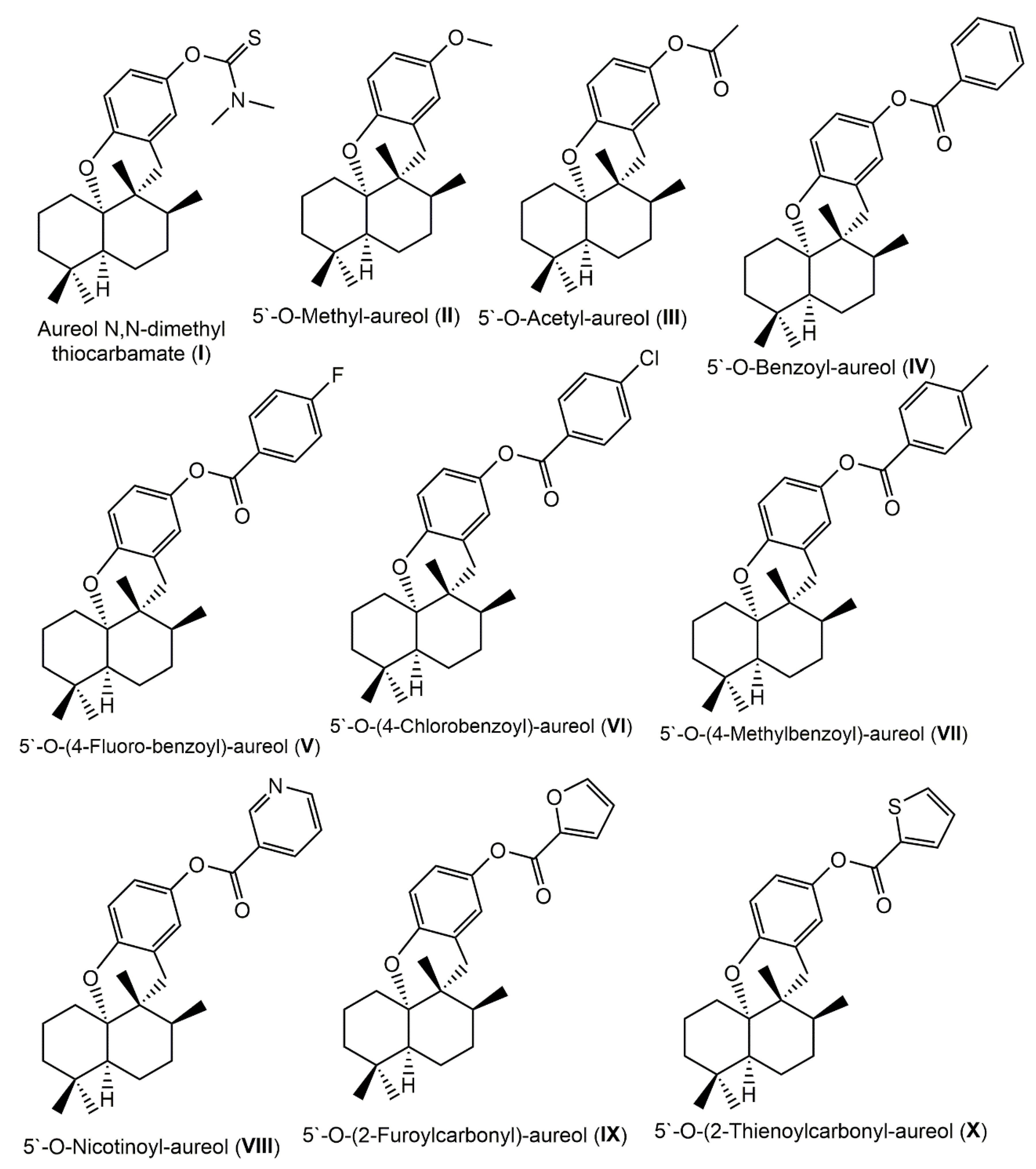
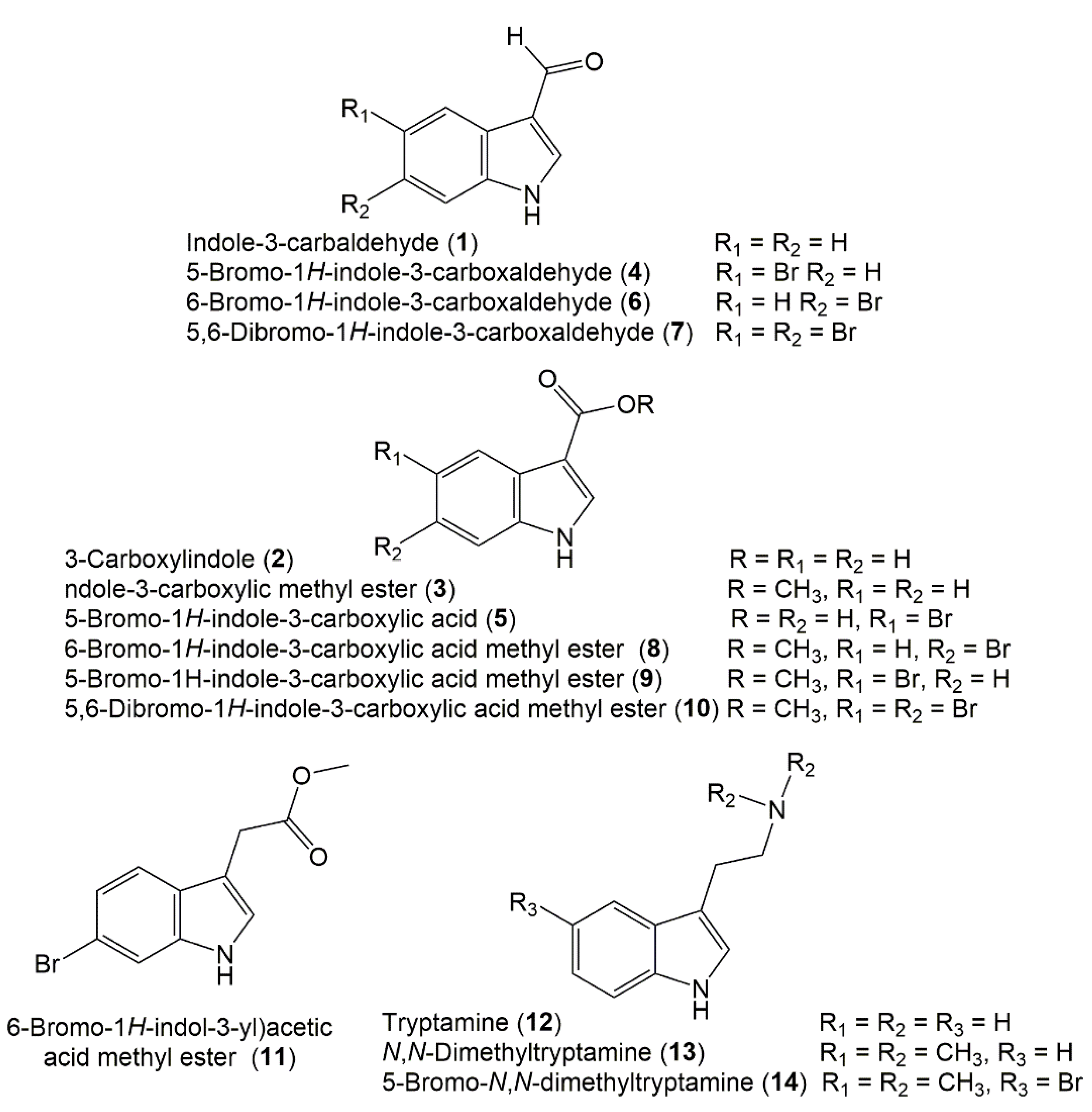
3.1. Indole Derivatives
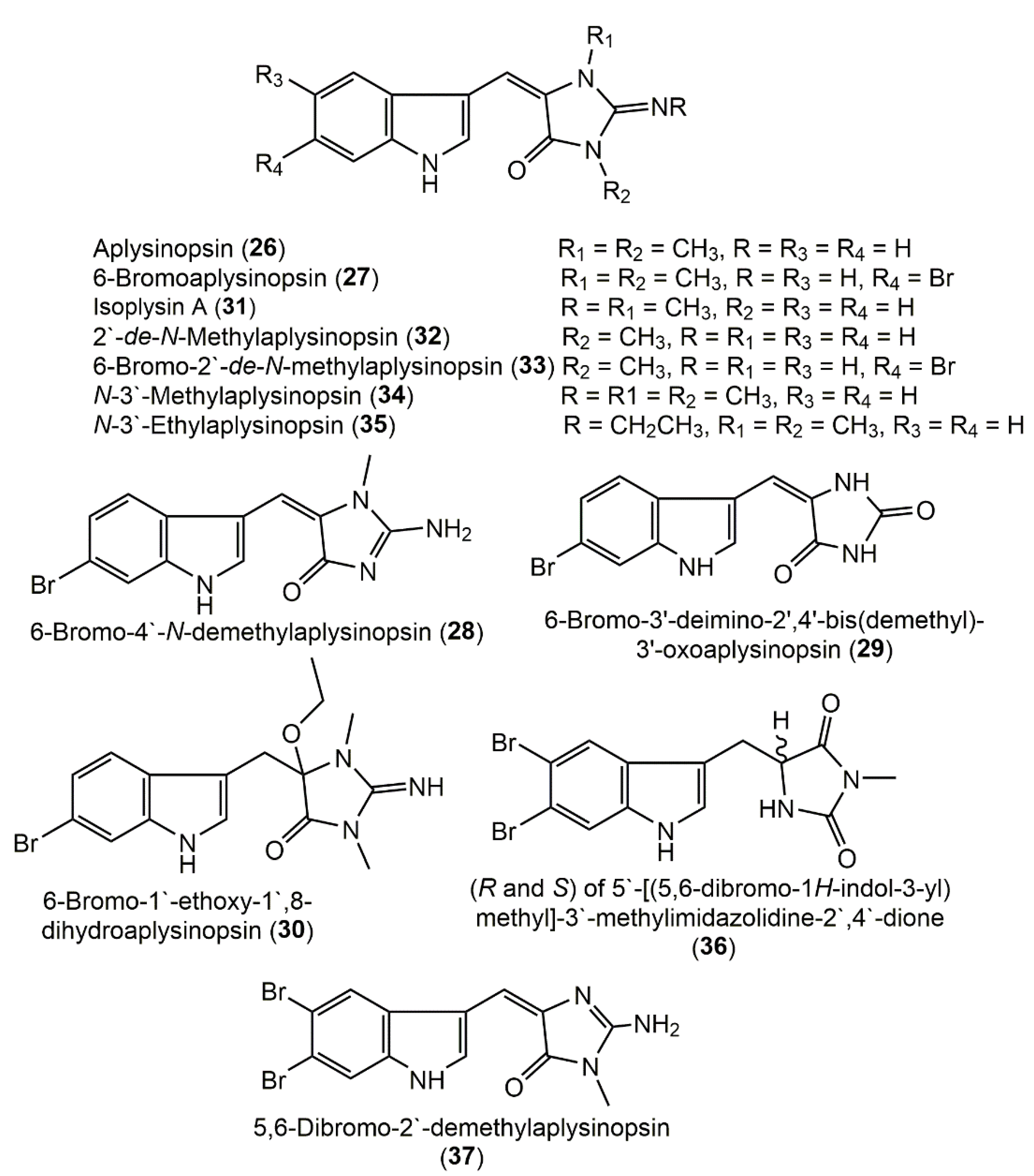
3.2. Aplysinopsin Derivatives
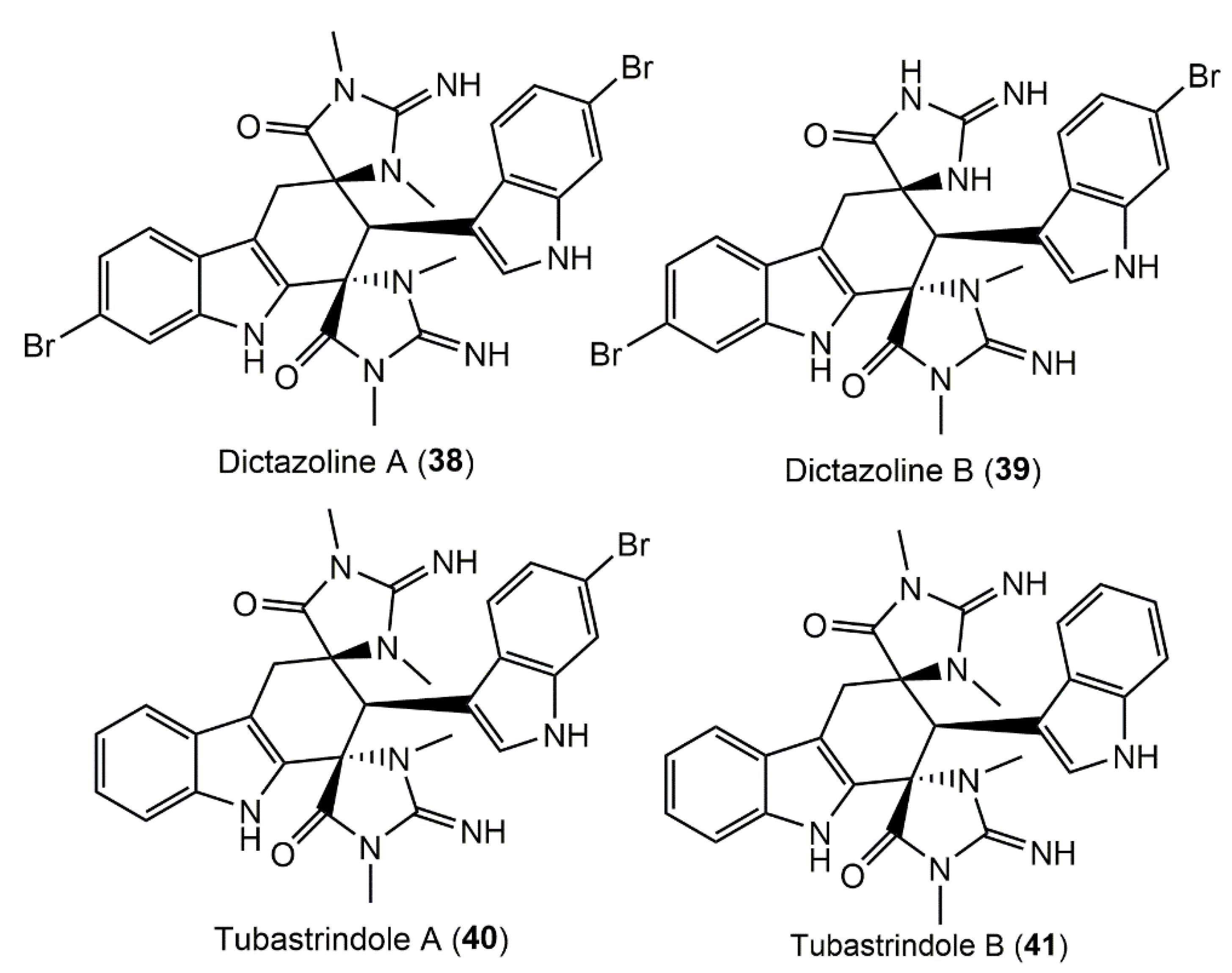
3.3. Bisspiroimidazolidinone Alkaloids
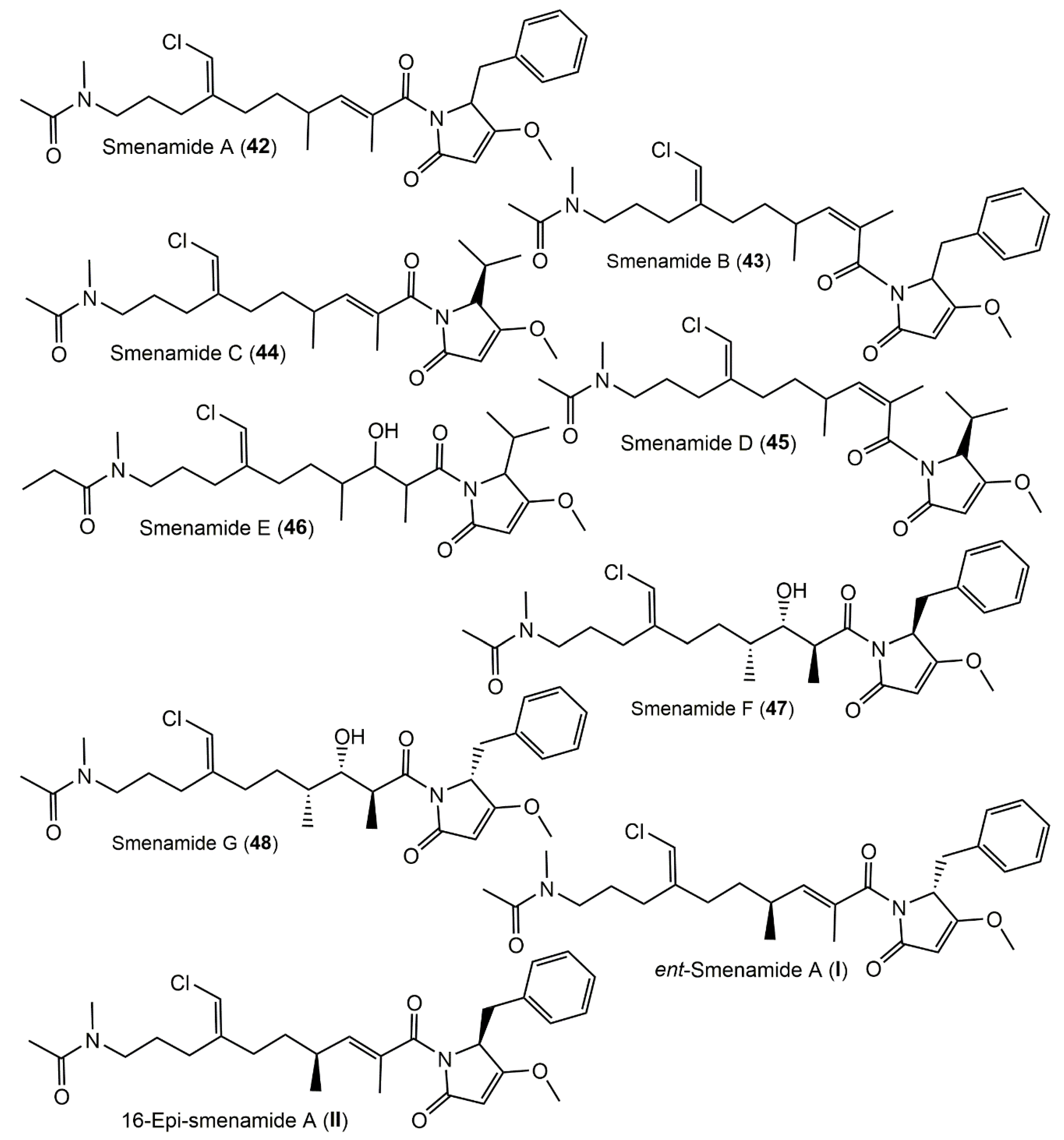
3.4. Polyketides
3.5. Terpenoids
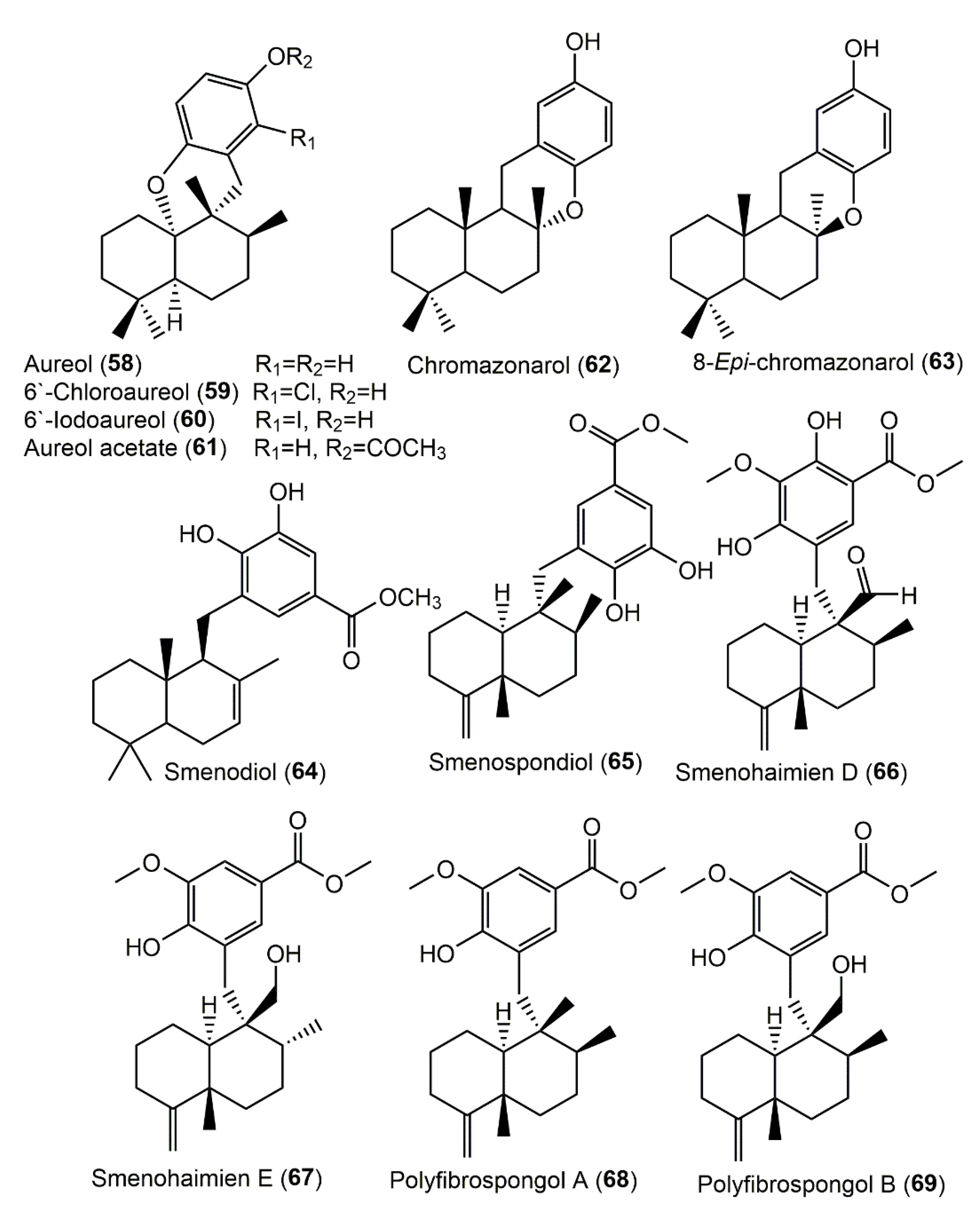
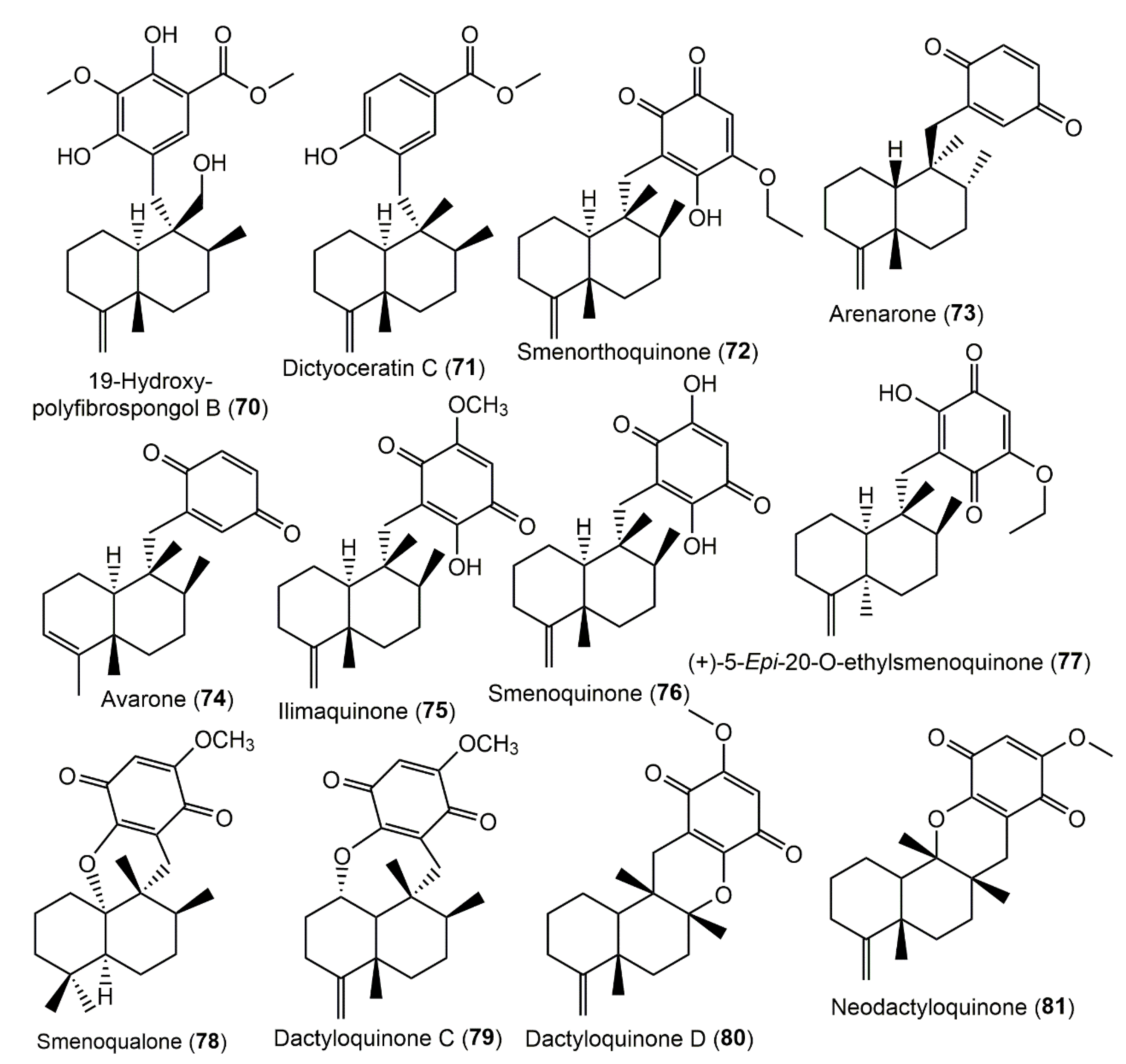
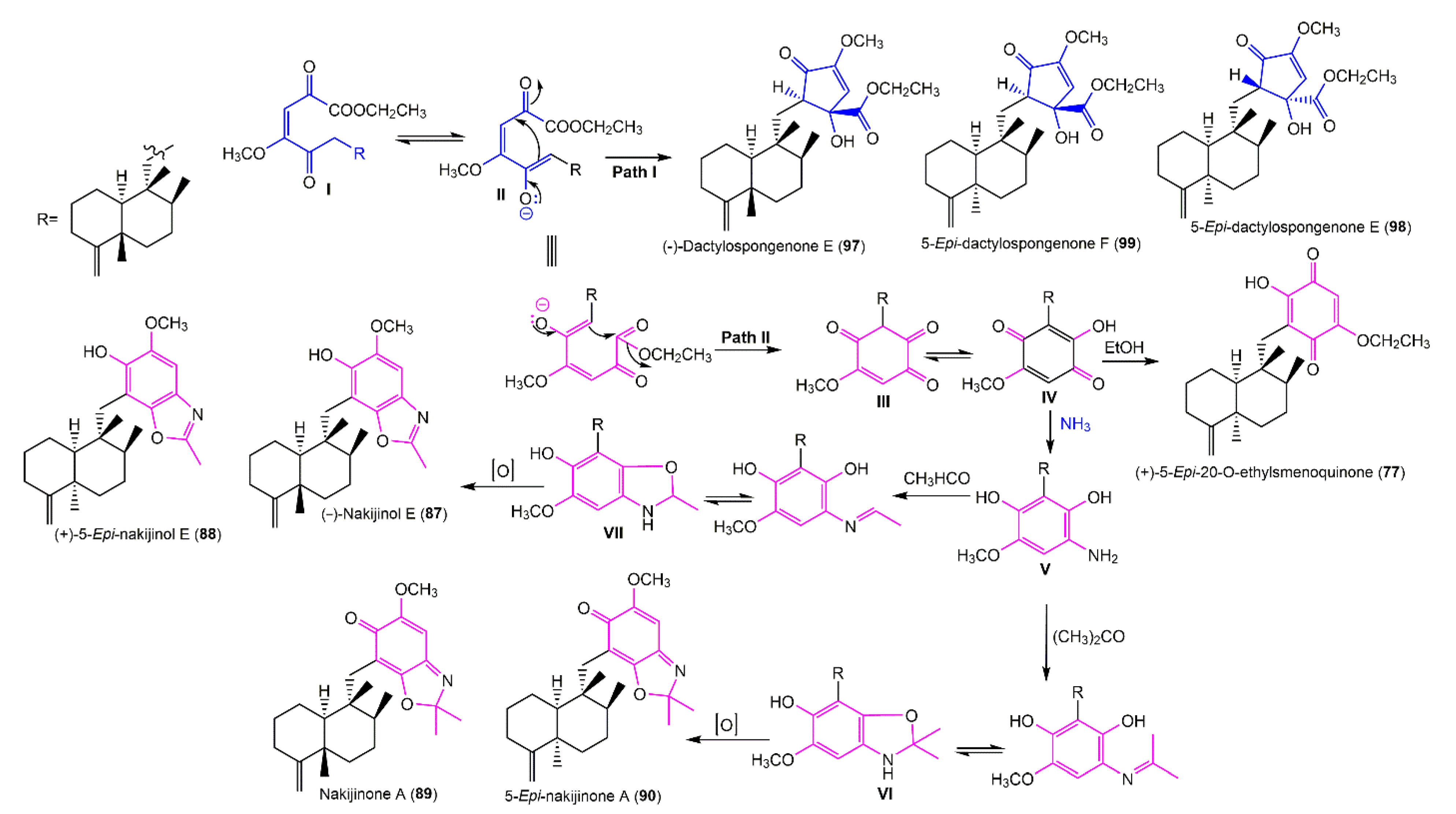
3.5.1. Hydroquinone and Quinone Sesquiterpenoids
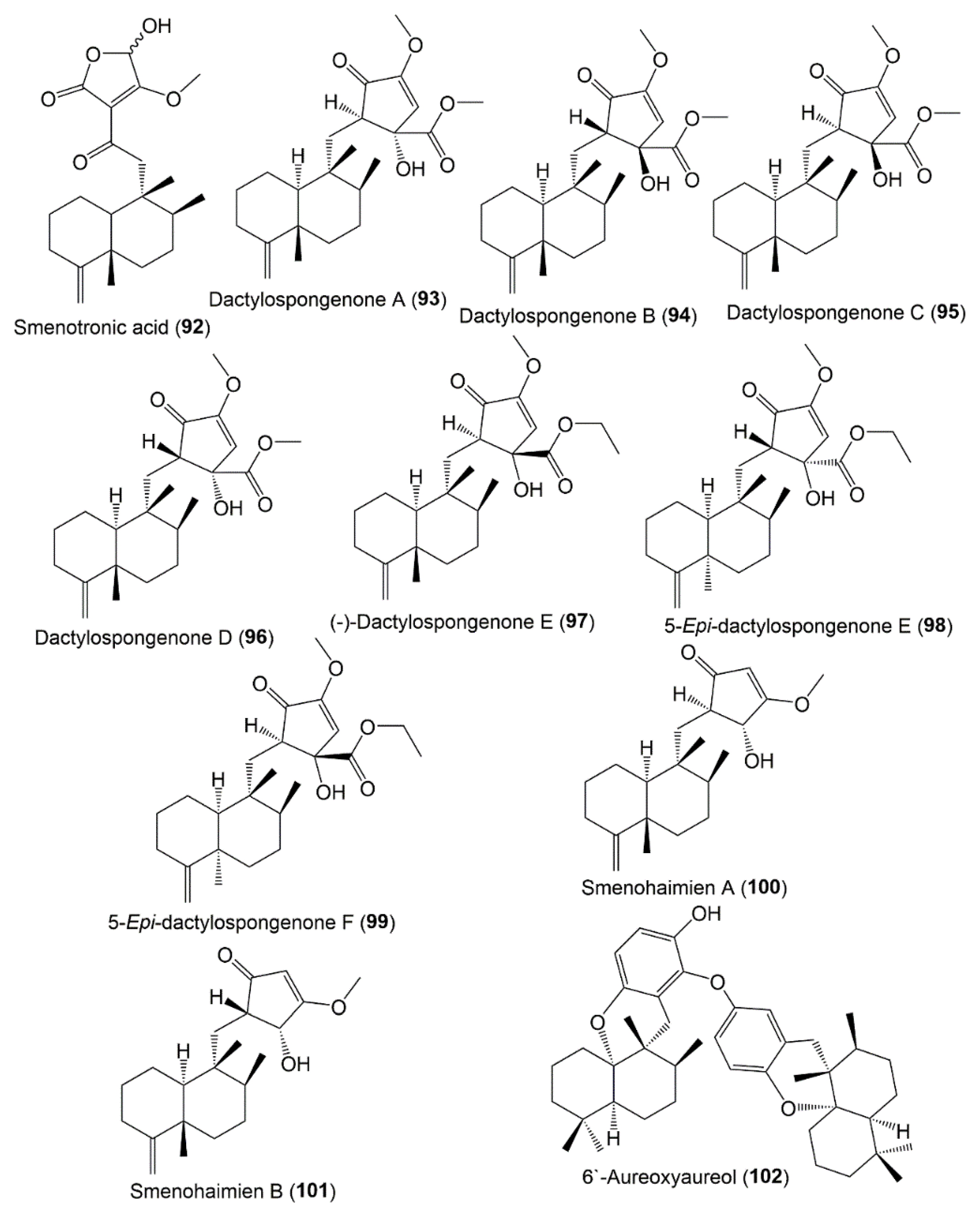
3.5.2. Tetronic Acid and Cyclopentenone Sesquiterpenoids
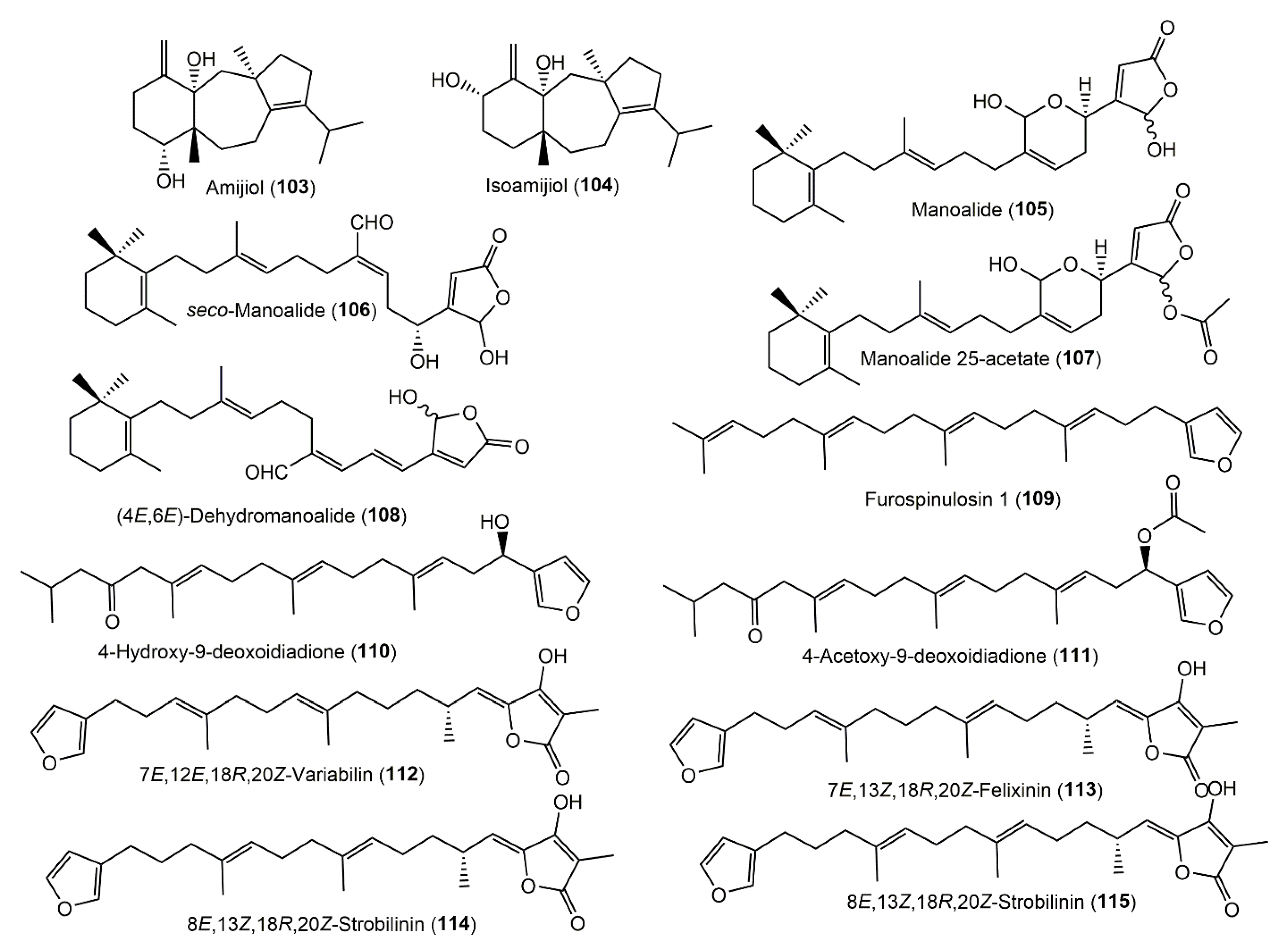
3.5.3. Diterpenoids
3.5.4. Sesterterpenoids
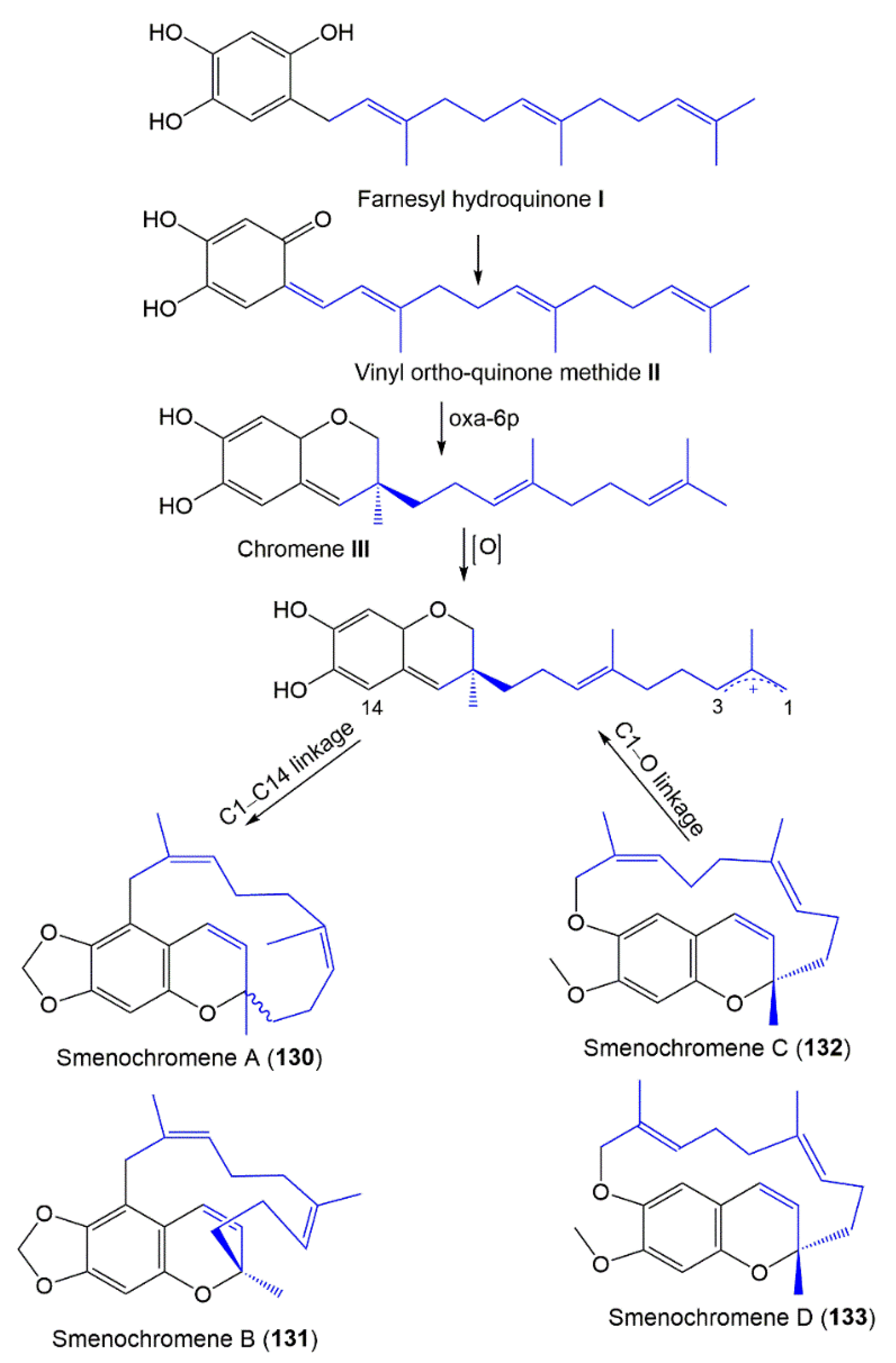
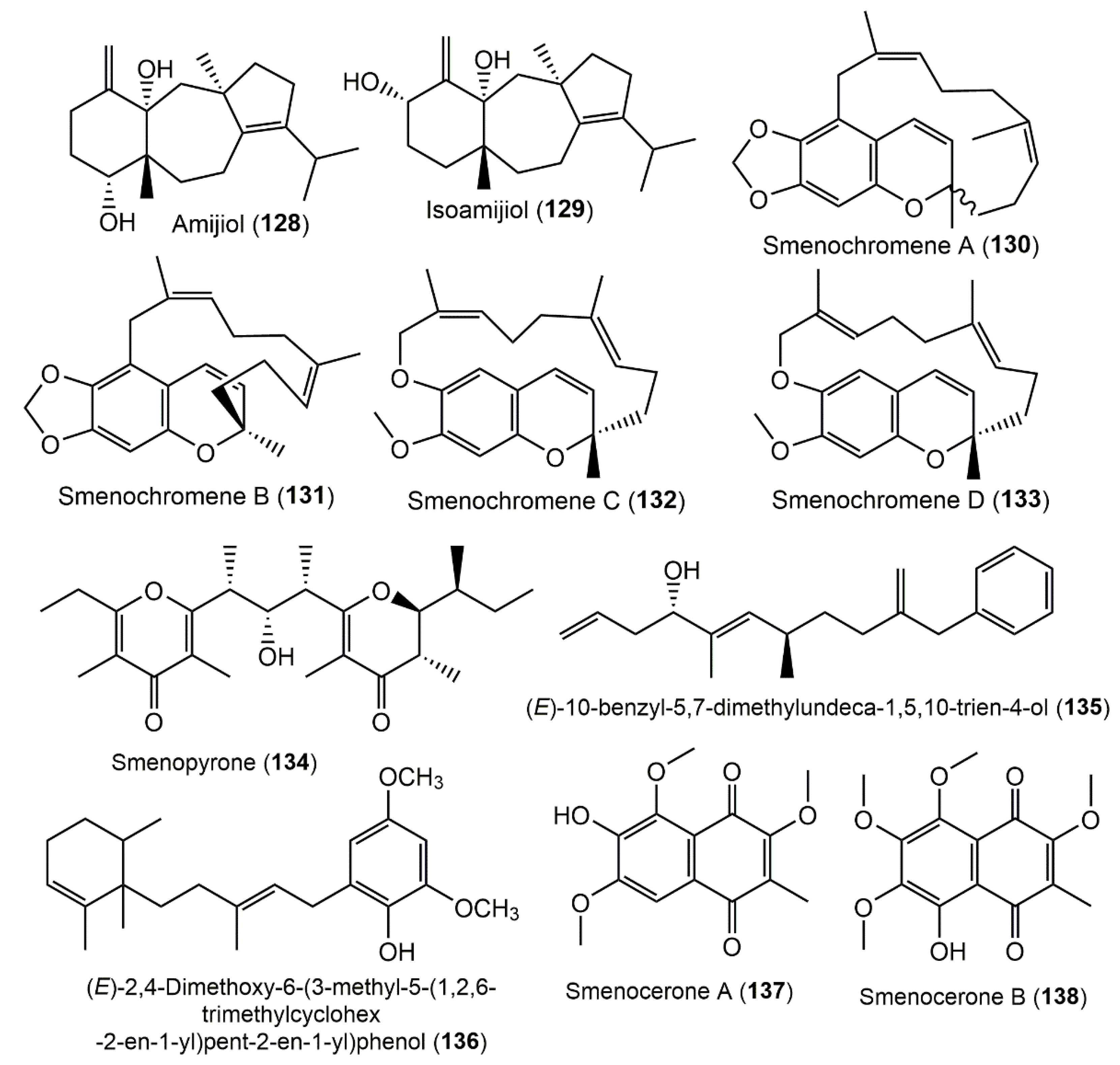
3.6. Chromene Derivatives
3.7. γ-Pyrone Derivatives
3.8. Phenyl Alkenes
3.9. Naphthoquinones
3.10. Fatty Acids, Sterols, and Phthalates
4. Biological Activity of Extracts
4.1. Antimicrobial Activity
4.2. Cytotoxic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A549 | Human lung adenocarcinoma epithelial cell line |
| A2780 | Human ovarian cancer cells line |
| AMPK | AMP-Activated protein kinase |
| ARPE-19 | Retinal pigment epithelial cell line |
| ATR | Adenosine triphosphate |
| BV2 | Microglia cells |
| BxPC-3 | Human pancreas adenocarcinoma cell line |
| Calu-1 | Human nonsmall-cell lung cancer cell line |
| CD | Circular dichroism |
| CH2Cl2 | Dichloromethane |
| CHCl3 | Chloroform |
| CHOP, CCAAT | Enhancer-binding protein homologous protein |
| CNV | Choroidal neovascularization |
| CRT | β-catenin response transcription |
| DLD-1 | Human colorectal cancer cell line |
| DR4 | Death receptor 4 |
| DR5 | Death receptor 5 |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| EC50 | Half-maximal effective concentration |
| ERK | Extracellular signal-regulated kinase |
| EtOH | Ethanol |
| EtOAc | Ethyl acetate |
| FST | Porsolt forced swim test |
| FRET | Fluorescence resonance energy transfer |
| FP | Fluorescence polarization |
| GCMS | Gas chromatography mass spectrometry |
| GIAO | Gauge-invariant atomic orbital |
| GI50 | The concentration for 50% of maximal inhibition of cell |
| HCT-116 | Human colon cancer cell line |
| HEK293 | Human embryonic kidney cell |
| HepG2 | Human liver cancer cell line |
| Hepa59T/VGH | Human liver carcinoma cell line |
| HeLa | Human cervical epitheloid carcinoma cell line |
| HL-60 | Human promyelocytic leukemia cell line |
| HPLC | High-performance liquid chromatography |
| HT-29 | Human colon cancer cell line |
| hTERT-RPE1 | Retinal pigment epithelial cell lines |
| HuCCA-1 | Human cholangiocarcinoma cell line |
| HUVECs | Human umbilical vein endothelial cell line |
| HUVSMCs | Human umbilical vein smooth muscle cells line |
| IC50 | Half-maximal inhibitory concentration |
| ICL | Microbial enzyme isocitrate lyase |
| K562 | Human immortalized myelogenous leukemia cell line |
| KB | Human oral epidermoid carcinoma cell line |
| L1210 | Mouse lymphocytic leukemia cell line |
| LC31 | Human lung squamous adenocarcinoma cell line |
| LC50 | Lethal concentration 50 |
| LC–MS–NMR | Liquid chromatography–mass spectrometry–nuclear magnetic resonance |
| LD50 | Half maximal lethal concentration |
| LPS | Lipopolysaccharide |
| LLC | Murine Lewis lung carcinoma |
| L-NMMA | Nitric oxide synthase inhibitor NG-monomethyl-L-arginine |
| LU-1 | Human lung carcinoma cell line |
| MCF-7 | Human breast cancer cell line |
| MDA-MB-231 | Human breast cancer cell line |
| MeOH | Methanol |
| MG-63 | Human osteosarcoma cell line |
| MIC | Minimum inhibitory concentration |
| MOLT-3 | Human T lymphoblast cell line |
| MTS | (3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazoliuminner salt) |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| nAMD | Neovascular age-related macular degeneration |
| n-BuOH | n-Butanol |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| p38 MAPK | p38 Mitogen-activated protein kinase |
| P 388 | Human leukemia cell line |
| PANC-1 | Human pancreas ductal carcinoma cell line |
| PARP | Poly-AD Pribose polymerase |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PDHA1 | Phosphorylation of its E1α subunit |
| RKO | Human colon cancer cell line |
| ROS | Reactive oxygen species |
| RTCA | xCELLigence system real-time cell analyzer |
| RP-18 | Reversed phase-18 |
| SRB | Sulforhodamine B |
| SiO2 CC | Silica gel column chromatography |
| SK-MEL-2 | Human melanoma cell line |
| SRB | Sulforhodamine B |
| SW480 | Human colorectal cancer cell line |
| TLC | Thin layer chromatography |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| UPLC–MS | Ultra performance liquid chromatography–tandem mass spectrometer |
References
- Ibrahim, S.R.; Mohamed, G.A. Marine pyridoacridine alkaloids: Biosynthesis and biological activities. Chem. Biodiv. 2016, 13, 37–47. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Fadil, S.A.; Fadil, H.A.; Hareeri, R.H.; Alolayan, S.O.; Abdallah, H.M.; Mohamed, G.A. Dactylospongia elegans—A promising drug source: Metabolites, bioactivities, biosynthesis, synthesis, and structural-activity relationship. Mar. Drugs 2022, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Esposito, R.; Federico, S.; Bertolino, M.; Zupo, V.; Costantini, M. Marine Demospongiae: A challenging treasure of bioactive compounds. Mar. Drugs 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Perdicaris, S.; Vlachogianni, T.; Valavanidis, A. Bioactive natural substances from marine sponges: New developments and prospects for future pharmaceuticals. Nat. Prod. Chem. Res. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Pawlik, J.R.; McMurray, S.E. The emerging ecological and biogeochemical importance of sponges on coral reefs. Ann. Rev. Mar. Sci. 2020, 12, 315–337. [Google Scholar] [CrossRef]
- Maslin, M.; Gaertner-Mazouni, N.; Debitus, C.; Joy, N.; Ho, R. Marine sponge aquaculture towards drug development: An ongoing history of technical, ecological, chemical considerations and challenges. Aquacult. Rep. 2021, 21, 100813. [Google Scholar] [CrossRef]
- Aguila-Ramírez, R.N.; Hernández-Guerrero, C.J.; González-Acosta, B.; Id-Daoud, G.; Hewitt, S.; Pope, J.; Hellio, C. Antifouling activity of symbiotic bacteria from sponge Aplysina gerardogreeni. Int. Biodeterior. Biodegrad. 2014, 90, 64–70. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Edrada-Ebel, R.; Mohamed, G.A.; Youssef, D.T.; Wray, V.; Proksch, P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. Arkivoc 2008, 12, 164–171. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoschke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.; Proksch, P. Callyaerins A–F and H, new cytotoxic cyclic peptides from the indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 2010, 18, 4947–4956. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Zayed, M.F.; Sayed, H.M. Ingenines A and B. Two new alkaloids from the indonesian sponge Acanthostrongylophora ingens. Drug Res. 2015, 65, 361–365. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, S.R.; Mohamed, G.A. Ingenines C and D, new cytotoxic pyrimidine-β-carboline alkaloids from the indonesian sponge Acanthostrongylophora ingens. Phytochem. Lett. 2016, 18, 168–171. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A. Pyridoacridine alkaloids from deep-water marine organisms: Structural elucidation. Bull. Fac. Pharm. Cairo Univ. 2016, 54, 107–135. [Google Scholar] [CrossRef][Green Version]
- Sim, C.J.; Lee, K.J.; Kim, Y.A. twelve new species of two genera Smenospongia and Cacospongia (Demospongia: Dictyoceratida: Thorectidae) from Korea. J. Spec. Res. 2016, 5, 31–48. [Google Scholar] [CrossRef]
- McKay, M.J.; Carroll, A.R.; Quinn, R.J.; Hooper, J.N. 1,2-Bis (1H-indol-3-Yl) ethane-1,2-dione, an indole alkaloid from the marine sponge Smenospongia sp. J. Nat. Prod. 2002, 65, 595–597. [Google Scholar] [CrossRef]
- Hang, D.T.T.; Nhiem, N.X.; Tai, B.H.; Anh, H.L.T.; Yen, P.H.; Van Dau, N.; Van Minh, C.; Van Kiem, P. Merosesquiterpenes from marine sponge Smenospongia Cerebriformis. Vietnam J. Chem. 2017, 55, 153. [Google Scholar]
- Hu, J.; Schetz, J.A.; Kelly, M.; Peng, J.; Ang, K.K.; Flotow, H.; Leong, C.Y.; Ng, S.B.; Buss, A.D.; Wilkins, S.P. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod. 2002, 65, 476–480. [Google Scholar] [CrossRef]
- Prawat, H.; Mahidol, C.; Kaweetripob, W.; Wittayalai, S.; Ruchirawat, S. Iodo–sesquiterpene hydroquinone and brominated indole alkaloids from the Thai sponge Smenospongia sp. Tetrahedron 2012, 68, 6881–6886. [Google Scholar] [CrossRef]
- Djura, P.; Stierle, D.B.; Sullivan, B.; Faulkner, D.J.; Arnold, E.V.; Clardy, J. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (Ident. Polyfibrospongia) echina. J. Org. Chem. 1980, 45, 1435–1441. [Google Scholar] [CrossRef]
- Tymiak, A.A.; Rinehart Jr, K.L.; Bakus, G.J. Constituents of morphologically similar sponges: Aplysina and Smenospongia species. Tetrahedron 1985, 41, 1039–1047. [Google Scholar] [CrossRef]
- Tasdemir, D.; Bugni, T.S.; Mangalindan, G.C.; Concepción, G.P.; Harper, M.K.; Ireland, C.M. Cytotoxic bromoindole derivatives and terpenes from the Philippine marine sponge Smenospongia sp. Z. Naturforsch. C 2002, 57, 914–922. [Google Scholar] [CrossRef]
- Teta, R.; Della Sala, G.; Esposito, G.; Via, C.W.; Mazzoccoli, C.; Piccoli, C.; Bertin, M.J.; Costantino, V.; Mangoni, A. A joint molecular networking study of a Smenospongia sponge and a cyanobacterial bloom revealed new antiproliferative chlorinated polyketides. Org. Chem. Front. 2019, 6, 1762–1774. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Fattorusso, E.; Menna, M. A new antibiotic chloro-sesquiterpene from the Caribbean sponge Smenospongia aurea. Z. Naturforsch. B 1993, 48, 209–212. [Google Scholar] [CrossRef]
- Esposito, G.; Della Sala, G.; Teta, R.; Caso, A.; Bourguet-Kondracki, M.; Pawlik, J.R.; Mangoni, A.; Costantino, V. Chlorinated thiazole-containing polyketide-peptides from the Caribbean sponge Smenospongia conulosa: Structure elucidation on microgram scale. Eur. J. Org. Chem. 2016, 2016, 2871–2875. [Google Scholar] [CrossRef]
- Shen, Y.; Liaw, C.; Ho, J.; Khalil, A.T.; Kuo, Y. Isolation of aureol from Smenospongia sp. and cytotoxic activity of some aureol derivatives. Nat. Prod. Res. 2006, 20, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, Y.; Faulkner, D.J.; Steiner, J.L.R.; Corcoran, E.; Clardy, J. Smenochromenes, unusual macrocyclic sesquiterpene hydroquinone derivatives from a Seychelles sponge of the genus Smenospongia. J. Org. Chem. 1991, 56, 6271–6274. [Google Scholar] [CrossRef]
- Kondracki, M.; Guyot, M. Biologically active quinone and hydroquinone sesquiterpenoids from the sponge Smenospongia sp. Tetrahedron 1989, 45, 1995–2004. [Google Scholar] [CrossRef]
- Van Kiem, P.; Hang, D.T.; Nhiem, N.X.; Tai, B.H.; Anh, H.L.T.; Van Cuong, P.; Quang, T.H.; Van Minh, C.; Van Dau, N.; Kim, Y. Sesquiterpene derivatives from marine sponge Smenospongia cerebriformis and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2017, 27, 1525–1529. [Google Scholar] [CrossRef]
- Hang, D.T.T.; Nhiem, N.X.; Tai, B.H.; Anh, H.L.T.; Yen, P.H.; Van Dau, N.; Van Minh, C.; Van Kiem, P. Sesquiterpene phenols from marine sponge Smenospongia cerebriformis. Vietnam J. Chem. 2017, 55, 148. [Google Scholar]
- Bourguet-Kondracki, M.; Guyot, M. A new sesquiterpene tetronic acid derivative from the marine sponge Smenospongia sp. Tetrahedron Lett. 1999, 40, 3149–3150. [Google Scholar] [CrossRef]
- Kwak, C.; Jin, L.; Han, J.H.; Han, C.W.; Kim, E.; Cho, M.; Chung, T.; Bae, S.; Jang, S.B.; Ha, K. Ilimaquinone induces the apoptotic cell death of cancer cells by reducing pyruvate dehydrogenase kinase 1 activity. Int. J. Mol. Sci. 2020, 21, 6021. [Google Scholar] [CrossRef]
- Hwang, I.H.; Oh, J.; Zhou, W.; Park, S.; Kim, J.; Chittiboyina, A.G.; Ferreira, D.; Song, G.Y.; Oh, S.; Na, M. Cytotoxic activity of rearranged drimane meroterpenoids against colon cancer cells via down-regulation of β-catenin expression. J. Nat. Prod. 2015, 78, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Bourguet-Kondracki, M.; Martin, M.; Guyot, M. Smenoqualone a novel sesquiterpenoid from the marine sponge Smenospongia sp. Tetrahedron Lett. 1992, 33, 8079–8080. [Google Scholar] [CrossRef]
- Huyen, L.T.; Hang, D.T.; Nhiem, N.X.; Tai, B.H.; Anh, H.L.T.; Quang, T.H.; Yen, P.H.; Van Minh, C.; Van Dau, N.; Van Kiem, P. Sesquiterpene quinones and diterpenes from Smenospongia cerebriformis and their cytotoxic activity. Nat. Prod. Commun. 2017, 12, 1934578X1701200402. [Google Scholar] [CrossRef]
- Kochanowska, A.J.; Rao, K.V.; Childress, S.; El-Alfy, A.; Matsumoto, R.R.; Kelly, M.; Stewart, G.S.; Sufka, K.J.; Hamann, M.T. Secondary metabolites from three Florida sponges with antidepressant activity. J. Nat. Prod. 2008, 71, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Murayama, T.; Ishibashi, M.; Kosuge, S.; Takamatsu, M.; Ohizumi, Y.; Kobayashi, H.; Ohta, T.; Nozoe, S.; Takuma, S. Hyrtiosins A and B, new indole alkaloids from the okinawan marine sponge Hyrtios erecta. Tetrahedron 1990, 46, 7699–7702. [Google Scholar] [CrossRef]
- Swain, S.P.; Mohanty, S. Imidazolidinones and imidazolidine-2,4-diones as antiviral agents. ChemMedChem 2019, 14, 291–302. [Google Scholar] [CrossRef]
- Dai, J.; Jiménez, J.I.; Kelly, M.; Barnes, S.; Lorenzo, P.; Williams, P. Dictazolines A and B, bisspiroimidazolidinones from the marine sponge Smenospongia cerebriformis. J. Nat. Prod. 2008, 71, 1287–1290. [Google Scholar] [CrossRef]
- Hwang, I.H.; Oh, J.; Kochanowska-Karamyan, A.; Doerksen, R.J.; Na, M.; Hamann, M.T. A novel natural phenyl alkene with cytotoxic activity. Tetrahedron Lett. 2013, 54, 3872–3876. [Google Scholar] [CrossRef]
- Le, T.H.; Hang, D.T.T.; Nhiem, N.X.; Yen, P.H.; Anh, H.L.T.; Quang, T.H.; Tai, B.H.; Van Dau, N.; Van Kiem, P. Naphtoquinones and sesquiterpene cyclopentenones from the sponge Smenospongia cerebriformis with their cytotoxic activity. Chem. Pharm. Bull. 2017, 65, 589–592. [Google Scholar]
- Caso, A.; Mangoni, A.; Piccialli, G.; Costantino, V.; Piccialli, V. Studies toward the synthesis of smenamide a, an antiproliferative metabolite from smenospongia aurea: Total synthesis of ent-smenamide A and 16-epi-smenamide A. Acs Omega 2017, 2, 1477–1488. [Google Scholar] [CrossRef]
- Esposito, G.; Teta, R.; Della Sala, G.; Pawlik, J.R.; Mangoni, A.; Costantino, V. Isolation of smenopyrone, a bis-γ-pyrone polypropionate from the Caribbean sponge Smenospongia aurea. Mar. Drugs 2018, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Caso, A.; Laurenzana, I.; Lamorte, D.; Trino, S.; Esposito, G.; Piccialli, V.; Costantino, V. Smenamide A analogues. synthesis and biological activity on multiple myeloma cells. Mar. Drugs 2018, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Via, C.W.; Glukhov, E.; Costa, S.; Zimba, P.V.; Moeller, P.D.; Gerwick, W.H.; Bertin, M.J. The metabolome of a cyanobacterial bloom visualized by MS/MS-based molecular networking reveals new neurotoxic smenamide analogs (C. D. and E). Front. Chem. 2018, 6, 316. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jeong, W.; Wang, N.; Lee, H.; Sim, C.J.; Oh, K.; Shin, J. Scalarane sesterterpenes from the sponge Smenospongia sp. J. Nat. Prod. 2008, 71, 1866–1871. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Wang, M.; Wang, J.; Wu, Y. A six-step synthetic approach to marine natural product (-)-aureol. Tetrahedron Lett. 2018, 59, 945–948. [Google Scholar] [CrossRef]
- Hwang, B.; Rho, J. Scalaran-type sesterterpenes from a marine sponge Smenospongia species showing the AMPK sctivation. J. Korean Magn. Reson. Soc. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Teta, R.; Irollo, E.; Della Sala, G.; Pirozzi, G.; Mangoni, A.; Costantino, V. Smenamides A and B, chlorinated peptide/polyketide hybrids containing a dolapyrrolidinone unit from the Caribbean sponge Smenospongia aurea. evaluation of their role as leads in antitumor drug research. Mar. Drugs 2013, 11, 4451–4463. [Google Scholar] [CrossRef]
- Esposito, G.; Teta, R.; Miceli, R.; Ceccarelli, L.S.; Della Sala, G.; Camerlingo, R.; Irollo, E.; Mangoni, A.; Pirozzi, G.; Costantino, V. Isolation and assessment of the in vitro anti-tumor activity of smenothiazole A and B, chlorinated thiazole-containing peptide/polyketides from the Caribbean sponge, Smenospongia aurea. Mar. Drugs 2015, 13, 444–459. [Google Scholar] [CrossRef]
- Rho, J.; Lee, H.; Shin, H.J.; Ahn, J.; Kim, J.; Sim, C.J.; Shin, J. New sesterterpenes from the sponge Smenospongia sp. J. Nat. Prod. 2004, 67, 1748–1751. [Google Scholar] [CrossRef]
- Wright, A.E.; Rueth, S.A.; Cross, S.S. An antiviral sesquiterpene hydroquinone from the marine sponge Strongylophora hartmani. J. Nat. Prod. 1991, 54, 1108–1111. [Google Scholar] [CrossRef]
- Carballelra, N.M.; Emiliano, A.; Rodriguez, J.; Reyes, E.D. Isolation and characterization of novel 2-hydroxy fatty acids from the phospholipids of the sponge Smenospongia aurea. Lipids 1992, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Abd-Alla, H.I.; Hassan, A.Z.; Aly, H.F.; Ghani, M.A. Chemical characterization, antioxidant and inhibitory effects of some marine sponges against carbohydrate metabolizing enzymes. Org. Med. Chem. Lett. 2012, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Zjawiony, J.K. Aplysinopsins-marine indole alkaloids: Chemistry, bioactivity and ecological significance. Mar. Drugs 2009, 7, 166–183. [Google Scholar] [CrossRef]
- Caso, A.; Esposito, G.; Della Sala, G.; Pawlik, J.R.; Teta, R.; Mangoni, A.; Costantino, V. Fast detection of two smenamide family members using molecular networking. Mar. Drugs 2019, 17, 618. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, M.; Guyot, M. Smenospongine: A cytotoxic and antimicrobial aminoquinone isolated from Smenospongia sp. Tetrahedron Lett. 1987, 28, 5815–5818. [Google Scholar] [CrossRef]
- Do, M.T.; Na, M.; Kim, H.G.; Khanal, T.; Choi, J.H.; Jin, S.W.; Oh, S.H.; Hwang, I.H.; Chung, Y.C.; Kim, H.S. Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to trail-induced apoptosis through activation of ROS-ERK/p38 MAPK–CHOP signaling pathways. Food Chem. Toxicol. 2014, 71, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Atas, E.; Oberhuber, M.; Kenner, L. The implications of PDK1–4 on tumor energy metabolism, aggressiveness and therapy resistance. Front. Oncol. 2020, 10, 583217. [Google Scholar] [CrossRef]
- Son, Y.; Lim, D.; Park, S.; Song, I.; Kim, J.; Shin, S.; Jang, H.; Liu, K.; Yuseok, O.; Song, G. Ilimaquinone inhibits neovascular age-related macular degeneration through modulation of Wnt/Β-catenin and p53 pathways. Pharmacol. Res. 2020, 161, 105146. [Google Scholar] [CrossRef]
- López, M.D.; Quiñoá, E.; Riguera, R.; Omar, S. Dactyltronic acids from the sponge Dactylospongia elegans. J. Nat. Prod. 1994, 57, 992–996. [Google Scholar] [CrossRef]
- Rosa, C.P.; Kienzler, M.A.; Olson, B.S.; Liang, G.; Trauner, D. Total synthesis of smenochromene B through ring contraction. Tetrahedron 2007, 63, 6529–6534. [Google Scholar] [CrossRef]
- Lin, Z.; Torres, J.P.; Ammon, M.A.; Marett, L.; Teichert, R.W.; Reilly, C.A.; Kwan, J.C.; Hughen, R.W.; Flores, M.; Tianero, M.D. A bacterial source for mollusk pyrone polyketides. Chem. Biol. 2013, 20, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Yao, L.; Li, J.; Gavagnin, M.; Guo, Y. Marine bis-Γ-pyrone polypropionates of onchidione family and their effects on the XBP1 gene expression. Bioorg. Med. Chem. Lett. 2018, 28, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Segraves, N.L.; Crews, P. Investigation of brominated tryptophan alkaloids from two Thorectidae sponges: Thorectandra and Smenospongia. J. Nat. Prod. 2005, 68, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Antifouling terpenes and steroids against barnacle larvae from marine sponges. Biofouling 1997, 11, 283–291. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.R.M.; Fadil, S.A.; Fadil, H.A.; Hareeri, R.H.; Abdallah, H.M.; Mohamed, G.A. Genus Smenospongia: Untapped Treasure of Biometabolites—Biosynthesis, Synthesis, and Bioactivities. Molecules 2022, 27, 5969. https://doi.org/10.3390/molecules27185969
Ibrahim SRM, Fadil SA, Fadil HA, Hareeri RH, Abdallah HM, Mohamed GA. Genus Smenospongia: Untapped Treasure of Biometabolites—Biosynthesis, Synthesis, and Bioactivities. Molecules. 2022; 27(18):5969. https://doi.org/10.3390/molecules27185969
Chicago/Turabian StyleIbrahim, Sabrin R. M., Sana A. Fadil, Haifa A. Fadil, Rawan H. Hareeri, Hossam M. Abdallah, and Gamal A. Mohamed. 2022. "Genus Smenospongia: Untapped Treasure of Biometabolites—Biosynthesis, Synthesis, and Bioactivities" Molecules 27, no. 18: 5969. https://doi.org/10.3390/molecules27185969
APA StyleIbrahim, S. R. M., Fadil, S. A., Fadil, H. A., Hareeri, R. H., Abdallah, H. M., & Mohamed, G. A. (2022). Genus Smenospongia: Untapped Treasure of Biometabolites—Biosynthesis, Synthesis, and Bioactivities. Molecules, 27(18), 5969. https://doi.org/10.3390/molecules27185969